Abstract
Background and objective
The relationship between hospitalisation with an eosinophilic acute exacerbation of COPD (AE-COPD) and future relapses is unclear. We aimed to explore this association by following 152 patients for 12 months after hospital discharge or until their first moderate or severe flare-up.
Methods
Patients hospitalised with AE-COPD were divided into eosinophilic and non-eosinophilic groups based on full blood count results on admission. All patients were treated with a course of systemic corticosteroid. The Cox proportional hazards model was used to study the association with the time to first re-exacerbation; a generalised linear regression model was applied to identify clinical variables related to the recurrence of relapses.
Results
We did not find a difference in the time to the next moderate or severe exacerbation between the eosinophilic (≥2% of total leukocytes and/or ≥200 eosinophils·µL−1, n=51, median (interquartile range): 21 (10–36) weeks) and non-eosinophilic groups (n=101, 17 (9–36) weeks, log-rank test: p=0.63). No association was found when other cut-off values (≥3% of total leukocytes and/or ≥300 eosinophils·µL−1) were used for the eosinophilic phenotype. However, the higher number of past severe exacerbations, a lower forced expiratory volume in 1 s (FEV1) at discharge and higher pack-years were related to shorter exacerbation-free time. According to a subgroup analysis (n=73), 48.1% of patients with initial eosinophilic exacerbations had non-eosinophilic relapses on readmission.
Conclusions
Our data do not support an increased risk of earlier recurring moderate or severe relapses in patients hospitalised with eosinophilic exacerbations of COPD. Eosinophilic severe exacerbations present a variable phenotype.
Short abstract
Shorter time to the next relapse after severe COPD exacerbation is related to the number of prior hospitalisations, smoking history and more severe airflow limitation. Blood eosinophils are not predictive of the recurrence of moderate or severe relapses. https://bit.ly/2VkbBNC
Introduction
Acute exacerbations of COPD (AE-COPD) are the leading cause of COPD-associated mortality and are contributing to a great extent to the high economic burden of the disease, especially when hospitalisation is required [1–3]. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations, one of the main goals of the pharmacological therapy is to reduce the number and severity of acute exacerbations [4]. The best predictor of an exacerbation is a past event [5], and certain clinical factors have been associated with an early relapse [6, 7]. However, it is less clear if certain inflammatory phenotypes of a flare-up predispose to earlier relapses.
Exacerbations are usually characterised by neutrophilic airway inflammation, but in a subgroup of patients elevated sputum and blood eosinophil granulocyte ratios are observed [8, 9]. Patients with elevated eosinophil counts during exacerbation respond more favourably to systemic corticosteroid treatment and have lower rates of early treatment failure [10–12]. However, this phenotype is also associated with increased COPD-related costs in the year after the relapse [13], and it is less clear whether an increased blood eosinophil count during AE-COPD is related to re-exacerbations. Few studies have investigated the relationship between eosinophil counts or ratios and exacerbation frequency, and results have been inconclusive [11, 14, 15]. In addition, these studies were limited or biased by enrolling patients either with concomitant pneumonia [14] or those receiving previous systemic corticosteroid [11] or antibiotic treatments [15].
Our aim was to study whether the eosinophilic subgroup of COPD exacerbations (defined by ≥2% of blood leukocytes or ≥200 cells·µL−1 and also ≥3% of blood leukocytes or ≥300 cells·µL−1) requiring hospitalisation shows earlier re-exacerbations in the 12 months after the index event. We also analysed the associations of clinical factors with the occurrence of future events.
Methods
Subjects
All patients admitted with the primary diagnosis of COPD exacerbation (acute worsening of respiratory symptoms in the past 72 h including increased dyspnoea, chest tightness, sputum production and sputum purulence) to the Department of Pulmonology, Semmelweis University, Budapest, Hungary between February 15, 2017 and August 15, 2018 were screened (supplementary figure S1). COPD had been previously diagnosed by a respiratory specialist according to the GOLD recommendations [4]. Exclusion criteria included asthma or a previous positive reversibility test (>200 mL or 12% increase in post-bronchodilator forced expiratory volume in 1 s (FEV1)), concurrent chronic pulmonary disease other than COPD, history of pulmonary malignancy in the past 3 years, concomitant pneumonia during the current hospitalisation, need for invasive ventilatory support and systemic corticosteroid or antibiotic treatment for any reason 4 weeks prior to admission. Corticosteroid use on admission was only allowed when a single dose of methylprednisolone was administered by the ambulance or at the emergency care department (<4 h before blood sample taking). The choice of hospital treatment was the responsibility of the attending physician, and all patients received systemic corticosteroids during hospitalisation. Some patients were prescribed low-dose oral corticosteroids post-discharge, which was tapered, and the treatment stopped within 1 week after discharge.
