Abstract
Management of complex left ventricular outflow tract obstruction (LVOTO) can be achieved with a Konno or Modified Konno procedure to enlarge the LVOT. We hypothesized that patients who undergo a Modified Konno procedure would have a higher rate of LVOT re-intervention compared to the Konno procedure. Patients who underwent a Konno or Modified Konno procedure for LVOTO at a single tertiary care center between 1990 and 2014 were retrospectively reviewed. The primary outcome was LVOT re-intervention post-discharge from index Konno or Modified Konno procedure. Cox regression and Kaplan–Meier estimates were used for time-to-event analysis of LVOT re-interventions, any unplanned re-interventions, and transplant-free survival. The study included 122 patients: 51 (41.8%) in the Konno group and 71 (58.2%) in the Modified Konno group. Median age at surgery was 8.2 (IQR 3–16) years in the Konno group and 3.9 (IQR 1.5–11) years in the Modified Konno group. Multiple left heart lesions were less prevalent in Modified Konno patients. There were 36 (29.5%) patients with LVOT re-interventions: 8 (16%) in the Konno group and 28 (39.4%) in the Modified Konno group (p = 0.01). Transplant-free survival at five years was 87.2% for the Konno group and 93.5% for the Modified Konno group. A higher rate of LVOT re-intervention was found in the Modified Konno group although the Konno and Modified Konno techniques were applied to different patient populations. This finding suggests that careful preoperative decision-making can direct therapy appropriately and that fundamental diagnosis affects procedure choice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00246-020-02522-9.
Keywords: Konno procedure, Modified Konno procedure, Left ventricular outflow tract, Aortic valve replacement
Introduction
Left ventricular outflow tract (LVOT) abnormalities represent 1–2% of all congenital anomalies. Most common known associations of LVOT obstruction (LVOTO) include (a) hypertrophic cardiomyopathy, (b) interrupted aortic arch, hypoplastic aortic arch, coarctation of the aorta, with or without ventricular septal defects, and (c) atrioventricular canal defects, particularly in certain unbalanced forms of atrioventricular canal defects [1–5]. Treatment options have evolved over time: less complicated forms of LVOTO may only require subaortic stenosis (SubAS) resection, while the more complex forms may benefit from a Modified Konno procedure with preservation of the native aortic valve, or the Konno procedure, which involves aortic valve replacement (with autograft or prosthetic valve) as part of LVOT enlargement [6, 7].
The Konno procedure, which was first described by Konno et al. in 1975 [8], aims to relieve LVOTO via aortoventriculoplasty and aortic valve replacement. This procedure has proven to be effective in the surgical management of small aortic annuli with multilevel LVOTO and recurrent SubAS. It is employed less often for LVOTO following Rastelli-type operations for transposition of the great arteries, LVOTO in patients with hypertrophic cardiomyopathy, and tunnel-like forms of SubAS [2, 9–15]. The Modified Konno procedure is also performed to treat LVOTO, but differs from the Konno procedure in that it is intended to preserve the native aortic valve [16]. The Modified Konno procedure has been used to treat patients with LVOTO related to the following: discrete subaortic fibrous ring, milder forms of tunnel-like SubAS, SubAS associated with atrioventricular septal defects or hypertrophic cardiomyopathy, SubAS in DORV (double outlet right ventricle) following biventricular repair and diffuse narrowing of the LVOT [3, 4, 9–11, 17, 18].
The purpose of this study was to compare outcomes of the Konno procedure to the Modified Konno procedure at our center. We hypothesized that the Modified Konno procedure would have a greater proportion of re-interventions on the LVOT.
Materials and Methods
Patient Population
This is a retrospective cohort study of consecutive patients who underwent a Konno or Modified Konno procedure at Boston Children’s Hospital between 1990 and 2014. This study was performed with waiver of consent and institutional review board approval. Patients who had their Konno or Modified Konno procedure at other institutions, but subsequent LVOT re-intervention at our center, were excluded as information on preoperative predictors was not available for these patients. Demographic, clinical, surgical, imaging, and follow-up data were obtained by reviewing clinical charts and echocardiographic databases.
Patients were grouped into four categories to evaluate outcomes based on fundamental diagnostic group: (1) isolated aortic valve and subaortic stenosis, (2) multiple left heart lesions, (3) atrioventricular canal defects/DORV/Tetralogy of Fallot-like lesions, and (4) transposition-like lesions.
Primary Predictor
The primary predictor was the type of index LVOT intervention (Konno versus Modified Konno).
Outcomes and Covariates
The primary outcome variable was LVOT re-intervention post-discharge from index surgery. Secondary outcomes included (1) non-LVOT re-interventions (mitral valve repair or replacement, arch interventions, right ventricle-pulmonary artery (RV-PA) conduit change, pulmonary valve replacement), (2) transplant-free survival from index surgery, and (3) a composite of post-discharge re-intervention and transplant-free survival from index surgery. Other outcomes included (a) major adverse events and (b) postoperative hospital length of stay (PHLOS) at index hospitalization. Covariates included age, gender, height, weight, body surface area, history of prematurity, neonate, number of prior cardiac surgeries, preoperative extracorporeal membrane oxygenation (ECMO) and associated intracardiac abnormalities.
Definitions
The index surgery was defined as the first Konno or Modified Konno procedure performed for the patient. Post-discharge re-interventions were defined as (1) surgical re-interventions including aortic valve interventions, Konno procedure (in Modified Konno group), redo Konno (in Konno group), RV-PA conduit change, permanent pacemaker or pacemaker generator change, arch interventions, mitral valve interventions, and other surgeries or (2) catheter re-interventions including balloon dilation (BD) and/or stenting of the aortic arch, LVOT, RV-PA conduit, branch pulmonary arteries and other catheter re-interventions. Major adverse events were defined as postoperative (1) mediastinitis, (2) ECMO, (3) re-exploration for bleeding, (4) atrioventricular block requiring a permanent pacemaker, (5) ventilator support greater than 7 days, (6) post-discharge re-admissions within 30 days of surgery and (7) in-hospital mortality. PHLOS was defined in days from date of surgery to date of discharge.
Statistical Analysis
Patient characteristics, perioperative data, and in-hospital (early) and post-discharge (late) outcomes are presented as medians with interquartile ranges for continuous variables and as percentages and frequencies for categorical variables. Continuous variables were compared between the two groups using Fisher’s exact test (2-tailed), while categorical variables were compared using Pearson Chi-square test. Predictors with a p value ≤ 0.1 were included in multivariable models using backward elimination. Multivariable cox regression and Kaplan–Meier methodology were used to analyze unplanned re-interventions and transplant-free survival between the two groups. In-hospital outcomes were analyzed using logistic regression for adverse events and Cox regression for PHLOS. Statistical analyses were performed with IBM SPSS Statistics for Windows, version 24.
Results
Clinical records of 122 patients were reviewed and included 51 (41.8%) in the Konno group and 71 (58.2%) in the Modified Konno group. Baseline characteristics and the outcomes of the two groups are displayed in Table 1.
Table 1.
