Abstract
The meninges serve as a functional barrier surrounding the brain, critical to the immune response, and can be compromised following head trauma. Meningeal enhancement can be detected on contrast-enhanced MRI in patients presenting with acute traumatic brain injury, even when head CT is negative. Following head trauma, gadolinium-based contrast appears to extravasate from the vasculature, enhancing the dura within minutes, and later permeates the subarachnoid space. The aims of this study were to characterize the initial kinetics of the uptake of contrast agent after injury and the delayed redistribution of contrast enhancement in the subarachnoid space in hyperacute patients. Neuroimaging was obtained prospectively in two large ongoing observational studies of patients aged 18 years or older presenting to the emergency department with suspected acute head injury. Dynamic contrast-enhanced MRI studies in a cohort of consecutively enrolling patients with mild traumatic brain injury (n = 36) determined that the kinetic half-life of dural-related meningeal enhancement was 1.3 ± 0.6 min (95% enhancement within 6 min). The extravasation of contrast into the subarachnoid space was investigated in a cohort of CT negative mild traumatic brain injury patients initially imaged within 6 h of injury (hyperacute) who subsequently underwent a delayed MRI, with no additional contrast administration, several hours after the initial MRI. Of the 32 patients with delayed post-contrast imaging, 18 (56%) had conspicuous expansion of the contrast enhancement into the subarachnoid space, predominantly along the falx and superior sagittal sinus. Patients negative for traumatic meningeal enhancement on initial hyperacute MRI continued to have no evidence of meningeal enhancement on the delayed MRI. These studies demonstrate that (i) the initial enhancement of the traumatically injured meninges occurs within minutes of contrast injection, suggesting highly permeable meningeal vasculature, and that (ii) contrast in the meninges redistributes within the subarachnoid space over the period of hours, suggesting a compromise in the blood–brain and/or blood-cerebrospinal barriers. Data from the parent study indicate that up to one in two patients with mild traumatic brain injury have traumatic brain injury on acute (<48 h) MRI, with a higher prevalence seen in patients with moderate or severe traumatic brain injury. The current study’s findings of traumatic meningeal enhancement and the subsequent delayed extravasation of contrast into the subarachnoid spaces indicate that a substantial percentage of patients with even mild traumatic brain injury may have a transient disruption in barriers separating the vasculature from the brain.
Keywords: traumatic brain injury, neuroimaging, MRI, blood–brain barrier, blood-cerebrospinal fluid barrier
After mild traumatic brain injury, traumatic meningeal enhancement and extravasation of contrast into subarachnoid space can be detected within hours of injury. The presence of these novel neuroimaging markers suggests that blood-meningeal barriers can be compromised in the acute phase, even in patients with no other findings of intracranial injury.
Graphical Abstract
Graphical Abstract.
Introduction
Complex barriers between the central nervous system and the peripheral circulation were first detected over a century ago through the failure of intravenous dyes to cross into the brain (Engelhardt and Sorokin, 2009). First viewed as passive barriers shielding the brain parenchyma from peripheral immune responses, the blood–brain and blood-cerebrospinal fluid barriers are now recognized as part of a system of neurovascular coupling and of active surveillance and coordinated immune responses (Engelhardt et al., 2017; Mastorakos and McGavern, 2019). Studies elucidating the biology of the brain’s immune response implicate disruption of these barriers as contributors to the neuronal and cognitive dysfunction that occurs in normal aging, vascular cognitive impairment, Alzheimer’s disease, multiple sclerosis and other neurodegenerative disorders (Iadecola, 2017).
The meninges reside in the space between the skull and the brain parenchyma’s glial limitans, the outer layer of the blood–brain barrier. In traumatic brain injury (TBI), traumatic vascular injury may occur in the brain parenchyma (e.g. intraparenchymal haemorrhage or microbleeds) or in vessels traversing the meninges (e.g. subdural haematoma or subarachnoid haemorrhage) (Kenney et al., 2016). As arterioles and venules travel from the parenchyma through the subarachnoid space to the dura, they are highly susceptible to mechanical shearing and stretching forces. Traumatic meningeal enhancement (TME), associated with meningeal injury, can be observed on contrast-enhanced MRI after acute TBI (Roth et al., 2014; Ricciardi et al., 2017; Davis et al., 2020) and at 2 weeks after injury (Kim et al., 2014; Russo et al., 2018). TME, in the absence of concurrent subdural or subarachnoid haemorrhage, represents meningovascular injury of sufficient severity to permit leakage of contrast, but not erythrocytes, into the potential subdural space and is consistent with the radiological construct of an acute subdural effusion.
TBI shares many pathologic features similar to stroke, including blood–brain barrier disruption (Roth et al., 2014; Russo et al., 2018). Gadolinium, which primarily binds to albumin, ordinarily cannot cross an intact blood–brain barrier and acts a surrogate for leakage of proteins and other macromolecules when detected outside of the vasculature. This disruption can be visualized by the delayed enhancement of cerebrospinal fluid space after administration of gadolinium-based contrast agents on fluid-attenuated inversion recovery (FLAIR) MRI in both ischaemic (Warach and Latour, 2004; Barr et al., 2010) and haemorrhagic (Kidwell et al., 2011) stroke. This phenomenon, known as hyperintense acute reperfusion marker (HARM), results from the leakage of gadolinium-based contrast agent across a damaged blood–brain barrier into the cerebrospinal fluid space (Warach and Latour, 2004; Kohrmann et al., 2012). HARM was first detected on early follow-up scans in stroke patients, suggesting that it takes time for intravascular contrast to leak from blood vessels into the subarachnoid space. The presence of TME, with known meningeal injury, raises the possibility that a phenomenon similar to HARM may occur after TBI.
The present study provides insight into the pathophysiology of the meningeal/vascular injury by investigating the temporal pattern of enhancement on post-contrast FLAIR MRI in two studies of acute mild TBI patients to provide insight into the pathophysiology of the meningeal/vascular injury. The aim of ‘Study 1—Rapid Kinetics of TME’ was to define the kinetics of gadolinium-based contrast agent extravasation that allow the visualization of TME. The aim of “Study 2—Extravasation of Contrast into the Subarachnoid Space (ECSAS)” was conducted to explore delayed ECSAS on FLAIR MRI in hyperacute TBI. Assuming that disruption would most likely occur early after injury, analogous to HARM seen in stroke, mild TBI patients imaged within 6 h of their initial injury were enrolled for Study 2.
Materials and methods
Population
Patients in this analysis were enrolled and imaged under one of the two ongoing prospective TBI studies (ClinicalTrials.gov identifiers NCT01132937 and NCT01287156) with both National Institutes of Health and local site Institutional Review Board approval at a Level 1 trauma centre (MedStar Washington Hospital Center in Washington, District of Columbia) and a Level 2 trauma centre (Suburban Hospital in Bethesda, Maryland). In accordance with the Declaration of Helsinki, written informed consent was obtained for all patients prior to initiation of study procedures. Demographics, history, injury details, presentation and standard of care imaging results were prospectively collected as previously described (Griffin et al., 2019). To be included in the cohort for retrospective analysis presented here, patients had to be over 18 years old and have presented to the emergency room with acute head injury and suspected TBI, met American Congress of Rehabilitation Medicine criteria for ‘mild TBI’ (Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine, 1993), and been able to undergo a research MRI with a gadolinium-based contrast agent. Two cohorts were defined and studied.
Study 1—Rapid kinetics of TME
Patients received baseline research MRI within 96 h of head injury and underwent dynamic contrast-enhanced FLAIR imaging obtained over a period of ∼8 min following injection. The dynamic contrast-enhanced FLAIR was an optional component of the research MRI and was not performed unless (i) contrast could be administered, (ii) patients were agreeable and (iii) logistics permitted. A positive head CT scan was not an exclusion criteria for Study 1.
