Abstract
In the gonadotropin‐releasing hormone (GnRH) antagonist protocol, it is necessary to reinforce contraceptive guidance assuming that luteinizing hormone surge is not detected by measurement of serum level and ovulation is not suppressed by GnRH antagonist.
Keywords: gonadotropin‐releasing hormone (GnRH) antagonist, ovarian hyperstimulation syndrome (OHSS), pregnancy, premature LH surge, premature ovulation
In the gonadotropin‐releasing hormone (GnRH) antagonist protocol, it is necessary to reinforce contraceptive guidance assuming that luteinizing hormone surge is not detected by measurement of serum level and ovulation is not suppressed by GnRH antagonist.

1. INTRODUCTION
Premature ovulation may occur during controlled ovarian stimulation. Usually, this is noticed and treatment is canceled before ovum pick‐up (OPU). In this case, a 24‐year‐old woman naturally conceived during the stimulation and OPU was successfully performed afterward. Despite developing severe ovarian hyperstimulation syndrome, she delivered a healthy baby.
Gonadotropin‐releasing hormone (GnRH) antagonists are currently widely used for controlled ovarian stimulation (COS). GnRH antagonists have the advantage of preventing severe ovarian hyperstimulation syndrome (OHSS) by using GnRH agonists as the final oocyte maturation trigger instead of human chorionic gonadotropin (hCG), which has a long half‐life. 1 , 2
Preventing premature ovulation (PO) completely is difficult. However, Wu et al 3 reported three cases of pregnancy by oocyte retrieval from residual follicles after PO. Additionally, Vicdan et al 4 reported five cases of PO that were converted from in vitro fertilization (IVF) to intrauterine insemination (IUI) and two became pregnant. In most cases of PO, it is noticed and the IVF cycle is canceled. Therefore, there have been few reports of successful cases of pregnancy after PO.
We report a case of spontaneous pregnancy due to PO in a GnRH antagonist protocol. No signs of a premature luteinizing hormone (LH) surge or PO were observed, despite monitoring LH levels and the follicle count during the COS. Additionally, luteal function was maintained even after the GnRH agonist trigger so the pregnancy continued, and a healthy live neonate was delivered.
2. CASE PRESENTATION
2.1. First consultation for assisted reproductive technology
A 24‐year‐old woman, gravida 0, para 0 had no special medical history and a regular 28‐day menstrual cycle. Her body mass index was 20.8 kg/m2. After five cycles of timed intercourse and one cycle of IUI at another hospital, she did not become pregnant and decided to visit our hospital. Her anti‐Mullerian hormone level was 5.34 ng/mL. After the first visit, IUI was performed for two cycles, but she did not become pregnant. The decision was then made to apply assisted reproductive technology (ART).
2.2. Clinical course of ART
COS with follicle‐stimulating hormone (FSH) (gonal‐f; follitropin‐alpha; EMD serono) was started on the menstrual cycle day (CD) 3 and GnRH antagonist ; cetrorelix acetate (cetrotide; Merckbiopharma) from CD 14. If LH level at the start day of controlled ovarian stimulation is above 2 mIU/mL, FSH is selected to be given for stimulation. At second consultation (this case is COS day 3), human menopausal gonadotropin (hMG) (ferring; Ferring Pharma) is selected to be administered. If LH is not over 10 mIU/mL, GnRH antagonist was administered in a flexible method, 5 and the criterion of start of GnRH antagonist is when the leading follicle diameter is 14‐16 mm or more, or when onset of LH surge is predicted to start, and when LH is 10 mIU/mL or more. Steep LH rise was not detected during the controlled ovarian stimulation, and LH concentration kept below 7.3 mIU/mL throughout the cycle. Low dose of human chorionic gonadotropin (hCG) (30 IU/day) was added to hMG so as to compensate for the decrease of LH due to the effect of GnRH antagonist. 