Patient characteristics and outcomes (N = 123)
| All (n = 122) | Konno (n = 51) | Modified Konno (n = 71) | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 6.1 (1.9,11.8) | 8.2 (3,15.9) | 3.9 (1.5,10.8) | |
| Gender (female) | 60 (49.2%) | 19 (37.7%) | 41 (57.7%) | 0.03 |
| Height (cm) | 112 (82.8,146.5), n = 113 | 121.7 (90.4,151.5), n = 46 | 96 (81,142), n = 67 | |
| Weight (kg) | 18.7 (10.4,40.5) | 23 (12.1,50) | 15.1 (9.9,31) | |
| BSA (kg/m2) | 0.7 (0.5,1.3), n = 120 | 0.87 (.55,1.5), n = 50 | 0.63 (.47,1.1), n = 70 | |
| Prematurity (< 37 weeks) | 3 (3.1%), n = 98 | 1 (2.4%), n = 41 | 2 (3.5%), n = 57 | |
| Neonate | 0 (0%) | 4 (7.8%) | 0 (0%) | 0.02 |
| Cardiac characteristics | ||||
| Number of prior surgeries | 1 (0,2), n = 121 | 1 (0,2) | 1 (0,2), n = 70 | |
| PreOP LVOT gradient (mmHg) | 70 (60.8,87.3), n = 120 | 75 (57.2,86.3), n = 50 | 70 (63.8,88.5), n = 70 | |
| PreOP ECMO | 2 (1.6%) | 1 (2%) | 1 (1.4%) | |
| Prior cardiac operationa | 70 (57.9%), n = 121 | 34 (66.7%) | 36 (51.4%), n = 70 | 0.09 |
| Intracardiac abnormalityb | 78 (62.4%) | 33 (61.1%) | 45 (63.4%), n = 69 | |
| Operative details | ||||
| CPB (minutes) | 159 (120,228.5), n = 69 | 208 (156,273), n = 29 | 135 (105,174.3), n = 40 | |
| CCT (minutes) | 102 (73,150), n = 69 | 129 (106.5,161.5), n = 29 | 85 (63.3,110.3), n = 40 | |
| In-hospital outcomesc | ||||
| Early mortality | 7 (5.7%) | 4 (7.8%) | 3 (4.2%) | |
| Major adverse events | 34 (27.9%) | 18 (35.3%) | 16 (22.5%) | |
| Pacemaker | 13 (10.7%) | 6 (11.8%) | 7 (9.9%) | |
| Any re-intervention | 16 (13.1%) | 7 (13.7%) | 9 (12.7%) | |
| Surgical re-intervention | 13 (10.7%) | 6 (11.8%) | 7 (9.9%) | |
| Catheter re-intervention | 4 (3.3%) | 2 (3.9%) | 2 (2.8%) | |
| ICU LOS (days) | 3.5 (2.4,6.6) | 3.7 (2.4,6.5) | 3.5 (2.4,6.7) | |
| Hospital LOS (days) | 7.3 (5.4,10.7) | 7.6 (6.5,13.8) | 6.5 (5.2,8.7) | 0.01 |
| Late outcomesd | ||||
| Any re-intervention | 56 (45.9%) | 20 (39.2%) | 36 (50.7%) | |
| Surgical re-intervention | 44 (36.1%) | 15 (29.4%) | 29 (40.8%) | |
| Catheter re-intervention | 33 (27%) | 13 (25.5%) | 20 (28.2%) | |
| LVOT re-intervention | 36 (29.5%) | 8 (15.7%) | 28 (39.4%) | 0.01 |
| Surgical LVOT re-intervention | 31 (25.4%) | 8 (15.7%) | 23 (32.4%) | 0.04 |
| Catheter LVOT re-intervention | 11 (9%) | 2 (3.9%) | 9 (12.7%) | 0.10 |
| Aortic valve surgery | 17 (13.9%) | 4 (7.8%) | 13 (18.3%) | 0.10 |
| Konno/redo Konno | 9 (7.4%) | 4 (7.8%) | 5 (7%) | |
| PPM generator change | 9 (7.4%) | 3 (5.9%) | 6 (8.5%) | |
| Mitral valve surgery | 11 (9%) | 3 (5.9%) | 8 (11.3%) | |
| Aortic arch intervention | 5 (4.1%) | 2 (3.9%) | 3 (4.2%) | |
| RV/PA conduit/PVR | 12 (9.8%) | 8 (15.7%) | 4 (5.6%) | 0.07 |
| Aortic arch balloon dilation | 3 (2.5%) | 2 (3.9%) | 1 (1.4%) | |
| AV BD/replacement | 8 (6.6%) | 0 (0%) | 8 (11.3%) | 0.01 |
| SubAS BD | 1 (0.8%) | 0 (0%) | 1 (1.4%) | |
| Conduit BD or stent | 12 (9.8%) | 6 (11.8%) | 6 (8.5%) | |
| Branch PA BD | 2 (1.6%) | 0 (0%) | 2 (2.8%) | |
| Mitral valve BD | 4 (3.3%) | 4 (7.8%) | 0 (0%) | 0.02 |
| Othere | 15 (12.3%) | 6 (11.8%) | 9 (12.7%) | |
| Late mortality | 6 (4.9%) | 5 (9.8%) | 1 (1.4%) | 0.03 |
| Late transplant | 2 (1.6%) | 0 (0%) | 2 (2.8%) |
p values < 0.05 are given in bold
p values > 0.1 have not been included
Data presented as frequency (%) or medians (25th and 75th percentile values) as appropriate. Where complete data were not available, the appropriate frequency is provided next to the variable value
aPrior cardiac operations include prior interrupted aortic arch repair, aortic valvotomy, subaortic stenosis resection, coarctation repair, mitral valve replacement and valvotomy, VSD closure, ASD closure, Manougian aortic root enlargement, pacemaker placement
bIntracardiac abnormalities include VSD, ASD, bicuspid aortic valve, aortic stenosis, supramitral ring, mitral stenosis, double outlet right ventricle, patent foramen ovale, Tetralogy of Fallot, Transposition of the Great Arteries
cEarly outcomes indicate events prior to discharge from hospitalization following index surgery (either a Konno or Modified Konno)
dLate outcomes indicate events that occurred after hospital discharge following index surgery (either a Konno or Modified Konno)
eOther category in late outcomes includes interventions involving the coronary arteries, subaortic stenosis resection, tricuspid valve interventions, and several atrial and ventricular interventions (ex. ASD and VSD closure)
p p-value, Cm centimeters, kg kilograms, kg/m2 kilograms per meter squared, PreOP preoperative, LVOT left ventricular outflow tract, mmHg millimeters of mercury, ECMO extracorporeal membranous oxygenation, CPB cardiopulmonary bypass time, CCT cross clamp time, ICU intensive care unit, LOS length of stay, PPM permanent pacemaker, RV-PA right ventricle to pulmonary artery, PVR pulmonary valve replacement, BD balloon dilation, SubAS subaortic stenosis, PA pulmonary artery
There were 19 (37.3%) females in the Konno group and 41 (57.7%) females in the Modified Konno group. The median age was 8.2 years (range 3, 16) in the Konno group and 3.9 years (range 2, 11) in the Modified Konno group. A history of prematurity was found in 2.4% (n = 1) of the Konno group and 3.5% (n = 2) of the Modified Konno group. In the Konno group, 7.8% (n = 4) were neonates at time of surgery, as compared to 0% (n = 0) in the Modified Konno group. The median number of prior surgeries was 1.0 with a range between 0 and 2 for both the Konno and Modified Konno groups, with the commonest being SubAS resection, coarctation repair, and aortic valvotomy for the Konno group, and coarctation repair and SubAS resection for the Modified Konno group (supplemental Tables S11 and S12). ECMO prior to the index surgery was required in 2% (n = 1) of the Konno group vs. 1.4% (n = 1) of the Modified Konno group. Median follow-up was 8.5 years for the Konno group and 7.9 years for the Modified Konno group.
