Abstract
This report is the first to document TEN caused by nivolumab treatment in head and neck cancer. We believe this article can contribute significantly in understanding the principles of nivolumab treatment in patients with head and neck cancer.
Keywords: head and neck cancer, nivolumab, Stevens‐Johnson syndrome, toxic epidermal necrolysis
This report is the first to document TEN caused by nivolumab treatment in head and neck cancer. We believe this article can contribute significantly in understanding the principles of nivolumab treatment in patients with head and neck cancer.

1. INTRODUCTION
Nivolumab, an antiprogrammed death‐1 (PD‐1) antibody, is an immune‐checkpoint inhibitor. According to the results of the CheckMate‐141 study, which compared nivolumab and a physician‐selected treatment modality for recurrent/metastatic head and neck squamous cell carcinoma with a history of chemotherapy including platinum, nivolumab significantly increases the overall survival rate. 1 Considering the results of this report, nivolumab was approved in the United States in November 2016 and in Japan in March 2017 as a treatment modality for patients with recurrent/metastatic head and neck cancer, who had been previously treated with platinum‐containing drugs. Currently, the clinical benefits of nivolumab therapy have been recognized not only in head and neck cancers but also in other types of cancers such as malignant melanoma, non‐small cell lung cancer, renal cell cancer, gastric cancer, and Hodgkin's lymphoma. Various immune‐related adverse events associated with nivolumab have been reported, because the mechanism of action of the drug is different from that of conventional anticancer drugs. However, the frequency of severe skin complications is considerably less.
Toxic epidermal necrosis (TEN) is a disease with fever and widespread erythema, erosion, blisters, and mucosal rash on the skin. TEN is a rare, albeit serious disease with a mortality rate of approximately 30%. 2 We herein report a patient with squamous cell carcinoma of the tongue who developed TEN after nivolumab treatment.
2. CASE REPORT
A 76‐year‐old man with cancer of the right side of the tongue (cT3N2bM0) underwent hemiglossectomy of the right side and dissection of the right side of the neck followed by reconstruction using anterolateral thigh flap. Postoperative pathological examination showed pT3N2b and negative surgical margins and extranodal invasion. The patient was treated with 60 Gy/30 Fr postoperative radiation therapy, because a vertical margin of only 4 mm could be obtained. Five months after surgery, multiple cavitatory lesions were detected in the lungs in the computed tomography (CT) scans. Bronchoscopic biopsy revealed that the tongue cancer had metastasized to the lungs (Figure 1). Six months after the surgery, chemotherapy with cisplatin plus 5‐FU (PF, CDDP 60 mg/m2, 5‐FU 750 mg/m2, a reduced dosage was used due to renal dysfunction) was initiated. Considering the possibility of chronic obstructive pulmonary disease and cavitatory lesions in the lung, cetuximab was not administered. He developed febrile neutropenia 13 days after the initiation of PF treatment and was urgently hospitalized. CT scans showed the progression of the lung metastases. Considering the possibility of platinum resistance, the first dose of nivolumab (240 mg/body) was administered 7 months after the surgery and the second dose was administered 2 weeks later. A rash appeared on the trunk region on the 27th day after the administration of the first dose of Nivolumab. Since fever of more than 38°C also developed on the 28th day, we suspected drug eruption and antihistamines were administered to the patient. Due to the exacerbation of the skin rash and fever, he was hospitalized on the 29th day. On the 31st day, a dermatologist diagnosed the patient with Stevens‐Johnson syndrome (SJS) (Figure 2A). Pathological examination of a skin specimen showed the presence of necrotic keratinocytes and vacuolar degeneration in the epidermis (Figure 2B). There was no history of usage of any drug other than Nivolumab. Hence, nivolumab was suspected to be the cause of SJS. On the same day, steroid pulse therapy with 1000 mg methylprednisolone was initiated, and cefepime was administered to prevent secondary infection. Steroid pulse therapy was continued for 3 days, after which it was transferred to oral administration with 50 mg prednisolone. On the 35th day, the dermatologist diagnosed the patient with TEN considering the spread of erythema and erosion (Figure 2C). According to the diagnostic criteria for TEN, the presence of three major items (a) Blisters and erosions exceeding 10% of the body surface area, (b) Fever, (c) Staphylococcal scalded skin syndrome, toxic shock syndrome, contagious impetigo, acute generalized exanthematous pustulosis, or autoimmune blistering) and of four sub‐items (a) Flat, atypical target legions initially, (b) Mucosal lesions at the skin mucosa transition, (c) Objective generalized symptoms, subjective malaise, or eating disorders, and (d) Histopathological changes in the epidermis such as necrosis 200 times more than 10 epidermal cell necrosis in a visual field) was confirmed. From the 37th day, intravenous immunoglobulin 40 mg/kg/d was administered for 5 days. From the 43rd day, symptoms improved. On the 49th day, the reduction in dose of predonin was initiated, and on the 66th day, treatment with predonin was completed. All skin eruptions had epithelialized, and only pigmentations were evident. However, his respiratory condition worsened from the 42nd day, and treatment with morphine was initiated from the 44th day to relieve the respiratory distress. CT scan advised on the 44th day showed increased lung metastatic lesions and pleural effusion (Figure 3). We believed that the deterioration of respiratory function occurred due to increase in cancerous lesions. Hence, a ventilator was not used. His respiratory condition continued to worsen, and he died on the 67th day. The clinical course of the case is depicted in Figure 4.
