Abstract
It may need to pay attention to the sustention of moderate cardiotoxicity and delayed elevation of plasma 10‐hydroxynortriptyline level in severe amitriptyline overdose case.
Keywords: cardiopulmonary arrest, disposition, ischemic‐reperfusion, severe overdose, tricyclic antidepressants
It may need to pay attention to the sustention of moderate cardiotoxicity and delayed elevation of plasma 10‐hydroxynortriptyline level in severe amitriptyline overdose case.

1. INTRODUCTION
In an amitriptyline (AT) overdose case with brief cardiopulmonary arrest, the concentrations of AT and metabolites were measured. Consequently, physiological damage by ischemic‐reperfusion was not suggested to affect disposition of AT and nortriptyline (NT). However, the level of 10‐hydroxynortriptyline (10‐OH‐NT) was belatedly elevated, suggested as a cause of sustention of moderate cardiotoxicity.
AT overdose is frequent among tricyclic antidepressants (TCA)‐related toxicities and more fatal than overdose of other antidepressants, including selective serotonin reuptake inhibitors and noradrenergic and specific serotonin antidepressants. 1 Followings are the pharmacological properties that are reported as toxic effects of TCA: (a) inhibition of norepinephrine reuptake at nerve terminals; (b) direct blockade of α‐adrenergic receptors; (c) membrane stabilizing or quinine‐like effect on the myocardium; and (d) anticholinergic action. 2 Based on these effects, a patient with AT toxicity manifests several clinical complications including sinus tachycardia, prolonged QRS/QTc duration, vasodilation, hypotension, cardiogenic shock, ventricular fibrillation/tachycardia, coma, drowsiness, delirium, respiratory depression, and others. 2 These symptoms are more likely to occur at total blood concentrations of AT and NT >1000 ng/mL or total concentrations of NT and 10‐OH‐NT >300 ng/mL. 3 , 4 , 5 Regarding TCA, plasma concentrations >450 ng/mL tend to show cognitive or behavioral toxicities 6 and >2000 or 3000 ng/mL are fatal, 7 , 8 though therapeutic range is from 50 to 300 ng/mL. 6 Therefore, blood concentrations of AT, NT, and 10‐OH‐NT may provide useful information in assessing the level of toxicity and in predicting subsequent clinical outcomes in patients following an AT overdose. In drug overdose cases, information regarding ingested dose is often sparse and thus a poor predictor of clinical outcome. 2 Therefore, blood concentration data for the drug in question is extremely valuable.
Cardiopulmonary arrest (CPA) sometimes occurs in AT overdose cases. The resuscitation following CPA is often accompanied with ischemic‐reperfusion, during which reactive oxygen species are derived, inflicting injury to living grafts or cells. 9 Injury to hepatocytes or intestinal tissues can result in dysfunctional or unusual protein expression of cytochrome P450 (CYP) 2C19 or CYP2D6, alteration of enterohepatic recirculation, or a dysfunctional elimination process, which can in turn affect the metabolism, distribution, and elimination of AT, NT, and 10‐OH‐NT, resulting in alterations in the overall disposition and pharmacokinetic profile. 10 These alterations in drug disposition may also change the occurrence of both, the onset and duration of clinical features of toxicity in patients. Nonetheless, the effect of CPA on subsequent drug disposition and its clinical effects are rarely investigated and reported, despite numerous reports of AT overdose. 3 , 11 , 12
In this report, we measured the blood levels of AT, NT, and 10‐OH‐NT and evaluated the influence of CPA on the pharmacokinetics of these compounds in a patient who had taken a severe overdose of AT. We also evaluated the decontamination effect of activated charcoal (AC) to evaluate its effects in cases such as this with CPA.