All procedures were in accordance with the 1964 Helsinki declaration and its later amendments. The study was approved by the ethics committee at the Semmelweis University (study No. 191/2017) and a written informed consent was signed by all participants.
Design
Our study was an observational prospective cohort clinical study. All patients underwent blood tests on hospital admission, clinical data were collected and spirometry was done within 48 h if the patient could perform it.
Based on the full blood picture results on admission, patients were divided into eosinophilic (≥2% of leukocytes and/or ≥200 eosinophils·µL−1) and non-eosinophilic subgroups [15]. Additionally, data analysis was also performed using other cut-offs, i.e. ≥3% eosinophils or leukocytes and/or ≥300 eosinophils·µL−1 [16].
Patients were followed until the occurrence of the first moderate or severe relapse following the index exacerbation or for 12 months. Moderate exacerbations were defined as the need for outpatient treatment with systemic corticosteroid and/or antibiotics due to worsening symptoms of COPD, while severe exacerbations required hospital treatment [4]. Treatment failure was defined as readmission to hospital with respiratory symptoms within 4 weeks after discharge following the treatment of the index exacerbation. Patient follow-up was carried out by phone calls every 3 months or by personal interviews upon re-admission. The time (i.e. weeks after hospital discharge) and the severity of the exacerbations were recorded. Those patients who did not have at least the 3-month follow-up data were categorised as lost to follow-up and were excluded from the analysis.
Measurements
Venous blood samples were taken to measure white blood cell count and C-reactive protein (CRP) concentration (Sysmex XN-1000, Sysmex Corporation, Kobe, Japan and Beckman Coulter AU680, Beckman Coulter Inc., Indianapolis, IN, USA). Spirometry was performed by 114 subjects according to current guidelines (PDT-111, Piston, Budapest, Hungary) [17]. We used the Charlson Comorbidity Index to assess the burden of comorbidities [18].
Analysis
Data were analysed by TIBCO Statistica data analysis software system for Windows version 13 (TIBCO Software Inc., Palo Alto, CA, USA). Continuous variables were presented as mean±sd or median (interquartile range (IQR)) and were compared using t-test and Mann–Whitney U-test. Categorical variables were compared with Chi-squared test and two-tailed Fisher exact test. We used Cox proportional hazards model to investigate the association between eosinophil granulocyte count or ratio and the time to first re-exacerbation. Log-rank test was used to compare the time to the next exacerbation between the high and low eosinophilic subgroups. To assess risk factors of the occurrence of relapses, a generalised linear regression model of negative binomial distribution was used. To calculate the required sample size per group, we used the results of the COPD-related readmission rate from a previous study, which divided patients into eosinophilic (≥2% of leukocytes and/or ≥200 eosinophils·µL−1) and non-eosinophilic subgroups [15]. We also utilised the findings of the ECLIPSE study, where 47% of patients had ≥1 moderate or severe exacerbations in the year prior to recruitment [5]. The minimum sample size per group was 39 using the log-rank test (1-β=0.80, α=0.05).
Results
Patient characteristics
In total, 152 patients completed the study: 51 patients had an eosinophilic exacerbation as the index event and 101 experienced an initial non-eosinophilic relapse (table 1). Subjects in the eosinophilic group had less severe airflow limitation on admission as shown by higher FEV1/forced vital capacity (FVC) values and lower serum CRP concentration compared to patients with non-eosinophilic relapses. Other characteristics of the index event including antibiotic therapy, need for noninvasive ventilation, length of hospital stay and treatment failure were similar between the study groups.
TABLE 1.
