Abstract
Timely genetic testing leading to early diagnosis of A‐T is crucial due to its plethora of implications on clinical management, particularly in those who develop malignancies. Thus, clinicians have to be astute in identifying diagnostic clues of A‐T.
Keywords: ataxia‐telangiectasia, cancer genetics, cancer management
Timely genetic testing leading to early diagnosis of A‐T is crucial due to its plethora of implications on clinical management, particularly in those who develop malignancies. Thus, clinicians have to be astute in identifying diagnostic clues of A‐T.

1. INTRODUCTION
Ataxia‐telangiectasia (A‐T), a rare hereditary cancer syndrome, can present with a myriad of clinical manifestations. Here, we described a case whose diagnosis of A‐T was missed till a second malignancy was confirmed. We aim to highlight diagnostic clues of A‐T and discussed important considerations in management of malignancies in A‐T.
Ataxia‐telangiectasia (A‐T) (OMIM #208900) is a rare autosomal recessive disorder resulting from biallelic pathogenic variants in the Ataxia‐Telangiectasia mutated (ATM) gene (OMIM *607585). Classically, it is characterized by progressive cerebellar ataxia, cutaneous telangiectasia, immunodeficiency, cancer susceptibility and radiation sensitivity. 1 , 2 , 3 However, variant A‐T may have a myriad of presentations. The ATM gene encodes a serine/threonine protein kinase which plays a crucial role in the repair of DNA double‐stranded breaks 1 , 2 , 3 , 4 , 5 and when impaired leads to carcinogenesis. Studies estimate lifetime cancer risks of 25%‐40% 4 , 5 , 6 of both solid and hematological malignancies. 2 , 4 , 7 , 8 , 9 , 10 Here, we report a patient with sensorimotor polyneuropathy, metachronous T‐cell prolymphocytic leukemia (T‐PLL), and cervical carcinosarcoma who was eventually diagnosed with A‐T to highlight clinical pearls and important management considerations for clinicians.
2. CASE REPORT
A 34‐year‐old Chinese woman presented with urinary incontinence, intermittent abdominal discomfort, and menorrhagia. On physical examination, a necrotic bleeding vaginal mass was noted. Computed tomography (CT) of the abdomen and pelvis revealed a cervical lesion and right ovarian lesion measuring 8 × 7 cm and 9 × 6 cm, respectively Figure 1. Medical history was significant for possible cerebral palsy that was recently revised to possible Charcot‐Marie‐Tooth disease when she presented with progressively worsening weakness, with nerve conduction study and electromyography showing diffused sensorimotor axonal polyneuropathy. She was diagnosed with CD4/CD8 double‐positive T‐PLL Figure 2A with complex cytogenetics at age 31 after an incidental finding of leucocytosis. Bone marrow cytogenetics then showed an abnormal mosaic female chromosome analysis with a normal cell line and one with numerical and structural abnormalities. However, there were no deletions or missense variants involving the ATM locus 11q23, which is present in up to 65% of all cases of T‐PLL. 11 , 12 , 13 She was placed on expectant management given absence of cytopenia nor rapidly increasing lymphocytosis, B symptoms, lymphadenopathy, or end organ involvement, as per T‐PLL International Study Group (TPLL‐ISG) guidelines. 14 Moreover, given her comorbidities and functional status, she was a poor candidate for most cytotoxic treatments targeting T‐PLL or bone marrow transplant. Other comorbidities included type 2 diabetes mellitus, multiple ophthalmological issues and persistently raised alpha‐fetoprotein (AFP) with mild transaminitis since age 29 for which investigations were unyielding.
FIGURE 1.
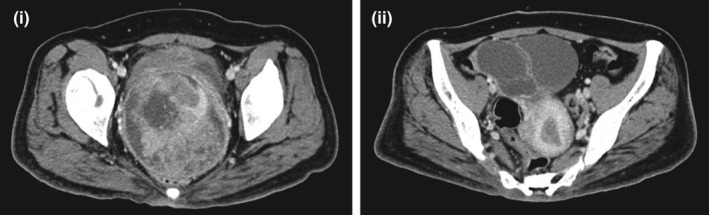
Computed tomography abdomen pelvis scan showing (i) Cervical tumor (ii) Ovarian metastases
FIGURE 2.

A, Mature‐looking T‐PLL lymphocyte with cytoplasmic blebs in peripheral blood film. B, Histology slides showing (i) Cervical adenosquamous carcinoma with complex glandular proliferation, papillae, and scattered keratinizing squamous whorls, (ii) Focal malignant cartilaginous nodules associated with cervical adenocarcinoma, (iii) Anastomosing cords of cells punctuated by small tubules, demonstrating cytoplasmic reactivity for synaptophysin on immunostain, and (iv) Ovarian metastasis with similar looking adenosquamous carcinoma with foci of keratinization (*) and some cells containing cytoplasmic mucin ( )
)
Tumor markers were normal apart from baseline elevated AFP: CEA 1.5 ug/L, CA 125 23.6 u/ml, Beta‐hCG < 0.6U/l, AFP 153 ug/L. Cervical biopsy showed squamous cell carcinoma (SCC) while right ovarian biopsy showed adenocarcinoma with focal mucinous differentiation. Our multidisciplinary consensus was that of at least FIGO stage IIB cervical SCC with a synchronous primary ovarian mucinous adenocarcinoma. Initial recommendation was made for definitive treatment with neoadjuvant chemotherapy followed by chemo‐radiotherapy for her cervical SCC and sequential debulking surgery for her ovarian adenocarcinoma. She received 2 cycles of paclitaxel (175 mg/m2) and carboplatin (AUC 5) at 3 weekly intervals but did not achieve adequate control of her symptoms of pain and per vagina bleeding. After restaging scans showed local progression of the ovarian mass, she underwent palliative open radical hysterectomy, bilateral salpingo‐oophorectomy, and bilateral pelvic lymphadenectomy. Histology revealed cervical carcinosarcoma with heterologous (cartilaginous) differentiation, predominantly comprised of adenosquamous carcinoma with focal neuroendocrine differentiation, admixed with a minor sarcomatous component. There was bilateral parametrium, upper vagina, lower uterine segment, and pelvic lymph node involvement. Histology of the right ovarian lesion revealed adenosquamous carcinoma, favoring metastasis from the cervical tumor as both had similar histology and immunoprofile Figure 2B.