2. CASE DESCRIPTION
A 55‐year‐old man (weight, 70.1 kg) with impaired awareness was found by his roommate in a warm room in his own house and transferred to our hospital. Rescue crews found 280 empty pills of 25 mg AT [corresponding to a total amount of 7 g (99.8 mg/kg)] beside him. A urine‐screening test using a commercial test kit (Triage® DOA, Sysmex Corp., Kobe, Japan) indicated TCA presence. Additionally, concurrent use of chlorpromazine, duloxetine, rabeprazole, trazodone, zolpidem tartrate, nitrazepam, and magnesium oxide was suspected. However, there was no evidence of excessive ingestion of these drugs, and the patient did not present with toxicity relevant to these drugs. Considering these observations and following comprehensive clinical assessments, we suspected that he likely ingested a massive dose of AT. The elapsed time of ingestion of the overdose could not be exactly estimated because the patient's awareness was impaired and there was no information available about anyone who had contacted him during the 12‐hours period before he was found. The roommate told us that she found him in a coma 12 hours after her last contact with him. Unfortunately, we did not have any other means of knowing if he had any pre‐existing disorders, other than depression. His comorbidity and past medical history were not available. On admission, the Glasgow Coma Scale (GCS) score was 3 (E1; V1; M1); systolic/diastolic blood pressure, 101/62 mm Hg; oxygen saturation, 94%; body temperature, 38.7°C; heart rate, 120/min; respiration rate, 20/min; blood pH, 7.022; QTc interval, 610 ms; and QRS interval, 270 ms (Figure 1A). Other relevant clinical data are included in the supporting information (Table S1). Atrial fibrillation (AF) with ventricular aberration or premature ventricular contraction, complete right bundle branch block, escape rhythm, and abnormal T wave were observed on the electrocardiogram (ECG; Figure 1A).
Figure 1.

Representative electrocardiograms obtained during hospitalization. (A) On admission; (B) 10 h later; (C) 12 h later; (D) 15 h later; (E) 60 h later; (F) 112 h later
Immediately after admission, a CPA following 10 seconds of clonus occurred, and 20 minutes later, he was resuscitated; however, his awareness remained impaired. The patient was thereafter intubated and treated for shock‐like hemodynamic status with the initiation of an infusion of 0.30 mg/h (approximately 0.07 μg/kg/min) of adrenaline, 0.60 mg/h of (approximately 0.14 μg/kg/min) of noradrenaline, and 15 mg/h (approximately 3.60 μg/kg/min) of dopamine. Totally, 1.1 mg/h (approximately 0.26 μg/kg/min) of adrenaline, 0.70 mg/h (approximately 0.17 μg/kg/min) of noradrenaline, and 30 mg/h (approximately 7.13 μg/kg/min) of dopamine were administered during the subsequent 11 hours period. The blood was alkalized by administering sodium bicarbonate (500 and 120 mL/d on the first and second hospital day, respectively). Additionally, 50 g of AC was repeatedly administered (five times in total) at 3, 14, 19, 27, and 35 hours after admission (Figure 1). Gastrointestinal decontamination other than AC administration was not conducted.
Approximately 15 hours after resuscitation from CPA, the drastically elongated QTc and QRS intervals were shortened to their near‐normal values (441 and 128 ms, respectively, Figure 1D), although their values remained near the upper limits of normal for several more hours. AF with ventricular aberration or premature ventricular contraction, nonspecific intraventricular conduction delay and temporal escape rhythm were also observed at some time point (Figure 1C,D). Clinical laboratory parameters of liver and renal function were elevated during the initial 10 hours; subsequently, the parameter values reduced (Figure S1). However, these values persistently remained at higher than normal levels, although there were no significant toxic effects observed. Approximately 60 hours after admission, the patient's awareness improved [GCS score of 14 (E4; V4; M6)]. The ECG showed the indications of sinus rhythm, although nonspecific intraventricular conduction delay was sometimes observed (Figure 1E). The creatinine clearance was almost normalized (approximately 80 mL/min) at 72 hours after admission. Nutritional provision was started with a small amount of enteral nutrition sometime after the end of catecholamine administration. During the 7th and 9th days of hospitalization, delirium was observed; however, the patient did not show any signed of orientation. ECG at 112 hours after admission indicated that sinus rhythm was maintained (Figure 1F), although premature ventricular contraction was infrequently observed. On the 10th day, he was transferred to the medical psychiatry unit. In the unit, the patient reported transient suicidal feelings that gradually dissipated. On the 23rd day, he was discharged.
