Abstract
Patients with sickle cell disease and COVID‐19 may not have a more dire outcome than the general population. Nevertheless, they may present with acute chest syndrome and other sickle cell crises which should be aggressively managed.
Keywords: acute chest syndrome, COVID–19, Ghana, SARS CoV‐2, sickle cell disease
Patients with sickle cell disease and COVID‐19 may not have a more dire outcome than the general population. Nevertheless, they may present with acute chest syndrome and other sickle cell crises which should be aggressively managed.
1. INTRODUCTION
Sickle cell disease (SCD) patients with coronavirus disease 2019 (COVID‐19) may not necessarily have a worse prognosis than patients without SCD. COVID‐19 may, however, trigger sickle cell crises even in the absence of severe COVID‐19 related respiratory symptoms and therefore this patient population should be closely monitored and appropriately managed
COVID‐19 has swept across the globe over the past 11 months, stretching health facilities to the limit.
COVID‐19 primarily affects the lungs and typically presents with symptoms such as fever, cough, breathlessness, myalgia. 1 Headache, rhinorrhoea, blocked nose, rigors, hemoptysis, anosmia, ageusia and conjunctivitis have all been considered as nonspecific symptoms of the disease. 2 Atypical presentations have also been reported including syncope, altered mental status, stroke, seizures, anorexia, abdominal pain, passage of loose stools (bloody and non bloody) and skin presentations such as necrosis, urticarial lesions and vesicular eruptions. 2 , 3 , 4 , 5 , 6 Risk factors for poor outcomes in patients with COVID‐19 include advanced age and comorbidities like diabetes mellitus, cardiovascular disease, malignancies and SCD. 7
SCD is a serious, hematological, inherited condition that affects millions of people globally. 8 It is due to point mutations in the Beta globin chain that result in deformities (sickling) in the morphology of red blood cells. 8 The homozygous genotype (HbSS) has very grave manifestations and results from substitution of glutamic acid by valine in position 6 of the Beta chain. 8 HbSC, one of the heterozygous genotypes, has a comparably less severe manifestation and is due to substitution of glutamic acid by lysine on the Beta chain. 8
In Ghana, an estimated 2% of newborns have SCD. 9 SCD patients are prone to developing vaso‐occlusive crises (VOC), acute chest syndrome (ACS) and thromboembolic conditions with sometimes fatal consequences. 7 , 10
VOC occurs when deoxygenated, sickled red blood cells adhere to the vascular endothelium and, along with adherent leukocytes and platelets, result in occlusion of the microcirculation, hypoxia and ischemic pain. 11 Triggers for this sickling and occlusion cascade include dehydration, infections and stress. 11 COVID‐19 may result in a pneumonia‐like illness and can thus lead to an ACS or trigger a VOC in this patient population. 12
SCD patients are prone to a prothrombotic state affecting both the arterial and venous systems. 10 The pronounced inflammation in COVID‐19 patients also predisposes them to a hypercoagulable state (COVID‐19–associated coagulopathy) with high mortality rates. 13 This state encompasses the various elements of Virchow's triad: damage to the endothelium, stasis due to decreased mobility especially in critically ill patients; and hypercoaguability from increased thrombogenic agents in the circulation such as factor V111, von Willebrand factor, D‐dimer and fibrinogen abnormalities. 13 , 14 Hypercoagulability may affect both the venous (deep vein thrombosis/pulmonary embolism) and arterial systems (ischemic stroke, acute occlusion of the arteries of the limbs, mesenteric ischemia). 13 Elevated levels of D‐dimer have been associated with increased severity and mortality. 13 Thus, SCD patients with COVID‐19 may be at high risk of developing thromboembolic events.