Study 2—Extravasation of contrast into the subarachnoid space (ECSAS)
Patients with negative head CT who received baseline research MRI within 6 h of their injury including an immediate post-contrast FLAIR ∼6 min after gadolinium-based contrast agent injection, underwent a delayed post-contrast FLAIR with no additional contrast administration ∼3 h after injection. This delayed scan was an optional component of the research study that was not performed unless (i) patients were agreeable and (ii) logistics permitted. To avoid confounding signal from blood, those with acute extra-axial haemorrhage detected on the research MRI were excluded from the quantitative analysis portion of Study 2. All patients were offered an additional MRI at follow-up ∼1-week post-injury.
Magnetic resonance imaging
Imaging was performed on two 3T MRI systems using commercially available sequences (Magnetom Skyra; Siemens Healthcare, Malvern, Pennsylvania and Achieva; Philips Medical Systems, Cleveland, Ohio). Patients received a standardized research MRI exam which, in addition to other sequences (Ricciardi et al., 2017), included a pre-contrast FLAIR acquisition (TR/TE/TI 9000/120/2500 ms), 40 contiguous interleaved slices, 3.5 mm thick with ∼1mm in plane resolution. A single dose of gadolinium-based contrast agent (0.1 mmol kg−1 gadobenate dimegulmine; Braco Diagnostics, or 0.2 mmol kg−1 gadopentetate dimeglumine; Bayer HealthCare, Washington, District of Columbia) was intravenously administered by power injection at 1 ml/s. A clinical neuroradiologist at each site reviewed all MRI exams to provide reports with clinical interpretation.
For Study 1 (Kinetics), a series of four FLAIR scans was obtained immediately following contrast injection, with ∼100 s per dynamic scan (Fig. 1A). For Study 2 (ECSAS), a post-contrast FLAIR was obtained 6 min after injection of gadolinium-based contrast agent (gadobenate dimegulmine or gadopentetate dimeglumine as described above) (Fig. 1B). Depending upon resource availability and clinical care needs, ∼3 h after contrast injection patients had an additional post-contrast FLAIR (delayed post-contrast FLAIR), obtained without additional administration of additional gadolinium-based contrast.
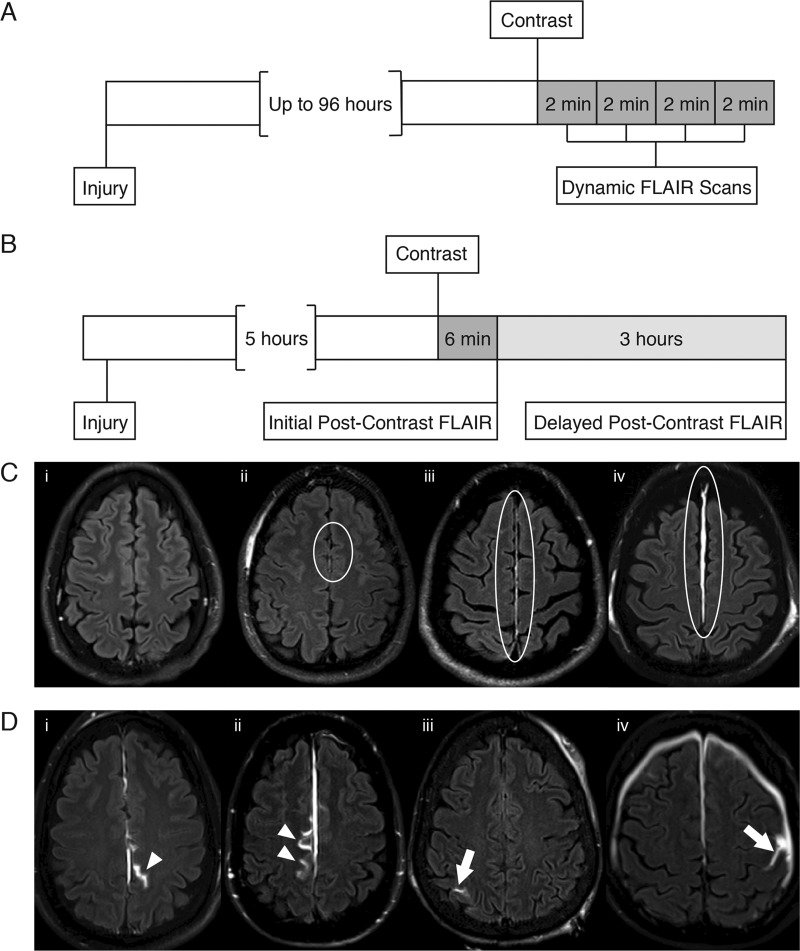
Figure 1.
Timelines of image acquisition for this study (A, B) and definitions for imaging analyses (C, D). (A) Study 1—Rapid Kinetics of Traumatic Meningeal Enhancement (TME): After contrast injection, a series of four dynamic FLAIR scans were obtained. (B) Study 2—Extravasation of Contrast into Subarachnoid Space (ECSAS): Immediate post-contrast FLAIR was acquired ∼5 h after injury and delayed post-contrast FLAIR acquired ∼3 h after contrast injection. (C) For grading of TME, examples in the falx are shown to demonstrate the grading system: (i) none, (ii) subtle, (iii) conspicuous and (iv) space-occupying. TME is within the area encompassed by the white ellipses. (D) Localization of ECSAS was classified as relative to the falx/parafalcine (white arrowheads) or convexity (white arrows).
Imaging analysis
Study 1 (Kinetics) imaging analysis
For each patient in the dynamic contrast-enhanced FLAIR set, the immediate post-contrast FLAIR was compared to the final post-contrast FLAIR. Raters were blinded to the order of scans and asked to identify the presence/absence of TME in each scan, if there was a visually detectable difference in enhancement, and, if so, in which image the TME was brighter.
For all scans identified as positive for TME, quantitative volume of interest analysis of DICOM images was performed in MIPAV (Medical Image Processing, Analysis and Visualization Software; Center for Information Technology, National Institutes of Health). Pre-contrast images were aligned to the mid-sagittal plane. Post-contrast images were aligned to the pre-contrast FLAIR using rigid registration with trilinear interpolation. Regions of maximal enhancement (volumes of interest) were defined by threshold-based segmentation from the fourth post-contrast FLAIR acquisition to create a mask of enhancing regions. Volumes of interest were manually grouped according to brain region (falx, convexity, cerebellum, vertex) and then transposed onto the preceding FLAIR acquisitions. The average voxel intensity for each volume of interest group was calculated for each FLAIR time point and then normalized to a volume of interest in the white matter.
Dynamics of contrast uptake were analysed in GraphPad Prism (Version 6) (GraphPad Software Inc., San Diego, California). Volume of interest voxel intensity data was normalized using min-max scaling with the pre-contrast voxel intensity set to 0 and the fourth post-contrast voxel intensity set to 1. A least-squares fit was used to estimate the exponential time constant (τ) of contrast uptake using
where S is the mean intensity of the volume of interest at time t from start of injection to start of each series.
Study 2 (ECSAS) imaging analysis
Two expert raters (with a third rater for tiebreaking), blinded to patient, TME status and immediate post-contrast FLAIR, independently reviewed only delayed post-contrast FLAIR for the presence/absence of contrast in the subarachnoid space (ECSAS) and for its location. Subsequently, expert raters, blinded to the results from the ECSAS read, reviewed the immediate post-contrast FLAIR to rate the presence, severity and location of TME. Severity of TME was graded on an ordinal scale; 1: none, 2: subtle, 3: conspicuous or 4: space-occupying (Fig. 1C). For those patients for whom a 1-week follow-up MRI was available, both pre- and post-contrast FLAIR obtained at that time were evaluated for the presence/absence of ECSAS and of TME.
Enhancement on delayed post-contrast FLAIR seen in the subarachnoid space was compared to the same, homologous region on the immediate post-contrast FLAIR using MIPAV. Mid-sagittal alignment was performed on both immediate and delayed post-contrast FLAIR, images were aligned using optimized automatic rigid registration with rotation and translation but no scaling or skew, and a subtraction image was calculated to visualize the pattern of contrast extravasation. Additionally, regions of positive enhancement were segmented and measured to determine the volume and intensity change.
