Abstract
Leflunomide (LF) represents the prototype member of dihydroorotate dehydrogenase (DHODH) enzyme inhibitors. DHODH is a mitochondrial inner membrane enzyme responsible for catalytic conversion of dihydroorotate into orotate, a rate-limiting step in the de novo synthesis of the pyrimidine nucleotides. LF produces cellular depletion of pyrimidine nucleotides required for cell growth and proliferation. Based on the affected cells the outcome can be attainable as immunosuppression, antiproliferative, and/or the recently gained attention of the antiviral potentials of LF and its new congeners. Also, protein tyrosine kinase inhibition is an additional mechanistic benefit of LF, which inhibits immunological events such as cellular expansion and immunoglobulin production with an enhanced release of immunosuppressant cytokines.
LF is approved for the treatment of autoimmune arthritis of rheumatoid and psoriatic pathogenesis. Also, LF has been used off-label for the treatment of relapsing-remitting multiple sclerosis. However, LF antiviral activity is repurposed and under investigation with related compounds under a phase-I trial as a SARS CoV-2 antiviral in cases with COVID-19.
Despite success in improving patients’ mobility and reducing joint destruction, reported events of LF-induced liver injury necessitated regulatory precautions. LF should not be used in patients with hepatic impairment or in combination with drugs elaborating a burden on the liver without regular monitoring of liver enzymes and serum bilirubin as safety biomarkers.
This study aims to review the pharmacological and safety profile of LF with a focus on the LF-induced hepatic injury from the perspective of pathophysiology and possible protective agents.
Keywords: Leflunomide, Immunomodulator, Anti-inflammatory, Antineoplastic, Antiviral, Drug-induced liver injury, COVID-19
Abbreviations: TAK1, transforming growth factor β-activated kinase 1; AMPK, adenosine monophosphate-activated protein kinase; ULK1, Unc-51-like autophagy activating kinase; PIM1 kinase, proto-oncogene serine/threonine protein kinase Pim-1; P56Lck, T-lymphocyte-specific protein tyrosine kinase; sEphrin-A1, soluble ephrin-A1; Eph-A2, ephrin-A2; JNK, c-Jun N-terminal kinases; LDH, lactate dehydrogenase; ERK1/2, extracellular signal-regulated kinases; Bcl2, B-cell lymphoma 2 protein; Bax, Bcl-2-associated X protein; PCNA, proliferating cell nuclear antigen; CCL4, carbon tetrachloride; COX2, cyclo-oxygenase 2; NO, nitric oxide; iNOS, inducible nitric oxide synthase; PGE2, prostaglandin E2; AMP, adenosine monophosphate
1. Introduction
Leflunomide (LF) is an immunomodulator and a member of the disease-modifying antirheumatic family of drugs (DMARDs). LF is used effectively in solo or as combined therapy in autoimmune arthritis [1]. Basic studies reported immunosuppressant, antirheumatic, antineoplastic, and antiviral potentials of LF [2], [3], [4], [5], [6]. While clinical studies secured an antirheumatic approval of LF in autoimmune arthritis, further studies on antineoplastic effectiveness have so far been inconclusive [7], [8], [9]. Additionally, clinical studies on the benefit of LF in treating refractory viral infections such as cytomegalovirus [10] and BK polyomavirus [11], [12], [13], [14] have also been undertaken without yielding a final decision to certify. Currently, a clinical trial is underway to study the effectiveness of LF in mild cases with COVID-19 [15].
Leflunomide was first launched at the end of 1998 with alarming events of drug-induced liver injury (DILI) ranging from a mild elevation of serum transaminases to life-threatening hepatitis [16], [17], [18], [19], [20], [21], [22], [23], [24]. In the early 2000s, the FDA had labelled LF with a precautionary regular hepatic function monitoring throughout the therapeutic regimen [25]. Studies-driving this decision involved professional and community concerns of LF-induced detrimental effects on the liver [16], [22], [24], [26], [27].
2. Chemistry and pharmacokinetics
LF is an isoxazole antirheumatic and immunosuppressant approved medication with antineoplastic and antimicrobial investigational situation [5], [9], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]. LF is used through the oral route in a formulation of 10, 20, or 100 mg per tablet. It is well absorbed, and upon exposure to first-pass intestinal and hepatic metabolism, LF is almost totally transformed into its active metabolite teriflunomide (A77-17226). In the liver, LF is a substrate of the hepatic microsomal enzymes CYP2C9, CYP3A4, and CYP2A1 [1], [40]. Cytochrome P450 enzymes are responsible for the opening of the LF isoxazole ring and the production of teriflunomide in two forms (E & Z), with the former having a higher potency [41]. Drugs that have an inducing or inhibitory effect on these hepatic microsomal enzymes carry the risk of adverse drug interactions with LF [1]. LF has a short plasma half-life (t1/2) of 3.5 h; however, teriflunomide t1/2 is ~360 h. The steady-state concentration (Css) of LF/teriflunomide is attainable after ~2.5 months. To hasten LF Css, a loading dose regimen of 100 mg/day for three days is followed by a maintenance dose of 10–20 mg/day onward. Teriflunomide is eliminated through the hepatobiliary route in unchanged form. In the case of LF toxicity, or the need for abrupt LF withdrawal, an accelerated drug elimination procedure using cholestyramine or activated charcoal should be followed to lower the teriflunomide plasma concentration to 0.02 mg/L, which would take two years to be accomplished without this procedure [1], [42], [43]. Teriflunomide has a 99% plasma protein-binding capacity and 11 L volume of distribution with an incapability of being dialyzed [44], [45].
