Abstract
Bronchodilator reversibility (BDR) is often used as a diagnostic test for adult asthma. However, there has been limited assessment of its diagnostic utility. We aimed to determine the discriminatory accuracy of common BDR cut-offs in the context of current asthma and asthma–COPD overlap (ACO) in a middle-aged community sample.
The Tasmanian Longitudinal Health Study is a population-based cohort first studied in 1968 (n=8583). In 2012, participants completed respiratory questionnaires and spirometry (n=3609; mean age 53 years). Receiver operating characteristic (ROC) curves were fitted for current asthma and ACO using continuous BDR measurements. Diagnostic parameters were calculated for different categorical cut-offs.
Area under the ROC curve (AUC) was highest when BDR was expressed as change in forced expiratory volume in 1 s (FEV1) as a percentage of initial FEV1, as compared with predicted FEV1. The corresponding AUC was 59% (95% CI 54–64%) for current asthma and 87% (95% CI 81–93%) for ACO. Of the categorical cut-offs examined, the European Respiratory Society/American Thoracic Society threshold (≥12% from baseline and ≥200 mL) was assessed as providing the best balance between positive and negative likelihood ratios (LR+ and LR−, respectively), with corresponding sensitivities and specificities of 9% and 97%, respectively, for current asthma (LR+ 3.26, LR− 0.93), and 47% and 97%, respectively, for ACO (LR+ 16.05, LR− 0.55).
With a threshold of ≥12% and ≥200 mL from baseline, a positive BDR test provided a clinically meaningful change in the post-test probability of disease, whereas a negative test did not. BDR was more useful as a diagnostic test in those with co-existent post-bronchodilator airflow obstruction (ACO).
Short abstract
Using the commonly adopted threshold, a positive BDR test provides a meaningful change in post-test probability of adult asthma, whereas a negative test does not. Discriminatory accuracy is much greater in those with coexistent post-BD airflow obstruction. https://bit.ly/3gPvlm8
Introduction
Asthma is a chronic inflammatory airways disease characterised by variable expiratory airflow limitation and respiratory symptoms which vary over time and in intensity [1]. A diagnosis of asthma is usually suspected based on clinical features, and tests of expiratory airflow are then used to confirm the diagnosis [1]. To avoid misdiagnosis and inappropriate treatment, international guidelines recommend a “test before treat” approach wherever possible. In more symptomatic individuals for whom early treatment is clinically indicated, spirometry is recommended within the first 1–3 months of treatment [1–3].
Of the available diagnostic tests for asthma, the Global Initiative for Asthma recommends spirometry to assess bronchodilator reversibility (BDR) as the first-line investigation [1]. BDR measures the increase in expiratory airflow in response to an inhaled short-acting bronchodilator and is usually based on the change in the forced expiratory volume in 1 s (ΔFEV1) [4]. “Significant reversibility” of FEV1 and/or the forced vital capacity (FVC) is considered a hallmark of current asthma and “rules in”, i.e. confirms, the diagnosis in most algorithms [1, 2]. However, it is estimated that ∼80% of those with current asthma do not exhibit significant reversibility [5, 6], and a negative BDR test typically warrants further investigations. Other recommended tests include nonspecific bronchial provocation or exercise challenge tests [1]. In addition, variable airflow limitation can be demonstrated over time or in response to controller treatment (e.g. inhaled corticosteroids (ICS)) to give a positive diagnosis [1].
Although BDR testing is commonly used, its clinical usefulness is still debated. A recent review of the historical development of recommendations for BDR testing has identified several important knowledge gaps [7]. These include a lack of consensus on which spirometric parameters BDR should be defined by and what constituted a significant response; insufficient data on sensitivities and specificities of common BDR cut-offs; and limited normative data derived from healthy general populations [7]. The latter point has more recently been addressed in an analysis of worldwide data from the Burden of Obstructive Lung Disease study and a pooled analysis of three large European population-based cohorts [6, 8] in which the prevalence of a significant response in healthy adults (defined as an increase in FEV1 of ≥12% from baseline) was reported to be 5.9% and 4.4%, respectively. Additionally, both studies provided data on BDR in different obstructive airways diseases, but defined current asthma based on symptoms and/or healthcare utilisation over the past 12 months. This definition, while common in epidemiological studies, may not be appropriate given the variable nature of asthma over time.
Current international guidelines recommend a cut-off for the diagnosis of asthma of ΔFEV1 ≥12% from baseline and ≥200 mL [9, 10]. In some cases, higher thresholds such as ΔFEV1 ≥15% from baseline and ≥400 mL are also used [9, 10]. However, there are still limited data on the diagnostic parameters (sensitivity and specificity) of these thresholds. While lower cut-offs such as ΔFEV1 ≥9% or ≥10% from baseline have been proposed and studied previously [11], they have not been widely adopted due to impractically high false-positive rates. Moreover, there is limited evidence on whether these thresholds are more useful in certain subgroups of asthma, such as those with asthma–COPD overlap, in whom the prevalence of BDR has been shown to be much higher [2].
In this study, we aimed to contribute additional normative data on BDR from an Australian population-based cohort and to examine the discriminatory accuracy of BDR for adult asthma, with and without fixed airflow obstruction. We examined the diagnostic parameters of different BDR measurements and cut-offs in both general and symptomatic samples.
