Abstract
Case series
Patients: Male, 13-year-old • Male, 33-year-old • Male, 34-year-old • Male, 35-year-old
Final Diagnosis: HNF1B nephropathy • HNF1B-MODY type 5
Symptoms: Diabetic ketoacidosis • elevated liver enzymes • hyperglycemia • hyperuricemia • hypomagnesemia • positive family history • renal cysts • renal magnesium wasting • weigh loss
Medication:—
Clinical Procedure: Genetic analysis • islet autoantibodies
Specialty: Endocrinology and Metabolic • Nephrology
Objective:
Congenital defects/diseases
Background:
Maturity onset diabetes of the young (MODY) usually presents in patients under the age of 25 years and is an autosomal dominant condition associated with mutations in the hepatocyte nuclear factor 1 alpha gene, glucokinase gene, or hepatocyte nuclear factor 4 alpha gene. This report is of a series of 4 cases from Poland of MODY type 5 associated with mutations in the hepatocyte nuclear factor 1 beta (HNF1B) gene, including a 13-year-old boy and adult men aged 33, 34, and 35 years.
Case Reports:
Three cases were diagnosed late, in patients in their mid-thirties. In two patients, the initial presentation was symptomatic diabetes complicated by ketoacidosis and hyperglycemic hyperosmolar state. Renal cysts were found in all patients, and pancreatic hypoplasia in 3 patients. All patients except 1 were negative for autoanti-bodies; 1 presented with hypomagnesemia. Insulin therapy was instituted in all cases. The combination of family history, imaging study results, and biochemical characteristics led to the decision to perform genetic analysis, which was conducted in 2 cases at diagnosis, and in the 2 remaining patients at 1 month and 2 years after diagnosis, respectively. Follow-up data revealed hypomagnesemia and/or hypermagnesuria in all patients.
Conclusions:
We present 3 young men over 25 years and 1 boy with HNF1B-MODY. Although rare, autosomal dominant gene associations should be considered in young patients with diabetes who present with renal/pancreatic anomalies and low serum magnesium. Unusual presentation and the presence of autoantibodies should not eliminate the possibility of a HNF1B defect.
Keywords: Case Reports, Diabetes Mellitus, Hepatocyte Nuclear Factor 1-beta, Magnesium
Background
Maturity onset diabetes of the young (MODY) is a rare disease, but constitutes the most common type of monogenic diabetes, with an estimated prevalence of 1 in 10 000 in adults [1]. Systematic population screening of the UK pediatric diabetes population identified 2.5% are MODY cases [2]. Despite ever-growing knowledge and clinician awareness of MODY, late diagnosis and misdiagnosis as type 2 or type 1 diabetes are not uncommon [3]. An autosomal dominant condition associated with a secretory defect of pancreas beta cells, MODY usually presents in patients below the age of 25 years [4]. As summarized by Peixoto-Barbosa et al, there are 14 known genes in which mutations result in MODY, of which the hepatocyte nuclear factor 1 alpha (HNF1A), glucokinase (GCK), and hepatocyte nuclear factor 4 alpha (HNF4A) gene mutations are the most frequently encountered [5]. According to the International Society for Pediatric and Adolescence Diabetes Guidelines, the diagnosis of MODY should be suspected in patients with a positive family history of diabetes who lack the characteristics of type 1 diabetes: no islet autoantibodies, low or no insulin requirements more than 5 years after diagnosis, and lack the characteristics of type 2 diabetes. Depending on the clinical picture of patients with, for example, mild stable fasting hyperglycemia, neonatal hypoglycemia, renal anomalies, or diabetes with an autosomal dominant trait, genetic testing should be proposed [5–7]. One of the less common causes of MODY is the mutation in the hepatocyte nuclear factor 1 beta (HNF1B) gene, the well-recognized cause of MODY type 5 (MODY5), which accounts for 5% to 10% of all MODY cases [5,8]. MODY5 develops during adolescence or early adulthood. In addition to insulin deficiency related to pancreatic hypoplasia, patients show some degree of hepatic insulin resistance, which explains why some of them do not respond to sulfonylurea treatment and need early insulin therapy [6].