6 Serum hCG concentration was measured in order to determine whether it keeps detectable concentration or not. We are determining the dose of FSH or hMG while keeping the FSH concentration at about 10 to around 35 mIU/mL. The consultation was performed twice or three times a week, so, in this case, the measurement of diameter of follicle and serum hormone was carried out at COS 1, 3, 7, 10, 13, 17, and 19 days. The maximum diameter of follicle at CD 9 (COS day 7) is right of 12 × 10 mm, left of 12 × 10 mm, respectively. Also, the maximum diameter of follicle at CD 12 (COS day 10) is right of 16 × 13 mm, left of 17 × 14 mm, respectively. No findings suggesting ovulation, such as deformity, disappearance, hemorrhage or luteinization of follicle, were found in any of the measurements by transvaginal ultrasonography during controlled ovarian stimulation cycle. On the 19th day of stimulation, under the consideration of oocyte maturation, we decided to retrieve the oocytes (ovum pick‐up [OPU]) 3 days later (cycle day 24, COS 22 days), and stimulation was continued until the 20th day of stimulation. The total duration of stimulation was 20 days, with a total of 6625 units of FSH + hMG administered (Figure 1). A final oocyte maturation trigger was performed with a GnRH agonist (leuprolide acetate [Lucrin]; AbbVie) (subcutaneous administration, 1 mg), and 35 hours later, the oocytes were retrieved with a transvaginal needle under venous anesthesia. A total of 31 oocytes (26 × meiosis‐2 stage [M2], 2 × meiosis‐1 stage [M1], 1 × germinal vesicle stage, and 2 × degenerated oocytes) were obtained. The unadjusted sperm volume was 3.2 mL, the motile sperm concentration was 5 M/mL, and the sperm motility was 17.9%, so insemination of the M2 oocytes was split. The fertilization result from intracytoplasmic sperm injection (ICSI) was 21 (12 × 2 pronuclear stage [PN], 1 × 1 PN, 5 × 3 PN, 3 × degeneration [Deg]) and that for conventional IVF was five (3 × 2 PN, 1 × 3 PN, 1 × Deg). Confirmation of fertilization showed 15 × 2 PN embryos and 1 × 1 PN embryo. Therefore, 8 × 2 PN embryos were cryopreserved and the remaining 7 × 2 PN and 1 × 1 PN embryos were cultured for blastocysts. Three blastocysts were formed by day 7 of culture (3AB, 4AA, 4BC by the Gardner classification).
FIGURE 1.
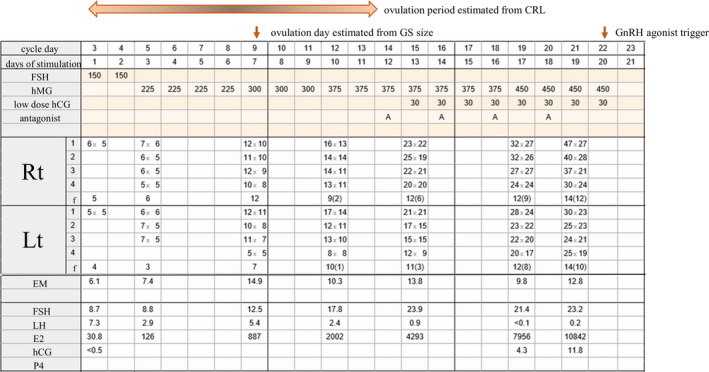
Clinical course during controlled ovarian stimulation. CRL, crown‐rump length; GS, gestational sac; GnRH, gonadotropin‐releasing hormone. Upper column (drugs used for controlled ovarian stimulation). FSH, follicular‐stimulating hormone (IU); hMG, human menopausal gonadotropin (IU); hCG, human chorionic gonadotropin (IU); A, GnRH antagonist 0.25 mg. Middle column (findings by transvaginal ultrasonography). 1‐4, follicle diameter (mm); f, number of 5 mm or larger follicle (14 mm or larger); EM, endometrium thickness (mm). Lower column (serum concentration of hormones). FSH, follicular‐stimulating hormone (mIU/mL); LH, luteinizing hormone (mIU/mL); E2, estradiol (pg/mL); hCG, human chorionic gonadotropin (mIU/mL); P4, serum progesterone concentration (ng/mL)
2.3. Clinical course after the OPU
Abdominal pain began the day after the OPU, and the patient lost consciousness twice on the 3rd day after the OPU, so she went to a hospital. A large amount of ascites, bilateral ovarian swelling (right: 56 × 69 mm, left: 62 × 45 mm), a heart rate of 110 bmp, and blood pressure of 70/50 mmHg were observed at the time of admission. The patient was immediately hospitalized and diagnosed with hypovolemic shock and severe OHSS.