Both studies imaging analysis
For both Study 1 and Study 2, pre- and post-contrast T1 and FLAIR, and gradient echo images were later examined by expert raters for the presence of parenchymal enhancement (Supplementary Fig. 1) and of traumatic microhaemorrhages (Supplementary Fig. 2), and, if seen, scored as in proximity to TME and/or ECSAS.
Statistical analysis
Study 1 (Kinetics) statistical analysis
Statistical analyses were performed in GraphPad Prism. Cohen’s kappa was used to measure inter-rater reliability for consensus reads. One-way repeated measure analysis of variance was used to assess TME time constants (τ) by brain region with P < 0.05 considered significant. Data reported are median (interquartile range) or mean (standard deviation) as appropriate.
Study 2 (ECSAS) statistical analysis
Statistical analyses for Study 2 were performed using either GraphPad Prism (Version 8) (GraphPad Software, San Diego, California) or SPSS (Version 22) statistical software (IBM, Armonk, New York). Categorical results were analysed through construction of contingency tables, with Cohen’s Kappa coefficient calculated as a measure of inter-rater reliability and interpreted using the Landis and Koch benchmark scale (Landis and Koch, 1977). NSI total scores, subgroup scores (Vestibular, Somatosensory, Cognitive and Affective), and Validity-10 scores were calculated per published procedures (Meterko et al., 2012; Vanderploeg et al., 2014; Lange et al., 2015; Vanderploeg et al., 2015; Lippa et al., 2016). Fisher’s exact tests and Mann–Whitney U tests were performed to characterize the population with/without subarachnoid space enhancement in the delayed post-contrast FLAIR. A repeated measures t-test was performed to determine the difference in volume of enhancement in the immediate and delayed post-contrast FLAIR.
Data availability
The authors confirm that the data supporting this study’s findings are available from the corresponding author upon reasonable request.
Results
Study 1—Rapid kinetics of TME
During the study period from November 2014 through June 2015, 102 consecutively enrolled patients were screened and 66 were excluded from this study (5 were outside the acute time window, 23 had moderate to severe TBI, 38 did not undergo the dynamic contrast-enhanced MRI). Of the 36 patients included in this analysis, 21 (58%) were female, 34 (94%) with Glasgow Coma Scale of 15 on presentation, 25 (69%) experienced post-traumatic loss of consciousness and the median age was 52 (33–60). The predominant mechanisms of injury were falls (47%) and acceleration/deceleration in motor vehicle collisions (33%). The median time from injury to MRI was 19 h (5.9–44).
Twelve out of 36 patients (33%) were positive for TME based on visual consensus (inter-rater agreement was excellent, kappa = 0.875 ± 0.085). Ten out of those 12 (83%) had visibly detectable increases in enhancement between first post-contrast and last post-contrast FLAIR in the dynamic contrast-enhanced sequence (kappa = 0.787 ± 0.115). Figure 2 shows an example of the dynamic contrast-enhanced FLAIR in a patient with TME and corresponding enhancement curves for the TME volumes of interest, as well as the results for quantitative volume of interest analysis from 30 discrete enhancing regions identified in 12 patients (11 falx, 6 vertex, 5 frontal, 4 temporal, 3 occipital, 1 cerebellar). Enhancement rates varied among regions and individuals (0.6≤ τ ≤ 3.6), with no identifiable systematic trend. One-way analysis of variance of enhancement constant (τ) by region showed no statistically significant differences. The average kinetic half-life of enhancement over all regions was 1.3 ± 0.6 min (95% enhancement within 6 min).
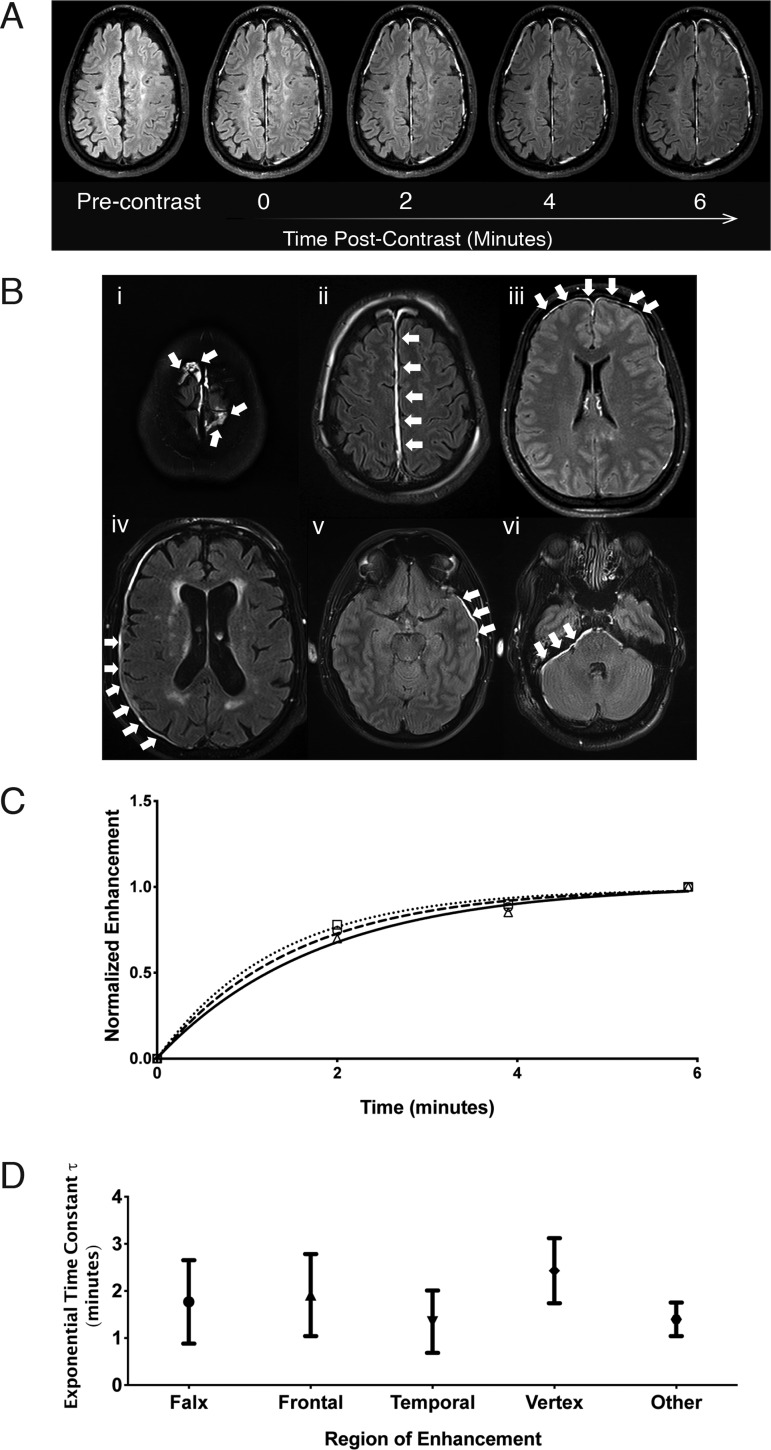
Figure 2.
Progression over time and characteristics of TME over time. (A) An example, indexed by approximate imaging time, of a patient showing progressive uptake of contrast in the meninges out to 6 min post-contrast. (B) Characteristics of TME in six common locations of enhancement: (i) vertex, (ii) falx, (iii) frontal convexity, (iv) parieto-occipital convexity, (v) temporal convexity and (vi) cerebellar tentorium. White arrows indicate TME in relevant areas. Representative images are from six separate patients. (C) Normalized average voxel intensity of three regions of the meninges of a single patient over 6 min after bolus contrast injection. The corresponding volumes of interest (VOI) are overlaid on the patient’s pre-contrast and post-contrast FLAIR images. τ for this individual = 1.3, >99% enhancement = 6.5 min. (D) The mean exponential time constant (τ) of enhancement for TME is similar across regions for patients with TME. Error bars represent ±1 standard deviation from the mean. One-way ANOVA F = 1.6, P-value = 0.2.