Representation of the Konno and Modified Konno procedures according to fundamental diagnostic group is shown in Table 2. Those patients with multiple left heart lesions were more likely to undergo a Konno procedure (p = 0.017) and patients with AV canal defects/DORV/VSD/TOF-like lesions were more likely to undergo a Modified Konno procedure (p = 0.001).
Table 2.
Konno and Modified Konno procedures according to fundamental diagnosis
| Fundamental diagnosis | Overall (122) | Konno (51) | Modified Konno (71) | p value |
|---|---|---|---|---|
| Isolated aortic valve/SubAS | 52 (42.6%) | 25 (49.0%) | 27 (38.0%) | 0.31 |
| Multiple left heart lesions | 35 (28.7%) | 21 (41.2%) | 14 (19.7%) | 0.017 |
| AVcanal/DORV/TOF-like | 28 (23.0%) | 4 (7.8%) | 24 (33.8%) | 0.001 |
| Transposition-like | 7 (5.7%) | 1 (2%) | 6 (8.5%) | 0.24 |
p values < 0.05 are given in bold
SubAS subaortic stenosis, AVcanal atrioventricular canal, DORV double outlet right ventricle, TOF Tetralogy of Fallot
Univariable analyses of the association between the predictors and outcomes of interest are described in Table 3. The findings of multivariable analysis are represented in Table 4, 5, 6, 7, 8.
Table 3.
Univariable comparisons to determine the factors for inclusion in multivariable model
| Post-DC LVOT RI | Post-DC RI | Postop Hospital LOS | Any mortality/transplant | Postop Adverse Events | |
|---|---|---|---|---|---|
| Group | 0.009 | 0.3 | 0.007 | 0.2 | 0.2 |
| Gender | 0.7 | 0.9 | 0.4 | 0.4 | 0.119 |
| Prematurity | 0.011 | 0.088 | 0.086 | 0.003 | 0.3 |
| Neonate | 0.2 | 0.9 | 0.001 | 0.4 | 0.031 |
| Preoperative ECMO | 0.4 | 0.2 | 0.9 | 0 | 0.021 |
| Prior surgery | 0.2 | 0.2 | 0.1 | 0.059 | 0.008 |
| Intracardiac abnormality | 0.2 | 0.3 | 0.3 | 0.010 | 0.2 |
| Age | 0.4 | 0.027 | 0.091 | 0.4 | 0.3 |
| Number of prior surgeries | 0.2 | 0.086 | 0.2 | 0.028 | 0.026 |
| Preoperative LVOT gradient | 0.2 | 0.5 | 0.7 | 0.6 | 0.2 |
| Weight | 0.6 | 0.062 | 0.053 | 0.2 | 0.4 |
| Height | 0.7 | 0.068 | 0.088 | 0.2 | 0.3 |
| BSA | 0.5 | 0.053 | 0.038 | 0.2 | 0.4 |
| CPB | 0.2 | 1.0 | 0.005 | 0.045 | 0.025 |
| CCT | 0.4 | 0.9 | 0.07 | 0.072 | 0.042 |
p values < 0.05 are given in bold
Only p values are provided. All variables with p ≤ 0.1 were included in final multivariable models
Postop postoperative, DC discharge, RI re-intervention, LVOT left ventricular outflow tract, ECMO extracorporeal membranous oxygenation, BSA body surface area, CPB cardiopulmonary bypass time, CCT cross clamp time
Table 4.
Multivariable Cox regression analysis to determine the factors associated with longer hospital length of stay
| Hazard ratio | Confidence interval | p value | |
|---|---|---|---|
| Modified Konno | 0.8 | 0.6, 1.0 | 0.017 |
| Age | 1.0 | 0.98, 1.0 | 0.57 |
| Intracardiac abnormality | 0.8 | 0.6, 1.0 | 0.072 |
| Prior surgery | 1.3 | 1.0, 1.6 | 0.053 |
p value < 0.05 is given in bold
Konno group served as the reference group
Table 5.
Multivariable logistic regression analysis to determine the factors associated with major postoperative adverse events
| Odds ratio | Confidence interval | p value | |
|---|---|---|---|
| Modified Konno | 0.5 | 0.2, 1.1 | 0.08 |
| Age | 1.0 | 1.0, 1.1 | 0.59 |
| Prior surgery | 0.3 | 0.1, 0.7 | 0.004 |
p value < 0.05 is given in bold
Konno group served as the reference group
Table 6.
Multivariable Cox regression analysis to determine the factors associated with post-discharge LVOT re-intervention
| Hazard ratio | Confidence interval | p value | |
|---|---|---|---|
| Modified Konno | 2.7 | 1.2, 5.9 | 0.012 |
| Age | 1.0 | 0.9, 1.0 | 0.42 |
| Prior surgery | 1.3 | 0.6, 1.3 | 0.45 |
p value < 0.05 is given in bold
Konno group served as the reference group
Table 7.
Multivariable Cox regression analysis to determine the factors associated with post-discharge re-intervention on the left ventricular outflow tract, mitral valve, tricuspid valve, right ventricular outflow tract, coronary arteries, atria, and ventricles
| Hazard ratio | Confidence interval | p value | |
|---|---|---|---|
| Modified Konno | 1.1 | 0.6, 2.0 | 0.69 |
| Age | 1.0 | 0.9, 1.0 | 0.004 |
| Prior surgery | 1.2 | 0.7, 2.1 | 0.54 |
p value < 0.05 is given in bold
Konno group served as the reference group
Table 8.
Multivariable Cox regression analysis to determine the factors associated with transplant-free survival
| Hazard ratio | Confidence interval | p value | |
|---|---|---|---|
| Modified Konno | 0.6 | 0.2, 1.7 | 0.32 |
| Prior surgery | 2.5 | 0.7, 8.9 | 0.16 |
| Intracardiac abnormality | 3.4 | 0.8, 15.2 | 0.11 |
Konno group served as the reference group
In-Hospital Outcomes
Median postoperative intensive care unit (ICU) length of stay was similar at 3.7 (IQR 2.4–6.5) days for the Konno group and 3.5 (IQR 2.4–6.7) days for the Modified Konno group. However, PHLOS was longer for the Konno group: 7.6 (IQR 6.5–13.8) days versus 6.5 (IQR 5.2–8.7) days for the Modified Konno group (p = 0.01).
There was no statistical difference in predischarge mortality: 7.8% (n = 4) for the Konno group and 4.2% (n = 3) for the Modified Konno group. Two patients required transplantation during index hospitalization: 2% (n = 1) of the Konno group and 1.4% (n = 1) of the Modified Konno group. Re-intervention prior to discharge was also not different between the groups: 13.7% (n = 7) of the Konno group and 12.7% (n = 9) of the Modified Konno group (supplemental Tables S13, S14). Major adverse events at any time point (including mortality) occurred in 35.3% (n = 18) of the Konno group, and 22.5% (n = 16) of the Modified Konno group. Postoperative atrioventricular block requiring pacemaker placement occurred in 11.8% (n = 6) of the Konno group and 9.9% (n = 7) of the Modified Konno group.