No abnormal findings were detected by head magnetic resonance imaging. A blood test showed the following: albumin level of 2.8 g/dL, creatinine 0.84 mg/dL, sodium 134 mEq/L, potassium 5.5 mEq/L, AST 44 U/L, ALT 20 U/L, WBC 15 300/μL, hemoglobin 18.7 g/dL, and Ht 54.3%. A heparin and dopamine drip infusion was administered.
Her ascites increased from the 8th day after the OPU (6th day of hospitalization), and a puncture and drainage procedure was performed twice. On day 11 after the OPU, transvaginal ultrasonography showed a 10‐mm gestational sac (GS) in the uterus, and her blood hCG level was 6990 mIU/mL. She was then discharged from the hospital on the 17th day after the OPU without any increase in ascites. After discharge, the course of her pregnancy was uneventful, and she gave birth to a female neonate (3136 g) at 40 weeks + 1. At a 1‐month postnatal examination, the infant was healthy without complications.
3. DISCUSSION
The definition of a premature LH surge differs depending on the study. In our case, if the definition of Kummer et al 7 (LH levels ≥ 10 mIU/mL and > 50% rise from baseline) was used, an accurate premature LH surge or ovulation could not be predicted or specified. Therefore, the spontaneous ovulation date of our case was estimated from two parameters, the GS and crown‐rump length (CRL). Assuming that the day when GS was detected at 10 mm was temporary menstrual day 40 8 (in this case, 11 days after OPU, 33 days from the start of COS and cycle day 35), so temporary menstrual day 14 (in this case, 26 days before GS detection, 7 days of COS, cycle day 9) was estimated to be the day of ovulation. The CRL was 15 mm 28 days after the OPU, and the estimated gestational age was 7 weeks + 4, 8 weeks + 2, and 9 weeks by 5, 50, and 95 percentile, respectively. Therefore, the estimated ovulation date (2weeks + 0) was CD 8 (3‐13), 8 so cycle day 8 or 9 is considered to be the highest chance of ovulation. Because the natural menstrual cycle in our case was 28 days, this suggested that ovulation might have occurred earlier than it should have in a natural cycle.
If normal COS is in progress, early luteinization and follicular atresia will occur after a premature LH surge. 9 However, in our case, fertilization and implantation occurred after the presumed spontaneous ovulation day, and follicular development was observed after this time. A total of 31 oocytes were retrieved by using a GnRH agonist trigger.
A previous study reported that the outcome of luteal phase ovarian stimulation (LU) was higher than that for follicular phase ovarian stimulation (FO) regarding the total dosage of hMG, M2 rate, 9 number of oocytes collected, fertilization rate, and the number of day 3 embryos. 10 Additionally, a study that compared FO only to FO + LU reported that the FO + LU group had a higher number of retrieved oocytes, M2 rate, and 2 PN rate. 11 Theoretically, continued stimulation after spontaneous ovulation is therefore possible for retrieving oocytes.
Although good results are obtained with LU, in this case, as a result of ICSI performed on 21 M2 oocytes in our case, 12 of 21 had 2 PN, but 5 had 3 PN. Higher peak estradiol levels, large oocyte yields, a high gonadotropin dose, and a lengthy stimulation procedure are considered to increase 3 PN. 12 , 13 Therefore, additional attention should be paid to stimulation after spontaneous ovulation.