Figure 1.
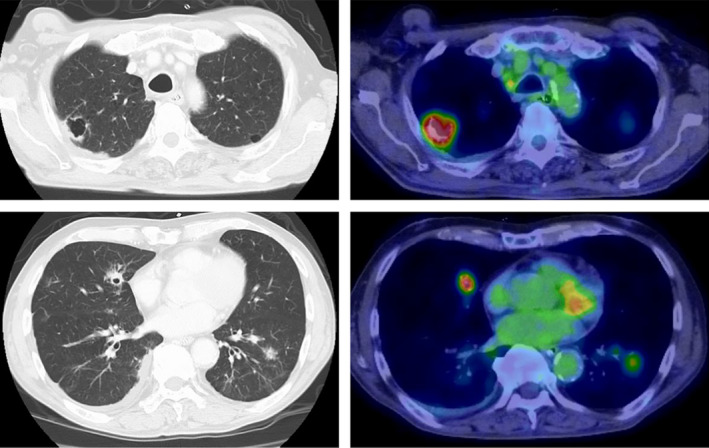
Computed tomography and positron emission tomography (PET) images of the lung metastases. Multiple metastases with cavity formation in the lungs. PET also shows fluorodeoxyglucose accumulation at the same sites
Figure 2.
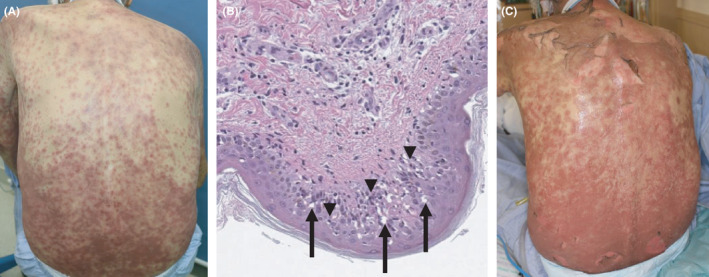
Skin photograph and histological image at the onset of Stevens‐Johnson syndrome (SJS)/ Toxic epidermal necrolysis (TEN). A, Skin photograph at the onset of SJS. B, Necrotic changes in epidermal keratinocytes, vacuolar degeneration in the epidermal layer (arrow), and lymphocyte infiltration into the epidermal layer (arrow head). C, Skin photograph at the onset of TEN
Figure 3.
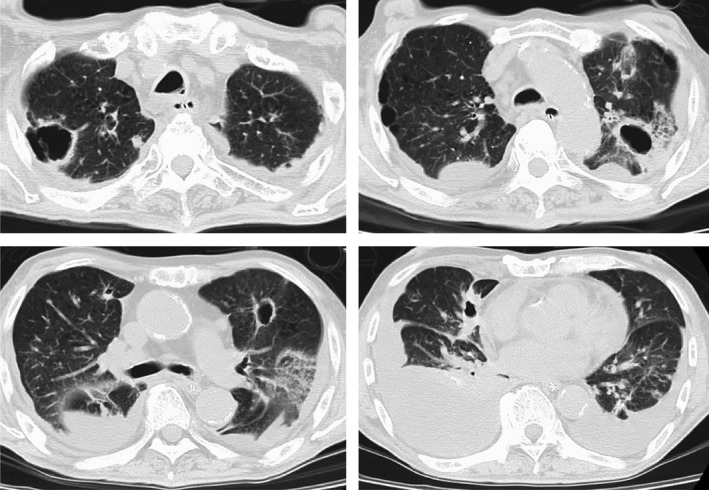
Computed tomography image of lungs on day 44. Increase in lung metastasis with cavitation and pleural effusion
Figure 4.