Multivariable cox regression for PHLOS (time to discharge) showed that patients who underwent a Konno procedure had a longer time to discharge (hazard ratio 0.8, 95% confidence interval 0.6, 1.0, p = 0.017) (Table 4). Multivariable logistic regression analysis for major postoperative adverse events showed no difference between the Konno and Modified Konno groups (Table 5).
Comparison by fundamental diagnostic group showed that patients who had multiple left heart lesions had a significantly higher rate of complications at 48.6% (n = 17, p = 0.01), overall re-interventions at 28.6% (n = 10, p = 0.01) and surgical re-interventions at 22.9% (n = 8, p = 0.04) prior to discharge. These patients also had a significantly longer ICU length of stay (LOS) at 5.7 days (IQR 3.3–9.6, p = 0.005) and a significantly longer PHLOS at 9.8 days (IQR 7.5–16.6, p = 0.003) (Table 9).
Table 9.
Outcomes based on fundamental diagnosis group
| Outcomes | All (n = 122) | Isolated AV and SubAS (n = 52) | Multiple left heart lesions (n = 35) | AV Canal Defects/DORV and VSD/TOF-like (n = 28) | Transposition-like lesions (n = 7) | p value |
|---|---|---|---|---|---|---|
| In-hospital outcomes | ||||||
| Complications | 34 (27.9%) | 11 (21.2%) | 17 (48.6%) | 4 (14.3%) | 2 (28.6%) | 0.01 |
| PPM | 13 (10.7%) | 4 (7.7%) | 7 (20%) | 1 (3.6%) | 1 (14.3%) | |
| Any RI | 16 (13.1%) | 5 (9.6%) | 10 (28.6%) | 1 (3.6%) | 0 (0%) | 0.01 |
| Surgical RI | 13 (10.7%) | 4 (7.7%) | 8 (22.9%) | 1 (3.6%) | 0 (0%) | 0.04 |
| Cath RI | 4 (3.3%) | 1 (1.9%) | 3 (8.6%) | 0 (0%) | 0 (0%) | |
| Mortality | 7 (5.7%) | 2 (3.8%) | 4 (11.4%) | 1 (3.6%) | 0 (0%) | |
| Complication/Mortality/RI | 34 (27.9%) | 11 (21.2%) | 17 (48.6%) | 4 (14.3%) | 2 (28.6%) | 0.01 |
| ICU LOS (days) | 3.5 | 2.8 (2.2, 4.8) | 5.7 (3.3, 9.6) | 2.8 (2.1, 6.6) | 3.6 (3.0, 3.7) | 0.005 |
| Hospital LOS (days) | 7.3 | 6.5 (5.4, 8.6) | 9.8 (7.5, 16.6) | 6.3 (5.2, 8.4) | 7.1 (6.5, 8.7) | 0.003 |
| Late outcomes | ||||||
| Any RI | 56 (45.9%) | 25 (48.1%) | 18 (51.4%) | 9 (32.1%) | 4 (57.1%) | |
| Surgical RI | 44 (36.1%) | 20 (38.5%) | 14 (40%) | 8 (28.6%) | 2 (28.6%) | |
| Cath RI | 33 (27%) | 13 (25%) | 12 (34.3%) | 4 (14.3% | 3 (57.1%) | 0.09 |
| LVOT RI | 36 (29.5%) | 20 (38.5%) | 8 (22.9%) | 6 (21.4%) | 2 (28.6%) | |
| Surgical LVOT RI | 31 (25.4%) | 17 (32.7%) | 7 (20%) | 6 (21.4% | 1 (14.3%) | |
| Cath LVOT RI | 11 (9%) | 7 (13.5%) | 3 (8.6%) | 0 (0%) | 1 (14.3%) | |
| AVR | 17 (13.9%) | 11 (21.2%) | 3 (8.6%) | 3 (10.7%) | 0 (0%) | |
| Konno | 9 (7.4%) | 7 (13.5%) | 1 (2.9%) | 1 (3.6%) | 0 (0%) | |
| PPM generator change | 9 (7.4%) | 1 (1.9%) | 5 (14.3%) | 1 (3.6%) | 2 (28.6%) | 0.02 |
| MV surgery | 11 (9%) | 1 (1.9%) | 7 (20%) | 3 (10.7%) | 0 (0%) | 0.03 |
| Arch intervention | 5 (4.1%) | 4 (7.7%) | 0 (0%) | 1 (3.6%) | 0 (0%) | |
| RV-PA CC/PVR | 12 (9.8%) | 3 (5.8%) | 7 (20%) | 1 (3.6%) | 1 (14.3%) | 0.09 |
| Arch BD | 3 (2.5%) | 2 (3.8%) | 1 (2.9%) | 0 (0%) | 0 (0%) | |
| AV BD/replacement | 8 (6.6%) | 5 (9.6%) | 3 (8.6%) | 0 (0%) | 0 (0%) | |
| SubAS BD | 1 (0.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (14.3%) | 0.001 |
| Branch PA BD | 2 (1.6%) | 1 (1.9%) | 0 (0%) | 0 (0%) | 1 (14.3%) | 0.05 |
| MV BD | 4 (3.3%) | 0 (0%) | 4 (11.4%) | 0 (0%) | 0 (0%) | 0.02 |
| Other | 15 (12.3%) | 6 (11.5%) | 5 (14.3%) | 3 (10.7%) | 1 (14.3%) | |
| Late mortality | 6 (4.9%) | 2 (3.8%) | 3 (8.6%) | 1 (3.6%) | 0 (0%) | |
| Late transplant | 2 (1.6%) | 1 (1.9%) | 1 (2.9%) | 0 (0%) | 0 (0%) | |
| Late mortality/transplant | 8 (6.6%) | 3 (5.8%) | 4 (11.4%) | 1 (3.6%) | 0 (0%) | |
| Readmission | 63 (51.6%) | 28 (53.8%) | 20 (57.1%) | 11 (39.3%) | 4 (57.1%) | |
| Any mortality/transplant | 15 (12.3%) | 5 (9.6%) | 8 (22.9%) | 2 (7.1%) | 0 (0%) | |
| Mortality/RI | 64 (52.5%) | 27 (51.9%) | 23 (65.7%) | 10 (35.7%) | 4 (57.1%) | |
| Mortality/LVOT RI | 44 (36.1% | 22 (42.3%) | 13 (37.1%) | 7 (25%) | 2 (28.6%) |
p values < 0.05 are given in bold
p values > 0.1 have not been included
Data presented as frequency (%) or medians (25th and 75th percentile values) as appropriate
Where complete data were not available, the appropriate frequency is provided next to the variable value
AV atrioventricular, SubAS subaortic stenosis, DORV double outlet right ventricle, VSD ventricular septal defect, TOF Tetralogy of Fallot, PPM permanent pacemaker, RI re-intervention, Cath catheter, ICU intensive care unit, LOS length of stay, LVOT left ventricular outflow tract, AVR aortic valve re-intervention MV mitral valve, RV-PA right ventricle to pulmonary artery, CC conduit change, PVR pulmonary valve replacement, BD balloon dilation, AV aortic valve, PA pulmonary artery
Post-Discharge Outcomes
Post-discharge surgical re-intervention occurred in 29.4% (n = 15) of patients in the Konno group and 40.8% (n = 29) of patients in the Modified Konno group (Supplemental Table S15). Catheter re-intervention post-discharge was performed in 25.5% (n = 13) of the patients in the Konno group and 28.2% (n = 20) of the patients in the Modified Konno group (Supplemental Table S16). LVOT re-interventions occurred in 15.7% (n = 8) of the Konno group and 39.4% (n = 28) of the modified Konno group. No patients in the Konno group required a heart transplant post-discharge, but 2.8% (n = 2) of the Modified Konno patients underwent transplant at 4 months and 18 years, respectively, after initial surgery. Post-discharge mortality was 9.8% (n = 5) for the Konno group and 1.4% (n = 1) for the Modified Konno group (Supplemental Table S17).