The plasma concentrations of AT at 14, 45, and 131 hours after admission and those of NT and its hydroxyl metabolites at 45 and 131 hours after admission were measured by liquid chromatography‐tandem mass spectrometry (Figure 2). Both AT and NT concentrations decreased over time after resuscitation following CPA. In contrast, the concentration of 10‐OH‐NT increased during this period. The elimination half‐lives of AT and NT estimated after 45 hours of admission were approximately 35 and 89 hours, respectively.
Figure 2.
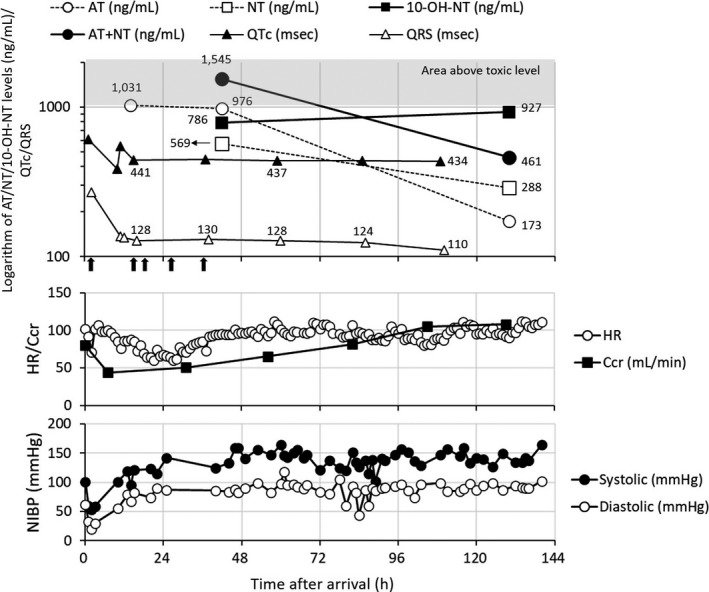
Time profiles of plasma levels of AT and its metabolites, QTc, QRS, heart rate, creatinine clearance, and blood pressure during the monitoring interval. AT, amitriptyline; NT, nortriptyline; 10‐OH‐NT, 10‐hydroxynortriptyline; HR, heart rate; Ccr, creatinine clearance; NIBP, noninvasive blood pressure. The black‐filled arrows under the x‐axis in top panel indicate AC administration at 3, 14, 19, 27, and 35 h after admission. The shaded area means the one above toxic concentration
3. DISCUSSION
In this overdose case with AT, AC was repeatedly administered (five times in total), following resuscitation 20 minutes after CPA. The subsequent concentration levels of AT were, then, reduced compared to the initial concentration. The repeated AC administration can complex with the TCA (doxepin) released following gradual disaggregation of the complex of doxepin with initially administered AC, thus suppressing the subsequent absorption of parent drug and metabolites secreted into the gastrointestinal tract. 13 This study also reported the repeated dosing effect of AC was observed despite the delay of three or more hours in AC administration following doxepin dosing. Although the delay between AT ingestion and AC initiation could not be confirmed in our case report, the initial measured concentration of AT (ie, 1031 ng/mL) was reduced after the repetitive AC administration. Thus, repeated AC administration and/or the clearance of the drug from the patient's system may have contributed to the reduction of AT concentrations. However, the reduction in AT concentrations between 14 and 45 hours after admission was extremely low (only 55 ng/mL). Additionally, the elimination half‐life estimated from this reduction was approximately 390 hours, while another study on AT overdose patients reported a half‐life of <10 hours when patients were treated with repeated AC administration. 14 There are several possible reasons for the discrepancy and slight reduction of AT concentration, including interruption of patient's individual drug clearance because of cellular damage by ischemic‐reperfusion; saturation of metabolic enzymes by the excessive amount of AT ingested; poor metabolism due to genetic polymorphism in CYP2C19 and CYP2D6, or anticholinergic suppression of elimination by AT and NT. Although it is unclear how the repeated AC administration and patient's clearance system effectively functioned, they may have at least partly contributed to the reduction of AT concentration. Thus, the further increase of the subsequent AT concentration would have not been shown. The repeated AC administration may have functioned as suppressing the elevation of peak concentration of AT, rather than accelerating the elimination process. Repetitive AC may have suppressed the potential increase in AT levels that could occur if the complex formed with the initial dose of AC is disaggregated. Additionally, AC may have also prevented the subsequent absorption/reabsorption of AT and its metabolites following their secretions into the gastrointestinal tract, as AT has been known to undergo enterohepatic circulation. 14 While further studies are needed, we suggest that repeated AC administration is a possible optional treatment in severe cases of AT overdose, even if CPA is presented and several hours have elapsed from the time of overdose.