3. Pharmacodynamics of LF
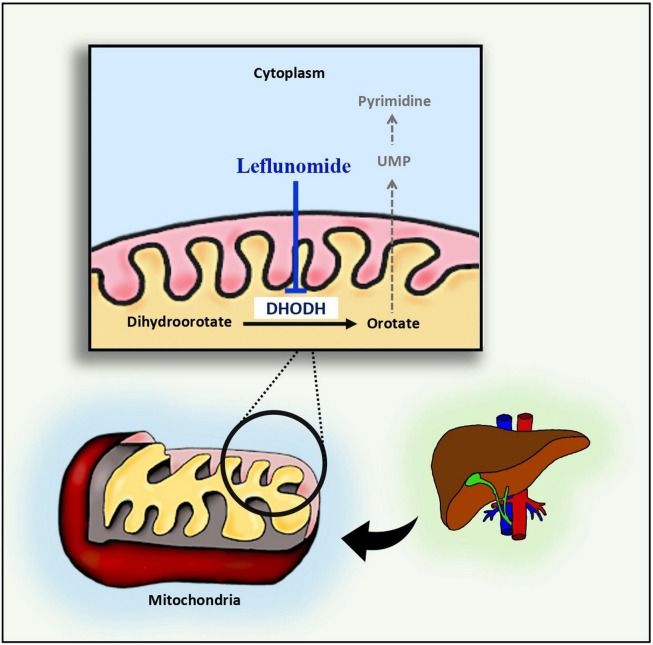
Teriflunomide is the biologically active mediator of LF actions [35], [46], [47]. LF acts through the inhibition of dihydroorotate dehydrogenase (DHODH), an inner mitochondrial membrane enzyme that catalyzes the rate-limiting step of the de novo pathway of pyrimidine biosynthesis [48]. Cellular regeneration and growth can be fulfilled through a salvage pathway with a two-fold coverage of pyrimidine nucleotide cellular requirements; however, the active proliferation of cells such as lymphocytes clonal expansion requires up to eightfold increase of pyrimidine nucleotides with a mandated dependence on the de novo pathway [49] Fig. 1 .
Fig. 1.
Leflunomide inhibits de novo synthesis of pyrimidine through inhibition of the mitochondrial enzyme DHODH.
3.1. Immunomodulator anti-inflammatory and antirheumatic
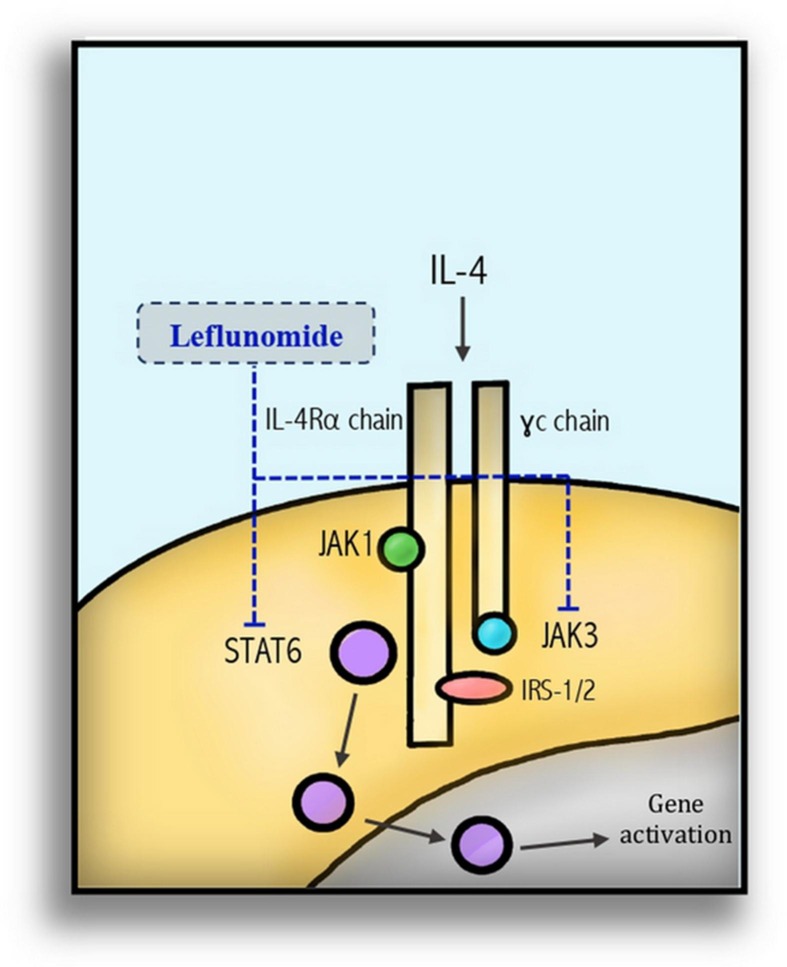
Immune-mediated disorders are associated with the active expansion of autoimmune lymphocytes and other innate immune cells such as monocytes and macrophages. The main and early characterized mode of action of LF is the cellular depletion of the pyrimidine nucleic acid building blocks with a milieu-dependent outcome such as inhibition of autoimmune lymphocyte expansion and, consequently, inhibition of immunokine and immunoglobulin production [2], [50]. Additionally, LF acts through the inhibition of the tyrosine kinase activity responsible for the signal transduction of many vital pathways in the immune response [3]. For instance, the inhibition of immunoglobulin class switching of IgM to IgG1, which is mediated through IL4-activated JAK3/STAT6 pathway [51] Fig. 2 . Similarly, the activation of T-cell proliferation by the T-cell growth factor IL2 was also inhibited with a deficiency of clonal expansion [52], [53]. LF inhibited signal transduction of the T-cell receptors stimulated by anti-CD3 mAb in Jurkat cells; this finding supports tyrosine phosphorylation inhibition as a mechanism of the immunosuppressive function of LF [54] Table 1 .
Fig. 2.
Leflunomide inhibits the immune system through inhibition of tyrosine kinase phosphorylation of the JAK3/STAT6 and immunoglobulin class switching.
Table 1.