In the absence of an effective, approved, drug therapy and vaccine against the virus, there is concern about the potential negative impact of the pandemic in SCD patients. Literature from France, the United Kingdom and America, 15 , 16 , 17 , 18 , 19 have described the presentation and outcomes of COVID‐19 in SCD patients. There is, however, a scarcity of data on the impact of COVID‐19 on patients in Africa where an estimated 75% of people affected by SCD reside. 7 We discuss the clinical evolution and outcomes of 3 patients with SCD and COVID‐19 pneumonia admitted at the Highly Infectious Isolation Unit (HIIU) of the Komfo Anokye Teaching Hospital (KATH) in Ghana. The patients were referred from peripheral facilities and had tested positive for COVID‐19 by real‐time reverse transcriptase polymerase chain reaction of the nasopharyngeal swabs, prior to admission to HIIU.
2. CASE 1
A postnatal, 30‐year‐old female with SCD (genotype HbSC) was admitted to the HIIU with a 2‐day history of dry cough, fever, vomiting and dark‐colored urine.
She had delivered in another facility 2 weeks before presentation and had a history of multiple transfusions during pregnancy.
At presentation, her temperature was 37.7°C but rose to 40°C on day 2 of admission. There was jaundice, pallor, pulse rate of 94 b/m and oxygen saturation (SpO2) of 96% in room air.
Chest X‐ray showed bilateral, peripheral, ground‐glass opacities in the middle and lower lobes suggestive of COVID‐19 pneumonia. ECG was normal with a QT interval (QTc) of 0.471 second.
Laboratory investigations revealed severe anemia, thrombocytopenia, deranged transaminases, elevated urea and creatinine (See Table 1) with elevated urea:creatinine of 11.6 and elevated serum bilirubin (29.0 micromol/l).
TABLE 1.
Laboratory results of patients
| Laboratory findings | Baseline | Day 6 | Day 8 | Day 10 | Baseline | Day 2 | Baseline | Day 5 |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | ||||||
| Hb (g/dL) [11.5‐16.5] | 4.8 | 4.8 | 4.3 | 6.2 | 6.1 | 7.3 | 5.3 | 8.1 |
| MCV (fl) [80‐100] | 78.2 | 81.5 | 82.1 | 79.7 | 66.4 | 77.5 | 87.7 | 82.4 |
| MCH (pg) [26‐38] | 26.8 | 26.1 | 26.5 | 25.7 | 23.3 | 23.9 | 29.6 | 27.5 |
| WBC (*103/microliter) [4‐10] | 38.65 | 6.25 | 8.07 | 9.49 | 22.14 | 21.26 | 19.3 | 11.0 |
| Neutrophil (*103/microliter) [1.5‐7] | Missing data | 2.81 | 4.16 | 6.92 | 7.77 | 7.77 | Missing data | 6.69 |
| Lymphocyte (*103/microliter) [1‐3.7] | Missing data | 2.93 | 3.16 | 2.16 | 12.19 | 12.19 | Missing data | 2.72 |
| PLT (*103/microliter) [140‐440] | 91 | 97 | 227 | 118 | 177 | 350 | 845 | 729 |
| ALT (U/L) [1‐41] | 236.2 | 24.2 | 20.6 | 63 | ||||
| AST (U/L) [1‐40] | 210.0 | 25.2 | 33.3 | 18 | ||||
| Urea (mmol/l) [2.5‐8.3] | 8.7 | 36.6 | 2.2 | 1.5 | ||||
| Creatinine (micromol/l) [44‐80] | 301 | 274 | 46 | 27 | ||||
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transferase; Hb, hemoglobin; MCH, mean cell hemoglobin; MCV, mean cell volume; PLT, platelets; WBC, white cell count.
Urine dipstick showed trace leukocytes and proteinuria (2+).
She was given intravenous meropenem 1 g 8 hourly, prophylactic dose of subcutaneous enoxaparin – 40 mg daily, oral hydroxychloroquine 200 mg 8 hourly × 10 days, oral azithromycin 500 mg stat, then 250 mg daily × 4 (in accordance with National Guidelines), oral zinc 40 mg daily, oral folic acid 5 mg daily and oral multivitamins one 12 hourly. She never required supplemental oxygen. She was also rehydrated adequately and hemotransfused with a total of 9 units of packed cells due to ongoing hemolysis (evidenced by jaundice and passage of dark‐colored urine) and recurrent fall in hemoglobin. After receiving the 7th unit of packed cells, oral prednisolone was prescribed on day 6 for a possible hyperhemolysis syndrome (HS). Hemolysis stopped after she received prednisolone and her hemoglobin rose to 6.2 g/dL on day 11 of admission.