In view of multiple primary cancers at a young age and uncertain underlying neurological condition, she was referred for genetic assessment upon her cervical cancer diagnosis. Born at term, independently ambulant and fully functional initially, she subsequently had difficulty walking and learning around age 9 and became wheelchair bound since age 23 Figure 3. Interview with patient's caregiver revealed that she was thought to have cerebral palsy, and her initial clinicians did not consider a diagnosis of A‐T. Unfortunately, specific details regarding her neurological deterioration and the workup then were not available as she was seen in a different institution. There was no family history of consanguinity nor developmental issues. Her father, a nonsmoker, died from lung cancer at age 47. Her paternal grandfather, a smoker, also died from lung cancer in his 30s while her paternal grandmother died from uterine cancer in her 30‐40s Figure 4. Saliva collected for germline clinical multi‐gene panel testing using next‐generation sequencing revealed two pathogenic variants in ATM (NM_000051.3): c. 2304_2305insTT (p.Glu769Leufs*9) and c. 9023G > A (p.Arg3008His). Cytogenetic testing performed on patient's blood revealed an increase in both spontaneous and Gamma‐Ray induced chromosome breakage, confirming the diagnosis of ataxia‐telangiectasia. Clinical examination did not reveal any cutaneous telangiectasia, although a broad face with coarse eyebrows and a few café‐au‐lait spots were noted. Subsequent testing revealed low IgG and IgA levels with gross pan‐cerebellar atrophy on magnetic resonance imaging of the brain in keeping with A‐T.
FIGURE 3.
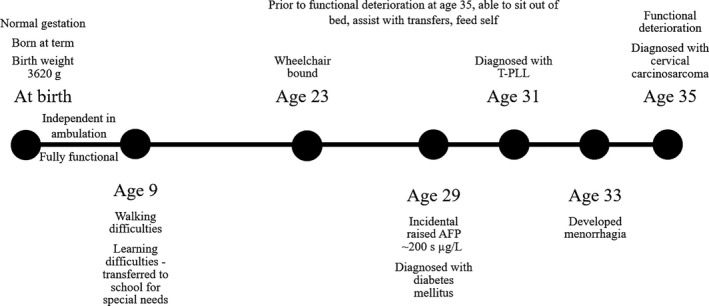
Timeline of events
FIGURE 4.
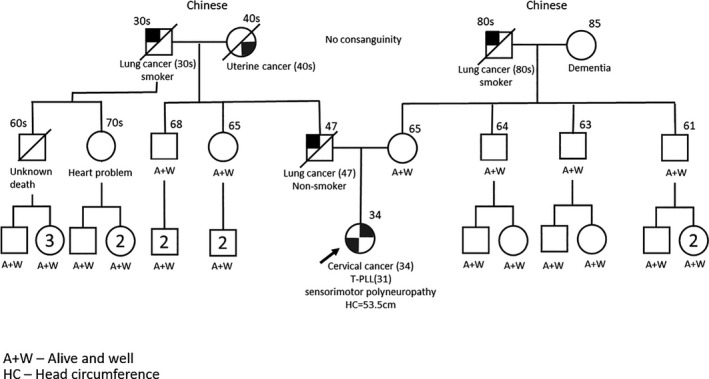
Pedigree
In view of her A‐T diagnosis, it was recommended to avoid radiation therapy in subsequent treatment. A postoperative CT revealed a new right lung nodule and a hepatic lesion likely representing metastases. Her diagnosis was revised to FIGO stage IVB cervical carcinosarcoma and she received a further 2 cycles of palliative chemotherapy with etoposide (300 mg/m2) and cisplatin (100 mg/m2), with a 50% dose reduction in view of a diagnosis of A‐T. Her disease progressed 3 months later, and she was placed on best supportive care prior to her demise shortly after.
3. DISCUSSION
This case highlights the potential for missed or delayed A‐T diagnoses, especially in cases of variant A‐T, and provides an impetus for clinicians to be aware of suggestive signs to facilitate earlier diagnosis. The first clue of our patient's A‐T diagnosis was the regressive loss of developmental milestones from age 9. Second, the early‐onset T‐PLL concurs with A‐T patients having a predisposition to T‐cell as opposed to B‐cell tumors 12 and at a significantly younger age of 20‐30s 4 , 10 , 12 , 15 , 16 compared to a median age of 69 in patients without A‐T. 10 Third, multiple primary cancers in patients with A‐T are not uncommon, with incidence ranging from 4% to 15%. 8 , 17 , 18 Of note, solid tumors mainly present in adulthood, with majority being breast, liver, gastric, thyroid, and esophageal carcinomas Appendix 1. 2 , 4 , 7 Finally, diabetes mellitus and particularly the raised AFP of unknown cause were also consistent with A‐T. 19 , 20 , 21
A range of phenotypes have been described in individuals with A‐T Table 1. Patients with variant A‐T have residual ATM kinase activity and thus a milder clinical course than classic A‐T. 22 , 23 Variant A‐T may present with extrapyramidal signs instead of cerebellar ataxia, milder neurological symptoms, and no lung disease or immunodeficiency. Although residual ATM kinase activity is protective against childhood tumors, variant A‐T are still at increased risk of developing cancers 22 , 23 especially solid malignancies given their longer lifespan compared to classic A‐T whose average life expectancy is approximately 25 years 24 , emphasizing the importance of timely genetic testing in this group who may present atypically. Although ATM kinase activity was not tested, based on clinical presentation, our patient is likely to have variant A‐T. Furthermore, residual ATM kinase activity has been demonstrated in another patient with the c. 9023G > A (p.Arg3008His) variant. 23 In comparison, A‐T heterozygotes often have a normal clinical phenotype. Although epidemiological studies report increased incidence of malignancies in blood relatives of A‐T patients 25 , 26 , 27 , 28 , 29 only the risk of breast cancer has been consistently shown to be raised, with lifetime risk of approximately 38%. 29 Female relatives who are A‐T heterozygotes should thus be offered surveillance with yearly mammography starting from age 40. 30
TABLE 1.