Leflunomide main actions and their mechanisms.
| Clinical use | MOA/action | Action | References |
|---|---|---|---|
| Immunomodulator and antirheumatic |
|
|
[54], [73], [106], [148], [149], [150], [151], [152] |
|
[4], [153], [154], [155], [156], [157] | ||
|
|
[71] | |
|
|
[3], [52], [83], [158], [159] | |
|
|
[61], [81], [82], [160] | |
| Antineoplastic |
|
|
[32], [35], [161], [162] |
|
|
[35], [163] | |
|
|
[35] | |
| Antiangiogenic |
|
|
[80] |
| Antiviral |
|
|
[5], [6], [164], [165], [166] |
Studies have highlighted the immunosuppressant activity of LF through the inhibition of the PI3K/Akt/mTOR [55]. Mammalian target of rapamycin (mTOR) is a serine/threonine kinase activated by the upstream effector phosphoinositide 3 kinase (PI3K). Downstream, mTOR induces cellular translational machinery in favor of cell growth, survival, and proliferation [55]. Akt and mTOR are important determinants of activated B-cell expansion and fate [56]. S6 Kinase 1 (S6K1)—a serine/threonine kinase—is a predominant downstream translational effector in the Akt/mTOR cell growth/survival pathway. LF and its metabolite teriflunomide inhibit S6K1 activity with the arrest of the cell cycle in the S phase and, hence inhibit cell proliferation [57]. Additionally, mast cells undertake major and versatile immune defense roles. Mast cells play a crucial role in the pathogenesis of autoimmune and inflammatory pathological conditions [58]. Mast cells are abundantly detected in the synovial membrane of joints in patients with RA [59]. LF is reported to inhibit PI3K/Akt stimulation with the induction of mast cell apoptosis [60].
Early in the development of LF, the anti-inflammatory benefits were reported [61], [62]. Manipulating innate immune responses is considered the anti-inflammatory gateway of LF. In a mouse model of lupus nephritis, LF inhibited the destructive tissue inflammatory pathway mediated through Toll-like receptor 9 (TLR9) signaling pathway with a reduction in the autoantibody production and immune complex deposition in the renal tissue [63]. LF anti-inflammatory activity is reported in a clinical study of patients with active rheumatoid arthritis. The main findings in this study are the reduction of the inflammatory joint destruction. IL1β and matrix metalloproteinases (MMP) such as MMP1 are reduced upon treatment with LF [64]. This may be explained by the inhibition of the TNF-α-dependent activation of NF-κB [65]. Furthermore, teriflunomide, the functioning metabolite of LF, was reported as being an inhibitor of neuroinflammatory events associated with HIV infection independent of viral replication which is attributed to the inhibition of the secretion of the proinflammatory mediators IL6, CXCL10, and CCL2 [66], [67], [68]. In a rat model of lung fibrosis-induced by bleomycin, LF reduced lung tissue expression of the inflammatory cytokines IL6, TNF-α, and NF-κB [69]. Additionally, LF anti-inflammation can be undertaken through the suppression of the trans-endothelial migration of blood mononuclear cells and the inhibition of the expression of adhesion molecule CD44 [70].
In patients with rheumatoid arthritis, increased levels of C-reactive protein (CRP) are correlated with joint destruction. Aryl hydrocarbon genomic activity induces a negative control on CRP expression. LF is an aryl hydrocarbon receptor agonist, which attenuates CRP expression and hence saves the structural integrity of the joints [71], [72] Table 1.
3.2. Antiproliferative activity
LF at low doses has a reversible antiproliferative action upon cell replenishment with pyrimidine [73], [74]. Meanwhile higher doses of LF showed irreversible antiproliferative activity [49]. Indeed, this action may carry a promising antineoplastic potential. In vitro studies reported dose- and time-dependent cytostatic effects of LF in transformed prostatic epithelial cells through pyrimidine depletion, mitochondrial bioenergetic disruption, and cytochrome c release with an apoptotic sequalae [32]. In neuroblastoma cells, LF induced cytostatic and apoptotic cellular fate attributed to the reduced expression of the DHODH enzyme at the transcriptional and translational levels [75]. In a melanoma cell line (A375), Dosacas and colleagues unveiled the inhibitory effect of LF/ A77-1726 (teriflunomide) on S6K1 with a resultant inhibition of its substrates S6, insulin receptor substrate-1 (IRS-1), and carbamoyl phosphate synthase 2, concluding an inhibition of cell proliferation [57]. Meanwhile, Xu et al, demonstrated the A77-1726-induced autophagy in vitro through releasing TAK1/AMPK/ULK1 pathway from the inhibitory effect of S6K1 [76]. In contrast, Cheng and colleagues (2020), reported the autophagy inhibitory action of LF, which is considered an enhancer of the cytotoxic effects on human bladder cancer cells. This was attributed to the inhibition of cancer cell escape mechanisms and the survival tendency through autophagy [35]. Furthermore, teriflunomide synergized Gemcitabine-induced growth inhibition of pancreatic cancer cells through PIM kinase-dependent inhibition of the c-myc tumorigenic signaling pathway [77]. Teriflunomide directly inhibits the entire PIM family, especially PIM-3 and PIM-1 [78]. Likewise, teriflunomide suppressed the proliferation of the murine leukemia cell line LSTRA through inhibition of the tyrosine kinase activity of p56lck [79]. Moreover, LF inhibited angiogenesis in an N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN)-induced bladder carcinogenesis animal model and a tumor xenograft model, as well as in bladder cancer cells in vitro via significant inhibition of the sEphrin-A1/EphA2 system [80] Table 1.
In contrast, low doses of leflunomide exhibited cell survival potential through the activation of PI3K/Akt signaling and reduced apoptosis, which was induced by anticancer agents in erythroleukemia cells. Also, LF inhibited p38/MAPK/JNK basal activity with the reduced apoptotic activity of caspase-3 [30].
3.3. Antiviral activity
LF has antiviral properties through the inhibition of viral nuclear material replication within the host cells, and the inhibition of protein tyrosine-kinase activity leading to inhibition of the phosphorylation of cellular proteins required for vital processes [81], [82], [83].
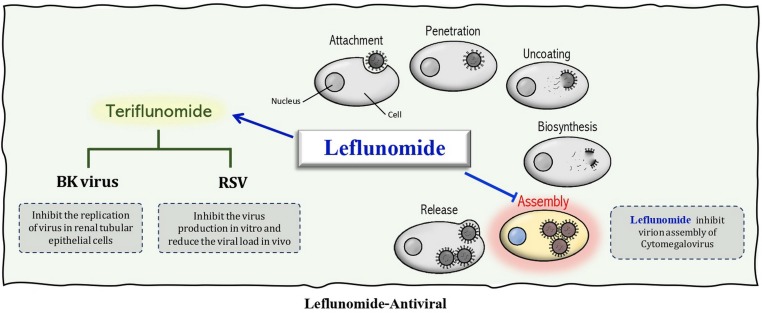
LF shows antiviral activity against many viruses such as Cytomegalovirus (CMV), Polyomavirus type BK, Herpes simplex virus, Respiratory syncytial virus (RSV), and SARS CoV2. LF suppressed CMV infection by inhibiting virion assembly rather than the synthesis of viral DNA [84]. With promising therapeutic potential, LF restrained CMV infection in vitro [85], in vivo [86], and in clinical research [10] Table 1 & Fig. 3 .