Patient characteristics
| Total | Eosinophilic exacerbation | Non-eosinophilic exacerbation | p-value | |
| Subjects N | 152 | 51 | 101 | |
| Male n (%) | 71 (46.7) | 23 (45.1) | 48 (47.5) | 0.86 |
| Age years | 66.2±8.7 | 65.9±9.1 | 66.3±8.6 | 0.81 |
| Charlson Comorbidity Index | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.93 |
| Current smoker n (%) | 98 (64.5) | 32 (62.7) | 66 (65.3) | 0.56 |
| Tobacco smoke exposition pack-years | 40 (30–50) | 40 (30–50) | 40 (30–50) | 0.94 |
| FEV1 (post-bronchodilator) % pred | 35.52±14.07 | 35.89±11.59 | 35.35±15.18 | 0.85 |
| FEV1/FVC | 0.47±0.11 | 0.51 ±0.10 | 0.45±0.11 | <0.01 |
| Time since COPD diagnosis years | 7 (3–12) | 7 (2–11) | 6.5 (3.5–12.5) | 0.52 |
| Hospital admission due to COPD in the previous year n | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.67 |
| Admission corticosteroid use n (%) | 43 (37.0) | 17 (41.4) | 26 (34.7) | 0.55 |
| Baseline ICS use n (%) | 93 (61.2) | 29 (56.9) | 64 (63.4) | 0.38 |
| Baseline LAMA use n (%) | 118 (77.6) | 38 (74.5) | 80 (79.2) | 0.40 |
| Baseline LABA use n (%) | 117 (77.0) | 38 (74.5) | 79 (78.2) | 0.53 |
| Post-discharge ICS use n (%) | 105 (69.1) | 33 (64.7) | 72 (71.3) | 0.46 |
| WBC count on admission 109 cells·L−1 | 10.88±4.17 | 11.20±4.10 | 10.72±4.22 | 0.50 |
| Eosinophil count cells·µL−1 | 91 (19–261) | 362 (251–524) | 37 (4–89) | <0.0001 |
| Eosinophil count % of WBC | 0.80 (0.20–2.45) | 3.50 (2.40–5.20) | 0.40 (0.03–0.80) | <0.0001 |
| C-reactive protein mg·L−1 | 9.7 (3.0–27.0) | 7.8 (2.6–17.5) | 14.4 (4.2–37.9) | 0.02 |
| Antibiotic use n (%) | 116 (77.9) | 37 (72.5) | 79 (80.6) | 0.30 |
| Need for noninvasive ventilation n (%) | 24 (15.8) | 5 (9.8) | 19 (18.8) | 0.24 |
| Length of hospitalisation days | 7 (5–10) | 7 (5–12) | 7 (6–10) | 0.89 |
| Treatment failure n (%) | 31 (20.6) | 13 (26.0) | 18 (18.0) | 0.29 |
Data are presented as mean±sd, and compared with t-test, or median (interquartile range), and analysed with Mann–Whitney U-test, unless otherwise stated. Categorical variables were analysed with Fisher exact test. FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; ICS: inhaled corticosteroid; LAMA: long-acting muscarinic antagonist; LABA: long-acting β2-agonist; WBC: white blood cell.
Re-exacerbations in the eosinophilic and non-eosinophilic groups
More severe exacerbations were recorded than moderate relapses after the index event. The proportion of patients with no relapse or with a moderate and severe re-exacerbation did not differ between the groups (Fisher exact test: p=0.84, figure 1).
FIGURE 1.
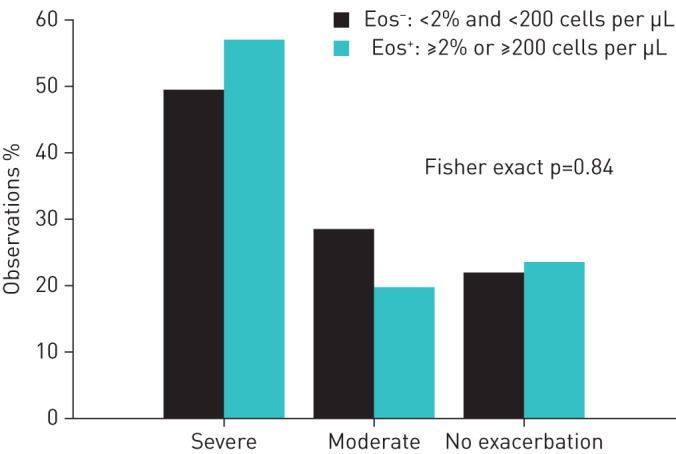
Patients with and without exacerbations in the eosinophilic (Eos+) and non-eosinophilic (Eos−) groups during the 12 months of follow-up.