| Classic A‐T | Variant A‐T | Heterozygotes A‐T carriers | |
|---|---|---|---|
| Neurology | Early‐onset cerebellar ataxia | Majority have symptom onset by 10 y of age | Phenotypically normal clinically |
| Usually, wheelchair bound by early second decade of life | Cerebellar ataxia may not be the predominant feature and tend to develop later in life if present | ||
| High incidence oculomotor apraxia | Most have a mixture of ataxia and/or peripheral neuropathy with extrapyramidal features | ||
| Slower progression of neurological disease with delayed loss of ability to walk | |||
| Oculomotor apraxia may not always be present, tend to develop at an older age if present | |||
| Oculocutaneous telangiectasia | Present | Present in approximately 60% of patients | |
| Pulmonary | Recurrent sinopulmonary infections | No significant pulmonary disease | |
| AFP | Elevated | Elevated | |
| Immunological manifestations | Commonly IgG/IgA immunodeficiency | No significant immunodeficiency requiring treatment | |
| May have elevated levels of IgM | |||
| Radiosensitivity | Increased sensitivity to ionizing radiation | Variable | Controversial |
| Malignancy | Increased risk of malignancy, ~25% lifetime risk | Increased risk of malignancy | Increased, mainly with regards to risk of breast cancer |
| High incidence of hematological malignancies at a young age | Later onset of malignancy | ||
| Adults susceptible to both lymphoid tumors and a variety of solid tumors including breast cancers |
To our knowledge, this is the first clinical report of an association between A‐T and cervical cancer though it has been reported in relatives of A‐T patients who are obligate heterozygous carriers of ATM variants. 9 , 27 , 28 , 31 The association between somatic alterations in ATM and risk of cervical cancer have also been reported. 32 , 33 Despite our patient's strong family history of young lung cancers, this has not been prominently reported in clinical literature on A‐T. Interestingly, up to 40% of lung adenocarcinomas have been reported to lack ATM protein expression due to somatic alterations. 34 ATM rs189037, rs664677, and rs664143 gene polymorphisms have also been reported as risk factors for lung cancer. 35 These ATM variants deserve further study with regards to their association with lung cancer, particularly in Asians where there is a higher incidence of adenocarcinomas in nonsmokers.
While radiation‐induced toxicities including death and secondary malignancies 5 , 36 are well established in A‐T, evidence is lacking for chemotherapy. Certain chemotherapeutic agents have been shown to have increased toxicities Appendix 2, whereas agents such as prednisone, 6‐mercaptopurine, asparaginase, and daunorubicin have been shown to be tolerable at normal doses. 37 There are currently no consensus guidelines with regard to dosing of chemotherapy in A‐T. Various approaches tried in multiple hematological and solid cancers are summarized in Table 2. Inferences that can be drawn are limited by the heterogeneity of primary malignancies reported over an extended time course whereby the standard dose/regime may have evolved. 37 , 38 , 39 , 40 In general, the most common strategy employed across studies is a 50% dose reduction of the standard regime. Some gradually up titrated the dose as tolerated while taking care to limit doses of certain agents, such as methotrexate and cyclophosphamide. Durable complete remissions have been successfully achieved with modified dose chemotherapy regimens. 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 The largest of these studies by Schoenaker et al 39 demonstrated no significant difference in remission rates for patients with T‐cell acute lymphoblastic leukemia receiving modified dose chemotherapy. Studies to better describe safety and efficacy of chemotherapeutic regimes in A‐T patients are needed. Ultimately, the decision regarding treatment regime and dosage should be a discussion among all managing healthcare professionals, patient and their family, and individualized based on patient's underlying comorbidities, functional status, and treatment goals.
TABLE 2.
Summary of dosages, toxicities and efficacy of chemotherapy in A‐T patients with cancer 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 54 , 55 , 56 , 57 , 58 , 59
| Tumor type | Tumor subtypes | Case report/Series | No. of cases on SD chemo | No. of cases on MD chemo | Stage | *Chemotherapy | % Dose reduction | Toxicities of note | Response rates | Overall survival (OS) |
|---|---|---|---|---|---|---|---|---|---|---|
| Non‐Hodgkin Lymphoma (NHL) | Burkitt's | Sandoval & Swift | 7 | 1 | I, II, IV | COP, CHOP, CP, COMV | Ranging from 33% to 75% of SD |
a 7 of 14 (50%) exposed to CPM ≥ 1200 mg/m2 had hemorrhagic cystitis a All 3 on bleomycin (both SD and RD) had pulmonary disease which was fatal in 2 |
Burkitt's: CR in 5 of 7 on SD, 1 on RD did not achieve remission a All study patients: CR in 1 of 11 on RD vs 16 of 21 on SD; P = .001 |
a Mean survival of SD vs RD: 12 (1‐162 + mo) vs 5 (0.5‐28 mo); P = .03 a Median survival with CR vs no CR on SD: 32.5 (1‐162 + mo) vs 5 (1‐22 mo); P = .01 |
| Ben Arush et al | 1 | 0 | COMP | Died of severe pneumonia 1 mo later | 1 mo | |||||
| Bienemann et al | 0 | 2 | III, IV | B‐NHL‐BFM 04 |
50% SD Increased to 75% after 2 cycles for 1 pt VP16 omitted for cycle 1, MTX at 0.5 g/m2 for 1 pt |
At least 1 with CCR | ||||
| Upadhyaya et al | 0 | 1 | I | CPM, VCR, DOX, Pred | VBL instead of VCR on D6 | Neutropenia, mucositis—2nd cycle DOX reduced to 75% of SD | EFS at least 6 y | |||
| Large cell | Sandoval & Swift | 7 | 4 | I‐IV | CHOP, COP, OH, OP, CHVP, HOP, CO, MTX | Ranging from 33% to 75% of SD | See above | CR in 5 of 7 on SD, 4 of 4 on RD did not achieve remission | See above | |
| Immunoblastic large cell | Ben Arush et al | 1 | 0 | COMP | Died 1 mo later of Acinetobacter sepsis | 1 mo | ||||
| Lymphoblastic | Sandoval & Swift | 2 | 1 | III, IV | CHOP, COP, OAra‐cTG | Ranging from 33% to 75% of SD | See above | CR in 1 of 2 on SD, CR in 1 on RD | See above | |