Fig. 3.
The antiviral activity of leflunomide and its active metabolite teriflunomide.
Also, LF can inhibit the replication of the BK polyomavirus in renal tubular epithelial cells through nonspecific pyrimidine depletion [12], [13], [14], [38]. Further, studies showed that the active metabolite of LF leads to the inhibition of RSV production in vitro and a reduction in viral load in vivo; this mechanism is uridine independent [87] Table 1 & Fig. 3.
Multiple sclerosis patients under treatment regimen with teriflunomide, and who developed COVID-19 showed better disease outcomes, which may be attributed to the immunosuppressant and antiviral activity of the drug [88].
The active form of LF was also found to be effective against Junín Virus. It can inhibit virus replication by inhibiting viral RNA synthesis through pyrimidine depletion in a dose-dependent manner. However, the addition of uridine or orotate reverses the inhibitory effect of LF [39].
Blocking of DHODH results in pyrimidine depletion which is very effective against rotavirus (responsible for dehydrating diarrhea). For that reason, LF anti-microbial activity was investigated against other organisms such as Helicobacter pylori, Plasmodium falciparum, and Schistosoma mansoni [89].
4. Clinical importance of LF
LF is FDA approved for the treatment of autoimmune arthritis of rheumatoid and psoriatic pathogenesis. LF can be used in solo, while in refractory cases it should be combined with other immunosuppressants. LF shows efficacy after four weeks of treatment with improved physical activity comparable to that of methotrexate. After two years, patients who received LF had no further increase in joint damage [90] Table 2 .
Table 2.
Leflunomide investigational uses.
| Clinical indication | Disease | Phase | Outcome | Reference |
|---|---|---|---|---|
| Antiviral | Mild COVID-19 patients | Phase I | Ongoing | [15] |
| HIV-1 | Phase I | Promising with safety concerns limited progress to further studies | [167] | |
| BK viremia associate nephropathy | Phase IV | Serum creatinine | [168], [169] | |
| Cancer | Smoldering multiple myeloma | Early phase I | Recruiting (to end June 2021) | [109] |
| Relapsed/refractory Multiple myeloma | Phase I/II | Completed | [110] | |
| Anaplastic astrocytoma | Phase II | NA | [7] | |
| Glioblastoma multiformans | Phase III | NA | [8] | |
| Advanced refractory prostate cancer | Phase II/III | NA | [9], [107] | |
| Mutant metastatic melanoma | Phase I | NA | [170] | |
| Metastatic triple negative breast cancer | Phase I/II | NA | [171] | |
| Autoimmune diseases | IgG4-related sclerosing disease | Phase IV Randomized Open label |
LF + glucocorticoid is significantly superior to glucocorticoids monotherapy | [172] |
| Lupus nephritis | Phase III Non-randomized Open label |
Low dose LF + prednisone effectiveness and safety | [173] |
4.1. Autoimmune and rheumatic conditions
LF has been initially approved for the treatment of autoimmune arthritis, mainly rheumatoid [1] and psoriatic types [91]. The target of therapy is to reduce the number of joints affected, which is scored clinically for pain and functionality [92]. The use of LF in other autoimmune conditions, such as systemic lupus and multiple sclerosis, is investigational but without an approval decision [93], [94], [95]. In multiple sclerosis, the use of LF active metabolite teriflunomide was approved in 2016 [96], [97]. This approval did not compare the efficacy of LF to teriflunomide in a clinical context and, thus, may require further clinical and pharmacoeconomic investigations. The approval of teriflunomide, which is >60 times as expensive as LF, has set aside the alternative off-label use of LF in multiple sclerosis. Revising the clinical profile of teriflunomide showed that an LF dose of 20 mg/tablet is equivalent to the 17 mg/tablet dose of teriflunomide [95], [96], [97].
The use of LF in systemic lupus erythematosus is still under investigation, some of these clinical trials have been suspended with no clinical data justifying this trial decision Table 2.
LF was also tried in other joint conditions such as ankylosing spondylitis. However, it did not show clinical promise except in patients with associating poly-arthropathy who did show an improvement. While it was reported that the spinal manifestations did not significantly improve, this may be attributed to the pathogenic mechanisms which are different from the joint ones [98].
4.2. Transplant rejection and graft versus host disease
LF is used for the prevention of graft rejection and graft-versus-host disease (GVHD). Studies reported the benefit of LF in different animal models of graft rejection [99] such as cardiac allograft [4], [100], intestinal transplantation [101], renal transplantation [102], corneal allograft [103], and fish-to-mouse pancreatic islets xenograft [104]. Also, LF is found to be of benefit in chronic musculoskeletal graft versus the host disease following an allogeneic hematopoietic stem cell transplant [105] Table 2.
4.3. Cancer
Recently, LF has been flagged as a promising antineoplastic drug through its pyrimidine nucleotide cellular deprivation activity [73], [106]. The limitation of LF antineoplastic clinical application may be attributed to the doubts surrounding its clinical efficacy due to the action reversal by uridine replenishment [73]. This may be true despite the protein tyrosine kinase inhibitory action, which was found to be of additional benefit. LF and its congeners are investigated in different in vitro and in vivo models of cancer including prostate [9], [32], [107], breast [34], neuroblastoma [8], [108], multiple myeloma [78], [109], [110], thyroid [33], leukemia [111], [112], and lymphoma [113] Table 2.
4.4. Antiviral
Recently, with the worldwide SARS CoV-2 pandemic, LF antiviral activity has been an appealing candidate fitting COVID-19 pathogenesis with a dual benefit. The first is the immunomodulatory and anti-inflammatory activity which may help to reduce the raged immune responses and cytokine storm. Secondly, the antiviral activity of the drug may have merit for handling the task [15]. A congener of LF, vidofludimus is currently under investigation in two phase-II/III trials in patients with COVID-19 [114] Table 2.