4. CONCLUSION
In conclusion, because the mechanisms of ovulation and oocyte maturation remain unclear, strict monitoring and proper contraceptive guidance are important during COS using the antagonist method. When oocyte retrieval is performed in a natural pregnancy, the risk of developing severe OHSS and threats to life increase. Therefore, guidance for contraception during infertility treatment seems contradictory, but it must be strictly used.
5. AUTHOR CONTRIBUTORS
DI: drafted the manuscript. YA: reviewed the manuscript. TA: reviewed the manuscript as an attending doctor of OHSS.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest regarding the publication of this case report.
ACKNOWLEDGMENTS
We thank Ellen Knapp, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. Informed consent was provided by the patient.
Inoue D, Asada Y, Ando T. Successful outcome of a pregnancy derived from premature ovulation in a gonadotropin‐releasing hormone antagonist protocol: A case report. Clin Case Rep.2021;9:883–886. 10.1002/ccr3.3689
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article.
REFERENCES
- 1. Mourad S, Brown J, Farquhar C. Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;1:CD012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang R, Lin S, Wang Y, Qian W, Zhou L. Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal Ovarian reserve: a systematic review and meta‐analysis. PLoS One. 2017;24:e0175985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu FS‐Y, Lee RK‐K, Hwu Y‐M. Encountering premature ovulation during controlled ovarian hyperstimulation in IVF/ICSI cycles. Taiwan J Obstet Gynecol. 2012;51:56‐59. [DOI] [PubMed] [Google Scholar]
- 4. Vicdan K, Akarsu C, Sözen E, Buluç B, Üstündağ DK, Biberoğlu K. Two successful pregnancies achieved by converting an in vitro fertilization cycle to an intrauterine insemination cycle in five cases with documented premature ovulation. J Turk Ger Gynecol Assoc. 2016;17:233‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang W, Xie D, Zhang H, et al. Cumulative live birth rates after the first ART cycle using flexible GnRH antagonist protocol vs. standard long GnRH agonist protocol: a retrospective cohort study in women of different ages and various Ovarian reserve. Front Endocrinol. 2020;11:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang S, Kuang Y. The effects of low‐dose human chorionic gonadotropin combined with human menopausal gonadotropin protocol on women with hypogonadotropic hypogonadism undergoing ovarian stimulation for in vitro fertilization. Clin Endocrinol. 2018;88:77‐87. [DOI] [PubMed] [Google Scholar]
- 7. Kummer NE, Weitzman VN, Benadiva CA, Schmidt DW, Engmann LL, Nulsen JC. In vitro fertilization outcomes in patients experiencing a premature rise in luteinizing hormone during a gonadotropin‐releasing hormone antagonist cycle. Fertil Steril. 2011;95:2592‐2594. [DOI] [PubMed] [Google Scholar]
- 8. Leveno KJ, Spong CY, Dashe JS, et al. Williams Obstetrics. New York: McGraw‐Hill Education, 2018. Appendix III. [Google Scholar]
- 9. Zhang W, Wang M, Wang S, et al. Luteal phase Ovarian stimulation for poor ovarian responders. JBRA Assist Reprod. 2018;22:193‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin L‐T, Vitale SG, Chen S‐N, et al. Ovarian stimulation may improve oocyte retrieval and oocyte quality in poor ovarian responders undergoing in vitro fertilization: preliminary results from a single‐center prospective pilot study. Adv Ther. 2018;35:847‐856. [DOI] [PubMed] [Google Scholar]
- 11. Sfakianoudis K, Simopoulou M, Maziotis E, et al. Evaluation of the second follicular wave phenomenon in natural cycle assisted reproduction. A key option for poor responders through luteal phase oocyte retrieval. Medicina. 2019;55:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dale B, DeFelice L. Polyspermy prevention: facts and artifacts? J Assist Reprod Ganet. 2011;28:199‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bianchi E, Wright GJ. Izumo meets juno: preventing polyspermy in fertilization. Cell Cycle. 2014;13:2019‐2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.