A significant shortening of QRS interval from 124 to 110 ms was observed after approximately 72 hours from admission. Simultaneously, the total concentrations of AT and NT were estimated to be below the toxic range (total of 1000 ng/mL), suggesting the importance of monitoring the total concentrations of AT and NT in AT overdose cases. However, despite the reduction in total concentrations of AT and NT to less than toxic levels at 110 hours after admission, QTc and QRS intervals remained relatively high values. The QRS interval, particularly, did not fall below the upper limit of normal (100 ms). In parallel with these cardiac effects, elevation of the concentration of 10‐OH‐NT was observed, although the creatinine clearance recovered to a normal level, suggesting that 10‐OH‐NT elimination was not impaired. 15 A previous study demonstrates the cardiotoxic potential of 10‐OH‐NT, although it was less toxic than NT. 16 Additionally, the total concentrations of 10‐E‐hydroxynortriptyline and NT of > 300 ng/mL were associated with QRS prolongation. 4 Therefore, we speculated that the high levels of 10‐OH‐NT may have contributed to this sustained prolonged QTc and QRS intervals. Several factors may have contributed to the delayed elevation of 10‐OH‐NT concentration. One of these may be the genetic polymorphism in CYP2C19 and CYP2D6. Then, we estimated the phenotype of our patient, based on previous reports by Mifsud et al and Franssen et al, although the cases reported by Mifsud et al were treated with a normal dose (10 mg daily) of AT. 12 , 17 In a case with a “normal phenotype” of both CYP2C19 and CYP2D6 (ie, case example III in the report by Mifsud et al), the metabolite‐to‐parent rate (MPR; ie, NT/AT) was reported as 0.4. On the other hand, the case with concomitant use of only CYP2C19 inhibitor (ie, omeprazole) but with normal phenotype in both CYP2C19 and CYP2D6 showed the MPR as 0.2. However, another case with “normal phenotype'” in CYP2C19 and CYP2D6 (ie, case example V in the report of Mifsud et al) and concomitant use of inhibitors for CYP2C19 and CYP2D6 (omeprazole and paroxetine, respectively) indicated an MPR as 0.7, which was close to the MPR of our case (calculated as approximately 0.6). AT itself has an inhibitory effect on both CYP2C19 and CYP2D6, although CYP2D6 inhibition is reported to be clinically insignificant at a normal dose. 6 However, in the current case, a massive dose of AT was ingested, suggesting that the inhibitory effect on CYP2D6 may have somewhat functioned. In addition, considering the similarity of MPR between ours (approximately 0.6) and case's mentioned above (0.7), in which the patient had normal phenotype but was concomitantly administered with inhibitors for CYP2C19 and CYP2D6, our patient may also have possessed a normal phenotype and hers CYP2C19 and CYP2D6 may have somewhat inhibited due to the extremely high dose of AT. We also considered the possibility that the patient was a rapid metabolizer of CYP2C19, and this rapid activity was suppressed to normal levels due to the inhibitory effect of the large amount of AT ingested. However, as shown in case example I reported by Mifsud et al, the rapid metabolizer of CYP2C19 indicated a high MPR of 2.0, despite receiving a concurrent CYP2C19 inhibitor (ie, omeprazole). Therefore, with regard to CYP2C19, our patient (with MPR of 0.6) was not considered to correspond to a rapid metabolizer. Regarding CYP2D6, we focused on the ratio of 10‐OH‐NT/NT. In our case study, the ratio was calculated to be approximately 1.4 at 45 hours after admission, which was relatively close to the estimated one in case example III (approximately 3.5) and example IV (approximately 2.0) in the report by Mifsud et al 17 In these