5. Common adverse effects
Antirheumatic agents are among the most commonly used drugs associated with hepatotoxic effects ranging from acute drug-induced liver injury (DILI) to chronic hepatic ailments and even drug-induced autoimmune hepatitis. Based on the National Data Bank for Rheumatic Diseases it has been estimated that leflunomide-related events leading to hospitalization (without formal causality assessment) occur in 2 out of 1000 patients per year [115].
LF is generally considered to be a safe drug with respect to the reported adverse effects. The main adverse effects of LF are gastrointestinal disturbances such as diarrhea (17%), nausea (9%), abdominal pain (5%), and increased hepatic enzymes (5–10%) [116], [117]. Hepatic injury represents a serious drawback of LF based on the issued report by the European Agency for the Evaluation of Medicinal Products (EMEA) in 2001. In this report, 129 cases of LF-induced hepatotoxicity were reported, which included two patients with hepatic cirrhosis, 15 presented with acute hepatic failure, and a 60% fatality rate [118]. Accordingly, a community petition was addressed to the FDA, advocating for the withdrawal of LF from the U.S. market [119], [120]. The FDA declined this petition based on the fact that the benefits outweighed the hazards with imposing a black-boxed warning on the pack of LF stating the need for a regular monitoring of hepatic enzymes and a restriction of use in patients with advanced hepatic diseases [1].
Furthermore, some cases with severe liver injury were reported with fatality outcomes [16], [19], [121]. This has been documented as occurring within six months of the start of LF in patients with risk factors for developing hepatotoxicity [122], [123]. LF combined with methotrexate enhanced hepatic damage with elevated liver enzymes reaching > 3 times the upper limits of normal (ULN). One case of liver injury was reported in a patient with liver cirrhosis who had received a combination of both LF and methotrexate [90]. Liver damage associated with LF therapy is commonly noted as alimentary tract symptoms, including nausea and abdominal distention; hence, liver transaminases must be monitored throughout the therapeutic regimen [124].
On the other hand, doses of LF (4, 12, 36 mg/kg) were found to significantly decrease the serum transaminase (ALT, AST) activity and improve antioxidant and anti-inflammatory mediated hepatic injury [125].
6. Studies reporting the drawbacks of leflunomide on the liver
The use of LF can be applied in solo or as a combined regimen with other immune-suppressing drugs like methotrexate. Liver toxicity is rare in rheumatoid arthritis patients using combination therapy with LF (20 mg/day) and methotrexate (20–25 mg/week) [126]. Meanwhile, animal studies using the combined therapy of LF and methotrexate showed high antiarthritic benefit but with the possibility of a hepatotoxic effect. In the same study, LF (10 mg/kg/day) and an LF/methotrexate combination showed the greatest degree of liver fibrosis [127]. Accordingly, in any patient with hepatic impairment, this combination is contraindicated. Furthermore, LF clinical guidelines recommend monthly monitoring of hepatic enzymes within the first six months of therapy with further trimonthly monitoring later. ALT levels greater than three times ULN without an increase in bilirubin have been identified as sensitive, but are not necessarily a specific signal of liver toxicity [128].
Clinical studies and basic research reported on the hepatotoxicity of LF, which was found to be dose and time-dependent [129], [130], [131], [132]. For the former, the use of LF doses of higher than 20 mg/day is associated with a higher incidence of hepatic injury, which may be asymptomatic or of a fulminating nature with a life-threatening hazard [1], [16], [126].
LF causes hepatotoxicity, which is presented as increased liver enzymes > 2–3 times ULN. In a human study involving 46 participants, 30% of patients continued LF therapy without dose diminution. However, 20% of the patients required the discontinuation of LF with normalized liver enzymes within 4–6 weeks afterward [26]. In the case of proof of LF-induced liver injury, a withdrawal maneuver is recommended with the use of cholestyramine, or activated charcoal for a faster washout of LF [1], [118].
LF is considered a therapeutic choice in patients with autoimmune hepatitis while this may be cautiously considered due to the hepatotoxic nature of the drug itself. However, the idiosyncratic nature of LF hepatotoxicity supports the metabolic idiosyncratic notion [133]. For instance, in APAP-induced hepatotoxicity, LF inhibited JNK1/2 activation and prevented mitochondrial permeability transition pore opening, thus offering protection from cell death induced by toxic concentrations of APAP [134]. While, in immune-mediated hepatitis induced by concanavalin A, LF inhibited T-cell mediated hepatic injury through the inhibition of NF-κB, TNF-α, and caspase-mediated apoptosis [135].
6.1. Pathogenesis of LF-induced liver injury (Table 3)
Table 3.
Leflunomide mechanisms of hepatotoxicity.
| Pathogenesis | Mechanism |
|---|---|
| Mitochondrial stress |
|
| Endoplasmic reticulum stress |
|
| Metabolic stress and inflammatory pathway |
|
| Hepatic fibrosis | |
| CYP450 polymorphism |
The liver is the main detoxifying tissue in the body with its exposure to chemical and toxicant liabilities, it requires stable energy resources and continuously replenished tissue antioxidant mechanisms. The metabolic products of xenobiotics represent a major threat with population variations based on their environmental and genetic makeup [136]. The main pathogenesis in LF hepatotoxicity involves the hepatic oxidant burden and metabolic and tissue energy derangements leading to cellular damage. LF-induced DILI is mediated through different mechanisms including an inflammatory pathway ending with tissue damage.
In animal studies, LF-induced-inflammatory liver injury occurs through dose-dependent upregulation of the TLR4/PI3K/mTOR pathway and the cellular apoptotic marker caspase 3 [129]. Also, LF and its active metabolite teriflunomide exhibited mitochondrial toxicity in human hepatic HepG2 cells. LF caused dose-dependent depletion in cellular ATP through the inhibition of mitochondrial oxidative phosphorylation complexes mainly complex V (F1FO ATP synthase), LDH leakage, and cell death [131]. Furthermore, LF-induced cytotoxicity in HepG2 cells was mediated by endoplasmic reticulum stress and the enhancement of the JNK and ERK1/2 of the MAPK signaling pathways [130].