Because HNF1B is expressed in many organs, predominantly in the liver, intestine, pancreas, kidney, and urogenital tract, the spectrum of clinical phenotypes in patients harboring gene mutations can vary enormously, ranging from isolated MODY5 or kidney disease to multi-organ disorder [9,10]. Therefore, selecting the appropriate patients with diabetes for genetic testing remains a challenge [11]. The knowledge of a wide spectrum of renal and extra-renal features associated with HNF1B mutations is essential in terms of cost-effective molecular testing, accurate diagnosis, and subsequent appropriate management. Faguer et al introduced selection criteria for genetic testing called the HNF1B score, in which MODY5, renal, urogenital, and pancreatic anomalies are highly weighted features; such a clinical tool can facilitate our decisions in day-to-day practice [12]. In this respect, a broader approach to a patient with hyperglycemia or diabetes mellitus is required. Attention should be paid not only to family history but should also be paid to renal involvement and extra-renal features, including biochemical markers.
This report is of a series of 4 cases of MODY5 associated with mutations in the HNF1B gene. These cases of a 13-year-old boy and adult men aged 33, 34, and 35 years describe unusual presentations of this disorder. The aim of this report is to facilitate the accurate diagnosis of new patients.
Case Reports
We describe 4 patients from 3 families with HNF1B-MODY, whose initial presentations were diabetes. The patients presented at a single tertiary center in Poland. In all cases, written informed consent was obtained from patients. Mutational analysis (targeted Sanger sequencing and MPLA analysis) of the HNF1B gene was performed in the certified Laboratory for Molecular Diagnostics (DIN EN ISO 9001: 2015, TÜV Süd standards, Germany), as described elsewhere [13]. All HNF1B mutations were classified as pathogenic, and of these, 1 is novel (c.1046-15T>A). This rare intronic sequence variant is predicted (using the Alamut visual tool) to create a new donor site in intron 4 at position c.1046-15 and to diminish the natural acceptor site at position c.1046.
The clinical characteristics and laboratory data at diagnosis and follow-up are outlined in the Table 1. The glomerular filtration rate was estimated (eGFR) in adults using the Modification of Diet in Renal Disease criteria, and for the patient < 18 years, using the Schwartz formula.
Table 1.
Summary of clinical and laboratory data.
| Clinical characteristics | Case 1 Family 1 | Case 2 Family 2 | Case 3 Family 3 | Case 4 Family 1 | |
|---|---|---|---|---|---|
| Sex | Male | Male | Male | Male | |
| Age of clinical diagnosis (years) | 13 | 33 | 34 | 35 | |
| Genotype | c.742C>T; p.Q248 | 17q12 deletion | c.1046-15T>A | c.742C>T; p.Q248 | |
| Renal phenotype* | C | d# | C | C | |
| Extra-renal phenotype (during observation) | |||||
| Pancreas anomaly | Y | Y | Y | N/A | |
| Elevated liver enzymes | N | Y | N | Y | |
| Hypomagnesemia | Y | Y | Y | N | |
| Hyperuricemia | Y | N/A | Y | Y | |
| At diagnosis | sMg | N/A | 0.44 | N/A | N/A |
| sUA | 9.7 | N/A | 6.1 | N/A | |
| eGFR | 187 | 97 | 79 | 69 | |
| HbA1c | 5.91 | 20.7 | 10.4 | 12.1 | |
| Fasting insulin level | 10.6 | N/A | N/A | N/A | |
| Fasting C-peptide | 1.41 | 0.71 | 0.90 | 1.43 | |
| Autoantibodies (ICA, GAD, IA2) | Negative | Negative | Negative | GAD, ICA (negative), IA2 (positive) | |
| At last follow-up | Age (years) | 22 | – | 36 | 46 |
| sMg | 0.61 | 0.59 | 0.71 | ||
| sUA | 5.72 | 7.2 | 6.4** | ||
| eGFR | 95 | 47 | 61 | ||
| FEMg | 11.4 | 24.3 | 11 | ||
| HbA1c | 5.9 | 7.1 | 8.3 | ||
eGFR – estimated glomerular filtration rate (ml/min/1.73 m2); FEMg – fractional excretion of Mg2+; HbA1c – glycated haemoglobin (%); m, maternal; N/A – not available; p – paternal; sMg – serum magnesium (mmol/l); sUA – serum uric acid (mg/dl); ICA – islet cel autoantibodies; GAD – glutamic acid decarboxylase autoantibodies; IA2 – insulinoma-associated autoantibodies. Laboratory abnormalities are in bold. Hypomagnesemia was considered when sMg <0.7 mmol/l; hyperuricemia when sUA >7 mg/dl or when on allopurinol. Reference values: C-peptide: 1.1–4.4 ng/ml; eGFR >90 ml/min/1.73 m2; HbA1c <6%; FEMg <4%; insulin: 3–17 uU/ml. FEMg was calculated using the following formula: (urine Mg×serum creatinine/0.7×serum Mg2+×urine creatinine)×100%.