two cases, the CYP2D6 and CYP2C19 phenotypes both identified as normal, although the case example IV concomitantly received a CYP2C19 inhibitor (omeprazole). Another case (case example V in the report by Mifsud et al) with concurrent use of CYP2D6 inhibitor (paroxetine) indicated a lower 10‐OH‐NT/NT ratio (approximately 0.3), while a different case with ultra‐rapid metabolism in CYP2D6 indicated a higher 10‐OH‐NT/NT ratio (approximately 8.0). These estimates were far from the 10‐OH‐NT/NT ratio in our patient. Taken together, our analysis indicates that the patient may have possessed a “normal phenotype” in CYP2D6 as well. Levels of AT and its metabolite (ie, NT) following AT overdose may inhibit both CYP2C19 and CYP2D6 in the initial phase. Then, as concentrations of AT and NT decreased with time, the inhibitory effects on CYP may have gradually weakened, while concentrations of 10‐OH‐NT may have been elevated in a delayed manner. Besides, other factors including saturation of metabolic enzymes, dysfunction of metabolic enzymes or elimination systems by ischemic‐reperfusion damage, insufficient alkalization of urine or anticholinergic effect of AT, might have contributed in a complex manner to the delayed elevation of 10‐OH‐NT levels. Although some potential drugs that inhibit CYP2C19 (ie, duloxetine and chlorpromazine) seemed to have been prescribed, their inhibitory effects can be ignored because there was no evidence of their massive ingestion, and the patient did not present relevant toxic effects. The delayed elevation of 10‐OH‐NT levels over 100 hours after admission was also indicated in a fatal case with AT overdose by Franssen et al, 12 supporting our finding. Monitoring the level of 10‐OH‐NT may also be favorable, if possible, in severe AT overdose cases because it has toxic effects on cardiac function and its concentration can be elevated in later.
In this case report, the elimination half‐life of AT and NT was calculated to be approximately 34 and 88 hours, respectively, from the levels measured at 45 and 131 hours. A previous study reported that the elimination half‐lives of nine cases with AT overdose ranged from 15 to 43 hours in patients treated with gastric lavage and 50 g of AC. 18 Even at normal doses, the elimination half‐life of AT is reported to range from 10 to 46 hours. 6 The elimination half‐life of AT (ie, 45 hours) in our patient was approximately same with the ones reported. For NT in the case with normal dose, elimination half‐life has been reported to range from 13 to 90 hours, although shorter in ultra‐metabolizers (13‐35 hours). 6 A case of NT overdose reported an elimination half‐life of 50 hours based on levels measured from 50 to 110 hours after admission, 12 whereas another case showed a half‐life of 184 hours and sustained elevated levels of NT (468 ng/mL) until 6 days after admission. 19 Although both previous overdose cases were not treated with AC, the elimination half‐life of NT (ie, 88 hours) in our patient treated with AC was compatible with the reported ones. The elimination half‐life of 10‐OH‐NT could not be calculated in this study because its elimination phase was not observed during our evaluation period, although its half‐life has been reported to be approximately 8‐10 hours. 20 Thus, taken these, the damage, possibly induced by ischemic‐reperfusion, would have not induced any meaningful anomalous effect on the disposition of AT or NT, despite physiological damage was suspected by the sustained elevation of lactate dehydrogenase (Figure S1). 21 , 22 As long as CPA is recovered within approximately 20 minutes, as seen in our study, and appropriate treatment is provided, a patient with AT/NT overdose can survive and be managed clinically.