Methods
Tasmanian Longitudinal Health Study
Methods of the baseline study and subsequent follow-ups have been described elsewhere [12, 13]. In brief, the Tasmanian Longitudinal Health Study (TAHS) is a population-based cohort of children born in 1961 and attending school in Tasmania, Australia in 1968 (n=8583). Large-scale follow-ups were conducted in 1974 (n=7380) and 2002 (n=5729). Between 2012 and 2016, when participants were aged 53 years, the cohort was traced and participants completed respiratory questionnaires (n=3609). Of these, 2646 (73%) opted to participate in a clinical study and spirometry. The current analysis includes participants in the 2012 follow-up.
Data collection
Spirometry was performed according to the joint American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines [14]. Lung function was assessed before and 15 min after inhalation of 300 µg salbutamol administered via a spacer. Predicted values for spirometry were derived from reference equations published by the Global Lung Initiative [15].
Definitions
Current doctor-diagnosed asthma (“current asthma”) was defined as asthma-related symptoms, healthcare or medication utilisation in the past 1 month in participants who reported doctor-diagnosed asthma and did not have post-bronchodilator (BD) airflow obstruction. Post-BD airflow obstruction consistent with COPD (“COPD”) was defined as post-BD FEV1/FVC ratio below the lower limit of normal. “Asthma–COPD overlap” (ACO) was defined by the combined criteria of current doctor-diagnosed asthma and post-BD airflow obstruction. The three disease categories were mutually exclusive.
Participants not meeting the criteria for current asthma, COPD or ACO were defined as having no airways disease. Of those without airways diseases, a reference sample of healthy adults was delineated using the following criteria: 1) never-asthma based on prospective data collected in 1968, 1974 and 2004; 2) never-smokers; and 3) no respiratory symptoms in the past 12 months (wheeze, shortness of breath or chest tightness). Normative data (i.e. mean±sd and upper limits of normal of BDR) were obtained from this reference sample.
BDR measurements and cut-offs
Three continuous BDR measurements were examined: 1) absolute volume change in FEV1 in mL (ΔFEV1); 2) ΔFEV1 as a percentage of the initial FEV1 (ΔFEV1initial), and (3) ΔFEV1 as a percentage of the predicted FEV1 (ΔFEV1pred) [7].
Two cut-offs recommended by ATS/ERS were examined: 1) ΔFEV1 ≥12% from baseline and ≥200 mL; and 2) ΔFEV1 ≥15% from baseline and ≥400 mL [1, 16]. These cut-offs were compared to cut-offs based on the reference sample upper limits of normal (ULN; 95th percentile) of the three continuous BDR measurements.
Statistical analysis
Receiver operating characteristic (ROC) curves were fitted for current asthma and ACO using the three continuous BDR measurements (ΔFEV1, ΔFEV1initial and ΔFEV1pred) as diagnostic classifiers against participants with no airways disease. When analyses were performed for current asthma, ACO participants were excluded, and vice versa.
For each categorical cut-off, the following diagnostic parameters were calculated: sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio and diagnostic odds ratio. The diagnostic odds ratio is a single indicator of diagnostic test performance calculated as the ratio between the positive and negative likelihood ratios [17]. Two statistical cut-offs, the Youden and Liu indexes, were examined for reference; methods for this approach are presented in supplementary methods E1.
All analyses were first conducted in the general population sample (whole TAHS cohort) to evaluate the discriminatory accuracy of BDR in nonselected settings (figure 1). Analyses were then repeated in the symptomatic subsample of the TAHS cohort, consisting of participants who self-reported one or more respiratory symptoms in the past 12 months (wheeze, shortness of breath or chest tightness). Details of the survey questionnaire used to define the symptomatic sample are provided in supplementary methods E2.
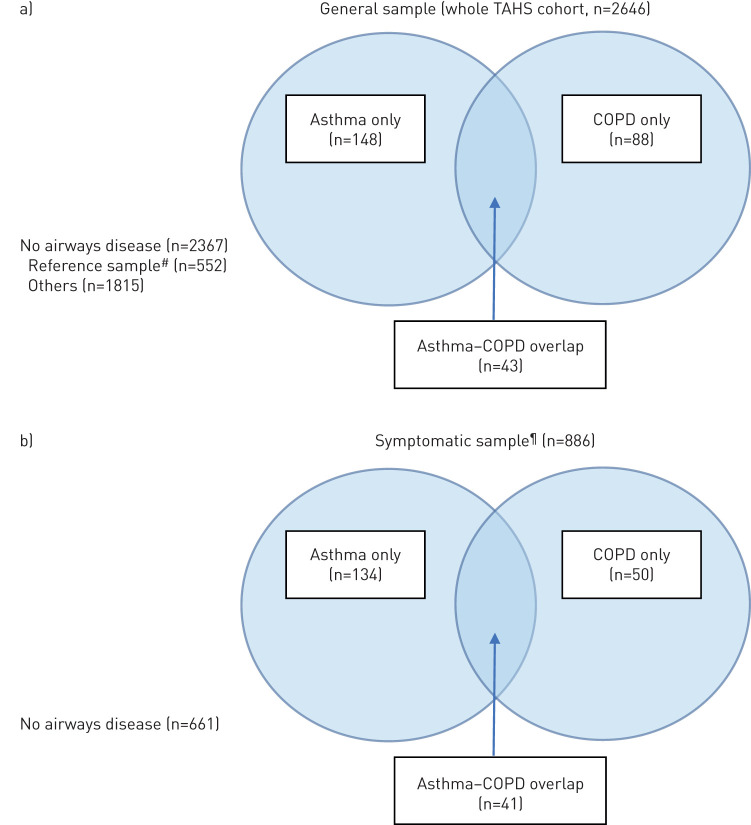
FIGURE 1.