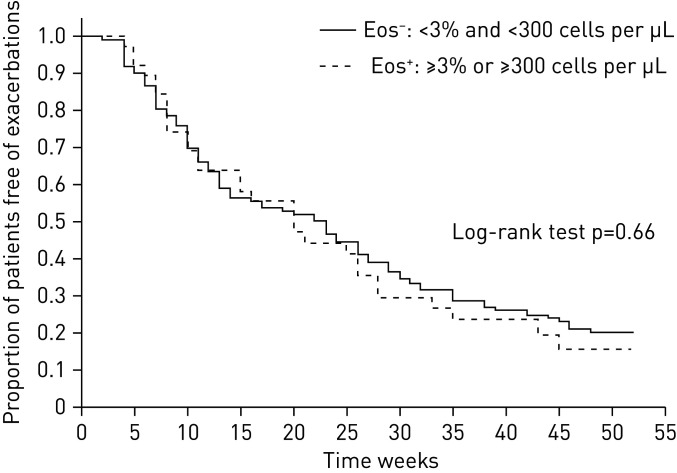
We did not find a difference in the time to the first exacerbation between the eosinophilic (≥2% and/or ≥200 eosinophils·µL−1) and non-eosinophilic groups (21 (10–36) weeks versus 17 (9–36) weeks, Mann–Whitney U-test: p=0.48, log-rank test: p=0.63) as shown in a Kaplan–Meier function plot (figure 2). The Cox proportional hazard model did not reveal an altered adjusted hazard ratio (HR) for the time to the first exacerbation for the eosinophilic group (HR: 1.10, 95% CI 0.75–1.61, p=0.64). When events were separated into severe and moderate exacerbations, no difference was found in the time of the relapse between the groups (log-rank test for severe exacerbations: p=0.90, for moderate exacerbations: p=0.51). No significant difference could be detected between the groups when we excluded the patients from the analysis who received systemic corticosteroids before the analysis of full blood count (supplementary figure S2).
FIGURE 2.
Recurrence of moderate or severe exacerbations in the eosinophilic (Eos+) and non-eosinophilic (Eos−) groups.
More patients in the eosinophilic (Eos+) group (n=39) were discharged from hospital on oral corticosteroids to complete the required course of treatment than in the non-eosinophilic (Eos−) group (n=58, p=0.03). However, there was no difference in the time to the first relapse between these subgroups of Eos+ and Eos− patients either (log-rank test: p=0.13).
Other cut-off values for the eosinophilic phenotype
We tested our hypothesis using other cut-off values for blood eosinophil ratios and counts [16]. Thirty-nine subjects (26% of all patients) had blood eosinophil granulocytes ≥3% of total leukocytes and/or ≥300 eosinophils·µL−1. Around three-quarters of patients had at least one exacerbation during the follow-up period (Eos+: 76.9%, Eos−: 77.9%), and in most cases patients required hospitalisation (no relapse/moderate exacerbation/severe exacerbation Eos+: n=9/7/23; Eos−: n=25/32/56, Chi-squared test p=0.42). The overall rate of COPD-related readmissions was similar in the two groups (Fisher exact test p=0.53). The eosinophilic subjects did not have an elevated risk of re-exacerbation based on a Cox proportional hazard model (HR: 0.91, 95% CI 0.60–1.38, p=0.66), and the time to the first re-exacerbation was also similar (Eos+: 20 (8–33) weeks versus Eos−: 22 (10–38) weeks, Mann–Whitney U-test p=0.54; log-rank test p=0.66), as seen on the Kaplan–Meier plot (figure 3).
FIGURE 3.
Recurrence of moderate or severe exacerbations in the eosinophilic (Eos+) and non-eosinophilic groups (Eos+) using the alternative cut-off for the eosinophilic phenotype of ≥3% of total leukocytes and/or ≥300 eosinophils·µL−1.
We also divided patients into tertiles based on the eosinophil ratios (<0.4% n=50, 0.4–1.8% n=51, >1.8% n=51). No difference was detected among the groups in median exacerbation-free periods (Eos low: 23 (10–52) weeks; Eos medium: 14 (7–35) weeks; Eos high: 20 (10–35) weeks, analysis of variance p=0.21; more details in the supplementary material and supplementary figure S3.)
Risk factors associated with the time to recurrence of exacerbations
We explored the risk factors related to the time to the next acute exacerbation including clinical parameters and either blood eosinophil percentage or count as inputs (tables 2 and 3). The higher number of previous severe exacerbations in the past 12 months, a more severe airflow limitation (% predicted post-bronchodilator FEV1 at discharge) and stronger exposure to cigarette smoke (expressed in pack-years) were significantly associated with shorter exacerbation-free time. However, stepwise regression analysis showed that the previous exacerbation history was the strongest predictor for future events.
TABLE 2.