| Bienemann et al | 0 | 1 | III, NB‐RG | NHL‐BFM 86 | 50% SD in protocol I, protocol M stopped because of severe toxicity, protocol II omitted | Toxicity experienced, not elaborated | ||||
| DLBCL | Sandlund et al | 0 | 5 | III, IV | LMB‐89 | Group C patients treated according to Group B arm (max MTX dose was 3 g/m2) | 2 sepsis, 1 pneumonitis, 2 multi‐organ failure, 1 severe VCR peripheral sensory neuropathy, 1 severe pulmonary leak with Ara‐C |
2 achieved CR 1 induction failure |
||
| Yamada et al | 0 | 1 | 9104 Standard risk protocol by Tokai Pediatric Oncology Study Group | 50% of SD | Nil side effects apart from mild reversible liver damage | Remained in CR 32 mo after diagnosis | ||||
| Rossi et al | 0 | 1 | IV‐B | Modified dose of R‐CHOP | 100% rituximab and prednisolone, 40% CPM, 30% DOX, 70% VCR | Remained in CR 24 mo after diagnosis | ||||
| Bienemann et al | 0 | 8 | II‐III | B‐NHL BFM 90, 95, 04 |
Mostly 50% SD, some gradually uptitrated to 75% and 100% Some limit MTX dose to 0.5‐1 g/m2 VP16, IFO, VCR & CPM omitted in some cases |
1 died from treatment‐associated toxicity at the end of the fourth course | At least 4 achieved CCR | |||
| Upadhyaya et al | 0 | 1 | IIIB | Modified LMB protocol | Induction phase—50% SD for CPM, DOX, IV MTX, VBL instead of VCR | Fungemia, transaminitis; MTX and DOX further dose reduced due to neutropenia and mucositis | ||||
| T cell | Overberg‐Schmidt et al | 1 | 0 | Acute lymphoblastic leukemia‐Berlin, Frankfurt, Munster 86 protocol | Hepatotoxicity, diarrhea, and recurrent varicella and herpes simplex infection; chemo stopped after 5 mo due to life‐threatening complications | Achieved CR |
EFS ~ 3 y OS 3 ye 8 mo |
|||
| NOS | Sandoval & Swift | 1 | 0 | IV | CHOPB | See above | Achieved CR | See above | ||
| Hodgkin disease (HD) | Nodular sclerosis (NS) | Tamminga et al | 0 | 1 | IIA | Reduced dose OPPA + involved field RT |
Procarbazine omitted 1st course 1/3 of SD 2nd course 2/3 of SD 3rd course full dose |
Tolerated 1st and 2nd course well NCI grade 3 BM suppression and NCI grades 2‐3 paralytic ileus with 3rd course |
Achieved CR |
EFS: 3 mo (biopsy non‐conclusive) OS: 10 mo, due to generalized progressive lymphadenopathy and pneumonia |
| Upadhyaya et al | 0 | 1 | IVB |
VAMP/COP Salvage ICE (MD) |
VBL instead of VCR, reduced dose of CPM, DOX, MTX | Prolonged myelosuppression, suspected splenic fungal lesions; worsening ataxia with ICE | Achieved CR | 2.5 y—due to relapse | ||
| 2 NS, 2 lymphocyte depleted | Sandoval & Swift | 0 | 4 | IIIB, IVB | HOP/ChVPPr, P + VP, ABVD | Ranging from 33% to 75% of SD | See above | All 4 patients did not achieve remission | See above | |
| 1 NS, 1 NOS | Ben Arush et al | 0 | 2 | COPP/ABV |
75% of SD VBL instead of VCR was given after the 1st cycle for 1 patient due to toxicity |
Severe SIADH and convulsions after first cycle—either due to VCR or CPM | Both achieved CR | |||
| Mixed cellularity | Irsfeld et al | 0 | 2 | IIA, IVB | German Group of Pediatric Oncology—HD 1990 for 1 patient, HD 1995 for 1 patient |
1 pt received 3 courses of OPPA instead of 2 in place of radiotherapy 1 pt had ABVD instead of COPP to avoid use of CPM |
1 had neurological deterioration? related to VCR 1 had CMV pneumonia presumably due to underlying immunodeficiency |
1 achieved CR | ||
| Classical HD | Bienemann et al | 0 | 1 | IVB | Only prednisolone | Only prednisolone | 1 mo | |||
| Acute Lymphoid Leukemia (ALL) | T‐PLL | Geling Li et al | 1 | Alemtuzumab 30 mg 3x/wk, pentostatin 4 mg/m2, tofacitinib | Tofacitinib dose adjusted according to renal function | Pentostatin stopped after acute renal failure requiring hemodialysis despite aggressive hydration, switched to tofacitinib | ~4.5 mo | |||
| ALL | Sandoval & Swift | 4 | 1 | CHOPB, POD, POLasp, PODLasp, PO + 6‐MP, P | Ranging from 33% to 75% of SD | See above | CR in 4 of 4 on SD, 1 on RD did not achieve remission | See above | ||
| Toledano & Lange | 20 | 0 | A variety of regimes, mainly with VCR, L‐asp, MTX, 6‐MP, DNM, prednisolone/prednisone | NA | 2 developed severe infections, 1 had neurological deterioration ?related to VCR | |||||
|
18 T‐ALL, 2 B‐cell precursor ALL |
Schoenaker et al | 11 | 9 (both B‐cell ALL received MD) |
A variety of regime:
|
Not standardized Those mentioned include omission of alkylating agents, reduction of MTX dose |
4 of 11 on upfront SD had severe toxicity (infections, neuropathy, hemorrhagic cystitis, leukopenia) 2 on upfront MD had sepsis Both B‐cell precursor ALL had toxicities despite upfront MD—1 persistent leukopenia, 1 sepsis |
No sig difference in CR rates between upfront SD and MD in T‐ALL: CR in 10 of 11 with upfront SD CR in 7 of 7 with upfront MD |
73% vs 57% on upfront SD vs upfront MD | ||
| T‐ALL | Ussowicz et al | 0 | 1 |
ALL IC‐BFM 2002 protocol For high‐risk chemo then allo‐SCT in view of poor prednisone response on D8 of induction therapy |
SD for induction (protocol I) SD for dexamethasone, VCR, L‐asp, DNR 50% dose of CPM and IFO 20% dose of MTX 75% dose of cytarabine Omit Vepesid Modified conditioning chemo pre–allo‐SCT |
Toxicities after SCT: Grade IV leucopenia with agranulocytosis, grade II mucositis, multiple viral infections, BKV hemorrhagic cystitis, EBV lymphoproliferative disorder |
Remained in complete hematological remission 3.5 y after SCT | |||
| Bienemann et al | 4 | 2 |
ALL‐BFM MR DEXA ALL‐BFM MR PRED ALL‐BFM HR PRED |
50%‐75% dose for DNR, CPM, DOX, VCR, ASP, ARA‐C, MP Dose reduction up to 20% SD for MTX |
Both on MD died of treatment‐associated toxicities | At least 3 of 4 on SD achieved CCR | ||||
| Pre‐B ALL | Brummel et al | 0 | 1 | Modified intermediate‐risk group ALL‐BFM‐2000 study protocol |
Start with 50% SD Increase to 66% SD for DNR Increase to 75% SD for Ara‐C Increase to 100% SD for VCR, L‐asp Limit CPM to 50% SD, DOX to 66% SD, IV MTX to 20% SD Dexamethasone, IT MTX at 100% SD |
Persistent neutropenia with IV MTX Developed pneumonia, candida pelliculosa sepsis, generalized seizures and mutism due to parainfectious encephalitis, recurrent bronchitis |
CR on day 15 of therapy Remained in CR > 1 y after end of maintenance therapy |
|||