Sample sizes of the disease subgroups in the a) general and b) symptomatic samples. #: never-asthma, never-smoker, no respiratory symptoms in the past 12 months; ¶: limited to participants who responded positively to one of five survey questions related to wheeze, shortness of breath or chest tightness in the past 12 months.
Two sensitivity analyses were performed. In the first sensitivity analysis, we excluded all participants who were on regular ICS to assess whether treatment status influenced on diagnostic utility. In the second sensitivity analysis, we used an amended definition of current asthma (based on symptoms, healthcare or medication utilisation in the past 12 months, rather than 1 month) more commonly used in epidemiological studies (see supplementary methods E3 for further details). All analyses were performed using STATA (version 15.1; Stata Corporation 2019, College Station, TX, USA).
Results
Participant characteristics
Of the 3609 participants in the 2012 TAHS follow-up, 2646 (73%) completed both pre- and post-BD spirometry. Of these, 2367 (89%) had no airways disease, 148 (6%) met the criteria for current asthma with self-reported doctor-diagnosed asthma, 88 (3%) met the criteria for spirometrically defined COPD and 43 (2%) met the criteria for ACO. The basic characteristics of these groups are presented in table 1 (general sample) and supplementary table E5 (symptomatic sample).
TABLE 1.
Basic characteristics of the disease subgroups in the 2012 Tasmanian Longitudinal Health Study study (general sample)
| No airways disease (n=2367)# | Asthma | COPD | ACO | ||
| Reference sample | Others | ||||
| Participants | 552 | 1815 | 148 | 88 | 43 |
| Age years | 52.5±0.8 | 52.7±0.8 | 52.9±0.8 | 52.8±0.7 | 52.8±0.8 |
| BMI kg·m−2 | 27.7±5.1 | 28.8±5.4 | 30.3±6.7 | 27.1±5.8 | 28.8±7.2 |
| Female | 285 (52) | 937 (52) | 77 (52) | 43 (49) | 19 (44) |
| Smoking history | |||||
| Never | 549 (100) | 567 (32) | 77 (53) | 15 (17) | 8 (19) |
| Former | 909 (51) | 56 (38) | 24 (28) | 17 (40) | |
| Current | 324 (18) | 13 (9) | 48 (55) | 18 (42) | |
| Asthma history | |||||
| Early-onset | 75 (51) | 19 (44) | |||
| Current ICS use | 76 (51) | 1 (1) | 18 (42) | ||
| Asthma severity | |||||
| Intermittent | 17 (13) | 1 (4) | |||
| Mild persistent | 33 (25) | 8 (29) | |||
| Moderate–severe persistent | 82 (62) | 19 (68) | |||
| Pre-BD spirometry | |||||
| FEV1 % pred | 102.0±11.8 | 98.7±13.2 | 91.6±15.1 | 78.5±15.1 | 64.7±18.8 |
| FVC % pred | 102.5±11.8 | 100.6±12.4 | 96.7±14.5 | 99.6±15.3 | 90.0±18.7 |
| FEV1/FVC % pred | 99.2±6.1 | 97.9±6.5 | 94.5±7.4 | 78.3±8.4 | 70.7±11.5 |
| Post-BD spirometry | |||||
| FEV1 % pred | 104.8±11.8 | 101.9±12.8 | 96.0±13.8 | 82.5±14.3 | 72.4±19.0 |
| FVC % pred | 102.3±11.7 | 100.8±12.1 | 98.1±13.4 | 102.8±14.9 | 96.7±17.5 |
| FEV1/FVC % pred | 102.2±5.2 | 100.8±6.0 | 97.8±6.4 | 79.8±7.0 | 73.8±11.6 |
| BDR indices | |||||
| ΔFEV1 mL | 91.9±121.0 | 102.1±132.2 | 145.7±159.3 | 127.5±199.2 | 257.4±188.0 |
| ΔFEV1 % of initial FEV1 | 2.9±3.7 | 3.4±4.4 | 5.4±6.7 | 6.0±8.8 | 13.5±11.6 |
| ΔFEV1 % of predicted FEV1 | 2.8±3.6 | 3.1±3.9 | 4.4±4.9 | 4.0±6.1 | 7.7±5.1 |
Data are presented as n, mean±sd or n (%). Complete data were obtained in 2625 (99%) for smoking history, 160 (84%) for asthma severity. ACO: asthma–COPD overlap; BMI: body mass index; ICS: inhaled corticosteroid; BD: bronchodilator; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; BDR: bronchodilator reversibility. #: reference sample criteria: never-asthma, never-smoker, no respiratory symptoms in the past 12 months.
The mean±sd ΔFEV1 in the reference sample was 91.9±121.0 mL, the ΔFEV1initial was 2.9±3.7% and the ΔFEV1pred was 2.8±3.6%. The corresponding ULN values were 280 mL, 9.3% and 8.8%, respectively. Compared to the reference sample, BDR was significantly higher in adults with each form of obstructive airways disease (supplementary table E1; p<0.002 for all comparisons). The magnitude of BDR was significantly higher in adults with ACO compared to both current asthma (mean difference (MD) +111.7 mL; p<0.001) and COPD (MD +129.9 mL; p<0.001), probably related to a lower baseline FEV1 in the ACO group (65% pred). In contrast, BDR was not significantly different between current asthma and COPD (MD −18.2 mL; p=0.44), despite a much lower baseline FEV1 in adults with COPD (92% versus 79% pred, respectively). Among adults with either current asthma or ACO, those with current ICS use had greater BDR compared to those who were untreated (supplementary table E2).