Generalised linear model of risk factors and time to first re-exacerbation including blood eosinophil percentage
| Effect | Regression coefficient | Standard error | 95% Confidence interval | p-value | |
| Lower | Upper | ||||
| Eosinophil % | −0.034 | 0.041 | −0.115 | 0.046 | 0.40 |
| % predicted post-bronchodilator FEV1 at discharge | 0.015 | 0.006 | 0.003 | 0.028 | 0.01 |
| Post-bronchodilator FEV1/FVC ratio in % at discharge | −1.320 | 0.764 | −2.818 | 0.177 | 0.08 |
| Charlson Comorbidity Index | 0.010 | 0.068 | −0.123 | 0.143 | 0.89 |
| Smoking history pack-years | −0.008 | 0.003 | −0.014 | −0.001 | 0.03 |
| Number of severe exacerbations in the past 12 months | −0.103 | 0.047 | −0.195 | −0.010 | 0.03 |
| Time since COPD diagnosis years | 0.001 | 0.014 | −0.026 | 0.028 | 0.92 |
| Age | 0.003 | 0.010 | −0.016 | 0.022 | 0.74 |
| Sex (male) | 0.019 | 0.084 | −0.145 | 0.183 | 0.82 |
| Smoking habit (current smoker) | −0.130 | 0.088 | −0.302 | 0.043 | 0.14 |
| Need for noninvasive ventilation | −0.011 | 0.111 | −0.229 | 0.206 | 0.92 |
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity. Bold font indicates statistical significance.
TABLE 3.
Generalised linear model of risk factors and time to first re-exacerbation including blood eosinophil count
| Effect | Regression coefficient | Standard error | 95% Confidence interval | p-value | |
| Lower | Upper | ||||
| Absolute eosinophil count | −0.001 | 0.000 | −0.001 | 0.000 | 0.12 |
| % predicted post-bronchodilator FEV1 at discharge | 0.016 | 0.006 | 0.004 | 0.028 | 0.01 |
| Post-bronchodilator FEV1/FVC ratio in % at discharge | −1.288 | 0.758 | −2.773 | 0.197 | 0.09 |
| Charlson Comorbidity Index | 0.001 | 0.067 | −0.131 | 0.133 | 0.99 |
| Smoking history pack-years | −0.008 | 0.003 | −0.014 | −0.001 | 0.03 |
| Number of severe exacerbations in the past 12 months | −0.102 | 0.046 | −0.193 | −0.011 | 0.03 |
| Time since COPD diagnosis years | <0.001 | 0.014 | −0.026 | 0.027 | 0.98 |
| Age | 0.002 | 0.010 | −0.016 | 0.021 | 0.80 |
| Sex (male) | 0.021 | 0.083 | −0.142 | 0.183 | 0.80 |
| Smoking habit (current smoker) | −0.137 | 0.088 | −0.308 | 0.035 | 0.12 |
| Need for noninvasive ventilation | −0.020 | 0.110 | −0.235 | 0.195 | 0.85 |
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity. Bold font indicates statistical significance.
Phenotypes of the next exacerbation
Seventy-three patients had blood eosinophil data of the next exacerbation on hospital admission. By the index exacerbation, 27 patients had an eosinophilic relapse (≥2% of total leukocytes and/or ≥200 eosinophils·µL−1) and 46 patients were assigned a non-eosinophilic event (<2% of total leukocytes and <200 eosinophils·µL−1). On readmission, 75.3% of these exacerbations (n=55) were of similar type to that of the index event. However, 48.1% of patients with an index eosinophilic exacerbation had a non-eosinophilic relapse as the next episode, and 10.9% of patients with an index non-eosinophilic relapse suffered a subsequent eosinophilic exacerbation (supplementary table S1). The rate of eosinophilic exacerbations was lower on readmission than during the first relapse (p<0.001, Fisher's exact test).
Discussion
This is the first prospective study to evaluate the relationship between the eosinophilic acute severe COPD exacerbation and future relapses. Our results indicate that patients with an eosinophilic exacerbation do not have an increased risk of earlier recurrences of moderate or severe relapses. In line with the literature, the history of previous severe exacerbation was the strongest predictor for future relapses.
We explored the recurrence of both severe and moderate exacerbations [4]. Although moderate relapses do not necessitate hospitalisation, they have negative effects on health status with similar magnitude to that of severe exacerbations. It has been proven that similarly to reported exacerbations, unreported and moderate exacerbations are associated with increased symptoms, airflow limitation and increased levels of inflammatory markers, although the time to resolution of all symptoms is shorter [19–21].
Increasing evidence suggests that blood eosinophil level (either count or percentage) can be a signal of corticosteroid response, and it can also guide corticosteroid therapy [10, 22]. Blood eosinophil percentage is a surrogate marker of sputum eosinophilia during an exacerbation [8], and it also correlates with small airways inflammation [9]. Strategies directed to normalise sputum eosinophil count improved airflow limitation, reduced symptoms and decreased the number of severe exacerbations [23–25], also implying a biological role for these cells in COPD.