Post-discharge outcomes based on fundamental diagnostic group demonstrated that patients with transposition-like lesions were more likely to undergo pacemaker generator change (n = 2, 28.6%, p = 0.02), and those patients with multiple left heart lesions were more likely to undergo mitral valve surgery (n = 7, 20%, p = 0.03) and mitral valve balloon dilation (n = 4, 11.4%, p = 0.02) (Table 9).
Figure 1 is a Kaplan–Meier analysis curve for the overall freedom from post-discharge LVOT re-interventions (primary outcome). The Konno group had a lower rate of post-discharge LVOT re-interventions [6 (11%) at 10 years for the Konno group versus 33 (47%) at 10 years for the Modified Konno group, log rank p = 0.002]. On multivariable cox modeling, the Modified Konno group had a significantly higher rate of LVOT re-interventions (hazard ratio 2.7, 95% confidence interval 1.2–5.9, p = 0.012) (Table 6).
Fig. 1.

Kaplan–Meier curve of freedom from unplanned LVOT re-interventions post-discharge from index surgery. Konno is represented in blue and Modified Konno in red. Numbers at risk at each time point are provided below the curve
Figure 2 is a Kaplan–Meier analysis curve for the overall freedom from post-discharge re-interventions. This curve demonstrates that there was no difference between the two groups for overall re-interventions [22 (44%) patients at 10 years in the Konno group versus 43 (60%) at 10 years in the Modified Konno group, log rank p = 0.144]. Multivariable Cox regression analysis for any post-discharge re-interventions showed no difference between the Konno and modified Konno groups (Table 8).
Fig. 2.
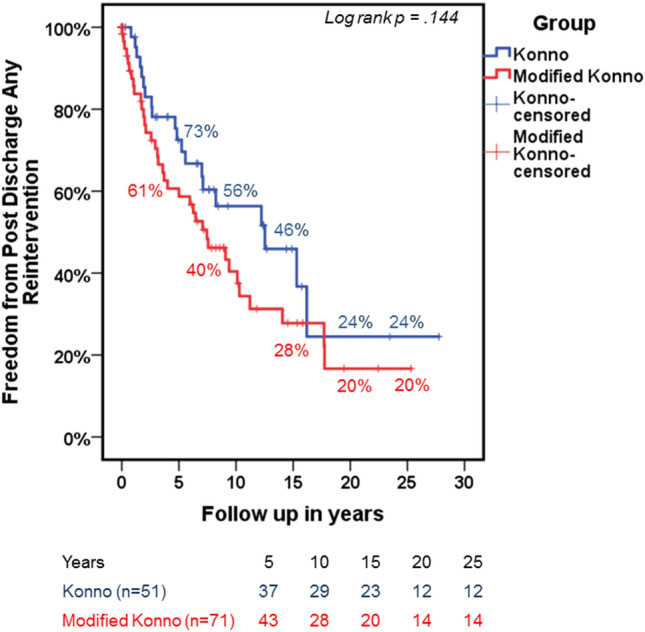
Kaplan–Meier curve of freedom from unplanned re-interventions post-discharge from index surgery. Konno is represented in blue and Modified Konno in red. Numbers at risk at each time point are provided below the curve
Overall Outcomes
Figure 3 represents the Kaplan–Meier curve for transplant-free survival following index operation; there was no significant difference between the Konno and Modified Konno groups [65 (92%) at 10 years for Modified Konno group versus 43 (85%) at 10 years for the Konno group, log rank p = 0.188]. Also, there was not a significant difference between the Konno and modified Konno groups for transplant-free survival on multivariable cox modeling (Table 9).
Fig. 3.

Kaplan–Meier curve of freedom from mortality and transplant from index surgery. Konno is represented in blue and Modified Konno in red. Numbers at risk at each time point are provided below the curve
Figure 4 provides a detailed flow diagram of outcomes in the Konno and Modified Konno groups based on anatomical area of re-intervention.
Fig. 4.
Flow diagram representing outcomes in Konno and Modified Konno groups
When compared based on age at index surgery, those patients who were younger at the time of the index Modified Konno procedure had a statistically shorter time to LVOT re-intervention (p = 0.04, Supplemental Fig. S5). There was not a significant correlation between age and time to LVOT re-intervention for those patients who had a Konno procedure, although younger patients did have a somewhat shorter time to LVOT re-intervention (p = 0.42, Supplemental Fig. S5). Also, there was not a significant correlation between age at index surgery and time to mortality for patients in the Modified Konno or Konno groups (p = 0.21 and p = 0.11, respectively, Supplemental Fig. S6). Overall, those patients who had a Modified Konno procedure were significantly younger than those who had a Konno procedure (p = 0.045, Supplemental Fig. S7).
When a composite measure, including post-index surgery mortality or transplant and any pre- or post-discharge intervention on the LVOT, was combined, there was a statistically significant difference between the Konno and Modified Konno groups (supplemental Fig. S8), with Modified Konno patients doing worse (log rank p = 0.03).
Discussion
There are several key findings in our study. We found that patients who had a Konno procedure were less likely to require re-intervention on the LVOT than those who had a Modified Konno procedure. Also, we found that there was no difference in overall re-interventions or transplant-free survival between the two groups. Finally, considering in-hospital outcomes, we found that patients in the Konno group had a longer PHLOS compared to those who had a Modified Konno procedure. A summary of the findings of similar outcomes in other studies involving the Konno and Modified Konno procedures is shown in Table 10.
Table 10.