Genetic polymorphic cytochrome P450 enzymes were investigated in 105 patients with rheumatic arthritis to examine the relationship between patients’ CYP1A2*1F, CYP2C19*17, CYP2C9*2, and CYP2C9*3 alleles and LF toxicity. Forty-three patients discontinued LF therapy within the first year due to toxicity. Patients with CYP1A2*1F were at a 9.7-fold higher risk than patients who only carried the allele. However, patients with CYP2C19 and CYP2C9 had no relationship [137]. Other genetic studies showed a correlation between the slow CYP2C9*3 allele and LF-induced hepatitis in rheumatoid arthritis patients [16], [138].
6.2. Preventive and therapeutic agents for LF-induced liver injury
Drug-induced liver injury (DILI) anticipation and prevention represent an elusive target for health and pharmaceutical bodies. DILI is classified as intrinsic such as paracetamol- and alcohol-induced DILI. On the other hand, idiosyncratic DILI is unpredictable and, hence, difficult to avoid. Without an understanding of the molecular pathogenesis of idiosyncratic DILI, it will be difficult to prevent or specifically manage. The possible protective agents for minimizing LF-induced liver injury may rely on antagonizing the oxidant stress, metabolic derangements, and inflammatory character of its pathogenesis.
6.2.1. Using antioxidant hepatoprotective agents
Rheum Palmatum L. showed hepatoprotective effects through anti-inflammatory, antioxidant and antiapoptotic mechanisms in mice by inhibiting NF-κB, nitric oxide, IL-1β, Caspase 3 and Caspase 8 in the liver tissue. Also, it reduced iNOS, COX-2, and Bax and enhanced the expression of Bcl-2 and PCNA [139]. Further, the silymarin and propolis hepatoprotective effect in CCL4 hepatotoxicity in rats is mediated through antioxidant properties [140]. In vivo studies on rats, showed that LF causes portal fibrosis, sinusoidal congestion, and infiltration with periductal inflammatory cells. Meanwhile, the use of LF combined with β-caryophyllene reduced the hepatotoxic effect of LF. β-caryophyllene acts through antioxidant activity that leads to the inhibition of hydroxyl anions, lipid peroxides, and superoxide anions [141]. Oenanthe Javanica—a Chinese medicinal herb—is an aquatic perennial herb cultivated in East Asian countries. Total phenolics from Oenanthe Javanica in 125, 250, and 500 mg/kg doses showed hepatoprotective effects in D-galactosamine-induced liver injury in mice through antioxidant and anti-inflammatory actions detected as decreased iNOS, COX2, NO, and PGE2 [142].
6.2.2. Using mitochondrial and metabolic equilibrating agents
5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) is an analog of AMP and an activator of AMP-dependent protein kinase (AMPK). AICAR prevents/reverses drug-induced mitochondrial and hepatocellular damage by regulating mitochondrial fusion and mitophagy in acetaminophen and diclofenac-induced hepatic cell toxicity of murine and human origin [143].
6.2.3. Using anti-inflammatory agents
The hepatoprotective effect of vitamin D3 (1,25(OH)2D3) [low-dose 0.025 μg/kg/day, moderate dose 0.15 μg/kg/day, and high dose 0.3 μg/kg/day) was investigated in diabetic-induced hepatic injury in rats. After four months of therapy, the high dose of vitamin D3 down-regulated TLR4, NF-κB, ALT, and LDL and improved hepatic tissue architecture with reduced inflammatory cell infiltrates [144].
Aqueous extract of the Chinese herb Aconitum Carmichaelii Debeaux showed anti-inflammatory and antiapoptotic potential in the treatment of acute liver failure induced by D-galactosamine in rats. The hepatoprotective effects are presented as a decrease of hepatic pathological scores, reduced expressions of TLR4, NF-κB, HMGB1, and caspase-3, and increased PCNA cell regeneration marker [145].
Total glucoside of paeony, which is a Chinese herb, was used as an adjuvant with methotrexate and leflunomide in doses of 0.6 and 1.8 g/day for 12–24 weeks. This combination showed the increased antirheumatic effectiveness of the LF/methotrexate combination in rheumatoid arthritis patients with hepatoprotective advantages. This combination ameliorates liver fibrosis and decreases the progression of liver disease in a non-alcoholic patient through IL13 regulation. [146].
In support, assessment of the therapeutic effect of total glucosides of peony for juvenile idiopathic arthritis was detected through the extraction of data from eight electronic databases that concluded total glucosides of peony as a unique nonbiologic disease-modifying antirheumatic drug (nonbiologic DMARD) with good efficacy and minimal adverse effects [147].
In conclusion, the current study represents a comprehensive revision of the clinical importance of LF as a multitask therapeutic agent not only in the approved application in autoimmune arthritis but also as an antineoplastic and antimicrobial candidate. With the ongoing dilemma of the SARS CoV2 pandemic and the life-threatening conditions of COVID-19 patients, LF should be investigated in patients with COVID-19 as an anti-inflammatory immunomodulator due to its advantageous antiviral activity. LF adverse effects are highlighted with an emphasis on LF-induced hepatotoxicity molecular pathogenesis and the possible hepatoprotective agents which can be considered as a supportive treatment during long-term therapeutic regimen on LF.
Declaration of Competing Interest
The authors declare that they have no knowSn competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors would like to acknowledge Mr. Jalal Munir AlJoundi (MSc. Ed, and ESOL certified Blended Learning Program and Curriculum Unit Supervisor at the Institute of Languages, University of Tabuk) for his generosity and proofreading of this manuscript.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contribution
RDA, MAE, GAA, SMA, SSA & SHE all contributed to the conceptualization, database searches, article collection, revision, and data extraction. SMA prepared the illustrating figures of the pathways. MAE & SHG prepared the tables. SHG supervised the work and prepared the final manuscript.
References
- 1.Sanofi-aventis: ARAVA® Tablets (leflunomide) 10 mg, 20 mg, 100 mg http://products.sanofi.us/arava/Arava.html, June 2020. In. Edited by Aventis S, vol. LEF-FPLR-SL-FEB16: Sanofi Aventis, 2016, pp. 1–25.