In our study, the distribution of patients with an eosinophilic exacerbation (≥2% and/or ≥200 eosinophils·µL−1) was similar to that reported in other investigations [14, 15]. Like other studies [14, 26], we found that patients with an eosinophilic exacerbation present with a lower serum CRP level suggesting a lower rate of infections. The eosinophilic type of relapses showed less severe airflow limitation characterised by higher FEV1/FVC, which is consistent with the findings of a secondary analysis of the ECLIPSE study, where FEV1 % predicted was higher in the eosinophilic group [27]. Other authors found no difference in baseline lung function [11, 14, 15], while Ko et al. [28] reported higher improvement in FEV1 values (both absolute and % predicted) in patients with blood or sputum eosinophilia.
The time to first moderate or severe exacerbation was similar after hospital treatment of an eosinophilic and non-eosinophilic exacerbation suggesting that the published cut-off values for blood eosinophil count or percentage are not biomarkers for re-admissions which has also been shown by others [11, 14]. In contrast, Couillard et al. [15] found in a post hoc analysis that the number of re-admissions was increased and the time to the first readmission was shorter in patients after an eosinophilic exacerbation. This discrepancy might be explained by the different outcome parameters (i.e. moderate and severe exacerbations were analysed together in our study), the pre-treatment of patients with systemic corticosteroid before blood tests or the more severe airflow limitation in our patient population compared to the other studies [11, 15, 28]. Of note, in our cohort the rate of corticosteroid therapy on admission was lower than in the study by Bafadhel et al. [14], but higher than that of Couillard et al. [15], where eosinophil count was determined before the initiation of a systemic corticosteroid therapy.
We did not find a difference in the length of hospital stay or the rate of treatment failure between the eosinophilic and non-eosinophilic groups. This supports the results of Couillard et al. [15], who also reported a similar length of hospitalisation, but our findings contradict other studies showing shorter hospital stay in the biomarker positive group [11, 14, 28]. Furthermore, our data are in line with the report of Prins et al. [11] who showed that the rate of late treatment failure (11–30 days post-discharge) was similar after eosinophilic or non-eosinophilic exacerbations.
Our data demonstrated that a subgroup of patients with an increased number of severe COPD exacerbations in the past have a shorter time till the recurrence of the next moderate or severe relapse. These results corroborate the findings of large-scale studies showing that the best predictor of an exacerbation is the positive history for prior events [5, 29]. We observed that exacerbation-free time also shortens with increasing severity of airflow limitation, which is in line with previous studies [5, 29, 30]. However, there is not enough evidence to use FEV1 alone as a predictor of exacerbation risk in COPD [31]. Interestingly, the stronger exposure to cigarette smoke negatively affected the time to the next flare-up. This may be explained by the relationship between smoking and FEV1 decline [32]. In addition, the incidence of lower respiratory infections is higher in current smokers, which can precipitate exacerbations [33, 34] and deteriorates lung function [35].
In an exploratory subgroup analysis, we investigated for the first time the stability of the eosinophilic type of severe exacerbation. Our data demonstrate that the eosinophilic exacerbation is a variable phenotype, while non-eosinophilic exacerbators show a more consistent timeline, which can be, at least partly, explained by the systemic corticosteroid treatment during the first relapse. This observation is also in line with data in stable COPD, i.e. patients with higher blood eosinophil counts on enrolment (≥0.34×109 cells·L−1) showed less stability within 1 year than patients with lower eosinophil counts (<0.34×109 cells·L−1) [36].
Our single-centre study has limitations. Approximately one-third of patients received a single dose of systemic corticosteroid before blood collection, which could have influenced results on eosinophil count. However, in real life settings treatment is often initiated in severe cases already out of hospital and finding clinically relevant biomarkers is also of importance in this group. In addition, the study has not been powered to analyse the effect of covariates (i.e. previous treatment, post-discharge oral corticosteroid use) on our findings in detail. Furthermore, data on past exacerbations were limited to hospitalisations (46% of all patients had at least one severe relapse in the year prior to study entry). However, as events with moderate severity outnumber severe relapses on a yearly basis [5], it can be speculated that most patients in our study had a positive exacerbation history.
In summary, eosinophilic exacerbations of COPD as defined by known blood eosinophil cut-off values do not increase the risk of earlier recurrence of moderate and severe relapses. However, the increased number of prior hospitalisations, smoking history and lower FEV1 % predicted are associated with a shorter exacerbation-free time with the strongest parameter being the previous exacerbation history. Our data add further knowledge on the clinical interpretation of eosinophilic AE-COPD and can facilitate the development of targeted interventions to prevent recurring exacerbations.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00543-2020.SUPPLEMENT (231.6KB, pdf)
Footnotes
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: B. Csoma has nothing to disclose.