Summary of findings in reference studies
| Title | Author | Year | Konno patient | Modified konno patients | Outcomes for konno patients | Outcomes for modified konno patients |
|---|---|---|---|---|---|---|
| Aortic valve replacement with concomitant aortoventriculoplasty in children and young adults: long-term follow-up | Fleming et al. | 1987 | 16 | 0 |
1 early death 1 late death 2 late reoperations: replacement of tissue aortic valves with mechanical aortic valves Mean follow-up: 66 months |
N/A |
| Diffuse subaortic stenosis: modified Konno procedures with aortic valve preservation | Vouhé et al. | 1993 | 0 | 11 | N/A |
1 early death 0 late deaths 1 early reoperation: aortic valvuloplasty for iatrogenic injury to the right coronary artery leaflet 1 late reoperation: VSD closure and mitral valvuloplasty Mean follow-up: 3.8 years |
| Konno aortoventriculoplasty with mechanical prosthesis in dealing with small aortic root: a good surgical option | Cobanoglu et al. | 1997 | 20 | 0 |
1 early death 1 late death 2 late reoperations: 1 cardiac transplant and 1 repeat Konno procedure 90% survival at 10 years 89% reoperation free survival at 10 years Mean hospital stay: 9.4 days Mean follow-up: 61 months |
N/A |
| Modified Konno-Rastan Procedure for Subaortic Stenosis: Indications, Operative Techniques, and Results | Roughneen et al. | 1998 | 0 | 16 | N/A |
0 early deaths 1 late death 1 late reoperation: redo Modified Konno 1 patient awaiting reoperation for aortic incompetence Mean follow-up: 62 months |
| Surgical treatment of subaortic stenosis: A seventeen-year experience | Serraf et al. | 1999 | 3 | 7 |
2 deaths 0 reoperations 1 patient with AV block |
0 deaths 0 reoperations |
| The Ross/Konno procedure in neonates and infants: intermediate-term survival and autograft function | Ohye et al. | 2001 | 10 | 0 |
0 early deaths 0 late deaths 0 late reoperations for aortic valve disease 3 patients with late reoperations: 1 MVR, 1 RV-PA allograft replacement and MVR, 1 RV-PA allograft replacement × 2 Median hospital length of stay: 13 days Median follow-up: 48 months |
N/A |
| Konno aortoventriculoplasty in children and adolescents: from prosthetic valves to the Ross operation | Erez et al. | 2002 | 60 | 0 |
11 early deaths 6 late deaths 16 late reoperations: 16 AVR, 2 MVR, 1 RVAD and cardiac transplant, 1 TVR, 1 PVR, 1 VSD closure Mean follow-up: 9.7 years |
N/A |
| The modified Konno procedure for complex left ventricular outflow tract obstruction | Caldarone et al. | 2003 | 0 | 18 | N/A |
0 early deaths 0 late deaths 0 reoperations Median follow-up: 3.1 years |
| The Ross-Konno Procedure in Children: Outcomes, Autograft and Allograft Function, and Reoperations | Brown et al | 2006 | 14 | 0 |
1 early death 1 late death 86% survival at 10 years 4 late reoperations: 3 patients had a conduit replacement, and 1 patient had an aortic root replacement (freedom from reoperation at 10 years = 69%) Mean length of hospital stay: 14 days Mean follow-up: 5.7 years |
N/A |
| Long-term results of the Konno procedure for complex left ventricular outflow tract obstruction | Suri et al | 2006 | 53 | 0 |
4 early deaths 86% survival at 10 years 16 late aortic reoperations in 15 patients, 3 PVR, 2 MVR, 2 TVR, 3 residual VSD’s and 1 cardiac transplant |
N/A |
| Modified Konno operation for diffuse subaortic stenosis | Metton et al. | 2008 | 0 | 31 | N/A |
0 early deaths 1 late death 1 early reoperation: aortic valve repair 2 late reoperations: Ross-Konno and aortic valve replacement Mean follow-up: 95 months |
| Long-term results of Konno procedure for complex left ventricular outflow tract obstruction | Sakamoto et al. | 2008 | 63 | 0 |
1 early death 6 late deaths 91.9% survival at 10 years 5 patients with late AVR, 3 patients with MVR, 2 patients with CABG, 1 patient with grafting of descending aorta (9 patients with reoperation overall) |
N/A |
| Ross and Ross-Konno Procedures in Infants, Childrenand Adolescents: A 13-Year Experience | Piccardo et al. | 2009 | 9 | 0 |
1 early death (diffuse brain ischemia) 0 late deaths Mean follow-up: 5.5 years |
N/A |
| Ross-Konno operation for patients with shone complex | Aeba et al. | 2010 | 2 | 0 |
0 early deaths 0 late deaths |
N/A |
| Ross-Konno procedure in children: midterm results | Hraska et al. | 2010 | 29 | 0 |
0 early deaths 1 late death (bacterial endocarditis) 96% survival at 7 years 3 early reoperations: 2 patients had diaphragm plication for diaphragm paresis, and 1 patient had a MVR 9 late reoperations: 4 conduit exchanges, 1 MVR, 1 LIMA graft, 2 pulmonary homograft replacements, and 1 septal patch aneurysm repair Median follow-up: 2.4 years |
N/A |
| Long-term results of the modified Konno procedure in high-risk children with obstructive hypertrophic cardiomyopathy | Laredo et al. | 2018 | 0 | 79 |
5 early deaths: refractory heart failure 3 late deaths: 1 acute MI, 1 from regurgitation post-MVR, and 1 from severe heart failure 8 late reoperations: 3 cardiac transplants, 3 residual VSD closures, 1 operation for aortic valve regurgitation, and 1 MVR Median hospital stay: 10 days Mean follow-up: 6 years |
|
| Twenty-year experience with the Konno operation: Konno incision does not impair LV function | Matsuzaki et al. | 2018 | 63 | 0 |
1 early death 9 late deaths 87.5% survival at 10 years 5 late reoperations on the aortic valve, 3 MVR, 2 CABG, 1 descending aortic replacement, 1 hemiarch replacement, 1 Bentall procedure, 1 BD re-coarctation, 5 patients with 7 catheter re-interventions Avoidance of LVOT reoperation at 10 years: 94.7% Avoidance of all events: 83% at 10 years Mean follow-up: 20.6 years |
N/A |
| Modified Ross–Konno procedure in children: subcoronary implantation technique with Konno incision for annular and subannular hypoplasia | Murin et al | 2018 | 13 | 0 |
1 Early death 0 Late deaths 0 Late reoperations ore catheter re-interventions Median follow-up: 20 months |
N/A |
| The Konno operation Is a durable option for relief of aortic stenosis in patients with complex left ventricular outflow tract obstruction—a single-center 20-Year experience | Matsushima et al. | 2019 | 21 | 0 |
2 Early deaths 4 Late deaths 72% Survival at 10 years 2 Early reoperations: mitral valve replacement 2 Late reoperations: 1 MVR and one cardiac transplant Avoidance of reaoperation: 89% at 10 years Mean follow-up: 7.6 years |
N/A |
N/A not applicable, VSD ventricular septal defect, LVOTO left ventricular outflow tract obstruction, PVR pulmonary valve replacement, MVR mitral valve replacement, TVR tricuspid valve replacement, AVR aortic valve replacement, CABG coronary artery bypass graft, LIMA left internal mammary artery, AV atrioventricular, MI myocardial infarction
In the Konno group of our study, 17% of the patients had a LVOT re-intervention post-discharge, and 39.6% of patients required a re-intervention overall. Post-discharge mortality was 9.4%. In the Modified Konno group of our study, 38.6% of patients required post-discharge re-intervention for LVOTO, and 50% patients required a re-intervention overall. Post-discharge mortality was 1.4%.
The reported LVOT re-intervention rate ranged from 0–14% for the Konno procedure and 0%-12.5% for the Modified Konno procedure [5, 10, 17–21]. The overall re-intervention rate ranged from 0–32% for the Konno procedure and 0–12.5% for the Modified Konno procedure [5, 10, 17–23]. Mortality rate ranged from 0–19% for the Konno procedure and 0–6.3% for the Modified Konno procedure [10, 14, 17–29]. The median follow-up time for these studies ranged from 1.7–4 years (Table 10). The differences in reported outcomes between our study and other studies may be attributed to smaller sample sizes, shorter follow-up times, or specific study populations depending on the report. In addition, our study included both catheter and surgical re-interventions, which were only definitively included in two previous studies [25, 26]. Some of the variation, particularly in length of stay, may be attributable to improvements in postoperative care in the more recent era.