- 2.Bartlett R.R., Schleyerbach R. Immunopharmacological profile of a novel isoxazol derivative, HWA 486, with potential antirheumatic activity–I. Disease modifying action on adjuvant arthritis of the rat. Int. J. Immunopharmacol. 1985;7(1):7–18. doi: 10.1016/0192-0561(85)90003-7. [DOI] [PubMed] [Google Scholar]
- 3.Mattar T., Kochhar K., Bartlett R., Bremer E.G., Finnegan A. Inhibition of the epidermal growth factor receptor tyrosine kinase activity by leflunomide. FEBS Lett. 1993;334(2):161–164. doi: 10.1016/0014-5793(93)81704-4. [DOI] [PubMed] [Google Scholar]
- 4.Williams J.W., Xiao F., Foster P.F., Chong A., Sharma S., Bartlett R., Sankary H.N. Immunosuppressive effects of leflunomide in a cardiac allograft model. Transpl. Proc. 1993;25(1 Pt 1):745–746. [PubMed] [Google Scholar]
- 5.Chong A.S., Zeng H., Knight D.A., Shen J., Meister G.T., Williams J.W., Waldman W.J. Concurrent antiviral and immunosuppressive activities of leflunomide in vivo. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transplant Surgeons. 2006;6(1):69–75. doi: 10.1111/j.1600-6143.2005.01152.x. [DOI] [PubMed] [Google Scholar]
- 6.Josephson M.A., Williams J.W., Chandraker A., Randhawa P.S. Polyomavirus-associated nephropathy: update on antiviral strategies. Transplant Infect. Dis. Off. J. Transplant. Soc. 2006;8(2):95–101. doi: 10.1111/j.1399-3062.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 7.Hannah AL: Leflunomide in Treating Patients With Anaplastic Astrocytoma in First Relapse. In.: https://ClinicalTrials.gov/show/NCT00003775, 2006.
- 8.A.L.P. Hannah, SUGEN“: SU-101 Compared With Procarbazine in Treating Patients With Glioblastoma Multiforme. In.: https://ClinicalTrials.gov/show/NCT00003293. June 2020, 2012.
- 9.Ko Y.J., Small E.J., Kabbinavar F., Chachoua A., Taneja S., Reese D., DePaoli A., Hannah A., Balk S.P., Bubley G.J. A multi-institutional phase ii study of SU101, a platelet-derived growth factor receptor inhibitor, for patients with hormone-refractory prostate cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2001;7(4):800–805. [PubMed] [Google Scholar]
- 10.Ehlert K., Groll A.H., Kuehn J., Vormoor J. Treatment of refractory CMV-infection following hematopoietic stem cell transplantation with the combination of foscarnet and leflunomide. Klin. Padiatr. 2006;218(3):180–184. doi: 10.1055/s-2006-933412. [DOI] [PubMed] [Google Scholar]
- 11.Araya C.E., Garin E.H., Neiberger R.E., Dharnidharka V.R. Leflunomide therapy for BK virus allograft nephropathy in pediatric and young adult kidney transplant recipients. Pediatr. Transplant. 2010;14(1):145–150. doi: 10.1111/j.1399-3046.2009.01183.x. [DOI] [PubMed] [Google Scholar]
- 12.Bernhoff E., Tylden G.D., Kjerpeseth L.J., Gutteberg T.J., Hirsch H.H., Rinaldo C.H. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J. Virol. 2010;84(4):2150–2156. doi: 10.1128/JVI.01737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faguer S., Hirsch H.H., Kamar N., Guilbeau-Frugier C., Ribes D., Guitard J., Esposito L., Cointault O., Modesto A., Lavit M., et al. Leflunomide treatment for polyomavirus BK-associated nephropathy after kidney transplantation. Transplant Int. Off. J. Eur. Soc. Organ Transplant. 2007;20(11):962–969. doi: 10.1111/j.1432-2277.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 14.Jung Y.H., Moon K.C., Ha J.W., Kim S.J., Ha I.S., Cheong H.I., Kang H.G. Leflunomide therapy for BK virus allograft nephropathy after pediatric kidney transplantation. Pediatr. Transplant. 2013;17(2):E50–E54. doi: 10.1111/petr.12029. [DOI] [PubMed] [Google Scholar]
- 15.M. Millis, L. Perea, Leflunomide in Mild COVID-19 Patients. In.: https://ClinicalTrials.gov/show/NCT04361214, 2020.
- 16.Sevilla-Mantilla C., Ortega L., Agundez J.A., Fernandez-Gutierrez B., Ladero J.M., Diaz-Rubio M. Leflunomide-induced acute hepatitis. Digest. Liver Dis. Off. J. Italian Soc. Gastroenterol. Italian Association Study Liver. 2004;36(1):82–84. doi: 10.1016/j.dld.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Gupta R., Gupta S.K. Severe hepatotoxicity in a rheumatoid arthritis patient switched from leflunomide to methotrexate. MedGenMed Medscape General Med. 2005;7(3):9. [PMC free article] [PubMed] [Google Scholar]
- 18.van Roon E.N., Jansen T.L., Houtman N.M., Spoelstra P., Brouwers J.R. Leflunomide for the treatment of rheumatoid arthritis in clinical practice: incidence and severity of hepatotoxicity. Drug Saf. 2004;27(5):345–352. doi: 10.2165/00002018-200427050-00006. [DOI] [PubMed] [Google Scholar]
- 19.Chávez-López M.A., Ramírez-González A., Martínez-Guevara M.A. Acute hepatitis associated to leflunomide in a patient with rheumatoid arthritis. Gastroenterol. Hepatol. 2007;30(7):430. doi: 10.1157/13108810. [DOI] [PubMed] [Google Scholar]
- 20.Mladenovic V., Domljan Z., Rozman B., Jajic I., Mihajlovic D., Dordevic J., Popovic M., Dimitrijevic M., Zivkovic M., Campion G., et al. Safety and effectiveness of leflunomide in the treatment of patients with active rheumatoid arthritis. Results of a randomized, placebo-controlled, phase II study. Arthritis Rheum. 1995;38(11):1595–1603. doi: 10.1002/art.1780381111. [DOI] [PubMed] [Google Scholar]
- 21.Garcia Rodriguez L.A., Duque A., Castellsague J., Perez-Gutthann S., Stricker B.H. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br. J. Clin. Pharmacol. 1999;48(6):847–852. doi: 10.1046/j.1365-2125.1999.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis J.R., Beukelman T., Onofrei A., Cassell S., Greenberg J.D., Kavanaugh A., Reed G., Strand V., Kremer J.M. Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann. Rheum. Dis. 2010;69(1):43–47. doi: 10.1136/ard.2008.101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siva C., Eisen S.A., Shepherd R., Cunningham F., Fang M.A., Finch W., Salisbury D., Singh J.A., Stern R., Zarabadi S.A. Leflunomide use during the first 33 months after food and drug administration approval: experience with a national cohort of 3,325 patients. Arthritis Rheum. 2003;49(6):745–751. doi: 10.1002/art.11452. [DOI] [PubMed] [Google Scholar]
- 24.Weinblatt M.E., Dixon J.A., Falchuk K.R. Serious liver disease in a patient receiving methotrexate and leflunomide. Arthritis Rheum. 2000;43(11):2609–2611. doi: 10.1002/1529-0131(200011)43:11<2609::AID-ANR32>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 25.US-FDA: Highlights of Prescribing Information: Arava (leflunomide) In., 2015.