4. CONCLUSION
It would be desired to monitor not only the concentration of AT and NT but also those of its hydroxyl metabolites, especially in severe AT/NT overdose because 10‐OH‐NT has toxic effects on cardiac function and its blood concentration can increase behind the reduction of AT and NT concentrations. Although further studies are needed, repeated AC administration may be a possible optional treatment in severe cases of AT overdose, even after several hours have elapsed from overdose. If the patient experiences CPA, as long as appropriate treatment is provided, AT/NT‐poisoned patient can survivable and may be clinically managed like a typical AT/NT overdose case. These experiences would be beneficial for other workers if they encounter a similar AT overdose case with CPA.
CONFLICT OF INTEREST
No conflicts of interest have been declared.
AUTHORS’ CONTRIBUTION
MA, RT, and ST measured the concentrations of amitriptyline, nortriptyline, and 10‐hydroxynortriptyline and evaluated their relationship with the patient's clinical outcome. MY, KS, and MS provided technical support in quantification by a validated method. KA provided several useful medical considerations as an emergency physician. RT and SN provided several useful pharmaceutical considerations as pharmacists engaged in the treatment of this patient. SF, NS, and TH provided helpful guidance and pharmacokinetic expertise in the area of pharmaceutical sciences. All authors have read and approved the final manuscript.
ETHICAL APPROVAL
Through the provision of a chance to opt‐out, the study and reporting were approved by the ethics review board of our hospital (Approval #: k181033).
Supporting information
Supinfo
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for English language editing. Published with written consent of the patient.
Ando M, Tamura R, Nakasako S, et al. Plasma concentration of amitriptyline and metabolites after resuscitation from cardiopulmonary arrest following an overdose: A case report. Clin Case Rep.2021;9:805–811. 10.1002/ccr3.3656
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hawton K, Bergen H, Simkin S, et al. Toxicity of antidepressants: rates of suicide relative to prescribing and non‐fatal overdose. Br J Psychiatry. 2010;196(5):354‐358. 10.1192/bjp.bp.109.070219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kerr GW, McGuffie AC, Wilkie S. Tricyclic antidepressant overdose: a review. Emerg Med J. 2001;18(4):236‐241. 10.1136/emj.18.4.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hultén BA, Heath A, Knudsen K, Nyberg G, Starmark JE, Mårtensson E. Severe amitriptyline overdose: relationship between toxicokinetics and toxicodynamics. J Toxicol Clin Toxicol. 1992;30(2):171‐179. 10.3109/15563659209038629 [DOI] [PubMed] [Google Scholar]
- 4. Hedges JR, Otten EJ, Schroeder T, Tasset TJ. QRS duration in acute overdose of tricyclic antidepressants. N Engl J Med. 1986;314(15):988‐989. 10.1056/NEJM198604103141512 [DOI] [PubMed] [Google Scholar]
- 5. Biggs JT, Spiker DG, Petit JM, Ziegler VE. Tricyclic antidepressant overdose: incidence of symptoms. JAMA. 1977;238(2):135‐138. [PubMed] [Google Scholar]
- 6. Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151(6):737‐748. 10.1038/sj.bjp.0707253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schulz M, Schmoldt A. Therapeutic and toxic blood concentrations of more than 800 drugs and other xenobiotics. Pharmazie. 2003;58(7):447‐474. [PubMed] [Google Scholar]
- 8. Petit JM, Spiker DG, Ruwitch JF, Ziegler VE, Weiss AN, Biggs JT. Tricyclic antidepressant plasma levels and adverse effects after overdose. Clin Pharmacol Ther. 1977;21(1):47‐51. 10.1002/cpt197721147 [DOI] [PubMed] [Google Scholar]