Our study involves different patient populations, as evidenced by our fundamental diagnostic groups. Although the Konno procedure was performed more frequently in those patients with multiple left heart lesions, and the Modified Konno procedure was performed more frequently in those patients with AV canal defects or DORV/VSD/TOF-like lesions, our study provides useful information for comparing the two procedures overall. In the future, when larger patient cohorts are available, we will consider the outcomes of the Konno and Modified Konno procedures for each fundamental diagnostic group.
Those patients with a fundamental diagnosis of multiple left heart lesions appear to have worse in-hospital outcomes, with a greater number of complications, overall re-interventions and surgical re-interventions. They also had a longer ICU LOS and PHLOS. This may be attributed to fact that their surgeries often involved both the aortic valve and the mitral valve. Post-discharge, these patients were more likely to require intervention on the mitral valve. This is expected, as many of the left heart lesions involved the mitral valve. Notably, there were no significant differences in post-discharge LVOT re-interventions or mortality between the fundamental diagnostic groups.
Our study found that patients who had a Modified Konno procedure were younger at the time of index surgery, and younger patients in both the Modified Konno and Konno groups had a shorter time interval between their index surgery and any subsequent LVOT interventions. In younger patients, there is a surgical preference for preserving the native aortic valve, and our findings suggest that a Modified Konno procedure was favored over a Konno procedure in this patient population. Each patient must therefore be carefully considered to determine the most appropriate surgery for LVOTO, as well as optimal timing for intervention.
Study Limitations and Future Recommendations
This retrospective study had inherent issues of missing data in both groups. Clinical charts and echocardiographic databases before 2000 had incomplete information. Preoperative patient selection may be biased by clinician preference and it is likely that the patient groups are not completely comparable. Patients undergoing Konno operations may represent those with forms of LVOTO that are at the more severe end of the diagnostic spectrum. Further prospective multicenter investigation may help resolve these limitations.
Conclusion
Overall, this study demonstrated that Modified Konno patients were at greater risk for LVOT re-intervention and Konno patients have a longer PHLOS, but other in-hospital and late outcomes did not differ. Morphology of the LVOT should be considered when deciding on choice of procedure for complex LVOTO. Fundamental diagnosis is a significant factor when deciding if a Konno or Modified Konno procedure is appropriate for each patient.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (DOCX 16 kb)
Electronic supplementary material 4 (DOCX 16 kb)
Electronic supplementary material 5 (DOCX 15 kb)
Electronic supplementary material 6 (DOCX 15 kb)
Electronic supplementary material 7 (DOCX 20 kb)
Electronic supplementary material 8 (DOCX 18 kb)
Electronic supplementary material 9 (DOCX 14 kb)
Supplemental Fig. 5 Time from index surgery to LVOT re-intervention based on age at index surgery Electronic supplementary material 10 (TIF 82 kb)
Electronic supplementary material 11 Supplemental Fig. 6 Time from index surgery to death based on age at index surgery (TIF 68 kb)
Electronic supplementary material 2 Supplemental Fig. 7 Box plot comparing age at index surgery for Konno and Modified Konno groups (TIF 49 kb)
Electronic supplementary material 3 Supplemental Fig. 8 Kaplan–Meier curve for the composite outcome of mortality/transplant or LVOT re-intervention post-index surgery for Konno or Modified Konno groups (TIF 88 kb)
Funding
There was departmental funding for this work.
Compliance with Ethical Standards
Conflicts of interests
There are no disclosures to report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mahwish Haider, Laura Carlson have contributed equally to this work.
References
- 1.Maron BJ, Henry WL, Clark CE, Redwood DR, Roberts WC, Epstein SE. Asymetric septal hypertrophy in childhood. Circulation. 1976;53(1):9–19. doi: 10.1161/01.cir.53.1.9. [DOI] [PubMed] [Google Scholar]
- 2.Quinones JA, DeLeon SY, Vitullo DA, Hofstra J, Cziperle DJ, Shenoy KP, Fisher EA, et al. Regression of hypertrophic cardiomyopathy after modified konno procedure. The Annals of Thoracic Surgery. 1995;60(5):1250–1254. doi: 10.1016/0003-4975(95)00585-9. [DOI] [PubMed] [Google Scholar]
- 3.Stulak JM, Burkhart HM, Dearani JA, Schaff HV, Cetta F, Barnes RD, Puga FJ. Reoperations After Initial Repair of Complete Atrioventricular Septal Defect. The Annals of Thoracic Surgery. 2009;87(6):1872–1878. doi: 10.1016/j.athoracsur.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 4.Pontailler M, Capderou A, Lebret E, Vergnat M, Ly M, Roussin R, Belli E. Subaortic Area at Risk for Development of Obstruction After Surgical Repair of Atrioventricular Septal Defect. World Journal for Pediatric and Congenital Heart Surgery. 2015;6(3):407–412. doi: 10.1177/2150135115588335. [DOI] [PubMed] [Google Scholar]
- 5.Laredo M, Khraiche D, Raisky O, Gaudin R, Bajolle F, Maltret A, Chevret S, Bonnet D, Vouhé PR. Long-term results of the modified Konno procedure in high-risk children with obstructive hypertrophic cardiomyopathy. The Journal of Thoracic and Cardiovascular Surgery. 2018;156(6):2285–2294.e2. doi: 10.1016/j.jtcvs.2018.06.040. [DOI] [PubMed] [Google Scholar]
- 6.Mavroudis C, Mavroudis CD, Jacobs JP. The Ross, Konno, and Ross-Konno operations for congenital left ventricular outflow tract abnormalities. Cardiol Young. 2014;24(6):1121–1133. doi: 10.1017/s1047951114002042. [DOI] [PubMed] [Google Scholar]
- 7.Erez E, Kanter KR, Tam VK, Williams WH. Konno aortoventriculoplasty in children and adolescents: from prosthetic valves to the ross operation. The Annals of Thoracic Surgery. 2002;74(1):122–126. doi: 10.1016/s0003-4975(02)03595-6. [DOI] [PubMed] [Google Scholar]
- 8.Konno S, Imai Y, Iida Y, Nakajima M, Tatsuno K. A new method for prosthetic valve replacement in congenital aortic stenosis associated with hypoplasia of the aortic valve ring. J Thorac Cardiovasc Surg. 1975;70(5):909–917. doi: 10.1016/S0022-5223(19)39673-4. [DOI] [PubMed] [Google Scholar]
- 9.van Son JA, Schaff HV, Danielson GK, Hagler DJ, Puga FJ. Surgical treatment of discrete and tunnel subaortic stenosis. Late survival and risk of reoperation. Circulation. 1993;88(5 Pt 2):159–169. [PubMed] [Google Scholar]