| Bienemann et al | 0 | 1 | ALL‐BFM MR DEXA | 50%‐66% SD for VCR, DNR, ASP, CPM, Ara‐C, MP, DOX, CPM; 20% SD for MTX | Remained in CCR 3.5 y after diagnosis | |||||
| Acute Myeloid Leukemia (AML) | Schoenaker et al | 1 | 2 | POG‐AML97A prot (SD), ECM‐HCEI course (MD), Oral 6‐MP (palliative) | Both SD and palliative patients died of sepsis |
SD—did not achieve remission MD—achieved CR |
||||
| Onoda et al | 0 | 1 |
Low‐dose induction therapy ‐ VP16 100 mg/m2 D1‐3, Ara‐C 150 mg/m2 D1‐3, DNR 25 mg/m2 D1, IT MTX 15 mg/dose D1 Dose optimized due to increased AML blasts ‐ 2 courses induction + 3 courses intensification based on high dose cytarabine (HiDAC) with CNS prophylaxis |
Alpha‐hemolytic streptococcal sepsis and pneumonia during second induction, transiently requiring non‐invasive positive pressure ventilation, irreversible unilateral pleural effusion | Hematological remission after induction phase | ~1 y, due to respiratory failure and leukemia relapse | ||||
| Solid tumors | Nephroblastoma | Perez‐Villena et al | 0 | 1 | III | SIOP‐TW‐01 protocol | 25% dose reduction, omitted radiotherapy |
Staphylococcus epidermidis bacteremia Septic shock after topotecan given for relapse |
Relapse at 34th week of treatment (during last cycle) | 40 wk |
| Dysgerminoma | Koksal et al | 0 | 1 | Carboplatin 450 mg/m2 D1, VP16 100 mg/m2 D1‐3, bleomycin 10 mg/m2 D2 | Regime as stated instead of first‐line PEB to avoid use of cisplatin |
Developed pneumonia after 1st cycle Lung function deterioration—bleomycin stopped after 1 cycle |
No evidence of residual or recurrence mass at second year of diagnosis | |||
| deVries & Kaplan | 1 | 0 | IIIc | Cisplatin + vinblastine x 2 cycles | No sign of recurrence at 24 mo postdiagnosis | |||||
| Endodermal sinus of the ovary | Pecorelli et al | 0 | 1 | Ic | Cis‐platinum, vinblastine, bleomycin x 5 courses postoperatively | 50% of SD | WHO grade 2 neurotoxicity at the 4th course of treatment | Remained in remission 20 mo after treatment |
Abbreviations: 6‐MP, 6‐mercaptopurine; ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; Ara‐C, cytarabine; CCR, complete clinical remission; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CHOPB, cyclophosphamide, doxorubicin, vincristine, prednisone, bleomycin; CHVP, cyclophosphamide, doxorubicin, etoposide; CO, cyclophosphamide, vincristine; COP, cyclophosphamide, vincristine, prednisone; COVM, doxorubicin, vincristine, vinblastine, methotrexate; CPM, cyclophosphamide; CR, complete remission; DNM, daunomycin; DNR, daunorubicin; DOX, doxorubicin; EFS, event‐free survival; HOP, doxorubicin, vincristine, prednisone; HOP/ChVPPr, doxorubicin, vincristine, prednisone/chlorambucil, vinblastine, prednisone, procarbazine; IFO, ifosfamide; L‐asp, L‐asparaginase; M, methotrexate; MD, modified dose; MP, Mercaptopurine; MTX, methotrexate; NOS, not otherwise specified; OAra‐cTG, vincristine, cytarabine, thioguanine; OH, vincristine, doxorubicin; OP, vincristine, prednisone; OPPA, vincristine, prednisone, procarbazine, doxorubicin; P, prednisone; PEB, bleomycin, etoposide, cisplatin; PODLasp, prednisone, vincristine, daunomycin, asparaginase; RD, reduced dose; SD, standard dose; VBL, vinblastine; VCR, vincristine; VP, P, etoposide, prednisone; VP16, etoposide.
Based on all patients in the study (regardless of tumor types).
Given significant considerations in the management of malignancies, early diagnosis of A‐T, prior to that of malignancy should there be, is of critical importance. Although there are no guidelines for cancer screening in A‐T, early diagnosis and hence knowledge of the underlying genetic disorder will allow patients/families to be cognizant of symptoms and prompt clinicians to do the necessary screening and workup, hopefully enabling detection of malignancy, if any, at a more favorable stage. Additionally, allogeneic hematopoietic stem cell transplantation has been shown to correct immunodeficiency and potentially retard deterioration of neurological function in case reports 50 , 51 which may be considered in selected cases. Regardless, early diagnosis of A‐T also allows for earlier introduction to a multidisciplinary care team, 7 , 52 with the aim to reduce associated morbidities, such as reducing contractures and maintaining functional activity, improving airway clearance, reducing aspiration risk, appropriate treatment of infections especially if recurrent, earlier detection and management of endocrinopathies, ultimately improving quality of life.
4. CONCLUSION
There is a need to improve the general genetic literacy of all clinicians. Ataxia‐telangiectasia is one of the important hereditary cancer syndromes that clinicians should not only be aware of, but also be astute in identifying the diagnostic clues. Any co‐occurrence of neurodevelopmental diagnosis must trigger a consideration for timely genetic testing. Also, AFP should be measured to rule out A‐T in children and patients with progressive neurological decline. Early diagnosis is critical as it may significantly alter management, treatment approach in individuals diagnosed with cancer and allow for interventions that may potentially reduce associated morbidities.
CONFLICT OF INTEREST
We have no conflict of interest to disclose.
AUTHOR CONTRIBUTION
JC: wrote the manuscript. RT: revised the manuscript and contributed to the interpretation of data. STL: revised the manuscript and contributed to the interpretation of genetic data. EC: revised the manuscript and contributed to the interpretation of genetic data. RG: revised the manuscript and contributed to the interpretation of pathological data. EF: revised the manuscript and contributed to the interpretation of data. KS: revised the manuscript. RN: revised the manuscript. JN: contributed to the interpretation of data, revised, and oversaw the writing of the manuscript.
INFORMED CONSENT
Our patient verbally consented to the publication of this case report. Written consent was provided by patient's mother on behalf of patient due to physical disability.
EDITORIAL POLICIES AND ETHICAL CONSIDERATIONS
Approval by our Centralised Institutional Review Board is not required for case report.