- 9. Brass CA, Narciso J, Gollan JL. Enhanced activity of the free radical producing enzyme xanthine oxidase in hypoxic rat liver. Regulation and pathophysiologic significance. J Clin Invest. 1991;87(2):424‐431. 10.1172/JCI115013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryu S, Park S, Lee JH, et al. A Study on CYP2C19 and CYP2D6 Polymorphic Effects on Pharmacokinetics and Pharmacodynamics of Amitriptyline in Healthy Koreans. Clin Transl Sci. 2017;10(2):93‐101. 10.1111/cts.12451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paksu S, Duran L, Altuntas M, et al. Amitriptyline overdose in emergency department of university hospital: evaluation of 250 patients. Hum Exp Toxicol. 2014;33(9):980‐990. 10.1177/0960327113520019 [DOI] [PubMed] [Google Scholar]
- 12. Franssen EJF, Kunst PWA, Bet PM, Strack van Schijndel RJM, van Loenen AC, Wilhelm AJ. Toxicokinetics of nortriptyline and amitriptyline: two case reports. Ther Drug Monit. 2003;25(2):248‐251. 10.1097/00007691-200304000-00018 [DOI] [PubMed] [Google Scholar]
- 13. Scheinin M, Virtanen R, Iisalo E. Effect of single and repeated doses of activated charcoal on the pharmacokinetics of doxepin. Int J Clin Pharmacol Ther Toxicol. 1985;23(1):38‐42. [PubMed] [Google Scholar]
- 14. Swartz CM, Sherman A. The treatment of tricyclic antidepressant overdose with repeated charcoal. J Clin Psychopharmacol. 1984;4(6):336‐340. [PubMed] [Google Scholar]
- 15. Young RC, Alexopoulos GS, Dhar AK, Kutt H. Plasma 10‐hydroxynortriptyline and renal function in elderly depressives. Biol Psychiatry. 1987;22(10):1283‐1287. 10.1016/0006-3223(87)90039-4 [DOI] [PubMed] [Google Scholar]
- 16. Pollock BG, Everett G, Perel JM. Comparative cardiotoxicity of nortriptyline and its isomeric 10‐hydroxymetabolites. Neuropsychopharmacology. 1992;6(1):1‐10. [PubMed] [Google Scholar]
- 17. Mifsud Buhagiar L, Sammut C, Chircop Y, et al. Practical liquid chromatography‐tandem mass spectrometry method for the simultaneous quantification of amitriptyline, nortriptyline and their hydroxy metabolites in human serum. Biomed Chromatogr. 2019;33(12):e4679 10.1002/bmc.4679 [DOI] [PubMed] [Google Scholar]
- 18. Hultén BA, Heath A, Knudsen K, Nyberg G, Svensson C, Mårtensson E. Amitriptyline and amitriptyline metabolites in blood and cerebrospinal fluid following human overdose. J Toxicol Clin Toxicol. 1992;30(2):181‐201. 10.3109/15563659209038630 [DOI] [PubMed] [Google Scholar]
- 19. Elsamadisi P, Sclafani A, Eche IM. Delayed cardiotoxicity from a massive nortriptyline overdose requiring prolonged treatment. J Pharm Pract. 2020;33(4):543‐547. 10.1177/0897190019838700 [DOI] [PubMed] [Google Scholar]
- 20. Nordin C, Bertilsson L. Active hydroxymetabolites of antidepressants. Emphasis on E‐10‐hydroxy‐nortriptyline. Clin Pharmacokinet. 1995;28(1):26‐40. 10.2165/00003088-199528010-00004 [DOI] [PubMed] [Google Scholar]
- 21. Zhang S, Zhang R, Wu F, Li X. MicroRNA‐208a regulates H9c2 cells simulated ischemia‐reperfusion myocardial injury via targeting CHD9 through Notch/NF‐kappa B signal pathways. Int Heart J. 2018;59(3):580‐588. 10.1536/ihj.17-147 [DOI] [PubMed] [Google Scholar]
- 22. Chakraborty M, Kamath JV. Pharmacodynamic interaction of green tea extract with hydrochlorothiazide against ischemia‐reperfusion injury‐induced myocardial infarction. J Adv Pharm Technol Res. 2014;5(3):134‐139. 10.4103/2231-4040.137428 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supinfo
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