C bilateral cysts; d# unilateral dysplasia+contralateral cysts;
on allopurinol.
Case 1
A 13-year-old boy was referred to the pediatric department for evaluation owing to fasting hyperglycemia. A glucose level of 216 mg/dL (after 2 h) during an oral glucose tolerance test confirmed the diagnosis of diabetes. The patient was born with asymptomatic spina bifida occulta of the lumbo-sacral region, posterior urethral valves, and a left pelviureteric junction obstruction. At the age of 21 months, he underwent surgical treatment for these conditions. During a thorough work-up, diabetes mellitus, hypertension, severe hypomagnesemia, hyperuricemia, proteinuria, and hypercholesterolemia were discovered. There were no islet cell antibodies (ICA), glutamic acid decarboxylase (GAD) antibodies, or insulinoma-associated (IA-2) autoantibodies found. The patient’s renal function was preserved. Ultrasound examination showed bilateral cortical renal cysts. The patient’s family history was positive for diabetes on the father’s side: the father (case 4) and grandmother, who both also had renal cysts, and 3 other relatives. The combination of renal cysts, diabetes, and positive family history raised a suspicion of HNF1B mutation, which led to molecular confirmation of MODY5 based on a heterozygous mutation (c.742C>T) in the HNF1B gene.
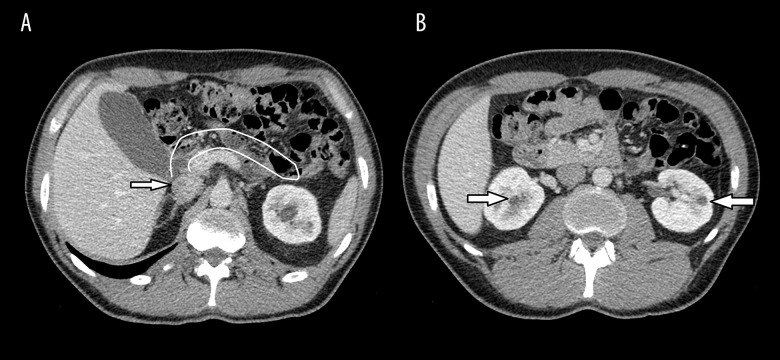
During consecutive follow-up, an abdominal computed tomography (CT) was carried out and pancreatic body and tail agenesis was noted (Figure 1). In addition, hypomagnesemia with hypermagnesuria and features of metabolic syndrome were discovered. The patient’s renal function was stable. He was initially treated with metformin, but, owing to poor glycemic control of diabetes, insulin was added. Because of the patient’s hyperuricemia and hypertension, he is being treated with allopurinol, bisoprolol, and ramipryl.
Figure 1.
Axial contrast-enhanced computed tomography image showing the absence of the pancreatic body and tail. (A) The arrow shows the pancreatic head; (B) arrows identify the renal cysts.
Case 2
A 33-year-old man was admitted to the emergency department and was found to have diabetic ketoacidosis (serum glucose of 600 mg/dL). He had been experiencing symptoms of diabetes mellitus (polyuria, polydipsia, polyphagia, and weight loss of 20 kg) over the previous 3 months. He had been addicted to alcohol for 10 years. Abdominal ultrasound and CT scans showed an atrophic left kidney and numerous cysts in the right kidney. Pancreatic calcification and agenesis of the pancreas tail were also found on the same examination. The patient’s family history was positive for diabetes in the father, who had an unspecified urinary tract malformation, and paternal grandfather. A single panel of laboratory tests taken with the patient in stable condition showed slightly elevated aminotransferase activity (ALT 69 U/L, AST 69 U/L), marked hypomagnesemia, and normal renal function. There were no ICA, GAD, or IA-2 autoantibodies found. The association of renal and pancreatic anomalies in the setting of diabetes prompted us to perform HNF1B testing. Subsequent genetic analysis discovered a complete deletion of the HNF1B gene. The patient was started on insulin therapy.