ACKNOWLEDGMENTS
We would like to thank our patient for consenting to the publication of the case report. We would also like to express our gratitude to the Lee foundation for their generous donations to the Lee Kong Chian NCCS Cancer Genetics Service fund that help subsidize the cost of testing for many of our patients.
Appendix 1. Cancer spectrum of A‐T individuals
|
Majority of malignancies in childhood are leukemias and lymphomas
Young adult A‐T patients predisposed to T‐PLL Adult A‐T patients are still at risk of lymphoid tumors but also has increased incidence of solid tumors |
Appendix 2. Chemotherapeutic agents of concern in A‐T and recommendations
| Chemotherapeutic agents | Concerns | Suggested approaches from studies |
|---|---|---|
| Alkylating agents | Acts by inducing DNA breaks | Avoid use or consider dose reduction |
| Bleomycin | Increased risk of pulmonary toxicity despite being administered at a reduced dose | Avoid use |
| Cyclophosphamide/ifosfamide | Higher risk of hemorrhagic cystitis, thought to be related to the presence of telangiectasia in the bladder | Limit dose to <1200 mg/m2, ensure vigorous hydration and concurrent mesna administration |
| Methotrexate | Neutropenia and infections. Gastrointestinal tract toxicities in children | Consider starting at reduced dose, up titrate as tolerated. Aggressive hydration, appropriate alkalinization of urine to optimize clearance and use of rescue leucovorin. Close monitoring of methotrexate levels |
| Topoisomerase II inhibitors | Acts by inhibiting repair of DNA double‐stranded breaks | Consider dose reduction |
| Vinca alkaloids | May worsen or confound progression of underlying neurological status | Consider alternatives, reduced dose and omission in event of neurological deterioration |
Cao J, Tan RYC, Li S‐T, et al. Identifying ataxia‐telangiectasia in cancer patients: Novel insights from an interesting case and review of literature. Clin Case Rep.2021;9:995–1009. 10.1002/ccr3.3543
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Choi M, Kipps T, Kurzrock R. ATM mutations in cancer: therapeutic implications. Mol Cancer Ther. 2016;15(8):1781‐1791. 10.1158/1535-7163.MCT-15-0945 [DOI] [PubMed] [Google Scholar]
- 2. Rothblum‐Oviatt C, Wright J, Lefton‐Greif MA, McGrath‐Morrow SA, Crawford TO, Lederman HM. Ataxia telangiectasia: a review. Orphanet J Rare Dis. 2016;11(1):159 10.1186/s13023-016-0543-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taylor AMR. Molecular pathology of ataxia telangiectasia. J Clin Pathol. 2005;58(10):1009‐1015. 10.1136/jcp.2005.026062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suarez F, Mahlaoui N, Canioni D, et al. Incidence, presentation, and prognosis of malignancies in ataxia‐telangiectasia: a report from the french national registry of primary immune deficiencies. J Clin Oncol. 2015;33(2):202‐208. 10.1200/JCO.2014.56.5101 [DOI] [PubMed] [Google Scholar]
- 5. Yanofsky RA, Seshia SS, Dawson AJ, et al. Ataxia‐telangiectasia: atypical presentation and toxicity of cancer treatment. Can J Neurol Sci. 2009;36(4):462‐467. 10.1017/S0317167100007794 [DOI] [PubMed] [Google Scholar]
- 6. Bielorai B, Fisher T, Waldman D, et al. Acute lymphoblastic leukemia in early childhood as the presenting sign of ataxia‐telangiectasia variant. Pediatr Hematol Oncol. 2013;30(6):574‐582. 10.3109/08880018.2013.777949 [DOI] [PubMed] [Google Scholar]
- 7. Bhatt JM, Bush A, van Gerven M, et al. ERS statement on the multidisciplinary respiratory management of ataxia telangiectasia. Eur Respir Rev. 2015;24(138):565‐581. 10.1183/16000617.0066-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrell D, Cromartie E, Swift M. Mortality and cancer incidence in 263 patients with ataxia‐telangiectasia. J Nat Cancer Inst. 1986;77(1):89‐92. 10.1093/jnci/77.1.89 [DOI] [PubMed] [Google Scholar]
- 9. Olsen JH, Hahnemann JM, Borresen‐Dale A‐L, et al. Cancer in patients with ataxia‐telangiectasia and in their relatives in the Nordic Countries. J Nat Cancer Inst. 2001;93(2):121‐127. 10.1093/jnci/93.2.121 [DOI] [PubMed] [Google Scholar]
- 10. Taylor AMR, Metcalfe JA, Thick J, Mak Y‐F. Leukemia and lymphoma in ataxia telangiectasia. Blood. 1996;87(2):423‐438. 10.1182/blood.V87.2.423.bloodjournal872423 [DOI] [PubMed] [Google Scholar]
- 11. Kiel MJ, Velusamy T, Rolland D, et al. Integrated genomic sequencing reveals mutational landscape of T‐cell prolymphocytic leukemia. Blood. 2014;124(9):1460‐1472. 10.1182/blood-2014-03-559542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stilgenbauer S, Schaffner C, Litterst A, et al. Biallelic mutations in the ATM gene in T‐prolymphocytic leukemia. Nat Med. 1997;3(10):1155‐1159. 10.1038/nm1097-1155 [DOI] [PubMed] [Google Scholar]
- 13. Vofechovsky I, Luo L, Dyer MJS, et al. Clustering of missense mutations in the ataxia‐telangiectasia gene in a sporadic T‐cellleukaemia. Nat Genet. 1997;17(1):96. [DOI] [PubMed] [Google Scholar]
- 14. Staber PB, Herling M, Bellido M, et al. Consensus criteria for diagnosis, staging, and treatment response assessment of T‐cell prolymphocytic leukemia. Blood. 2019;134(14):1132‐1143. 10.1182/blood.2019000402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang C‐H, Wu K‐H, Yang Y‐L, et al. Association between ataxia telangiectasia mutated gene polymorphisms and childhood leukemia in Taiwan. Chin J Physiol. 2011;54(6):413‐418. 10.4077/CJP.2011.AMM106 [DOI] [PubMed] [Google Scholar]
- 16. Yuille M, Coignet L, Abraham S, et al. ATM is usually rearranged in T‐cell prolymphocytic leukaemia. Oncogene. 1998;16(6):789‐796. 10.1038/sj.onc.1201603 [DOI] [PubMed] [Google Scholar]