Comparison of BDR measurements (ROC curves)
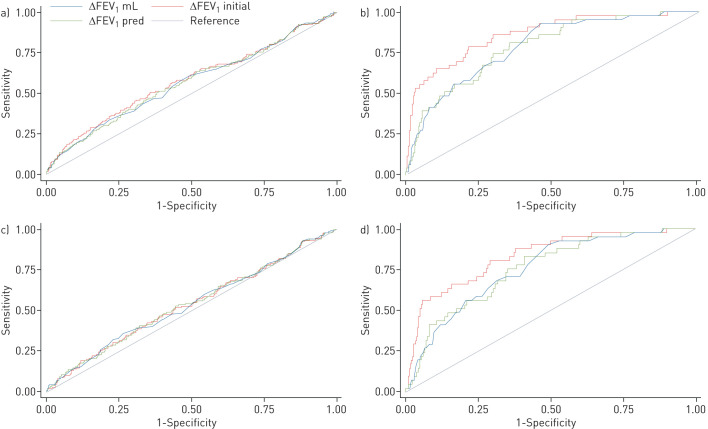
Whereas all continuous BDR measurements performed similarly for current asthma, the areas under the ROC curve (AUC) for ACO was highest when BDR was expressed as ΔFEV1initial as compared to ΔFEV1 or ΔFEV1pred (table 2 and figure 2). The corresponding AUC was 59% (95% CI 54–64%) for current asthma and 87% (95% CI 81–93%) for ACO. Findings were similar when ROC curves were developed for the symptomatic sample (table 2 and figure 2).
TABLE 2.
General sample: areas under the receiver operating characteristic curve (AUC) (95% CI) of bronchodilator reversibility measures as diagnostic classifiers for current doctor-diagnosed asthma and asthma–COPD overlap (ACO)
| Asthma % | ACO % | |
| General sample (whole cohort) | ||
| ΔFEV1 mL | 57 (52–62) | 79 (73–86) |
| ΔFEV1 % of initial FEV1 | 59 (54–64) | 87 (81–93) |
| ΔFEV1 % of predicted FEV1 | 57 (52–62) | 79 (73–86) |
| Symptomatic sample | ||
| ΔFEV1 mL | 56 (50–61) | 76 (69–83) |
| ΔFEV1% of initial FEV1 | 56 (50–61) | 84 (78–90) |
| ΔFEV1% of predicted FEV1 | 55 (50–61) | 77 (69–84) |
FEV1: forced expiratory volume in 1 s.
FIGURE 2.
Receiver operator characteristic curves of the bronchodilator reversibility measures for a,c) current doctor-diagnosed asthma and b,d) asthma–COPD overlap in the a,b) general and c,d) symptomatic samples. FEV1: forced expiratory volume in 1 s.
Diagnostic parameters of BDR cut-offs
All cut-offs investigated had low sensitivities, but high specificities for both current asthma and ACO (table 3). Cut-offs were generally more sensitive for ACO (12–60%) than for current asthma (5–20%), but were highly specific for both conditions (92–99%). The positive likelihood ratios ranged between 2.09 and 4.83 for current asthma, and between 5.40 and 16.05 for ACO. The negative likelihood ratios ranged between 0.87 and 0.96 for current asthma, and between 0.43 and 0.89 for ACO.
TABLE 3.
General sample: diagnostic parameters of bronchodilator reversibility cut-off points for current doctor-diagnosed asthma and asthma–COPD overlap (ACO)
| Sensitivity % (95% CI) | Specificity % (95% CI) | Positive predictive value % (95% CI) | Negative predictive value % (95% CI) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) | Diagnostic OR (95% CI) | |
| Asthma | |||||||
| ΔFEV1 (mL) ULN# | 16 (11–23) | 92 (91–93) | 11 (7–16) | 95 (94–96) | 2.09 (1.42–3.09) | 0.91 (0.85–0.98) | 2.30 (1.45–3.65) |
| ΔFEV1% of initial FEV1 ULN¶ | 20 (14–27) | 92 (91–93) | 13 (9–19) | 95 (94–96) | 2.58 (1.81–3.68) | 0.87 (0.80–0.94) | 2.97 (1.93–4.57) |
| ΔFEV1% of predicted FEV1 ULN+ | 15 (10–21) | 94 (93–95) | 13 (8–19) | 95 (94–96) | 2.40 (1.58–3.63) | 0.91 (0.85–0.97) | 2.64 (1.63–4.27) |
| ΔFEV1 12% of initial+200 mL | 9 (6–15) | 97 (96–98) | 16 (9–26) | 95 (94–96) | 3.26 (1.89–5.65) | 0.93 (0.89–0.98) | 3.50 (1.92–6.37) |
| ΔFEV1 15% of initial+400 mL | 5 (2–9) | 99 (99–99) | 23 (10–41) | 95 (94–95) | 4.83 (2.12–11.02) | 0.96 (0.93–1.00) | 5.02 (2.13–11.85) |
| ACO | |||||||
| ΔFEV1 (mL) ULN# | 42 (28–57) | 92 (91–93) | 9 (5–13) | 99 (98–99) | 5.40 (3.70–7.88) | 0.63 (0.49–0.81) | 8.56 (4.59–15.98) |
| ΔFEV1% of initial FEV1 ULN¶ | 60 (46–74) | 92 (91–93) | 12 (8–17) | 99 (99–100) | 7.96 (6.03–10.52) | 0.43 (0.30–0.62) | 18.62 (9.92–34.93) |
| ΔFEV1% of predicted FEV1 ULN+ | 40 (26–54) | 94 (93–95) | 10 (6–16) | 99 (98–99) | 6.37 (4.27–9.51) | 0.65 (0.51–0.82) | 9.89 (5.25–18.62) |
| ΔFEV1 12% of initial+200 mL | 47 (33–61) | 97 (96–98) | 22 (14–32) | 99 (99–99) | 16.05 (10.82–23.80) | 0.55 (0.42–0.73) | 29.14 (15.30–55.48) |
| ΔFEV1 15% of initial+400 mL | 12 (5–24) | 99 (99–99) | 17 (6–36) | 98 (98–99) | 11.87 (4.75–29.64) | 0.89 (0.80–1.00) | 13.30 (4.82–36.72) |
ΔFEV1: change in forced expiratory volume in 1 s; ULN: upper limit of normal. #: 280 mL; ¶: 9.2%; +: 8.8%.