She gradually improved clinically. Her COVID‐19 test was repeated after completion of hydroxychloroquine and was negative. ECG was normal with QTc of 0.431 second.
She was discharged after 14 days to continue follow up at the SCD clinic.
3. CASE 2
A 34‐year‐old female with SCD (HbSC genotype) presented to the HIIU with a 2‐week history of fever and cough and a 2‐day history of breathlessness and severe left thoracic pain. She had a history of a yearly occurrence of a VOC.
At presentation, her temperature was 37.0°C. She was pale, jaundiced and tachypnoeic (44 cycles per minute). SpO2 was 95% in room air. Chest X‐ray showed bilateral infiltrates involving peripheral zones as well as findings suggestive of lung infarction from an ACS (Figure 1).
FIGURE 1.
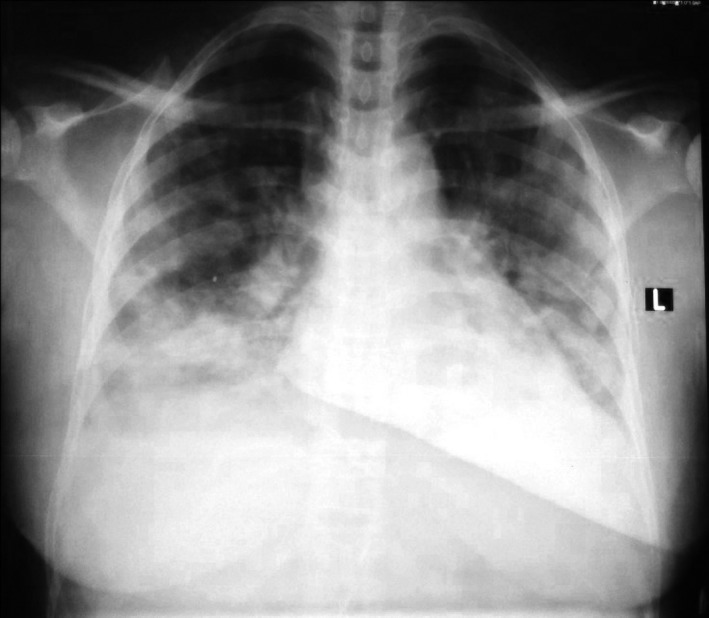
Chest X‐ray of patient 2
Her QTc was 0.447 second.
Laboratory investigations revealed microcytic, hypochromic anemia (See Table 1), hypoalbuminemia of 30.2 g/L and hyperbilirubinemia of 30.2 micromol/L.
Urine dipstick showed trace leukocytes and protein.
She was given intranasal oxygen at 5 liters/minute for a day, intravenous ceftriazone 2 g 12 hourly, oral hydroxychloroquine 200 mg 8 hourly, oral folic acid 5 mg daily, oral paracetamol, prophylactic subcutaneous enoxaparin, oral multivitamins one 12 hourly and adequately hydrated with intravenous and oral fluids.
She was also hemotransfused with one unit of packed cells.
On day 2 of admission, her respiratory rate declined to 29 cycles/min. It further declined to 18 cycles/min on day 3 with complete resolution of the chest pain.
She improved after 4 days at HIIU, so was transferred to a lower level hospital for further care from where she was subsequently discharged after a negative COVID‐19 test.
4. CASE 3
A 20‐year‐old female, with SCD (genotype HbSS) presented at HIIU with a week's history of breathlessness, following a cesarean section.
She had a history of hemotransfusion 4 years ago.
On examination, she was afebrile (36.5°C) and pale. Respiratory rate was 24 cycles per minute. She was hypoxic with SpO2 of 51% in room air and 98% on a nonrebreather mask at 15 liters/minute.
Her chest X‐ray showed massive cardiomegaly (probably from sickle cell cardiomyopathy) and bilateral, patchy, peripheral, ground‐glass opacities in the lower zones suggestive of COVID‐19 pneumonia.