Case 3
A 34-year-old man was referred for diagnostic evaluation because of significant weight loss over a 12-month period. During this work-up, hyperglycemia (serum glucose of 300 mg/dL) was discovered and a diabetes mellitus diagnosis was made. An ultrasound examination showed bilateral renal cysts with moderate impairment of renal function, corresponding to chronic kidney disease (CKD) stage II (eGFR 62 mL/min/1.73 m2). A positive family history of diabetes in the patient’s grandparents was noted. Three months later, the patient presented with jaundice and significantly elevated liver function tests (LFTs) results (total bilirubin 10.8 mg/dL, ALT 3333 U/L, AST 1559 U/L, GGTP 384 U/L, and ALP 135 U/L). Of note, a high level of testosterone and suppressed LH and FSH were disclosed.
Results of a viral hepatitis screen and antinuclear antibody test were negative. There were no evident changes in the liver on the abdominal ultrasound and CT (the liver was slightly enlarged). Oral prednisone was initiated, following which the LFTs started to normalize, and the jaundice subsided within a few weeks. This event was hypothesized to be due to anabolic steroid abuse.
As a part of the differential diagnostics, a contrast-enhanced abdominal CT was carried out. This revealed agenesis of the pancreas body and tail and confirmed renal cysts localized in both kidneys (Figure 1). On hospitalization, serum Mg2+ (sMg) was assessed for the first time, and hypomagnesemia was observed. There were no ICA, GAD, or IA-2 autoantibodies found. One month after his first presentation, the patient’s daughter was diagnosed with HNF1B nephropathy, which reverse guided the molecular diagnosis of HNF1B-MODY in our patient. A novel heterozygous mutation (c.1046-15T>A) was found in the HNF1B gene. During the follow-up period, the patient was recurrently seen on the ward because of severe abdominal pain. Porphyria was excluded. The patient was diagnosed with gastroesophageal reflux. The symptoms were relieved following treatment with a proton pomp inhibitor. On the last follow-up analysis, laboratory tests showed CKD progression to stage III, hyperuricemia, hypomagnesemia, and profound hypermagnesuria. The patient’s renal ultrasound image was stable. Intensive functional insulin therapy was initiated.
Case 4
A 35-year-old man presented with peripheral neuropathy. After clinical evaluation, he was diagnosed as being in a hyperglycemic hyperosmolar state. His blood glucose was >1000 mg/dL.
No diabetic ketoacidosis was present. Autoantibody screening revealed IA-2 autoantibodies, while results for ICA and GAD autoantibodies were negative. In addition, the patient was found to have hyperuricemia and hypertension. Ultrasound imaging showed bilateral hyperechogenic kidneys with single renal cysts. The patient’s renal function was moderately compromised (eGFR of 69 mL/min/1.73 m2). The underlying cause of diabetes remained unclear for 2 years until the patient’s son (case 1) was diagnosed with a HNF1B mutation. Results of genetic analysis identified a recurrent, heterozygous mutation (c.742C>T), confirming MODY5 in this patient. HNF1B mutations were also found on subsequent analysis of 2 other sons of the patient with congenital anomalies of the kidney and urinary tract. During his last follow-up visit, laboratory test results showed the following abnormalities: reduced eGFR (61 mL/min/1.73 m2), elevated LFTs, and hypercholesterolemia. Although his sMg remained normal (0.71 mmol/L), renal Mg2+ wasting, defined as the fractional excretion of Mg2+ (FEMg), was evidenced. At present, the patient is on insulin treatment. To treat proteinuria/hypertension and hyperuricemia, he is receiving ramipryl and allopurinol, respectively.