- 10.Serraf A, Zoghby J, Lacour-Gayet F, Houel R, Belli E, Galletti L, Planché C. Surgical treatment of subaortic stenosis: A seventeen-year experience. The Journal of Thoracic and Cardiovascular Surgery. 1999;117(4):669–678. doi: 10.1016/s0022-5223(99)70286-2. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Ma K, Hu S, Hua Z, Yang K, Yan J, Chen Q. Surgical outcomes of 380 patients with double outlet right ventricle who underwent biventricular repair. The Journal of Thoracic and Cardiovascular Surgery. 2014;148(3):817–824. doi: 10.1016/j.jtcvs.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Kosaka Y, Kurosawa H, Nagatsu M. Konno Procedure Using Atrioventricular Groove Patch Plasty After Arterial Switch Operation. The Annals of Thoracic Surgery. 2004;78(5):1854–1855. doi: 10.1016/j.athoracsur.2003.08.060. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto T, Matsumura G, Kosaka Y, Iwata Y, Yamamoto N, Saito S, Kurosawa H. Long-term results of Konno procedure for complex left ventricular outflow tract obstruction. Eur J Cardiothorac Surg. 2008;34(1):37–41. doi: 10.1016/j.ejcts.2008.03.065. [DOI] [PubMed] [Google Scholar]
- 14.Cobanoglu A. Konno-aortoventriculoplasty with mechanical prosthesis in dealing with small aortic root: a good surgical option. Eur J Cardiothorac Surg. 1997;12(5):766–770. doi: 10.1016/s1010-7940(97)00221-2. [DOI] [PubMed] [Google Scholar]
- 15.Suri RM, Dearani JA, Schaff HV, Danielson GK, Puga FJ. Long-term results of the Konno procedure for complex left ventricular outflow tract obstruction. The Journal of Thoracic and Cardiovascular Surgery. 2006;132(5):1064–1071.e2. doi: 10.1016/j.jtcvs.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Daniel T, Jokhadar M, Sahu A, Kogon B. Konno Aortoventriculoplasty Following Arterial Switch Operation Requires Pulmonary Valve Disruption and Right Ventricular Outflow Tract Reconstruction. Congenital Heart Disease. 2012;8(4):E115–E118. doi: 10.1111/j.1747-0803.2012.00693.x. [DOI] [PubMed] [Google Scholar]
- 17.Caldarone CA, Van Natta TL, Frazer JR, Behrendt DM. The modified Konno procedure for complex left ventricular outflow tract obstruction. The Annals of Thoracic Surgery. 2003;75(1):147–151. doi: 10.1016/s0003-4975(02)03985-1. [DOI] [PubMed] [Google Scholar]
- 18.Roughneen PT, DeLeon SY, Cetta F, Vitullo DA, Bell TJ, Fisher EA, Blakeman BP, Bakhos M. Modified Konno-Rastan Procedure for Subaortic Stenosis: Indications, Operative Techniques, and Results. The Annals of Thoracic Surgery. 1998;65(5):1368–1376. doi: 10.1016/s0003-4975(97)01421-5. [DOI] [PubMed] [Google Scholar]
- 19.Metton O, Ali WB, Raisky O, Vouhe PR. Modified Konno operation for diffuse subaortic stenosis. Multimedia Manual of Cardio Thoracic Surgery. 2008 doi: 10.1510/mmcts.2008.003426. [DOI] [PubMed] [Google Scholar]
- 20.Vouhe P, Ouaknine R, Poulain H, Vernant F, Mauriat P, Pouard P, Leca F, et al. Diffuse subaortic stenosis: modified Konno procedures with aortic valve preservation. Eur J Cardiothorac Surg. 1993;7(3):132–136. doi: 10.1016/1010-7940(93)90035-a. [DOI] [PubMed] [Google Scholar]
- 21.Ohye RG, Gomez CA, Ohye BJ, Goldberg CS, Bove EL. The Ross/Konno procedure in neonates and infants: intermediate-term survival and autograft function. The Annals of Thoracic Surgery. 2001;72(3):823–830. doi: 10.1016/s0003-4975(01)02814-4. [DOI] [PubMed] [Google Scholar]
- 22.Hraska V, Krajci M, Haun C, Ntalakoura K, Razek V, Lacour-Gayet F, Reichenspurner H, et al. Ross and Ross-Konno procedure in children and adolescents: mid-term results☆. Eur J Cardiothorac Surg. 2004;25(5):742–747. doi: 10.1016/j.ejcts.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Brown JW, Ruzmetov M, Vijay P, Rodefeld MD, Turrentine MW. The Ross-Konno Procedure in Children: Outcomes, Autograft and Allograft Function, and Reoperations. The Annals of Thoracic Surgery. 2006;82(4):1301–1306. doi: 10.1016/j.athoracsur.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Fleming WH, Sarafian LB. Aortic Valve Replacement with Concomitant Aortoventriculoplasty in Children and Young Adults: Long-term Follow-up. The Annals of Thoracic Surgery. 1987;43(6):575–578. doi: 10.1016/s0003-4975(10)60224-x. [DOI] [PubMed] [Google Scholar]
- 25.Murin P, Sinzobahamvya N, Schulz A, Lorenzen V, Ovroutski S, Berger F, Photiadis J, Cho M-Y. Modified Ross-Konno procedure in children: subcoronary implantation technique with Konno incision for annular and subannular hypoplasia†. Interactive CardioVascular and Thoracic Surgery. 2018;27(2):264–268. doi: 10.1093/icvts/ivy063. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzaki Y, Hiramatsu T, Sakamoto T, Nagashima M, Niinami H, Yamazaki K. Twenty-year experience with the Konno operation: Konno incision does not impair LV function. General Thoracic and Cardiovascular Surgery. 2018;66(5):270–275. doi: 10.1007/s11748-018-0896-z. [DOI] [PubMed] [Google Scholar]
- 27.Matsushima S, Burri M, Strbad M, Ruf B, Lange R, Hörer J, Cleuziou J. The Konno Operation Is a Durable Option for Relief of Aortic Stenosis in Patients With Complex Left Ventricular Outflow Tract Obstruction—A Single-Center 20-Year Experience. World Journal for Pediatric and Congenital Heart Surgery. 2019;10(6):678–685. doi: 10.1177/2150135119872476. [DOI] [PubMed] [Google Scholar]
- 28.Piccardo A, Ghez O, Gariboldi V, et al. Ross and Ross-Konno procedures in infants, children and adolescents: a 13-year experience. J Heart Valve Dis. 2009;18:76–82. [PubMed] [Google Scholar]
- 29.Aeba R, Okamoto K, Yozu R. Ross-Konno operation for patients with Shone complex. Tex Heart Inst J. 2010;37(2):240–241. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (DOCX 16 kb)
Electronic supplementary material 4 (DOCX 16 kb)
Electronic supplementary material 5 (DOCX 15 kb)
Electronic supplementary material 6 (DOCX 15 kb)
Electronic supplementary material 7 (DOCX 20 kb)
Electronic supplementary material 8 (DOCX 18 kb)
Electronic supplementary material 9 (DOCX 14 kb)
Supplemental Fig. 5 Time from index surgery to LVOT re-intervention based on age at index surgery Electronic supplementary material 10 (TIF 82 kb)
Electronic supplementary material 11 Supplemental Fig. 6 Time from index surgery to death based on age at index surgery (TIF 68 kb)
Electronic supplementary material 2 Supplemental Fig. 7 Box plot comparing age at index surgery for Konno and Modified Konno groups (TIF 49 kb)
Electronic supplementary material 3 Supplemental Fig. 8 Kaplan–Meier curve for the composite outcome of mortality/transplant or LVOT re-intervention post-index surgery for Konno or Modified Konno groups (TIF 88 kb)