Study 2—ECSAS
During the study period of January 2015 through August 2019, 347 patients were enrolled and screened for inclusion in this sub-study; 250 (72%) had a negative head CT, 85 of 250 (34%) received MRI within 6 h of injury, and 75 of 85 (88%) received MRI contrast agent. Of the 75 patients who received contrast, 44 (59%) were positive for TME and 58 were offered a second scan. Of those offered a second scan, 23 refused, 1 could not be completed due to clinical care needs and 2 could not be done secondary to scheduling conflicts. A total of 32 of 58 (55%) patients approached were included in Study 2.
Table 1 shows the demographics of Study 2 patients stratified by presence or absence of subarachnoid space enhancement. Factors such as age, sex, mechanism of injury, past medical history/comorbidities, Glasgow Coma Scale and Glasgow Outcome Scale-Extended were not significantly different between the two groups. ECSAS negative patients had higher total NSI scores, with higher NSI subgroup Somatosensory and Cognitive scores, at 1 week post-injury, in comparison to ECSAS positive patients. Twenty-three (72%) of Study 2 patients were TME positive. Inter-rater agreement was excellent for the presence/absence of TME on both immediate and delayed post-contrast FLAIR, enhancement along convexity, enhancement of falx and on the TME grading scale (Fig. 1C); kappa = 1.0, 0.94, 0.85 and 0.79, respectively.
Table 1.
Characterization of the population for Study 2
| ECSAS |
P-value | ||
|---|---|---|---|
| Positive (n = 18) | Negative (n = 14) | ||
| Age (years) (median, IQR) | 57 (41–67) | 44 (33–53) | 0.067 |
| Male | 12 (66.7%) | 7 (50.0%) | 0.473 |
| Time (mean ± SD) | |||
| Injury to ED Triage (h) | 0.55 ± 0.24 | 0.58 ± 0.41 | 0.836 |
| Injury to CT (h) | 1.23 ± 0.39 | 1.30 ± 0.54 | 0.677 |
| Injury to first MRI (h) | 4.3 ± 0.87 | 4.8 ± 0.67 | 0.098 |
| First MRI to second MRI (min) | 171 ± 48 | 204 ± 59 | 0.099 |
| Race/ethnicity | 0.021 | ||
| White | 15 (83.3%) | 7 (50.0%) | |
| Black | 0 (0.0%) | 5 (35.7%) | |
| Other* | 3 (16.7%) | 2 (14.2%) | |
| Severity of injury | 0.629 | ||
| GCS = 15 | 17 (94.4%) | 12 (85.7%) | |
| GCS = 14 | 1 (5.6%) | 2 (14.3%) | |
| Mechanism of injury | 0.522 | ||
| Acceleration/deceleration | 7 (38.9%) | 3 (21.4%) | |
| Direct impact to head | 6 (33.3%) | 5 (35.7%) | |
| Fall | 5 (27.8%) | 6 (42.9%) | |
| Comorbidity | |||
| History of TBI | 8 (44.4%) | 8 (57.1%) | 0.722 |
| History of migraines | 1 (5.5%) | 4 (28.6%) | 0.142 |
| History of anxiety | 3 (16.7%) | 4 (28.6%) | 0.669 |
| History of depression | 3 (16.7%) | 1 (7.1%) | 0.613 |
| GOSE (median, IQR) | |||
| 30-day visit (n) | 18 (100%) | 10 (71%) | |
| 30-day GOSE | 7.5 (5.75–8) | 6 (4.75–8) | 0.224 |
| 90-day visit (n) | 15 (83%) | 9 (64%) | |
| 90-day GOSE | 8 (7–8) | 7 (5–8) | 0.086 |
| 1 Year Visit (n) | 15 (83%) | 4 (29%) | |
| 1-year GOSE | 7 (7–8) | 6.5 (5–8) | 0.276 |
| NSI (median, IQR) | |||
| 1-week visit (n) | 18 (100%) | 14 (100%) | |
| 1-week NSI total | 6.5 (1.75–14.25) | 20 (5.75–31) | 0.027 |
| 30-day visit (n) | 18 (100%) | 10 (71%) | |
| 30-day NSI total | 3 (0.75–13.5) | 11 (1–38) | 0.219 |
| NSI subgroups (median, IQR) | |||
| 1-week vestibular | 0 (0–2.25) | 2 (0–5) | 0.159 |
| 30-day vestibular | 0.5 (0–2) | 1.5 (0–5.5) | 0.360 |
| 1-week somatosensory | 1.5 (0.75–4.5) | 6.5 (2.75–10) | 0.003 |
| 30-day somatosensory | 0.5 (0–4) | 4.5 (0.75–11.5) | 0.037 |
| 1-week cognitive | 0 (0–2) | 2 (0–10) | 0.016 |
| 30-day cognitive | 0 (0–2) | 1 (0–8) | 0.154 |
| 1-week affective | 1.5 (0.75–4.25) | 3 (1–9.75) | 0.234 |
| 30-day affective | 0 (0–5) | 3.5 (0–10.75) | 0.371 |
ECSAS = extravasation of contrast into subarachnoid space; ED = emergency department; GCS = Glasgow Coma Scale; GOSE = Glasgow Outcome Scale-Extended; IQR = interquartile range; NSI = Neurobehavioral Symptom Inventory; Other* = Hispanic/Latino/Asian/American Indian/Native Hawaiian; SD = standard deviation. P-values in bold are <0.05.
Of Study 2 patients, 18 (56%) were scored as positive for ECSAS on the delayed post-contrast FLAIR. Inter-rater reliability was moderate for presence/absence of ECSAS, along the convexity, and adjacent to the falx; kappa = 0.69, 0.55, 0.8, respectively. Examples of findings and locations on delayed post-contrast scan that would be classified as ECSAS are shown in Fig. 1D. The morphology of ECSAS can be better appreciated in sequential axial sections (Fig. 3) or in multi-planar views (Fig. 4), rather than in a single section.
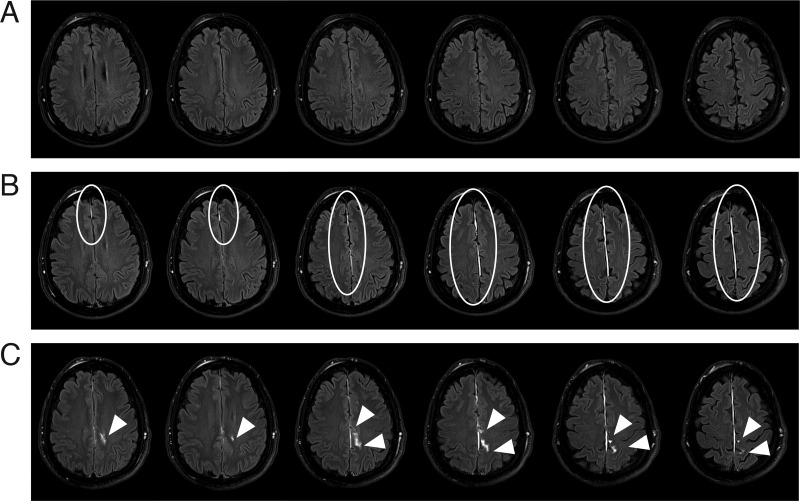
Figure 3.
Comparison of findings on pre-contrast, immediate post-contrast and delayed post-contrast FLAIR in a representative patient over sequential axial slices. (A) On pre-contrast FLAIR, the presence of FLAIR hyperintensities in subdural or subarachnoid spaces on the MRI of a patient with acute TBI can indicate acute subdural haematoma or acute subarachnoid haemorrhage. This patient has no evidence of FLAIR hyperintensities in either the subdural or the subarachnoid spaces prior to the administration of contrast. (B) Immediate post-contrast FLAIR of the same patient reveals TME in the falx (white ellipses), which appears in the potential subdural space. (C) The delayed post-contrast FLAIR of this patient shows persistent TME in the falx as well as the evolution of extravasation of contrast into subarachnoid space (ECSAS) (white arrowheads).