Conflict of interest: A. Bikov has nothing to disclose.
Conflict of interest: F. Toth has nothing to disclose.
Conflict of interest: G. Losonczy has nothing to disclose.
Conflict of interest: V. Müller reports consultation fees from AstraZeneca, Chiesi, Berlin-Chemie/A. Menarini, Boehringer Ingelheim International GmbH, Teva Pharmaceutical Industries Ltd and GlaxoSmithKline plc, outside the submitted work.
Conflict of interest: Z. Lazar has nothing to disclose.
Support statement: This publication was supported by the Janos Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00559/16) and the ÚNKP 19-4-SE-92 New National Excellence Program of the Hungarian Ministry for Innovation and Technology to Z. Lázár, and by the and ÚNKP 17-2-1-SE-22 New National Excellence Program of the Hungarian Ministry for Human Capacities to B. Csoma. A. Bikov is supported by the NIHR Manchester BRC. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. . Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. doi: 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibson GJ, Loddenkemper R, Lundbäck B, et al. . Respiratory health and disease in Europe: the new European Lung White Book. Eur Respir J 2013; 42: 559–563. doi: 10.1183/09031936.00105513 [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology 2016; 21: 14–23. doi: 10.1111/resp.12660 [DOI] [PubMed] [Google Scholar]
- 4.Vogelmeier CF, Criner GJ, Martinez FJ, et al. . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur Respir J 2017; 49: 1700214. doi: 10.1183/13993003.00214-2017 [DOI] [PubMed] [Google Scholar]
- 5.Hurst JR, Vestbo J, Anzueto A, et al. . Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 6.Hu WP, Lhamo T, Liu D, et al. . Development of a nomogram to predict the risk of 30-day re-exacerbation for patients hospitalized for acute exacerbation of chronic obstructive pulmonary disease. COPD 2019; 16: 160–167. doi: 10.1080/15412555.2019.1606187 [DOI] [PubMed] [Google Scholar]
- 7.Lau CS, Siracuse BL, Chamberlain RS. Readmission After COPD Exacerbation Scale: determining 30-day readmission risk for COPD patients. Int J Chron Obstruct Pulmon Dis 2017; 12: 1891–1902. doi: 10.2147/COPD.S136768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bafadhel M, McKenna S, Terry S, et al. . Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011; 184: 662–671. doi: 10.1164/rccm.201104-0597OC [DOI] [PubMed] [Google Scholar]
- 9.Lazar Z, Kelemen A, Galffy G, et al. . Central and peripheral airway nitric oxide in patients with stable and exacerbated chronic obstructive pulmonary disease. J Breath Res 2018; 12: 036017. doi: 10.1088/1752-7163/aac10a [DOI] [PubMed] [Google Scholar]
- 10.Bafadhel M, McKenna S, Terry S, et al. . Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2012; 186: 48–55. doi: 10.1164/rccm.201108-1553OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prins HJ, Duijkers R, Lutter R, et al. . Blood eosinophilia as a marker of early and late treatment failure in severe acute exacerbations of COPD. Respir Med 2017; 131: 118–124. doi: 10.1016/j.rmed.2017.07.064 [DOI] [PubMed] [Google Scholar]
- 12.Bafadhel M, Davies L, Calverley PM, et al. . Blood eosinophil guided prednisolone therapy for exacerbations of COPD: a further analysis. Eur Respir J 2014; 44: 789–791. doi: 10.1183/09031936.00062614 [DOI] [PubMed] [Google Scholar]
- 13.Poder TG, Carrier N, Belanger M, et al. . Eosinophil counts in first COPD hospitalizations: a 1-year cost analysis in Quebec, Canada. Int J Chron Obstruct Pulmon Dis 2018; 13: 3065–3076. doi: 10.2147/COPD.S170747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bafadhel M, Greening NJ, Harvey-Dunstan TC, et al. . Blood eosinophils and outcomes in severe hospitalized exacerbations of COPD. Chest 2016; 150: 320–328. doi: 10.1016/j.chest.2016.01.026 [DOI] [PubMed] [Google Scholar]