Using the diagnostic odds ratio as a single indicator of diagnostic performance, the best cut-off for current asthma was ΔFEV1 15% of initial+400 mL. However, even this cut-off performed relatively poorly, with a positive likelihood ratio of 4.83, negative likelihood ratio of 0.96 and diagnostic odds ratio of 5.02 (95% CI 2.13–11.85). In contrast, the optimal cut-off for ACO was ΔFEV1 12% of initial+200 mL, which provided a positive likelihood ratio of 16.05, a negative likelihood ratio of 0.55 and diagnostic odds ratio of 29.14 (95% CI 15.30–55.48).
Overall, the ATS/ERS cut-off (ΔFEV1 12% of initial+200 mL) was assessed as providing the best balance between positive and negative likelihood ratios, corresponding to sensitivities and specificities of 9% and 97%, respectively, for current asthma (positive likelihood ratio 3.26, negative likelihood ratio 0.93), and 47% and 97%, respectively, for ACO (positive likelihood ratio 16.05, negative likelihood ratio 0.55). Compared to the general sample, cut-offs in the symptomatic sample were marginally less specific, but had similar sensitivities (table 4); positive likelihood ratios were slightly lower, whereas negative likelihood ratios were largely unchanged.
TABLE 4.
Symptomatic sample: diagnostic parameters of bronchodilator reversibility cut-off points for current doctor-diagnosed asthma and asthma–COPD overlap (ACO)
| Sensitivity % (95% CI) | Specificity % (95% CI) | Positive predictive value % (95% CI) | Negative predictive value % (95% CI) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) | Diagnostic OR (95% CI) | |
| Asthma | |||||||
| ΔFEV1 (mL) ULN# | 17 (12–24) | 89 (86–91) | 23 (15–32) | 85 (82–88) | 1.54(1.01–2.36) | 0.93 (0.86–1.01) | 1.65 (1.00–2.74) |
| ΔFEV1% of initial FEV1 ULN¶ | 20 (14–28) | 88 (85–90) | 24 (17–33) | 85 (83–88) | 1.68 (1.14–2.49) | 0.91 (0.83–0.99) | 1.85 (1.15–2.99) |
| ΔFEV1% of predicted FEV1 ULN+ | 16 (10–23) | 91 (89–93) | 26 (17–36) | 85 (82–88) | 1.82 (1.15–2.89) | 0.92 (0.86–1.00) | 1.97 (1.16–3.37) |
| ΔFEV1 12% of initial+200 mL | 10 (6–17) | 95 (93–96) | 27 (16–42) | 85 (82–87) | 2.00 (1.11–3.60) | 0.95 (0.89–1.00) | 2.12 (1.11–4.04) |
| ΔFEV1 15% of initial+400 mL | 5 (3–10) | 98 (96–99) | 30 (13–53) | 85 (82–87) | 2.32 (0.97–5.52) | 0.97 (0.93–1.01) | 2.39 (0.96–5.92) |
| ACO | |||||||
| ΔFEV1 (mL) ULN# | 41 (28–57) | 89 (86–91) | 18 (11–27) | 96 (95–98) | 3.72 (2.45–5.66) | 0.66 (0.51–0.85) | 5.65 (2.91–10.97) |
| ΔFEV1% of initial FEV1 ULN¶ | 61 (46–74) | 88 (85–90) | 23 (15–32) | 98 (96–99) | 5.09 (3.71–6.98) | 0.44 (0.30–0.65) | 11.47 (5.89–22.35) |
| ΔFEV1% of predicted FEV1 ULN+ | 41 (28–57) | 91 (89–93) | 22 (13–33) | 96 (95–98) | 4.82 (3.12–7.45) | 0.64 (0.50–0.83) | 7.53 (3.83–14.77) |
| ΔFEV1 12% of initial+200 mL | 49 (34–64) | 95 (93–96) | 35 (23–49) | 97 (95–98) | 9.35 (6.00–14.57) | 0.54 (0.40–0.73) | 17.30 (8.62–34.70) |
| ΔFEV1 15% of initial+400 mL | 12 (5–26) | 98 (96–99) | 24 (8–47) | 95 (93–97) | 5.40 (2.08–14.02) | 0.90 (0.80–1.01) | 6.02 (2.09–17.34) |
ΔFEV1: change in forced expiratory volume in 1 s; ULN: upper limit of normal. #: 280 mL; ¶: 9.2%; +: 8.8%.
Sensitivity analyses
The findings of the sensitivity analyses are presented in the supplementary material. The AUC and diagnostic parameters of the BDR measurements and cut-offs were largely unchanged when participants on regular ICS were excluded from the analyses (supplementary tables E6–E8) or when a 12-month (instead of 1-month) definition of current asthma was used (supplementary tables E9–E12).