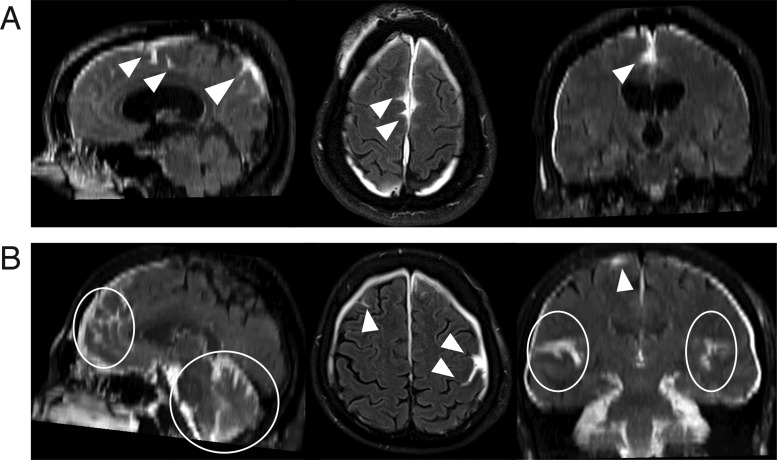
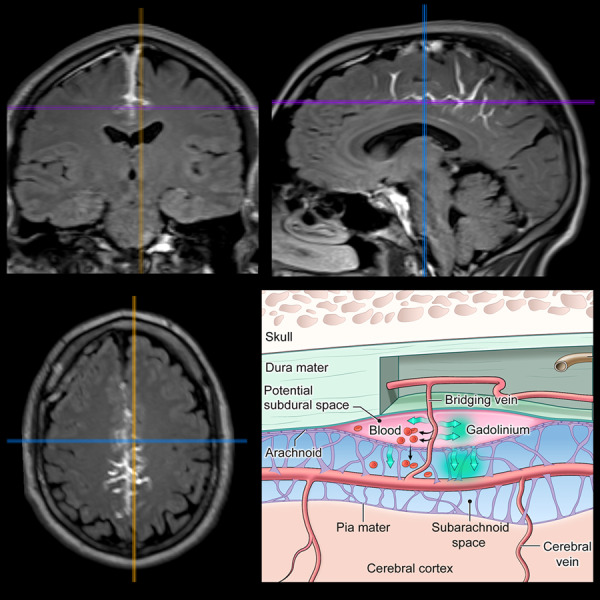
Figure 4.
Multiplanar views of extravasation of contrast into subarachnoid space (ECSAS), representing a breach of the blood-subarachnoid barrier. (A) A subject’s delayed post-contrast FLAIR shows ECSAS in the falx, with sagittal, axial and coronal views (left to right) clearly demonstrating the three-dimensional extravasation of contrast. (B) ECSAS can also occur along the bilateral convexities and cerebellum, as seen in another subject’s delayed post-contrast FLAIR. The white arrowheads and ellipses indicate areas of ECSAS.
ECSAS was not detected on the delayed post-contrast FLAIR in patients that were negative for TME on the immediate post-contrast FLAIR; the positive predictive value of TME for predicting ECSAS was 0.78. The association between TME and ECSAS on hyperacute MRI was significant (P < 0.001), as was the association between parafalcine TME and parafalcine ECSAS (P = 0.002). There was no association between TME in the convexity and ECSAS in the convexity. ECSAS was detected in 0 of 9, 2 of 4, 6 of 8 and 8 of 9 patients with none, subtle, conspicuous and space-occupying TME, respectively, suggesting a relationship between the severity of meningeal enhancement and leakage into the subarachnoid space (P < 0.001).
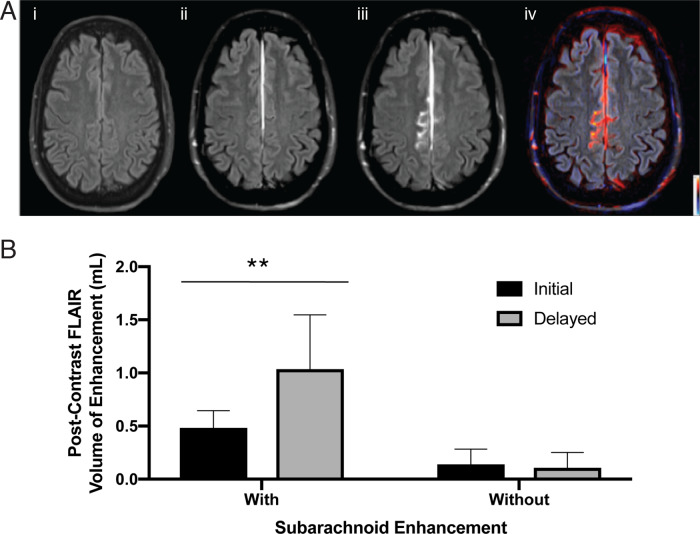
On quantitative analysis of ECSAS in the subset of ECSAS positive patients with no MRI evidence of extra-axial haemorrhage (n = 15), repeated measures t-test showed approximately a 2-fold increase in volume of enhancement in patients identified as having subarachnoid space enhancement (t(df)=3.73(6), P < 0.01) (Fig. 5). However, there was no significant difference in the immediate and delayed FLAIR sequences in patients identified as having no subarachnoid space enhancement.
Figure 5.
Quantitative analysis of ECSAS. (A) The initial FLAIR sequence (i) is prior to administration of contrast. The initial post-contrast FLAIR sequence (ii) demonstrates extravasation of contrast into the meningeal space ∼6 min after injection of gadolinium contrast. The delayed post-contrast FLAIR (iii) depicts leakage of contrast into the subarachnoid space ∼3 h after contrast injection. The subtraction image (iv) shows the pattern of contrast extravasation. (B) Comparison of volume of enhancement in immediate and delayed post-contrast FLAIR. Patients identified as having subarachnoid space enhancement (n = 7) had higher volume of enhancement compared to patients without subarachnoid enhancement (n = 8). (P < 0.01).
Twenty-eight of the Study 2 patients had 1-week follow-up MRI with and without gadolinium-based contrast agent. Neither TME nor ECSAS was visible on the pre-contrast FLAIR at 1-week follow-up (Fig. 6). However, 14 (50%) of the patients demonstrated persistent TME on post-contrast FLAIR obtained 6 min after gadolinium-based contrast agent administration at that visit.
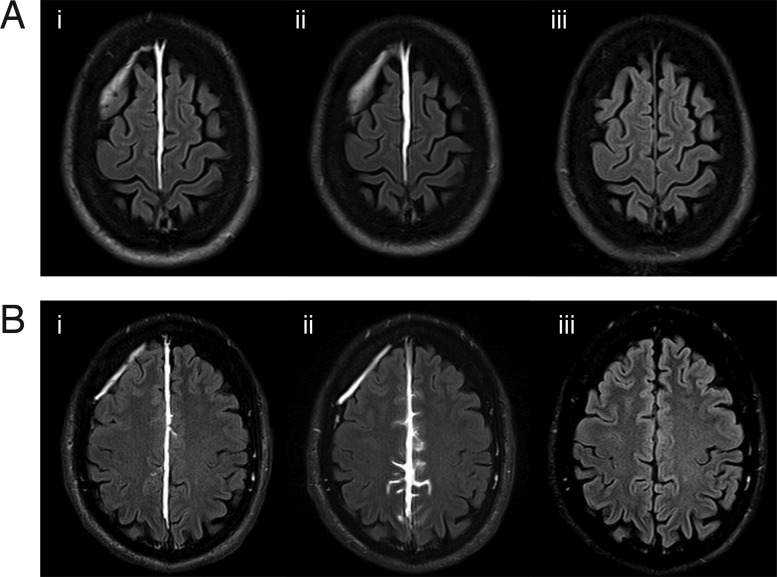
Figure 6.
Longitudinal findings on FLAIR relative to time after contrast injection. (A) Example of a patient with TME but without ECSAS; (B) Example of a patient with TME and with ECSAS. Comparison of (i) the immediate post-contrast FLAIR at 6 min, (ii) the delayed post-contrast FLAIR at 2 h and (iii) the FLAIR at 7 days demonstrates that the contrast enhancement seen in TME and ECSAS on the day of injury is absent 1 week after contrast injection.