Discussion
The diagnosis of MODY5 is a challenge for clinicians. A widespread availability of molecular diagnostics permits differential diagnoses, but cost and turnaround time of larger gene panels or diagnostic exomes is an issue in many national health systems. Targeted diagnostic approaches are comparatively inexpensive and fast, with no risk of additional genetic findings that require medical actions and constitute ethical problems. In this respect, an appropriate selection of those patients that likely have a high diagnostic yield for a targeted genetic test is crucial. Timely and accurate diagnosis offers opportunities for genetic counseling, early intervention, and implementation of a coordinated care plan. Here, we presented 4 cases of HNF1BMODY to familiarize clinicians with features that may be encountered in patients with this entity. We believe that knowledge of a broad and unusual presentation may assist clinicians in selecting candidates for testing for HNF1B gene mutations.
HNF1B is a transcription factor encoded by the HNF1B gene located on chromosome 17 and is involved in organogenesis of the kidneys and urinary tract, pancreas, liver, and reproductive organs. As summarized by Ferre et al [14] the HNF1B transcription factor is required for ureteric bud branching, initiation of nephrogenesis, and nephron segmentation. Thus, patients harboring HNF1B mutations develop heterogeneous renal abnormalities including multicystic dysplastic kidneys, glomerulocystic kidney disease, renal agenesis, renal hypoplasia, and renal interstitial fibrosis [9,12,16]. The mechanism of kidney cyst formation relies on downregulating the expression of multiple cystic disease genes, such as fibrocystin-1 (PKHD1), kinesin-like 12 (KIF12), suppressor of cytokine signaling 3 (SOCS3), and polycystic kidney disease 2 (PKD2). Furthermore, HNF1B transcription factor regulates ion transport in kidney by affecting expression of genes encoding tubular transporters. That explains why HNF1B mutations result in several electrolyte disorders, such as hypomagnesemia due to hypermagnesuria, hypocalciuria, or hypouricosuria [14].
A detailed literature review by Mateus et al [10] summarizes the latest information on the role of HNF1B in pancreas development. Pancreatic malformations and/or exocrine pancreatic dysfunction have been documented in 30% to 40% of mutation carriers [12]. A report of 20 fetal autopsy cases with HNF1B mutations described pancreatic agenesis in 2 of 20 and hypoplasia in 13 of 20 cases [16]. Importantly, pancreatic anomalies can be difficult to detect with ultrasound, so CT scanning or magnetic resonance imaging scan should be conducted. In our case series, pancreatic malformation was detected in 3 patients; in patients 1 and 2 by chance, as a CT scan was performed to document renal cysts in patient 1 and the malformation was detected during an episode of liver derangement in patient 2. Currently, there are no data to advocate for CT scanning in patients with diabetes for the detection of pancreatic anomalies. It seems, however, that fecal elastase 1 could be a feasible marker in HNF1B-related disease [17] and could possibly serve as a screening marker of pancreas involvement, thereby suggesting a HNF1B defect. Unfortunately, no results were available in our cases to assess patients’ pancreatic exocrine function.
Early reports have described an association between HNF1B mutations and MODY. Patients with HNF1B mutations often present with renal cysts and renal function decline that precede diabetes, hence HNF1B-MODY was initially referred to as renal cysts and diabetes (RCAD) syndrome. Currently, HNF1BMODY constitutes 5% to 15% of MODY cases [5]. However, it is now evident that many other renal and extra-renal phenotypes may occur [18] and any renal malformation in association with diabetes should prompt an investigation of HNF1B. When diabetes and any renal malformation are present, the likelihood of identification of a HNF1B mutation is estimated to be about 40% [11]. Since their first hospitalization, all our presented cases had shown renal cysts (bilaterally in 3 cases). Despite this, 2 cases remained unsolved until their off-spring were diagnosed by pediatricians. It is worth remembering that bilateral major renal anomalies, particularly cysts from unknown origins, have the highest prediction value for a HNF1B defect [19].
Considering the heterogeneous phenotype of HNF1B, a screening score has been developed that could be used as a tool for clinicians to select patients with suspicion of HNF1B before genetic testing. Perhaps not all clinicians will use this scoring system prior to requesting HNF1B mutation testing [12], as observed with our cases. The clinicians who cared for the patients were successful in providing prompt molecular diagnosis in 3 patients because of their clinical vigilance. They made diagnoses of HNF1B-MODY based on the co-existence of family, imaging, and laboratory characteristics. Only in patient 4 was there a 2-year delay in diagnosis, although he presented with RCAD syndrome, which should have led to an earlier diagnosis.