Discussion
Using data from a prospective Australian community-based cohort followed from age 7 to 53 years, we compared the discriminatory accuracy of common BDR measurements and cut-offs for adult asthma. Across a range of cut-off points, ΔFEV1, ΔFEV1initial and ΔFEV1pred performed similarly for current asthma, whereas ΔFEV1initial performed better than ΔFEV1 and ΔFEV1pred for ACO. The discriminatory accuracy of these measures were better for ACO compared to current asthma. All cut-offs examined in this study had low sensitivities, but high specificities for both current asthma and ACO, missing ≥80% of those with current asthma and approximately half of those with ACO.
The ULN of BDR in “healthy” nonsmokers in this middle-aged Australian cohort (ΔFEV1 280 mL, ΔFEV1initial 9.3%, ΔFEV1pred 8.8%) was consistent with previous reports [7]. In a recent review of seven population-based cohorts, ULN estimates ranged between 240 and 320 mL for ΔFEV1, between 5.9% and 13% for ΔFEV1initial, and between 8.7% and 11.6% for ΔFEV1pred [8, 18–23]. Consistent with a recent analysis of three European cohorts [6], we found that BDR distributions were similar in adults with current asthma and those with COPD, but significantly higher in ACO compared to either current asthma or COPD. While there is ongoing debate around the usefulness of BDR in distinguishing obstructive airways diseases [24, 25], our findings indicate that BDR has some phenotypic value in delineating ACO (current asthma in those with fixed airflow obstruction) from common COPD.
A long-standing issue surrounding the clinical application of BDR has been the lack of agreement on how it should be expressed [4, 16, 26]. ΔFEV1 and ΔFEV1initial are most commonly used; however, cut-offs based on these measures tend to be biased by age, sex and other factors [11]. While the impact of these factors has been argued to have minimal clinical impact by some authors [27], others have recommended the use of age-specific BDR cut-offs [11] or alternative measurements with theoretically less susceptibility to bias such as ΔFEV1pred [11, 21], change in FEV1 z-score (ΔzFEV1) [21], or change in volume-based parameters such as ΔFVC. The latter two approaches are not widely used, although there is some evidence that ΔFVC may be more clinically relevant in severe airways obstruction [21]. Our study was inherently standardised by age by nature of recruitment and found that across a range of thresholds, ΔFEV1initial provided a better diagnostic utility compared to ΔFEV1 and ΔFEV1pred, despite these theoretical advantages, and accounted for low baseline FEV1, especially relevant to those in the ACO subgroup.
ATS guidelines first recommended a cut-off for the diagnosis of asthma of ΔFEV1 ≥12% from baseline and ≥200 mL in 1991 [26], with these thresholds based on studies of small numbers of patients with obstructive airways defects [27–29]. In the years since, few studies have evaluated the sensitivities and specificities of common cut-offs for obstructive airways diseases [7]. An obstacle is that there is no single “gold standard” test for current asthma, and its diagnosis often requires a degree of clinical judgement. In an Australian population-based study, the sensitivities of BDR for current doctor-diagnosed asthma ranged between 7% and 18% depending on the cut-off (ΔFEV1 ≥400 mL versus ΔFEV1pred ≥9%) [11]. In a study of 190 participants with asthma-like symptoms, a cut-off of ΔFEV1initial >12% provided a sensitivity of 13% and specificity of 93% compared to clinical opinions from a panel of three respiratory physicians [30]. While the first study was performed in steroid-naïve patients, approximately half of the participants in the second study were on ICS treatment at the time of assessment. As observed in our study, participants on ICS had higher BDR responses than those who were untreated, consistent with these participants being more likely to have greater disease activity and/or severity [30].
All cut-offs examined in our study demonstrated low sensitivities, but high specificities for both current asthma and ACO. These findings imply that a positive test could assist with ruling in a diagnosis of asthma, whereas a negative test was unhelpful. Of note, positive and negative likelihood ratios provide a more robust measure of test performance with respect to pre- and post-test probabilities. A high positive likelihood ratio (>5) implies that a positive test provides a clinically meaningful increase in the post-test probability of the disease [31]. In contrast, a low negative likelihood ratio (<0.2) implies that a negative test would provide a clinically meaningful decrease in the post-test probability of the disease [31]. Most positive and negative likelihood ratios for adult asthma fell within the clinically unhelpful range of 0.2–5, indicating the test was of limited diagnostic value. A notable exception was the relatively high positive likelihood ratios achieved for ACO, particularly using the ATS/ERS guideline cut-off. Consequently, our results reaffirm that BDR testing is more relevant in those with more severe disease and lower baseline lung function.
As expected, the sensitivities, specificities, positive and negative likelihood ratios of the BDR cut-offs were mostly unchanged between the analyses in the general and symptomatic samples. In contrast, the changes in positive and negative predictive values between the two analyses probably reflected differences in the prevalence of disease between the two populations.
Strengths and limitations
There are a number of strengths to our study. Firstly, our study has good external validity as our participants were derived from the general population. Secondly, due to the nature of recruitment, all participants were of similar age and we were able to assess the diagnostic parameters of BDR largely independently of age effects. Thirdly, we had access to prospective data collected from the first to sixth decades of life, which allowed us to more accurately determine our participants' lifetime asthma histories and delineate the healthy reference sample compared to previous studies. Finally, we examined the diagnostic parameters of different BDR measurements and cut-offs in both general and respiratory symptom populations using both clinical (1-month) and epidemiological (12-month) definitions.