ECG showed sinus rhythm and normal QTc (0.417 second).
Laboratory investigations revealed anemia (Table 1), hypoalbuminemia of 26 g/L and hyperbilirubinemia of 27.1 micromol/L. Urine dipstick revealed protein (2+).
She was given intravenous dexamethasone 12 mg stat, 8 mg daily × 5, prophylactic dose of subcutaneous enoxaparin, oral hydroxychloroquine, oral furosemide 40 mg daily, oral multivitamins, oral folic acid and oral azithromycin. She was also adequately hydrated and transfused with 3 units of packed cells.
On day 3, her oxygen requirement improved and her saturation was 98% on intranasal prongs. On day 4, her temperature rose to 41°C. Samples were taken for blood and urine cultures and antipyretics and intravenous cefuroxime were administered. No organism was isolated from the cultures.
The fever resolved on day 6 and she was weaned off oxygen on day 9. She continued to improve clinically and was discharged on day 11 for review at the hematology clinic.
5. DISCUSSION
Fever, cough, fatigue, vomiting and breathlessness were among the admission symptoms reported by our patients. This is in agreement with what has been observed by other authors. 20 , 21
It is hypothesized that SCD patients coinfected with COVID‐19 would be at a higher risk of a more severe disease presentation and course since they usually have pre‐existing pulmonary compromise and are also at high risk of infections due to immune dysfunction and compromise. 22 , 23 In our case series, only patient 3 had a presentation indicative of severe COVID‐19 infection although admission to the intensive care unit and ventilation were not needed. Patients with the homozygous sickle cell gene tend to have severe immune dysfunction from autosplenectomy, impaired B and T cell function, abnormal chemotaxis and opsonization so making them vulnerable to developing severe viral and bacterial infections and this could explain the severe presentation in this patient. 24 Patients 1 and 2 presented with a hemolytic crises and ACS respectively probably triggered by COVID‐19. This illustrates that SCD patients may present with crises without evidence of severe COVID‐19 respiratory symptoms. Nur et al reported on 2 SCD patients who presented with VOC and ACS in the absence of typical COVID‐19 pulmonary symptoms. 12 Beerkens et al also documented VOC, ACS and hemolytic crises as part of the clinical features of a patient they reported on. 25
As a result of the prothrombotic state that SCD and COVID‐19 predispose patients to, some experts currently recommend treatment of asymptomatic SCD patients with mild COVID‐19 with prophylactic doses of low molecular weight heparin (such as enoxaparin 40 mg daily) while symptomatic patients with severe or critical COVID‐19 should be treated with therapeutic doses of 1 mg/kg 12 hourly barring any contraindications. 13 , 26 Evidence of the thrombogenic nature of COVID‐19 had not emerged at the time these patients were treated at HIIU and therefore patient 3 received only prophylactic doses of enoxaparin.
Patient 1 had been hemotransfused multiple times during her pregnancy. Despite transfusion of 7 units of packed cells at HIIU, her hemoglobin level failed to rise leading to suspicion of HS; a potentially fatal phenomenon seen in SCD patients with a past history of numerous transfusions (including use of compatible blood) as in this case. 27 The exact mechanism for this syndrome is unknown but it is postulated to be primarily due to bystander hemolysis where there is destruction of both the recipient's red blood cells (RBCs) and the transfused RBCs by the recipient's antibodies (alloantibodies) in the absence of the expression of red blood cell antigens against which the antibodies are targeted. 27 HS is a diagnosis of exclusion although laboratory tests can aid in the diagnosis including the direct antiglobulin test (usually negative in the acute stage), lactate dehydrogenase (elevated) and reticulocyte count (usually reticulocytopenia). 27 , 28 These tests could not be done for our patient due to resource constraints. Treatment is with steroids and, if severe, immunoglobulins as well. Additional transfusions should be avoided if possible. 28 Our patient responded favorably to oral steroids but it was needful to give 2 more units of blood due to the presence of severe, symptomatic anemia.