Diagnosis of MODY5 is an even greater challenge for clinicians with regards to its prevalence, inheritance, and clinical picture. It is widely accepted that a positive family history for diabetes and renal anomaly or CKD could be critical for considering HNF1B mutation [12]. Thus, irrespective of any abnormalities, clinicians should be alert to HNF1B mutation when there is a positive family history, as was the case in our patients. However, it should be remembered that in about 50% of cases, mutations occur de novo. In this context, a negative familial history should not preclude HNF1B mutation. Furthermore, several reports have shown that a significant number of MODY cases are missed or misdiagnosed. Shields et al in the United Kingdom estimate that >80% of MODY patients are not diagnosed by molecular testing [3]. As the authors suggest, it could reflect low clinician awareness of MODY or unequal access to genetic testing. Other possible explanations for this phenomenon could be an overlap between MODY and type 1 and type 2 diabetes phenotypes and late age of presentation. In our present cases, 3 men developed MODY5 far above the age of 25 years. Of note, the initial clinical presentation does not stay in line with well-recognized phenotypes of milder forms of diabetes [6,7]. Cases 2 and 4 from our study presented with ketoacidosis and a hyperglycemic hyperosmolar state, respectively. Data from several studies underline the fact that establishing the correct diagnosis of MODY is also crucial in terms of providing accurate prognosis and treatment, for example. With respect to the latter, patients with MODY usually have a low or no insulin requirement for years. However, this mainly applies to those with GCK, HNF1A, and HNF4A mutations [6,7,10]. Unfortunately, patients with MODY5 not only suffer from insulin deficiency related to pancreatic hypoplasia but also show some degree of hepatic insulin resistance, explaining why they often require insulin therapy to be instituted as a first line of treatment, as was observed in all our cases.
Regarding the biochemical markers, in contrast to type 1 diabetes, MODY is characterized by the absence of islet auto-antibodies, including GAD and IA-2 antibodies and detectable serum C-peptide. McDonald et al [20] observed that the prevalence of GAD and IA-2 in patients with MODY is the same as in control participants (<1%). Of note, finding islet autoantibodies, especially IA-2 antibodies, makes the diagnosis of MODY unlikely, with a negative likelihood ratio for identifying MODY from type 1 diabetes of 0.01 [21]. Thus, authors recommend molecular testing only if the clinical phenotype strongly suggests MODY rather than type 1 diabetes. Interestingly, our patient 4, who presented with IA-2 autoantibodies, is a rare MODY antibody-positive example in which renal anomalies and positive family history of diabetes supported genetic testing. With regard to serum C-peptide, its detectable level is observed in MODY and type 1 diabetes patients within the initial honeymoon period, which limits its use as a MODY predictor at first presentation. The aforementioned findings agree with the Mateus et al [10] case report, in which MODY5 was confirmed in 15-year-old girl who presented with symptomatic hyperglicemia (no ketosis). Results of a detailed clinical evaluation were negative for GAD, IA-2, and anti-zinc transporter 8 autoantibodies and a C-peptide level was detectable. Because of early antibody-negative diabetes development, concurrent renal hypoplasia, bilateral diminished corticomedullary differentiation, simple renal cysts, and positive family history of congenital anomalies of the kidney and urinary tract, HNF1B gene sequencing was requested. A similar diagnostic process occurred in our cases 1 and 2. Interestingly, our patient 4 presented with IA-2 autoantibodies, thereby being a rare example of antibody-positive MODY. In this patient, molecular diagnosis was made with 2 years of delay owing to confirmation of HNF1B mutation in the patient’s son.