There are several important limitations to the study. The narrow age-bracket of our participants potentially limits the generalisability of our results beyond a middle-aged adult population. Our gold-standard definition of asthma was based on self-reported features including a history of doctor-diagnosed asthma, which may have introduced misclassification (in cases of incorrect recall or incorrect diagnosis by the treating physician) and excluded those with undiagnosed asthma. Diagnoses of asthma in the community were likely to have been guided by existing ATS/ERS guidelines and thresholds. Finally, approximately half of our asthmatic participants were on ICS in the weeks prior to assessment. While these participants had higher BDR responses than those who were untreated, probably due to more severe disease, they may have had a reduced response compared to if they were ICS-naïve. Consequently, our results probably underestimate the sensitivity of BDR cut-offs in treatment-naïve adult asthma, despite findings being relatively unchanged in the sensitivity analysis which excluded ICS users. Therefore, future studies in patients with newly diagnosed, treatment-naïve patients are still required.
Conclusion
BDR remains a simple and inexpensive method of measuring expiratory airflow variability. Applying the thresholds examined in this study, a positive BDR test provides a clinically meaningful change in the post-test probability of asthma, whereas a negative test does not. In the presence of typical clinical features, a negative test therefore warrants further investigations. Overall, our findings identify important limitations of BDR testing, but support its use as an initial investigation in the work-up of suspected adult asthma and ACO, with an optimal threshold of ≥12% and ≥200 mL from baseline.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00042-2020.SUPPLEMENT (749.2KB, pdf)
Acknowledgements
We acknowledge the TAHS study participants and previous investigators, Heather Gibson, Bryan Gandevia, Harold Silverstone and Norelle Lickiss. We thank Mark Jenkins, Lyle Gurrin, Graham Giles, John Hopper (Centre for Epidemiology and Biostatistics, Victoria), Alan James (Sir Charles Gairdner Hospital, Nedlands, Western Australia), Peter Frith (Flinders University, Adelaide), Richard Wood-Baker (Tasmanian School of Medicine, Tasmania) and Iain Feather (Gold Coast Hospital, Queensland), who are TAHS investigators but not coauthors of this manuscript, for their assistance with obtaining funds and data collection. We also acknowledge the study site coordinators and respiratory scientists who collected data in lung function laboratories in Tasmania, Victoria, Queensland and New South Wales, the research interviewers and data entry operators; and the organisational roles of Cathryn Wharton (Centre for Epidemiology and Biostatistics) and Desiree Mészáros (Tasmanian School of Medicine). Furthermore, we thank the late Stephen Morrison (University of Queensland) for his assistance with obtaining funds and data collection.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Ethics approval: This study was approved by separate human ethics review committees at all participating institutions, principally the University of Melbourne (040375) and the University of Tasmania (H0012710). Written informed consent was obtained from all participants. All data used in this study were de-identified.
Conflict of interest: D.J. Tan has nothing to disclose.
Conflict of interest: C.J. Lodge has nothing to disclose.
Conflict of interest: A.J. Lowe has nothing to disclose.
Conflict of interest: D.S. Bui has nothing to disclose.
Conflict of interest: G. Bowatte has nothing to disclose.
Conflict of interest: D.P. Johns has nothing to disclose.
Conflict of interest: G.S. Hamilton has nothing to disclose.
Conflict of interest: P.S. Thomas reports having participated in advisory board meetings for GSK and AstraZeneca, unrelated to this work.
Conflict of interest: M.J. Abramson reports investigator-initiated grants for unrelated research from Pfizer and Boehringer Ingelheim, personal fees for unrelated consultancy and assistance with conference attendance from Sanofi, and a speaker's fee from GSK, outside the submitted work.
Conflict of interest: E.H. Walters has nothing to disclose.
Conflict of interest: J.L. Perret reports a travel grant from Boehringer Ingelheim, outside the submitted work.
Conflict of interest: S.C. Dharmage has nothing to disclose.
Support statement: This study was supported by the National Health and Medical Research Council (NHMRC) of Australia; the University of Melbourne, Clifford Craig Foundation; the Victorian, Queensland and Tasmanian Asthma Foundations; Royal Hobart Hospital; Helen MacPherson Smith Trust; and GlaxoSmithKline. The funding bodies had no direct role in the conduct of the study, the collection, management, statistical analysis and interpretation of the data, preparation or approval of the manuscript. D.J. Tan was funded by a NHMRC Postgraduate Scholarship and Royal Australian College of Physicians (RACP) Woolcock Scholarship. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention 2019. Available from: http://ginasthma.org. [Google Scholar]
- 2.National Institute for Health and Care Excellence (NICE) 2017. www.nice.org.uk/guidance/ng80. Asthma: Diagnosis, Monitoring and Chronic Asthma Management. NICE guideline [NG80] Date last updated: February 12, 2020. [Google Scholar]