Studies by Hussain et al and others mostly from outside Africa have shown good outcomes in SCD patients with COVID‐19. 17 , 18 , 23 Similarly, none of our patients had an adverse disease outcome. SCD patients have a chronic inflammatory state at healthy, baseline state due to high levels of circulating IL‐1, IL‐6 and TNF alpha. 29 These cytokines, notably IL‐6, have been implicated in the pathogenesis of the cytokine storm which has been shown to be responsible for lung damage and the multi‐organ failure seen in severe COVID‐19 pneumonia. It is possible that the usual baseline inflammatory state in SCD patients may have been protective against the cytokine storm since such patients are used to being in a persistent state of inflammation. 23 , 29 Nevertheless SCD patients with COVID‐19 coinfection should be monitored closely for features such as worsening hypoxia (which may be due to thromboembolic events, acute respiratory distress syndrome or acute chest syndrome), evidence of multi‐organ damage and for vaso‐occlusive or hemolytic crises and if these develop, aggressive management should be instituted.
6. CONCLUSION
In conclusion, the patient with SS genotype had a severe presentation of COVID‐19 respiratory features. The two other patients (SC) had hemolytic crises, VOC and ACS at presentation. All the patients, however, had a favorable clinical outcome and were successfully discharged home. This implies that COVID‐19 may not result in dire outcomes in SCD patients. However, it may trigger crises in these patients even in the absence of severe COVID‐19 related lung disease. This patient population should be closely monitored for sickle cell crises and if these develop they should be aggressively treated.
This is, however, a small case series and in‐depth studies with a larger population size are needed to enhance knowledge into the impact of COVID‐19 in SCD patients, especially those with the HbSS gene.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
All the authors were involved in the clinical management of the patients. YH and YA: designed the study, collected the needed data, and wrote the case series. DAYA, AOA, KHM, JAD, SAK, KAD, POB, and COA: critically reviewed the study and provided important input. All the authors have approved of the final version.
ETHICAL APPROVAL
Ethical approval (reference no. KATH‐IRB/AP/067/20) was obtained from the Institutional Review Board of the Komfo Anokye Teaching Hospital.
ACKNOWLEDGMENTS
The authors warmly extend appreciation to all the staff at the Highly Infectious Isolation Unit for their hard work and dedication in the care of COVID‐19 patients. We also acknowledge the patients who provided informed consent to have their data used.
Hardy YO, Amenuke DAY, Abukari Y, et al. Clinical presentations and outcomes of COVID‐19 infection in sickle cell disease patients: Case series from Komfo Anokye teaching hospital, Ghana. Clin Case Rep.2021;9:1018–1023. 10.1002/ccr3.3719
DATA AVAILABILITY STATEMENT
All data used for this case series are included in this published article.
REFERENCES
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet Lond Engl. 2020;395(10223):507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organisation . Coronavirus disease (COVID‐19) [Internet]. 2020 [cited 2020 Dec 2]. https://www.who.int/news‐room/q‐a‐detail/coronavirus‐disease‐covid‐19. Accessed December 2, 2020.