Both studies
Analysis of T1 and FLAIR pre- and post-contrast images demonstrated no evidence of parenchymal gadolinium enhancement in proximity to TME or ECSAS in patients in these studies (Supplementary Fig. 1). There was one patient with parenchymal enhancement adjacent to a traumatic microhaemorrhage, but not near TME. The spatial relationship between traumatic microhaemorrhages and TME/ECSAS was also examined (Supplementary Fig. 2). Nine of the patients in these studies had both traumatic microhaemorrhages and TME. In four patients, the traumatic microhaemorrhage was scored as in proximity (within adjacent parenchyma) to the TME; in five patients the traumatic microhaemorrhages were located in the contralateral hemisphere.
Discussion
This work demonstrates, in a population of CT negative patients with mild TBI presenting within hours of head injury, the occurrence of conspicuous enhancement of the subarachnoid space by extravasation of gadolinium-based contrast agent from the vasculature to cerebrospinal fluid-containing spaces. The enhancement represents a compromise in the blood-cerebrospinal fluid barrier and/or blood–brain barrier detectable in an individual patient with mild TBI, using routine MRI sequences already in clinical use. Of the patients imaged within 6 h of injury, three in five had immediate enhancement of the meninges. Of those with TME, three out of four also demonstrated delayed enhancement of the subarachnoid space. While this is an exploratory study with a limited number of patients, these findings suggest that up to one in two patients with mild TBI presenting to an emergency department may have a compromised blood-cerebrospinal fluid or blood–brain barrier.
The pattern of TME and ECSAS, with its predilection for the falx and proximity to the superior sagittal and other dural sinuses (Fig. 3), suggests that this phenomenon may have relevance to the brain’s lymphatic system and immune surveillance. In mice (Aspelund et al., 2015; Louveau et al., 2015) and humans (Absinta et al., 2017b), the brain’s lymphatics run alongside the dural venous sinuses. TME appears to result from contrast leaking into the potential subdural space (Fig. 7), similar to a subdural effusion. While the dura mater is fibrous and dense, its middle layer is a rich plexus of fenestrated vessels lacking tight junctions. Small solutes, including gadolinium-based contrast agent, may readily permeate dural vasculature but are excluded from the cerebrospinal fluid by the arachnoid membrane. We conjecture that trauma resulting in movement of the brain within the cranium may stress bridging veins (Yamashima and Friede, 1984), arachnoid trabecula, arachnoid villi and meningeal lymphatics adjacent to dural venous sinuses. ECSAS may represent a compromised blood-cerebrospinal fluid barrier from traumatic injury to the arachnoid membrane or the smaller structures linking the human glymphatic system within the brain parenchyma to the meningeal lymphatics (Ringstad et al., 2018; Meng et al., 2019).
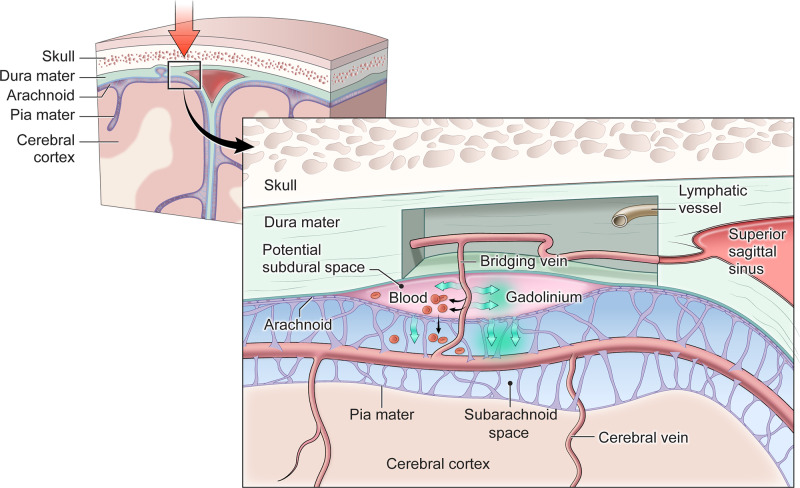
Figure 7.
Conceptual diagram demonstrating how TME and ECSAS might evolve after TBI. The base of the highly vascularized dura mater and the arachnoid lie in close proximity in uninjured meninges. Head trauma (symbolized with a large red arrow) may result in damage that allows either slippage of the dura mater and arachnoid membranes to open a potential subdural space, and/or for bridging veins crossing from the arachnoid space into the dura to be damaged. Subdural haematoma (indicated by red blood cells in the potential subdural space) may result when blood leaking from a damaged bridging vein accumulates in the potential space between the dura and arachnoid. Gadolinium-based contrast agents (indicated by green arrows) can pass through both tears large enough for red blood cells, as well as across smaller areas of damage to get into the potential subdural space, leading to the phenomenon of TME near areas of damaged meninges. In some cases, there may be traumatic damage to the arachnoid membrane itself, with delayed contrast extravasation from the area of TME into adjacent subarachnoid space resulting in the pattern of ECSAS. Damage to the recently discovered meningeal lymphatic vessels, which have been described to be in close association with the dural veins and sinuses, could also be involved the development of TME and/or ECSAS.
Disruption of the blood–brain and blood-cerebrospinal fluid barriers as indicated by TME and ECSAS may allow blood-borne substances normally limited to the dura to enter into the subarachnoid space. This may have therapeutic implications for drug delivery but also may permit peripheral immune cells and concomitant medications to enter the cerebrospinal fluid, for good or ill. When the damaged vessels heal quickly, there may be few long-term consequences; with more severe injury or repetitive traumatic exposure, clearance may be impaired. Furthermore, traumatic vascular injury, associated with the accumulation of hemosiderin-laden macrophages in perivascular spaces alongside vessels bridging the brain and the meninges (Griffin et al., 2019), may impact perivascular ‘glymphatic clearance’ with long-term consequences. Acute injury detected by MRI to these parafalcine structures, known to be involved in the biology of clearance and immune surveillance, may help establish a mechanistic link to the epidemiological association of TBI and neurodegenerative disorders such as chronic traumatic encephalopathy and dementia.
The first study presented demonstrated that in mild TBI patients who undergo acute pre- and post-contrast FLAIR MRI, gadolinium-based contrast agent extravasation into the potential subdural space reaches peak enhancement within 6 min of contrast administration. Maximal extravasation of gadolinium-based contrast agent into the subdural space was rapid, and nearly complete minutes after injection. However, in the second study extravasation continued beyond the potential subdural space, with enhancement slowly progressing over a period of hours in the subarachnoid space. The appearance of the delayed enhancement suggests gadolinium-based contrast agent tracks vascular-appearing structures from regions of enhanced dura into the cerebrospinal fluid-filled subarachnoid space. This is the first direct evidence of blood-cerebrospinal fluid barrier or blood–brain barrier disruption in patients with minor head injury.
TME after TBI is thought to result from gadolinium-based contrast agent extravasation into a meningeal fluid-filled space with a similar T1 relaxation time constant to cerebrospinal fluid (Mamourian et al., 2000; Roth et al., 2014). The pattern of enhancement differs from findings seen with gadolinium contrast extravasation across the blood–brain barrier into the parenchyma, which are readily seen on post-contrast T1 sequences alone. This type of classic parenchymal enhancement on post-contrast T1 sequences was not observed in proximity to TME or ECSAS in patients in the present study, nor was it seen on delayed post-contrast T1 (Supplementary Fig. 1). In comparison to post-contrast T1 sequences, post-contrast FLAIR MRI improves detection of extra-axial lesions associated with meningeal disease in adults (Mathews et al., 1999) and children (Goo and Choi, 2003). This may be secondary to the absence of slow-flow vessel contrast enhancement with FLAIR (Mathews et al., 1999). Findings of TME are also more conspicuous on post-contrast FLAIR than on post-contrast TI sequences (Davis et al., 2020).