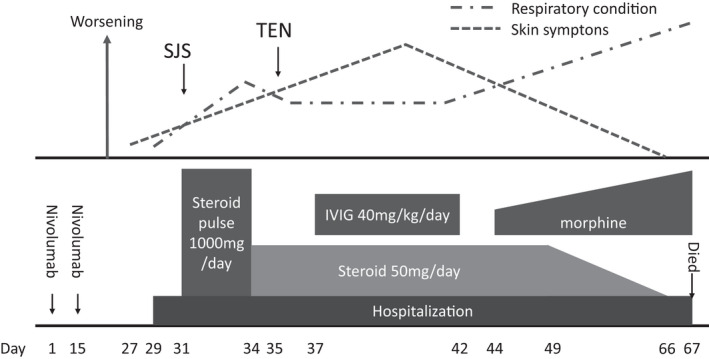
Schema of symptom transition. The upper row shows the course of the respiratory condition and skin symptoms of the patient. The bottom row outlines the details of the instituted treatment
3. DISCUSSION
SJS and TEN present as widespread erythema, erosion, blisters, mucosal rash, and fever. Frequently, they are caused by drugs, but can also be caused by infections from mycoplasma or certain viruses. 2 Apoptosis of epidermal and mucosal epithelial cells induced by CD8+ T cells is considered as the etiology of both SJS and TEN. However, the detailed mechanism of development of SJS and TEN has not been elucidated. These diseases are rare but serious. In particular, the mortality rate associated with the most severe form of TEN is as high as 30%. Most cases of TEN develop from SJS. According to the Japanese diagnostic criteria for SJS and TEN, SJS is diagnosed if erosion involves less than 10% body surface area, and TEN is diagnosed if it involves 10% or more. Our patient was initially diagnosed with SJS and subsequently with TEN after worsening of skin symptoms.
When SJS or TEN are caused by a drug, they develop within 1‐2 weeks from the beginning of drug therapy. In our patient, skin symptoms appeared on the 27th day after administration of the first dose of nivolumab. A relatively long time had passed from the administration of nivolumab until the appearance of skin symptoms. However, as no new drug therapy, apart from nivolumab, was ongoing, nivolumab was determined to be the causative drug for the onset of TEN. In ALDEN, an algorithm developed for determining the drug causality in SJS and TEN, a period of onset of 5 to 28 days after the drug administration is “suggestive,” which is consistent with the course of this patient. 3 However, we must understand that severe skin adverse events can occur even 4 weeks after drug administration.
SCORTEN is an assessment of the severity of SJS and TEN, and we performed a retrospective study on this case. 4 On admission, age and the presence of a malignancy were positive items, and the area of epidermal exfoliation, pulse, blood urea nitrogen (BUN), and blood glucose were negative items. HCO3 ‐ in the blood was not measured. As a result, SCORTEN in this case was 2 or 3, and the severity was judged to be at the mild to moderate level. In the RegiSCAR study, the mortality rate of acute SJS and TEN was determined to be 23%. 5 The acute cause of death is sepsis, which occurs due to secondary bacterial and fungal infections in the skin and mucous membrane lesions. Pneumonia is a major complication of SJS and TEN, with approximately half of the patients requiring a ventilator, which is often associated with a poor prognosis. 6
Our patient showed improvement in skin symptoms, but died due to worsening of the respiratory function. Increase in lung metastatic lesions and associated pleural effusion were the principal causes of respiratory deterioration. Furthermore, it was determined that respiratory function was further deteriorated due to pneumonia caused by SJS or TEN. However, it is difficult to arrange ventilators for patients with cancer that cannot be cured.
SJS and TEN caused by nivolumab have been reported in patients with melanoma, hepatocellular carcinoma, non‐small cell lung cancer, and lymphoma, but this is the first reported case of SJS or TEN associated with head and neck cancer. 7 , 8 , 9 , 10 SJS or TEN was not observed in adverse event reports in the CheckMate‐141 study. 1 In addition, only 2 cases of grade 3 or grade 4 skin adverse events were reported in the international randomized phase 3 study (n = 945) with nivolumab and ipilimumab for advanced melanoma. 11 Deaths caused by TEN have been reported with nivolumab use for metastatic melanoma and lymphoma. 7 , 9 The mechanism by which immune‐checkpoint inhibitors such as anti‐PD‐1 antibody and anticytotoxic T lymphocyte‐associated‐4 antibody cause SJS and TEN has not been elucidated. Several drugs such as antibacterial, antiepileptic, and nonsteroidal anti‐inflammatory drugs, and allopurinol are listed as high‐risk drugs for the development of SJS and TEN. Currently, molecular‐targeting drugs and immune‐checkpoint inhibitors used for cancer treatment are not classified as risk drugs but are expected to be assessed for risk after the documentation of adequate cases treated with these anticancer agents in the future.