One of the underestimated biochemical hallmarks suggestive of HNF1B disease is concurrent hypomagnesemia due to renal Mg2+ loss [12,13,19]. In the present case series, sMg was tested at diagnosis in only 1 patient (case 2). On follow-up, low sMg was found in another 2 patients when the diagnosis of HNF1B-MODY was known. Although the results of sMg for patient 3 on the last visit was within the normal range (just above the lower normal limit), Mg2+ renal wasting was noted. In fact, the hypermagnesuria in patients 2 and 4 was enormous. This observation provides evidence that the assessment of sMg and renal Mg2+ wasting could possibly be helpful for the diagnosis of HNF1B-MODY among patients with diabetes. Therefore, a paired assessment of sMg and FEMg is recommended in patients with suspected HNF1B-related disease. In adults, we propose using a sMg cut-off value of 0.7 mmol/L, while in children, all clinicians should be encouraged to utilize sMg age- and sex-specific intervals [13]. Importantly, one should be aware that hypomagnesemia is common among patients with diabetes (in 25%) [21], which may limit its usefulness as a predictor. Contributory mechanisms most likely are multifactorial [22]. Therefore, as previously mentioned, an assessment of sMg along with FEMg may provide information on whether hypomagnesemia results from renal Mg2+ loss. As shown by Kurstjens et al [23], mean FEMg in a cohort of patients with type 2 diabetes was 3.9±2.7%. Although 40.8% of the subjects suffered from renal Mg2+ wasting (defined as FEMg >4%), FEMg was not significantly different between patients with normomagnesemia (3.9±2.8%) and hypomagnesemia (3.9±2.5%). Since most patients had FEMg 9%, it seems that this cut-off value could be applicable when considering HNF1B defects in patients with diabetes. Preferably, an assessment of sMg and FEMg should be done in a steady state to exclude potential influences including electrolyte abnormalities, metabolic acidosis, and osmotic diuresis. [18]. The severity of CKD should also be taken into consideration, as FEMg tends to increase with renal function deterioration. It can thus be suggested that both sMg and FEMg may help in the differential diagnostics of patients with diabetes.
Conclusions
This report presents 3 young men over 25 years and one 13-year-old boy with MODY5 associated with mutations in the HNF1B gene. Although rare, autosomal dominant gene associations should be considered in young patients with diabetes who present with renal/pancreatic anomalies, low serum magnesium, and/or hypermagnesuria. Thus, we recommend the measurement of sMg and FEMg as simple, easily accessible markers for informing the diagnosis of HNF1B-related disease. Importantly, unusual clinical presentation, which includes a late and severe clinical presentation and the presence of islet autoantibodies, should not eliminate the possibility of HNF1B-MODY.
Acknowledgments
Genetic analyses were possible through the Polish Monogenic Diabetes Registry and the Polish Registry of Inherited Tubulopathies (POLtube). We thank Dr. Małgorzata Urbańska-Kosińska for her help with patient recruitment.
Footnotes
Conflict of Interest
None.
References:
- 1.Orphanet MODY. 2020. Orpha.net. https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=552.
- 2.Shepherd M, Shields B, Hammersley S, et al. Systematic population screening, using biomarkers and genetic testing, identifies 2.5% of the U.K. pediatric diabetes population with monogenic diabetes. Diabetes Care. 2016;39(11):1879–88. doi: 10.2337/dc16-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shields BM, Hicks S, Shepherd MH, et al. How many cases are we missing? Diabetologia. 2010;53(12):2504–8. doi: 10.1007/s00125-010-1799-4. [DOI] [PubMed] [Google Scholar]
- 4.Gat-Yablonski G, Shalitin S, Phillip M. Maturity onset diabetes of the young – review [published correction appears in Pediatr Endocrinol Rev,2007;5(1): 470] Pediatr Endocrinol Rev. 2006;3(Suppl. 3):514–20. [PubMed] [Google Scholar]
- 5.Peixoto-Barbosa R, Reis A, Giuffrida F. Update on clinical screening of maturity-onset diabetes of the young (MODY) Diabetol Metab Syndr. 2020;12:50. doi: 10.1186/s13098-020-00557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hattersley AT, Greeley SAW, Polak M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2018;19(Suppl. 27):47–63. doi: 10.1111/pedi.12772. [DOI] [PubMed] [Google Scholar]