- 15.Couillard S, Larivee P, Courteau J, et al. . Eosinophils in COPD exacerbations are associated with increased readmissions. Chest 2017; 151: 366–373. doi: 10.1016/j.chest.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Larivee P, Courteau J, et al. . Greater eosinophil counts at first COPD hospitalization are associated with more readmissions and fewer deaths. Int J Chron Obstruct Pulmon Dis 2019; 14: 331–341. doi: 10.2147/COPD.S187375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham BL, Steenbruggen I, Miller MR, et al. . Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med 2019; 200: e70–e88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan H, Li B, Couris CM, et al. . Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 19.Calderazzo MA, Trujillo-Torralbo MB, Finney LJ, et al. . Inflammation and infections in unreported chronic obstructive pulmonary disease exacerbations. Int J Chron Obstruct Pulmon Dis 2019; 14: 823–832. doi: 10.2147/COPD.S191946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijayasaratha K, Stockley RA. Reported and unreported exacerbations of COPD: analysis by diary cards. Chest 2008; 133: 34–41. doi: 10.1378/chest.07-1692 [DOI] [PubMed] [Google Scholar]
- 21.Bathoorn E, Liesker JJ, Postma DS, et al. . Change in inflammation in out-patient COPD patients from stable phase to a subsequent exacerbation. Int J Chron Obstruct Pulmon Dis 2009; 4: 101–109. doi: 10.2147/COPD.S4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivapalan P, Lapperre TS, Janner J, et al. . Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): a multicentre, randomised, controlled, open-label, non-inferiority trial. Lancet Respir Med 2019; 7: 699–709. doi: 10.1016/S2213-2600(19)30176-6 [DOI] [PubMed] [Google Scholar]
- 23.Brightling CE, Monteiro W, Ward R, et al. . Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2000; 356: 1480–1485. doi: 10.1016/S0140-6736(00)02872-5 [DOI] [PubMed] [Google Scholar]
- 24.Siva R, Green RH, Brightling CE, et al. . Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J 2007; 29: 906–913. doi: 10.1183/09031936.00146306 [DOI] [PubMed] [Google Scholar]
- 25.Brightling CE, Bleecker ER, Panettieri RA, Jr., et al. . Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir Med 2014; 2: 891–901. doi: 10.1016/S2213-2600(14)70187-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonald MI, Osadnik CR, Bulfin L, et al. . Low and high blood eosinophil counts as biomarkers in hospitalized acute exacerbations of COPD. Chest 2019; 156: 92–100. doi: 10.1016/j.chest.2019.02.406 [DOI] [PubMed] [Google Scholar]
- 27.Singh D, Kolsum U, Brightling CE, et al. . Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J 2014; 44: 1697–1700. doi: 10.1183/09031936.00162414 [DOI] [PubMed] [Google Scholar]
- 28.Ko FWS, Chan KP, Ngai J, et al. . Blood eosinophil count as a predictor of hospital length of stay in COPD exacerbations. Respirology 2020; 25: 259–266. doi: 10.1111/resp.13660 [DOI] [PubMed] [Google Scholar]
- 29.Mullerova H, Maselli DJ, Locantore N, et al. . Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest 2015; 147: 999–1007. doi: 10.1378/chest.14-0655 [DOI] [PubMed] [Google Scholar]
- 30.Bikov A, Lange P, Anderson JA, et al. . FEV1 is a stronger mortality predictor than FVC in patients with moderate COPD and with an increased risk for cardiovascular disease. Int J Chron Obstruct Pulmon Dis 2020; 15: 1135–1142. doi: 10.2147/COPD.S242809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soriano JB, Lamprecht B, Ramirez AS, et al. . Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2007 and 2011 staging systems: a pooled analysis of individual patient data. Lancet Respir Med 2015; 3: 443–450. doi: 10.1016/S2213-2600(15)00157-5 [DOI] [PubMed] [Google Scholar]
- 32.Scanlon PD, Connett JE, Waller LA, et al. . Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease – The Lung Health Study. Am J Respir Crit Care Med 2000; 161: 381–390. doi: 10.1164/ajrccm.161.2.9901044 [DOI] [PubMed] [Google Scholar]
- 33.Seemungal T, Harper-Owen R, Bhowmik A, et al. . Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 1618–1623. doi: 10.1164/ajrccm.164.9.2105011 [DOI] [PubMed] [Google Scholar]
- 34.Mallia P, Message SD, Gielen V, et al. . Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 2011; 183: 734–742. doi: 10.1164/rccm.201006-0833OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanner RE, Anthonisen NR, Connett JE, et al. . Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the Lung Health Study. Am J Respir Crit Care Med 2001; 164: 358–364. doi: 10.1164/ajrccm.164.3.2010017 [DOI] [PubMed] [Google Scholar]
- 36.Oshagbemi OA, Burden AM, Braeken DCW, et al. . Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am J Respir Crit Care Med 2017; 195: 1402–1404. doi: 10.1164/rccm.201701-0009LE [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00543-2020.SUPPLEMENT (231.6KB, pdf)