In head and neck cancer, nivolumab is approved as a treatment modality for patients with platinum‐resistant recurrence/metastasis. Several patients show distant metastasis or local recurrence with a compromised general medical condition. Prior chemotherapy is also a factor that can affect the general condition. If SJS or TEN develops in such patients, it will be fatal. In addition, treatment guidelines for SJS and TEN recommend plasma‐exchange therapy in cases of resistance to steroid pulse therapy. However, plasma‐exchange therapy should be carefully used in patients with cancer. In our patient, steroid pulse therapy was not effective immediately, and hence, plasma‐exchange therapy was considered. However, due to the increase in lung metastatic lesions and worsening of the respiratory condition, plasma‐exchange therapy was not initiated, and intravenous immunoglobulin was administered. Infliximab, an antitumor necrosis factor‐α antibody, may be administered for severe skin adverse events, but no reports on its use in patients with adverse skin events caused by nivolumab have been documented. 11 It is necessary to determine a suitable treatment modality for TEN considering the prognosis and disease control status of each case.
With the progress of immunotherapy for cancer, many patients are treated with immunotherapeutic agents worldwide. In the future, the number of patients developing severe skin adverse events, such as SJS or TEN, is expected to increase. For immunotherapy, the patient's general medical condition should be evaluated to manage the adverse events by early detection and appropriate treatment planning.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
KK: involved in conceptualization and writing; DS, MS, YM, SH, TS, and NK: involved in investigation and review; MN, TH: involved in supervision and review.
Funding information
This study was supported by JSPS KAKENHI, 19K18795.
ETHICAL APPROVAL
Written informed consent was obtained from the patient in the Japanese language to publish his clinical details including his photograph.
ACKNOWLEDGMENTS
Published with written consent of the patient.
Koshizuka K, Sakurai D, Sunagane M, et al. Toxic epidermal necrolysis associated with nivolumab treatment for head and neck cancer. Clin Case Rep.2021;9:848–852. 10.1002/ccr3.3695
REFERENCES
- 1. Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous‐Cell Carcinoma of the Head and Neck. N Engl J Med. 2016;375(19):1856‐1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56(2):181‐200. [DOI] [PubMed] [Google Scholar]
- 3. Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN, an algorithm for assessment of drug causality in Stevens‐Johnson Syndrome and toxic epidermal necrolysis: comparison with case‐control analysis. Clin pharmacol Ther. 2010;88(1):60‐68. [DOI] [PubMed] [Google Scholar]
- 4. Guégan S, Bastuji‐Garin S, Poszepczynska‐Guigné E, Roujeau JC, Revuz J. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006;126(2):272‐276. [DOI] [PubMed] [Google Scholar]
- 5. Sekula P, Dunant A, Mockenhaupt M, et al. Comprehensive survival analysis of a cohort of patients with Stevens‐Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013;133(5):1197‐1204. [DOI] [PubMed] [Google Scholar]
- 6. Lerch M, Mainetti C, Terziroli Beretta‐Piccoli B, Harr T. Current perspectives on stevens‐johnson syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol. 2018;54(1):147‐176. [DOI] [PubMed] [Google Scholar]
- 7. Vivar KL, Deschaine M, Messina J, et al. Epidermal programmed cell death‐ligand 1 expression in TEN associated with nivolumab therapy. J Cutan Pathol. 2017;44(4):381‐384. [DOI] [PubMed] [Google Scholar]
- 8. Salati M, Pifferi M, Baldessari C, et al. Stevens‐Johnson syndrome during nivolumab treatment of NSCLC. Ann Oncol. 2018;29(1):283‐284. [DOI] [PubMed] [Google Scholar]
- 9. Griffin LL, Cove‐Smith L, Alachkar H, Radford JA, Brooke R, Linton KM. Toxic epidermal necrolysis (TEN) associated with the use of nivolumab (PD‐1 inhibitor) for lymphoma. JAAD Case Rep. 2018;4(3):229‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dasanu CA. Late‐onset Stevens–Johnson syndrome due to nivolumab use for hepatocellular carcinoma. J Oncol Pharm Pract. 2019;25(8):2052‐2055. [DOI] [PubMed] [Google Scholar]
- 11. Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(13):1270‐1271. [DOI] [PubMed] [Google Scholar]