The TME observed on post-contrast FLAIR in TBI patients is also distinct from the MRI patterns seen on non-contrast FLAIR with subarachnoid haemorrhage and subdural haematoma (Stuckey et al., 2007; Le and Gean, 2009). In addition to trauma, this pattern of meningeal enhancement on post-contrast FLAIR can be seen as a delayed response after neurosurgery (Bozzao et al., 2003). The post-traumatic phenomena described here as TME and ECSAS, with broad meningeal regions affected, differs from the small focal area of leptomeningeal enhancement seen on post-contrast FLAIR in 25% of patients with multiple sclerosis versus 22% of patients without multiple sclerosis (Absinta et al., 2017a). The timing and pattern of ECSAS, with its predilection for the parafalcine area, are also distinct from that seen after experimental focused ultrasound-induced blood–brain barrier opening (Meng et al., 2019).
On delayed post-contrast FLAIR MRI, ECSAS may appear similar to HARM, a pattern of post-gadolinium enhancement of cerebrospinal fluid space first noticed in ischaemic stroke patients following mechanical embolectomy, ascribed to early blood–brain barrier disruption, and associated with reperfusion injury (Warach and Latour, 2004). While HARM can appear in untreated stroke patients, its occurrence is higher in treated groups, and its presence is associated with haemorrhagic transformation (Kidwell et al., 2008). HARM also occurs in intracerebral haemorrhage, with a different pattern than seen on classic post-contrast T1-weighted imaging, suggesting that HARM represents a blood-cerebrospinal fluid barrier disruption (Kidwell et al., 2011), not just blood–brain barrier disruption (Barr et al., 2010). However, unlike HARM which is most frequently seen in the vascular distribution of the stroke, ECSAS occurs adjacent to conspicuous meningeal injury, and in particular, along the falx and the vessels leading to the superior sagittal sinus.
These results suggest TME and ECSAS are imaging biomarkers for recent TBI, observable in the absence of other TBI-related imaging findings on CT or conventional MRI. The short time to peak enhancement indicates that when present, these findings can be readily detected in the time course of a clinically feasible MRI. This study confirms that TME can be detected in patients with TBI using an MRI protocol that is short enough to be feasible for an acute clinical population. The presence of TME in mild TBI patients with otherwise unremarkable CT and MRI may serve as a biomarker for identification of specific classes of mild TBI patients (TME positive and TME negative) for other clinical research. Efforts to develop automated processes to quantify TME have begun, but are limited by difficulties in detecting small areas of TME more readily identified by trained observers (Castro et al., 2016). Whether TME and/or ECSAS on acute MRI in TBI patients is associated with particular symptoms and/or worse outcomes is an area of active investigation. The present investigations were exploratory in nature and not specifically designed to address those questions, but the ongoing prospective study may provide some clues. Further research is underway to determine the long-term significance of TME in the TBI population.
The study of ECSAS in this population is constrained by several limitations. Whether this phenomenon occurs in a control population without TBI is unknown and could not be addressed on the current clinical protocol, which is not approved for contrast administration to healthy controls. However, ECSAS was not detected in mild TBI patients on delayed MRI in the absence of TME on the immediate post-contrast FLAIR, suggesting that without conspicuous injury to the meninges, leakage into the subarachnoid space is unlikely.
The study population who participated in the hyperacute arm of the study is also not necessarily representative of all TBI. Obtaining an MRI within 6 h of injury selects for patients that are medically stable for scanning in that time window and also biases towards the milder TBI population. Performing a second scan to detect ECSAS requires a cooperative and willing patient who can lie still in an MRI scanner for the second time in <3 h. Many TBI patients, particularly those with headaches or other injuries causing pain or discomfort, or who are experiencing other severe symptoms, are unable or unwilling to tolerate the second scan. While 71% of those agreeing to Study 2 had TME, only 59% of patients screened had TME. This suggests the one in two reported prevalence of ECSAS may be slightly overestimated based on Study 2.
Not all patients with TBI will be able to undergo MRI, given clinical constraints and scanner availability. However, the imaging biomarkers of TME and ECSAS may prove illustrative in combination with blood-based biomarkers for the identification of specific groups of TBI patients. For example, glial fibrillary acidic protein has recently been identified as a blood-based biomarker in TBI patients with head CT or brain MRI positive for intracranial trauma-related findings (Gill et al., 2018).
Key clinical questions in current TBI research include understanding the link between TBI and long-term consequences, including persistent post-concussive symptoms in some subsets of the TBI population, and understanding why some individuals appear to be at risk for the development of dementia (Kenney et al., 2018) and chronic traumatic encephalopathy (McKee and Robinson, 2014; Mez et al., 2017; Shively et al., 2017; Tagge et al., 2018). TME and ECSAS indicate that after trauma, substances that would normally be kept from the brain may breach disrupted blood–brain or blood-cerebrospinal fluid barriers. Prospective studies are needed to determine whether these new imaging markers are correlated with inflammation, as seen in mouse models of repetitive injury (Russo et al., 2018), and outcomes long term.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
We would like to thank the clinical and administrative teams who have and continue to support the Traumatic Head Injury Neuroimaging Classification (THINC) study, and the administrative team from the Center for Neuroscience and Regenerative Medicine who provided programmatic support. We are also greatly appreciative of the patients and families for their willingness to participate in the study.
Funding
Support for this work was provided by the United States Department of Defense through the Center for Neuroscience and Regenerative Medicine and the Intramural Research programme of the National Institutes of Health, United States Department of Health and Human Services.
Competing interests
The authors report no competing financial or non-financial interests.
Glossary
- ECSAS =
extravasation of contrast into the subarachnoid space
- FLAIR =
fluid-attenuated inversion recovery
- GCS =
Glasgow Coma Scale
- GOSE =
Glasgow Outcome Scale-Extended
- HARM =
hyperintense acute reperfusion marker
- IQR =
interquartile range
- NSI =
Neurobehavioral Symptom Inventory
- SD =
standard deviation
- TBI =
traumatic brain injury
- TME =
traumatic meningeal enhancement
Contributor Information
Lisa Christine Turtzo, Acute Cerebrovascular Diagnostics Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892, USA.
Neekita Jikaria, Center for Neuroscience and Regenerative Medicine, Bethesda, MD 20814, USA.
Martin R Cota, Center for Neuroscience and Regenerative Medicine, Bethesda, MD 20814, USA.
Joshua P Williford, Johns Hopkins Suburban Hospital, Bethesda, MD 20814, USA.
Victoria Uche, Center for Neuroscience and Regenerative Medicine, Bethesda, MD 20814, USA.
Tara Davis, Johns Hopkins Suburban Hospital, Bethesda, MD 20814, USA.
Judy MacLaren, Johns Hopkins Suburban Hospital, Bethesda, MD 20814, USA.
Anita D Moses, Center for Neuroscience and Regenerative Medicine, Bethesda, MD 20814, USA.
Gunjan Parikh, R Adams Shock Trauma Center, University of Maryland School of Medicine, Baltimore, MD 21201, USA; Division of Neurocritical Care and Emergency Neurology, University of Maryland School of Medicine, Baltimore, MD 21201, USA.
Marcelo A Castro, Center for Neuroscience and Regenerative Medicine, Bethesda, MD 20814, USA.
Dzung L Pham, Center for Neuroscience and Regenerative Medicine, Bethesda, MD 20814, USA; Department of Radiology and Radiological Sciences, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA.
John A Butman, Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD 20892, USA.
Lawrence L Latour, Acute Cerebrovascular Diagnostics Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892, USA; Center for Neuroscience and Regenerative Medicine, Bethesda, MD 20814, USA.