- 3.Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet 2018; 391: 350–400. doi: 10.1016/S0140-6736(17)30879-6 [DOI] [PubMed] [Google Scholar]
- 4.Brand PL, Quanjer PH, Postma DS, et al. Interpretation of bronchodilator response in patients with obstructive airways disease. The Dutch Chronic Non-Specific Lung Disease (CNSLD) Study Group. Thorax 1992; 47: 429–436. doi: 10.1136/thx.47.6.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA 2017; 317: 269–279. doi: 10.1001/jama.2016.19627 [DOI] [PubMed] [Google Scholar]
- 6.Janson C, Malinovschi A, Amaral AFS, et al. Bronchodilator reversibility in asthma and COPD: findings from three large population studies. Eur Respir J 2019; 54: 1900561. doi: 10.1183/13993003.00561-2019 [DOI] [PubMed] [Google Scholar]
- 7.Tuomisto LE, Ilmarinen P, Lehtimäki L, et al. Immediate bronchodilator response in FEV1 as a diagnostic criterion for adult asthma. Eur Respir J 2019; 53: 1800904. doi: 10.1183/13993003.00904-2018 [DOI] [PubMed] [Google Scholar]
- 8.Tan WC, Vollmer WM, Lamprecht B, et al. Worldwide patterns of bronchodilator responsiveness: results from the Burden of Obstructive Lung Disease study. Thorax 2012; 67: 718–726. doi: 10.1136/thoraxjnl-2011-201445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pennock BE, Rogers RM, McCaffree DR. Changes in measured spirometric indices. What is significant? Chest 1981; 80: 97–99. doi: 10.1378/chest.80.1.97 [DOI] [PubMed] [Google Scholar]
- 10.Sourk RL, Nugent KM. Bronchodilator testing: confidence intervals derived from placebo inhalations. Am Rev Respir Dis 1983; 128: 153–157. doi: 10.1164/arrd.1983.128.1.153 [DOI] [PubMed] [Google Scholar]
- 11.Appleton SL, Adams RJ, Wilson DH, et al. Spirometric criteria for asthma: adding further evidence to the debate. J Allergy Clin Immunol 2005; 116: 976–982. doi: 10.1016/j.jaci.2005.08.034 [DOI] [PubMed] [Google Scholar]
- 12.Matheson MC, Abramson MJ, Allen K, et al. Cohort profile: the Tasmanian Longitudinal Health Study (TAHS). Int J Epidemiol 2017; 46: 407–408i. [DOI] [PubMed] [Google Scholar]
- 13.Gibson HB, Silverstone H, Gandevia B, et al. Respiratory disorders in seven-year-old children in Tasmania. Aims, methods and administration of the survey. Med J Aust 1969; 2: 201–205. doi: 10.5694/j.1326-5377.1969.tb105698.x [DOI] [PubMed] [Google Scholar]
- 14.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 15.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 17.Glas AS, Lijmer JG, Prins MH, et al. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003; 56: 1129–1135. doi: 10.1016/S0895-4356(03)00177-X [DOI] [PubMed] [Google Scholar]
- 18.Lorber DB, Kaltenborn W, Burrows B. Responses to isoproterenol in a general population sample. Am Rev Respir Dis 1978; 118: 855–861. [DOI] [PubMed] [Google Scholar]
- 19.Dales RE, Spitzer WO, Tousignant P, et al. Clinical interpretation of airway response to a bronchodilator. Epidemiologic considerations. Am Rev Respir Dis 1988; 138: 317–320. doi: 10.1164/ajrccm/138.2.317 [DOI] [PubMed] [Google Scholar]
- 20.Kainu A, Lindqvist A, Sarna S, et al. FEV1 response to bronchodilation in an adult urban population. Chest 2008; 134: 387–393. doi: 10.1378/chest.07-2207 [DOI] [PubMed] [Google Scholar]
- 21.Quanjer PH, Ruppel GL, Langhammer A, et al. Bronchodilator response in FVC is larger and more relevant than in FEV1 in severe airflow obstruction. Chest 2017; 151: 1088–1098. doi: 10.1016/j.chest.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 22.Torén K, Bake B, Olin AC, et al. Measures of bronchodilator response of FEV1, FVC and SVC in a Swedish general population sample aged 50–64 years, the SCAPIS Pilot Study. Int J Chron Obstruct Pulmon Dis 2017; 12: 973–980. doi: 10.2147/COPD.S127336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe S, Renzetti AD Jr, Begin R, et al. Airway responsiveness to a bronchodilator aerosol. I. Normal human subjects. Am Rev Respir Dis 1974; 109: 530–537. [DOI] [PubMed] [Google Scholar]
- 24.Chhabra SK Acute bronchodilator response has limited value in differentiating bronchial asthma from COPD. J Asthma 2005; 42: 367–372. doi: 10.1081/JAS-200062992 [DOI] [PubMed] [Google Scholar]
- 25.Miravitlles M Diagnosis of asthma–COPD overlap: the five commandments. Eur Respir J 2017; 49: 1700506. doi: 10.1183/13993003.00506-2017 [DOI] [PubMed] [Google Scholar]
- 26.Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis 1991; 144: 1202–1218. doi: 10.1164/ajrccm/144.5.1202 [DOI] [PubMed] [Google Scholar]
- 27.Eliasson O, Degraff AC Jr. The use of criteria for reversibility and obstruction to define patient groups for bronchodilator trials. Influence of clinical diagnosis, spirometric, and anthropometric variables. Am Rev Respir Dis 1985; 132: 858–864. [DOI] [PubMed] [Google Scholar]
- 28.Criteria for the assessment of reversibility in airways obstruction. Report of the Committee on Emphysema American College of Chest Physicians. Chest 1974; 65: 552–553. doi: 10.1378/chest.65.5.552 [DOI] [PubMed] [Google Scholar]
- 29.Pellegrino R, Rodarte JR, Brusasco V. Assessing the reversibility of airway obstruction. Chest 1998; 114: 1607–1612. doi: 10.1378/chest.114.6.1607 [DOI] [PubMed] [Google Scholar]
- 30.Backer V, Sverrild A, Ulrik CS, et al. Diagnostic work-up in patients with possible asthma referred to a university hospital. Eur Clin Respir J 2015; 2: 27768. doi: 10.3402/ecrj.v2.27768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGee S Simplifying likelihood ratios. J Gen Intern Med 2002; 17: 646–649. doi: 10.1046/j.1525-1497.2002.10750.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00042-2020.SUPPLEMENT (749.2KB, pdf)