- 17. Spacey SD, Gatti RA, Bebb G. The molecular basis and clinical management of ataxia telangiectasia. Can J Neurol Sci. 2000;27(3):184‐191. 10.1017/S0317167100000822 [DOI] [PubMed] [Google Scholar]
- 18. Spector BD, Filipovich AH, Perry CS, Kersey KS. Epidemiology of cancer in ataxia telangiectasia In: Bridges BA, Harnden DC, eds. Ataxia telangiectasia‐a cellular and molecular link between cancer, neuropathology and immune deficiency. New York, NY: John Wiley and Sons; 1982:103‐138. [Google Scholar]
- 19. Donath H, Hess U, Kieslich M, et al. Diabetes in patients with ataxia telangiectasia: a national cohort study. Front Pediatr. 2020;8:317 10.3389/fped.2020.00317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schieving JH, de Vries M, van Vugt JMG, et al. Alpha‐fetoprotein, a fascinating protein and biomarker in neurology. Eur J Paediatr Neurol. 2014;18(3):243‐248. 10.1016/j.ejpn.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 21. Waldmann TA, McIntire KR. Serum‐alpha‐fetoprotein levels in patients with ataxiatelangiectasia. Lancet. 1972;300(7787):1112‐1115. 10.1016/S0140-6736(72)92717-1 [DOI] [PubMed] [Google Scholar]
- 22. Reiman A, Srinivasan V, Barone G, et al. Lymphoid tumours and breast cancer in ataxia telangiectasia; substantial protective effect of residual ATM kinase activity against childhood tumours. Br J Cancer. 2011;105(4):586‐591. 10.1038/bjc.2011.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schon K, Os N, Oscroft N, et al. Genotype, extrapyramidal features and severity of variant ataxia‐telangiectasia. Ann Neurol. 2019;85(2):170‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crawford TO. Survival probability in ataxia telangiectasia. Arch Dis Child. 2005;91(7):610‐611. 10.1136/adc.2006.094268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geoffroy‐Perez B, Janin N, Ossian K, et al. Cancer risk in heterozygotes for ataxia‐telangiectasia. Int J Cancer. 2001;93(2):288‐293. 10.1002/ijc.1329 [DOI] [PubMed] [Google Scholar]
- 26. Olsen JH, Hahnemann JMD, Børresen‐Dale A‐L, et al. Breast and other cancers in 1445 blood relatives of 75 Nordic patients with ataxia telangiectasia. Br J Cancer. 2005;93(2):260‐265. 10.1038/sj.bjc.6602658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swift M, Morrell D, Massey RB, Chase CL. Incidence of cancer in 161 families affected by ataxia–telangiectasia. N Engl J Med. 1991;325(26):1831‐1836. 10.1056/NEJM199112263252602 [DOI] [PubMed] [Google Scholar]
- 28. Thompson D, Duedal S, Kirner J, et al. Cancer risks and mortality in heterozygous ATM mutation carriers. J Nat Cancer Inst. 2005;97(11):813‐822. 10.1093/jnci/dji141 [DOI] [PubMed] [Google Scholar]
- 29. van Os NJH, Roeleveld N, Weemaes CMR, et al. Health risks for ataxia‐telangiectasia mutated heterozygotes: a systematic review, meta‐analysis and evidence‐based guideline: health risks for ataxia‐telangiectasia mutated heterozygotes. Clin Genet. 2016;90(2):105‐117. 10.1111/cge.12710 [DOI] [PubMed] [Google Scholar]
- 30. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf. Accessed 8 April 2020.
- 31. Swift M, Sholman L, Perry M, Chase C. Malignant neoplasms in the families of patients with ataxia‐telangiectasia. Cancer Res. 1976;36(1):209‐215. [PubMed] [Google Scholar]
- 32. Mazumder Indra D, Mitra S, Roy A, et al. Alterations of ATM and CADM1 in chromosomal 11q22.3–23.2 region are associated with the development of invasive cervical carcinoma. Hum Genet. 2011;130(6):735‐748. 10.1007/s00439-011-1015-8 [DOI] [PubMed] [Google Scholar]
- 33. Oliveira S, Ribeiro J, Sousa H, Pinto D, Baldaque I, Medeiros R. Genetic polymorphisms and cervical cancer development: ATM G5557A and p53bp1 C1236G. Oncol Rep. 2012;27(4):1188‐1192. 10.3892/or.2011.1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Villaruz LC, Jones H, Dacic S, et al. ATM protein is deficient in over 40% of lung adenocarcinomas. Oncotarget. 2016;7(36):57714‐57725. 10.18632/oncotarget.9757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yan Z, Tong X, Ma Y, et al. Association between ATM gene polymorphisms, lung cancer susceptibility and radiation‐induced pneumonitis: a meta‐analysis. BMC Pulm Med. 2017;17(1):205 10.1186/s12890-017-0555-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bergom C, West CM, Higginson DS, et al. The implications of genetic testing on radiation therapy decisions: a guide for radiation oncologists. Int J Radiat Oncol Biol Phys. 2019;105(4):698‐712. 10.1016/j.ijrobp.2019.07.026 [DOI] [PubMed] [Google Scholar]
- 37. Tamminga RYJ, Dolsma WV, Leeuw JA, Kampinga HH. Chemo‐ and radiosensitivity testing in a patient with Ataxia Telangiectasia and Hodgkin disease. Pediatr Hematol Oncol. 2002;19(3):163‐171. 10.1080/088800102753541314 [DOI] [PubMed] [Google Scholar]
- 38. Sandoval C, Swift M. Treatment of lymphoid malignancies in patients with ataxia‐telangiectasia. Med Pediatr Oncol. 1998;31:491‐497. [DOI] [PubMed] [Google Scholar]
- 39. Schoenaker MHD, Suarez F, Szczepanski T, Mahlaoui N, Loeffen JL. Treatment of acute leukemia in children with ataxia telangiectasia (A‐T). Eur J Med Genet. 2016;59(12):641‐646. 10.1016/j.ejmg.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 40. Toledano SR, Lange BJ. Ataxia‐telangiectasia and acute lymphoblastic leukemia. Cancer. 1980;45:1675‐1678. [DOI] [PubMed] [Google Scholar]
- 41. Ben Arush MW, Rosenthal J, Dale J, et al. Ataxia telangiectasia and lymphoma: an indication for individualized chemotherapy dosing—report of treatment in a highly inbred arab family. Pediatr Hematol Oncol. 1995;12(2):163‐169. 10.3109/08880019509029550 [DOI] [PubMed] [Google Scholar]