- 7.Tattersall RB, Fajans SS. A difference between the inheritance of classical juvenile-onset and maturity-onset type diabetes of young people. Diabetes. 1975;24(1):44–53. doi: 10.2337/diab.24.1.44. [DOI] [PubMed] [Google Scholar]
- 8.Bellanne-Chantelot C, Clauin S, Chauveau D, et al. Large genomic rearrangements in the hepatocyte nuclear factor-1 (tcf2) gene are the most frequent cause of maturity-onset diabetes of the young type 5. Diabetes. 2005;54(11):3126–32. doi: 10.2337/diabetes.54.11.3126. [DOI] [PubMed] [Google Scholar]
- 9.Bockenhauer D, Jaureguiberry G. HNF1B-associated clinical phenotypes: The kidney and beyond. Pediatr Nephrol. 2015;31(5):707–14. doi: 10.1007/s00467-015-3142-2. [DOI] [PubMed] [Google Scholar]
- 10.Mateus JC, Rivera C, O’Meara M, et al. Maturity-onset diabetes of the young type 5 a MULTISYSTEMIC disease: a CASE report of a novel mutation in the HNF1B gene and literature review. Clin Diabetes Endocrinol. 2020;6:16. doi: 10.1186/s40842-020-00103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sztromwasser P, Michalak A, Małachowska B, et al. A cross-sectional study of patients referred for HNF1B-MODY genetic testing due to cystic kidneys and diabetes. Pediatr Diabetes. 2020;21(3):422–30. doi: 10.1111/pedi.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faguer S, Chassaing N, Bandin F, et al. The HNF1B score is a simple tool to select patients for HNF1B gene analysis. Kidney Int. 2014;86(5):1007–15. doi: 10.1038/ki.2014.202. [DOI] [PubMed] [Google Scholar]
- 13.Kołbuc M, Leßmeier L, Salamon-Słowińska D, et al. Hypomagnesemia is underestimated in children with HNF1B mutations. Pediatr Nephrol. 2020;35(10):1877–86. doi: 10.1007/s00467-020-04576-6. [DOI] [PubMed] [Google Scholar]
- 14.Ferrè S, Igarashi P. New insights into the role of HNF-1β in kidney (patho) physiology. Pediatr Nephrol. 2019;34(8):1325–35. doi: 10.1007/s00467-018-3990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okorn C, Goertz A, Vester U, et al. HNF1B nephropathy has a slow-progressive phenotype in childhood – with the exception of very early onset cases: Results of the German Multicenter HNF1B Childhood Registry. Pediatr Nephrol. 2019;34(6):1065–75. doi: 10.1007/s00467-018-4188-8. [DOI] [PubMed] [Google Scholar]
- 16.Duval H, Michel-Calemard L, Gonzales M, et al. Fetal anomalies associated with HNF1Bmutations: Report of 20 autopsy cases. Prenat Diagn. 2016;36(8):744–51. doi: 10.1002/pd.4858. [DOI] [PubMed] [Google Scholar]
- 17.Clissold R, Fulford J, Hudson M, et al. Exocrine pancreatic dysfunction is common in hepatocyte nuclear factor 1β-associated renal disease and can be symptomatic. Clin Kidney J. 2018;11(4):453–58. doi: 10.1093/ckj/sfx150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verhave J, Bech A, Wetzels J, Nijenhuis T. Hepatocyte nuclear factor 1β-associated kidney disease: More than renal cysts and diabetes. J Am Soc Nephrol. 2015;27(2):345–53. doi: 10.1681/ASN.2015050544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raaijmakers A, Corveleyn A, Devriendt K, et al. Criteria for HNF1B analysis in patients with congenital abnormalities of kidney and urinary tract. Nephrol Dial Transpl. 2014;30(5):835–42. doi: 10.1093/ndt/gfu370. [DOI] [PubMed] [Google Scholar]
- 20.McDonald TJ, Colclough K, Brown R, et al. Islet autoantibodies can discriminate maturity-onset diabetes of the young (MODY) from type 1 diabetes. Diabet Med. 2011;28(9):1028–33. doi: 10.1111/j.1464-5491.2011.03287.x. [DOI] [PubMed] [Google Scholar]
- 21.Mather H, Nisbet J, Burton G, et al. Hypomagnesaemia in diabetes. Clin Chim Acta. 1979;95(2):235–42. doi: 10.1016/0009-8981(79)90364-4. [DOI] [PubMed] [Google Scholar]
- 22.Pham PC, Pham PM, Pham SV, et al. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2007;2(2):366–73. doi: 10.2215/CJN.02960906. [DOI] [PubMed] [Google Scholar]
- 23.Kurstjens S, de Baaij J, Bouras H, et al. Determinants of hypomagnesemia in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2017;176(1):11–19. doi: 10.1530/EJE-16-0517. [DOI] [PubMed] [Google Scholar]