References
- Absinta M, Cortese IC, Vuolo L, Nair G, de Alwis MP, Ohayon J, et al. Leptomeningeal gadolinium enhancement across the spectrum of chronic neuroinflammatory diseases. Neurology 2017. a; 88: 1439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 2017. b; 6: e29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2015; 212: 991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr TL, Latour LL, Lee KY, Schaewe TJ, Luby M, Chang GS, et al. Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke 2010; 41: e123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzao A, Floris R, Fasoli F, Fantozzi LM, Colonnese C, Simonetti G. Cerebrospinal fluid changes after intravenous injection of gadolinium chelate: assessment by FLAIR MR imaging. Eur Radiol 2003; 13: 592–7. [DOI] [PubMed] [Google Scholar]
- Castro MA, Williford JP, Cota MR, MacLaren JM, Dardzinski BJ, Latour LL, et al. Quantification of traumatic meningeal injury using dynamic contrast enhanced (DCE) fluid-attenuated inversion recovery (FLAIR) imaging. Proc SPIE 2016; 9788: 97882P. [Google Scholar]
- Davis TS, Nathan JE, Tinoco Martinez AS, De Vis JB, Turtzo LC, Latour LL. Comparison of T1-Post and FLAIR-Post MRI for identification of traumatic meningeal enhancement in traumatic brain injury patients. PLoS One 2020; 15: e0234881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 2009; 31: 497–511. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol 2017; 18: 123–31. [DOI] [PubMed] [Google Scholar]
- Gill J, Latour L, Diaz-Arrastia R, Motamedi V, Turtzo C, Shahim P, et al. Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI. Neurology 2018; 91: e1385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goo HW, Choi CG. Post-contrast FLAIR MR imaging of the brain in children: normal and abnormal intracranial enhancement. Pediatr Radiol 2003; 33: 843–9. [DOI] [PubMed] [Google Scholar]
- Griffin AD, Turtzo LC, Parikh GY, Tolpygo A, Lodato Z, Moses AD, et al. Traumatic microbleeds suggest vascular injury and predict disability in traumatic brain injury. Brain 2019; 142: 3550–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 2017; 96: 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney K, Amyot F, Haber M, Pronger A, Bogoslovsky T, Moore C, et al. Cerebral vascular injury in traumatic brain injury. Exp Neurol 2016; 275 (Pt 3): 353–66. [DOI] [PubMed] [Google Scholar]
- Kenney K, Iacono D, Edlow BL, Katz DI, Diaz-Arrastia R, Dams-O'Connor K, et al. Dementia after moderate-severe traumatic brain injury: coexistence of multiple proteinopathies. J Neuropathol Exp Neurol 2018; 77: 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell CS, Burgess R, Menon R, Warach S, Latour LL. Hyperacute injury marker (HARM) in primary hemorrhage: a distinct form of CNS barrier disruption. Neurology 2011; 77: 1725–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell CS, Latour L, Saver JL, Alger JR, Starkman S, Duckwiler G, et al. Thrombolytic toxicity: blood brain barrier disruption in human ischemic stroke. Cerebrovasc Dis 2008; 25: 338–43. [DOI] [PubMed] [Google Scholar]
- Kim SC, Park SW, Ryoo I, Jung SC, Yun TJ, Choi SH, et al. Contrast-enhanced FLAIR (fluid-attenuated inversion recovery) for evaluating mild traumatic brain injury. PLoS One 2014; 9: e102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrmann M, Struffert T, Frenzel T, Schwab S, Doerfler A. The hyperintense acute reperfusion marker on fluid-attenuated inversion recovery magnetic resonance imaging is caused by gadolinium in the cerebrospinal fluid. Stroke 2012; 43: 259–61. [DOI] [PubMed] [Google Scholar]
- Landis J, Koch G. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. [PubMed] [Google Scholar]
- Lange RT, Brickell TA, Lippa SM, French LM. Clinical utility of the Neurobehavioral Symptom Inventory validity scales to screen for symptom exaggeration following traumatic brain injury. J Clin Exp Neuropsychol 2015; 37: 853–62. [DOI] [PubMed] [Google Scholar]
- Le TH, Gean AD. Neuroimaging of traumatic brain injury. Mt Sinai J Med 2009; 76: 145–62. [DOI] [PubMed] [Google Scholar]
- Lippa SM, Lange RT, Bailie JM, Kennedy JE, Brickell TA, French LM. Utility of the Validity-10 scale across the recovery trajectory following traumatic brain injury. J Rehabil Res Dev 2016; 53: 379–90. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523: 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamourian AC, Hoopes PJ, Lewis LD. Visualization of intravenously administered contrast material in the CSF on fluid-attenuated inversion-recovery MR images: an in vitro and animal-model investigation. AJNR Am J Neuroradiol 2000; 21: 105–11. [PMC free article] [PubMed] [Google Scholar]
- Mastorakos P, McGavern D. The anatomy and immunology of vasculature in the central nervous system. Sci Immunol 2019; 4: eaav0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews VP, Caldemeyer KS, Lowe MJ, Greenspan SL, Weber DM, Ulmer JL. Brain: gadolinium-enhanced fast fluid-attenuated inversion-recovery MR imaging. Radiology 1999; 211: 257–63. [DOI] [PubMed] [Google Scholar]
- McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement 2014; 10: S242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Abrahao A, Heyn CC, Bethune AJ, Huang Y, Pople CB, et al. Glymphatics visualization after focused ultrasound-induced blood-brain barrier opening in humans. Ann Neurol 2019; 86: 975–80. [DOI] [PubMed] [Google Scholar]
- Meterko M, Baker E, Stolzmann KL, Hendricks AM, Cicerone KD, Lew HL. Psychometric assessment of the Neurobehavioral Symptom Inventory-22: the structure of persistent postconcussive symptoms following deployment-related mild traumatic brain injury among veterans. J Head Trauma Rehabil 2012; 27: 55–62. [DOI] [PubMed] [Google Scholar]
- Mez J, Daneshvar DH, Kiernan PT, Abdolmohammadi B, Alvarez VE, Huber BR, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 2017; 318: 360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J Head Trauma Rehabil 1993; 8: 86–7. [Google Scholar]
- Ricciardi MC, Bokkers RP, Butman JA, Hammoud DA, Pham DL, Warach S, et al. Trauma-specific brain abnormalities in suspected mild traumatic brain injury patients identified in the first 48 hours after injury: a blinded magnetic resonance imaging comparative study including suspected acute minor stroke patients. J Neurotrauma 2017; 34: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstad G, Valnes LM, Dale AM, Pripp AH, Vatnehol SS, Emblem KE, et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 2018; 3: e121537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature 2014; 505: 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MV, Latour LL, McGavern DB. Distinct myeloid cell subsets promote meningeal remodeling and vascular repair after mild traumatic brain injury. Nat Immunol 2018; 19: 442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively SB, Edgerton SL, Iacono D, Purohit DP, Qu BX, Haroutunian V, et al. Localized cortical chronic traumatic encephalopathy pathology after single, severe axonal injury in human brain. Acta Neuropathol 2017; 133: 353–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckey SL, Goh TD, Heffernan T, Rowan D. Hyperintensity in the subarachnoid space on FLAIR MRI. AJR Am J Roentgenol 2007; 189: 913–21. [DOI] [PubMed] [Google Scholar]
- Tagge CA, Fisher AM, Minaeva OV, Gaudreau-Balderrama A, Moncaster JA, Zhang XL, et al. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain 2018; 141: 422–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderploeg RD, Cooper DB, Belanger HG, Donnell AJ, Kennedy JE, Hopewell CA, et al. Screening for postdeployment conditions: development and cross-validation of an embedded validity scale in the neurobehavioral symptom inventory. J Head Trauma Rehabil 2014; 29: 1–10. [DOI] [PubMed] [Google Scholar]
- Vanderploeg RD, Silva MA, Soble JR, Curtiss G, Belanger HG, Donnell AJ, et al. The structure of postconcussion symptoms on the Neurobehavioral Symptom Inventory: a comparison of alternative models. J Head Trauma Rehabil 2015; 30: 1–11. [DOI] [PubMed] [Google Scholar]
- Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke 2004; 35: 2659–61. [DOI] [PubMed] [Google Scholar]
- Yamashima T, Friede RL. Why do bridging veins rupture into the virtual subdural space? J Neurol Neurosurg Psychiatry 1984; 47: 121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting this study’s findings are available from the corresponding author upon reasonable request.