- 42. Bienemann K, Burkhardt B, Modlich S, et al. Promising therapy results for lymphoid malignancies in children with chromosomal breakage syndromes (ataxia teleangiectasia or Nijmegen‐breakage syndrome): a retrospective survey. Br J Haematol. 2011;155(4):468‐476. 10.1111/j.1365-2141.2011.08863.x [DOI] [PubMed] [Google Scholar]
- 43. Brummel B, Bernbeck B, Schneider D. Complicated but successful treatment of a patient with ataxia telangiectasia and Pre‐B‐acute lymphoblastic leukemia. Klinische Pädiatrie. 2010;222(06):391‐394. 10.1055/s-0030-1267151 [DOI] [PubMed] [Google Scholar]
- 44. Irsfeld H, Korholz D, Janben G, Wahn V, Schroten H. Fatal outcome in two girls with Hodgkin disease complicating ataxia‐telangiectasia (Louis‐Bar syndrome) despite favorable response to modified‐dose chemotherapy. Med Pediatr Oncol. 2000;34:62‐64. [DOI] [PubMed] [Google Scholar]
- 45. Koksal Y, Caliskan U, Ucar C, et al. Dysgerminoma in a child with ataxia‐telangiectasia. Pediatr Hematol Oncol. 2007;24(6):431‐436. 10.1080/08880010701451434 [DOI] [PubMed] [Google Scholar]
- 46. Pecorelli S, Sartori E, Favalli G, Ugazio AG, Gastaldi A. Ataxia‐telangiectasia and endodermal sinus tumor of the ovary: report of a case. Gynecol Oncol. 1988;29(2):240‐244. 10.1016/0090-8258(88)90219-3 [DOI] [PubMed] [Google Scholar]
- 47. Rossi G, Zecca M, Marchi A, Stefano PD, Sammarchi L, Locatelli F. Modified chop‐chemotherapy plus rituximab for diffuse large b‐cell lymphoma complicating ataxia‐telangiectasia. Br J Haematol. 2003;120(2):369‐371. 10.1046/j.1365-2141.2003.04041_4.x [DOI] [PubMed] [Google Scholar]
- 48. Ussowicz M, Musiał J, Duszeńko E, Haus O, Kałwak K. Long‐term survival after allogeneic‐matched sibling PBSC transplantation with conditioning consisting of low‐dose busilvex and fludarabine in a 3‐year‐old boy with ataxia‐telangiectasia syndrome and ALL. Bone Marrow Transplant. 2013;48(5):740‐741. 10.1038/bmt.2012.207 [DOI] [PubMed] [Google Scholar]
- 49. Yamada Y, Inoue R, Fukao T, et al. Ataxia telangiectasia associated with B‐cell lymphoma: the effect of a half‐dose of the drugs administered according to the acute lymphoblastic leukemia standard risk protocol. Pediatr Hematol Oncol. 1998;15(5):425‐429. 10.3109/08880019809016571 [DOI] [PubMed] [Google Scholar]
- 50. Bakhtiar S, Woelke S, Huenecke S, et al. Pre‐emptive allogeneic hematopoietic stem cell transplantation in ataxia telangiectasia. Front Immunol. 2018;9:2495 10.3389/fimmu.2018.02495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Beier R, Sykora K‐W, Woessmann W, et al. Allogeneic‐matched sibling stem cell transplantation in a 13‐year‐old boy with ataxia telangiectasia and EBV‐positive non‐Hodgkin lymphoma. Bone Marrow Transplant. 2016;51(9):1271‐1274. 10.1038/bmt.2016.93 [DOI] [PubMed] [Google Scholar]
- 52. Taylor M. The A‐T Specialist Centre Nottingham City Hospital Campus Nottingham University Hospitals NHS trust. 32. 2014.
- 53. Verhagen MMM, Last JI, Hogervorst FBL, et al. Presence of ATM protein and residual kinase activity correlates with the phenotype in ataxia‐telangiectasia: a genotype‐phenotype study. Hum Mutat. 2012;33(3):561‐571. 10.1002/humu.22016 [DOI] [PubMed] [Google Scholar]
- 54. deVries CR, Kaplan GW. An unusual case of urinary incontinence, ataxiatelangiectasia, and metastatic dysgerminoma: case report and review of the literature. Urology. 1997;50(3):453‐455. 10.1016/S0090-4295(97)00244-6 [DOI] [PubMed] [Google Scholar]
- 55. Onoda T, Kanno M, Meguro T, et al. Successful treatment of acute myeloid leukaemia in a patient with ataxia telangiectasia. Eur J Haematol. 2013;91(6):557‐560. 10.1111/ejh.12186 [DOI] [PubMed] [Google Scholar]
- 56. Overberg‐Schmidt US, Baumgarten E, Stein H. Low‐grade non‐hodgkin's lymphoma after high‐grade non‐hodgkin's lymphoma in a child with ataxia teleangiectasia. Cancer. 1994;73(5):1522‐1525. [DOI] [PubMed] [Google Scholar]
- 57. Perez‐Villena A, Cormenzana M, de Prada I, Perez‐Martınez A, Aleo E. Ataxia‐telangiectasia and wilms tumor: reduced treatment but early relapse. J Pediatr Hematol Oncol. 2013;35(4):308‐310. [DOI] [PubMed] [Google Scholar]
- 58. Sandlund JT, Hudson MM, Kennedy W, Onciu M, Kastan MB. Pilot study of modified LMB‐based therapy for children with ataxia‐telangiectasia and advanced stage high grade mature B‐cell malignancies: pilot study for children with A‐T and cancer. Pediatr Blood Cancer. 2014;61(2):360‐362. 10.1002/pbc.24696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Upadhyaya SA, Mody R, Walkovich K, Hutchinson RJ, Sandlund JT, Connelly JA. Ataxia telangiectasia and cancer predisposition: challenges in management. J Pediatr Hematol Oncol. 2018;40(6):4. [DOI] [PubMed] [Google Scholar]
- 60. Caporossi D, Porfirio B, Nicoletti B, et al. Hypersensitivity of lymphoblastoid lines derived from ataxia telangiectasia patients to the induction of chromosomal aberrations by etoposide (VP‐16). Mutat Res. 1993;290(2):265‐272. 10.1016/0027-5107(93)90167-E [DOI] [PubMed] [Google Scholar]
- 61. Cohen JM, Cuckow P, Davies EG. Bladder wall telangiectasis causing life‐threatening haematuria in ataxia‐telangiectasia: a new observation. Acta Paediatr. 2008;97(5):667‐669. 10.1111/j.1651-2227.2008.00736.x [DOI] [PubMed] [Google Scholar]
- 62. Taylor AMR, Rosney CM, Campbell JB. Unusual sensitivity of Ataxia telangiectasia cells to Bleomycin. Cancer Res. 1979;39(3):1046‐1050. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