- 3. Singhania N, Bansal S, Singhania G. An atypical presentation of novel coronavirus disease 2019 (COVID‐19). Am J Med. 2020;133(7):e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study. Am J Gastroenterol. 2020;115:766‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prince G, Sergel M. Persistent hiccups as an atypical presenting complaint of COVID‐19. Am J Emerg Med. 2020;38(7):1546.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casas CG, Català A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;29(10):71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dexter D, Simons D, Kiyaga C, et al. Mitigating the effect of the COVID‐19 pandemic on sickle cell disease services in African countries. Lancet Haematol. 2020;7(6):e430‐e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. da Guarda CC, Yahouédéhou SCMA, Santiago RP, et al. Sickle cell disease: a distinction of two most frequent genotypes (HbSS and HbSC). PLoS ONE. 2020;15(1):e0228399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asare EV, Wilson I, Benneh‐Akwasi Kuma AA, Dei‐Adomakoh Y, Sey F, Olayemi E. Burden of sickle cell disease in Ghana: the Korle‐Bu experience [Internet]. Adv Hematol. 2018;2018:e6161270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naik RP, Streiff MB, Haywood C, Nelson JA, Lanzkron S. Venous thromboembolism in adults with sickle cell disease: a serious and under‐recognized complication. Am J Med. 2013;126(5):443‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Darbari DS, Sheehan VA, Ballas SK. The vaso‐occlusive pain crisis in sickle cell disease: definition, pathophysiology, and management. Eur J Haematol. 2020;105(3):237‐246. [DOI] [PubMed] [Google Scholar]
- 12. Nur E, Gaartman AE, van Tuijn CFJ, Tang MW, Biemond BJ. Vaso‐occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID‐19). Am J Hematol. 2020;95(6):725‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singhania N, Bansal S, Nimmatoori DP, Ejaz AA, McCullough PA, Singhania G. Current overview on hypercoagulability in COVID‐19. Am J Cardiovasc Drugs. 2020;20:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nascimento JHP, de Gomes BF, do Carmo Júnior PR, et al. COVID‐19 and hypercoagulable state: a new therapeutic perspective. Arq Bras Cardiol. 2020;114(5):829‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arlet J‐B, de Luna G, Khimoud D, et al. Prognosis of patients with sickle cell disease and COVID‐19: a French experience. Lancet Haematol. 2020;7(9):e632‐e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Luna G, Habibi A, Deux J, et al. Rapid and severe Covid‐19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. Am J Hematol [Internet]. 2020;95:876‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chakravorty S, Padmore‐Payne G, Ike F, Tshibangu V, Graham C, Stuart‐Smith S. COVID‐19 in patients with sickle cell disease ‐ a case series from a UK Tertiary Hospital. Haematologica. 2020;105:2691‐2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Azerad M‐A, Bayoudh F, Weber T, et al. Sickle cell disease and COVID‐19: atypical presentations and favorable outcomes. eJHaem. 2020;1(1):338‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramachandran P, Perisetti A, Kathirvelu B, et al. Low morbidity and mortality with COVID‐19 in sickle cell disease: a single center experience. eJHaem [Internet]. 2020;1:608‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li L‐Q, Huang T, Wang Y‐Q, et al. COVID‐19 patients' clinical characteristics, discharge rate, and fatality rate of meta‐analysis. J Med Virol. 2020;92(6):577‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shet A, Ataga K, Wun T, et al. COVID‐19 and sickle cell disease ‐ Hematology.org [Internet]. 2020. [cited 2020 Jul 9]. https://www.hematology.org:443/covid‐19/covid‐19‐and‐sickle‐cell‐disease. Accessed July 9, 2020.
- 23. Hussain FA, Njoku FU, Saraf SL, Molokie RE, Gordeuk VR, Han J. COVID‐19 infection in patients with sickle cell disease. Br J Haematol. 2020;189(5):851‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. Br J Haematol. 2014;166(2):165‐176. [DOI] [PubMed] [Google Scholar]
- 25. Beerkens F, John M, Puliafito B, Corbett V, Edwards C, Tremblay D. COVID‐19 pneumonia as a cause of acute chest syndrome in an adult sickle cell patient. Am J Hematol. 2020;95(7):E154‐E156. [DOI] [PubMed] [Google Scholar]
- 26. Alsayegh F, Mousa S. Challenges in the Management of Sickle Cell Disease During SARS‐CoV‐2 Pandemic. Clin Appl Thromb. 2020;26:107602962095524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gouveia M, Soares N, Santoro M, Azevedo F. Hyperhemolysis syndrome in a patient with sickle cell anemia: case report. Rev Bras Hematol E Hemoter. 2015;11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Win N. Hyperhemolysis syndrome in sickle cell disease. Expert Rev Hematol. 2009;2(2):111‐115. [DOI] [PubMed] [Google Scholar]
- 29. Tonen‐Wolyec S, Djang'eing'a RM, Kambale‐Kombi P, Tshilumba CK, Bélec L, Batina‐Agasa S. Vulnerability of sickle cell disease persons to the COVID‐19 in sub‐Saharan Africa. Hematology. 2020;25(1):280‐282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used for this case series are included in this published article.