- 26.Gupta R., Bhatia J., Gupta S.K. Risk of hepatotoxicity with add-on leflunomide in rheumatoid arthritis patients. Arzneimittelforschung. 2011;61(5):312–316. doi: 10.1055/s-0031-1296204. [DOI] [PubMed] [Google Scholar]
- 27.Thomasset S.C., Ong S.L., Large S.R. Post-coronary artery bypass graft liver failure: a possible association with leflunomide. Ann. Thoracic Surgery. 2005;79(2):698–699. doi: 10.1016/j.athoracsur.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S., Zheng Y., Jun X., Narla R.K., Mahajan S., Navara C., Mao C., Sudbeck E.A., Uckun F.M. Alpha-cyano-beta-hydroxy-beta-methyl-N-[4-(trifluoromethoxy)phenyl] propenamide: an inhibitor of the epidermal growth factor receptor tyrosine kinase with potent cytotoxic activity against breast cancer cells. Clinical Cancer Res. Off. J. American Assoc. Cancer Res. 1998;4(11):2657–2668. [PubMed] [Google Scholar]
- 29.Ghosh S., Narla R.K., Zheng Y., Liu X.P., Jun X., Mao C., Sudbeck E.A., Uckun F.M. Structure-based design of potent inhibitors of EGF-receptor tyrosine kinase as anti-cancer agents. Anticancer Drug Des. 1999;14(5):403–410. [PubMed] [Google Scholar]
- 30.Leger D.Y., Liagre B., Beneytout J.L. Low dose leflunomide activates PI3K/Akt signalling in erythroleukemia cells and reduces apoptosis induced by anticancer agents. Apoptosis Int. J. Programmed Cell Death. 2006;11(10):1747–1760. doi: 10.1007/s10495-006-9439-7. [DOI] [PubMed] [Google Scholar]
- 31.Uckun F.M. Chemosensitizing anti-cancer activity of LFM-A13, a leflunomide metabolite analog targeting polo-like kinases. Cell cycle (Georgetown, Tex) 2007;6(24):3021–3026. doi: 10.4161/cc.6.24.5096. [DOI] [PubMed] [Google Scholar]
- 32.Hail N., Jr., Chen P., Bushman L.R. Teriflunomide (leflunomide) promotes cytostatic, antioxidant, and apoptotic effects in transformed prostate epithelial cells: evidence supporting a role for teriflunomide in prostate cancer chemoprevention. Neoplasia (New York, NY) 2010;12(6):464–475. doi: 10.1593/neo.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alhefdhi A., Burke J.F., Redlich A., Kunnimalaiyaan M., Chen H. Leflunomide suppresses growth in human medullary thyroid cancer cells. J. Surg. Res. 2013;185(1):212–216. doi: 10.1016/j.jss.2013.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohamad Fairus A.K., Choudhary B., Hosahalli S., Kavitha N., Shatrah O. Dihydroorotate dehydrogenase (DHODH) inhibitors affect ATP depletion, endogenous ROS and mediate S-phase arrest in breast cancer cells. Biochimie. 2017;135:154–163. doi: 10.1016/j.biochi.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Cheng L., Wang H., Wang Z., Huang H., Zhuo D., Lin J. Leflunomide inhibits proliferation and induces apoptosis via suppressing autophagy and PI3K/Akt signaling pathway in human bladder cancer cells. Drug Des. Develop. Therapy. 2020;14:1897–1908. doi: 10.2147/DDDT.S252626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Humphries B.A., Cutter A.C., Buschhaus J.M., Chen Y.C., Qyli T., Palagama D.S.W., Eckley S., Robison T.H., Bevoor A., Chiang B., et al. Enhanced mitochondrial fission suppresses signaling and metastasis in triple-negative breast cancer. Breast Cancer Res. BCR. 2020;22(1):60. doi: 10.1186/s13058-020-01301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rinaldo C.H., Hirsch H.H. Antivirals for the treatment of polyomavirus BK replication. Expert Rev. Anti-infective Therapy. 2007;5(1):105–115. doi: 10.1586/14787210.5.1.105. [DOI] [PubMed] [Google Scholar]
- 38.Santeusanio A.D., Lukens B.E., Eun J. Antiviral treatment of BK virus viremia after kidney transplantation. Am. J. Health-Syst. Pharm. AJHP Off. J. Am. Soc. Health-Syst. Pharmacists. 2017;74(24):2037–2045. doi: 10.2146/ajhp160585. [DOI] [PubMed] [Google Scholar]
- 39.Sepulveda C.S., Garcia C.C., Damonte E.B. Antiviral activity of A771726, the active metabolite of leflunomide, against Junin virus. J. Med. Virol. 2018;90(5):819–827. doi: 10.1002/jmv.25024. [DOI] [PubMed] [Google Scholar]
- 40.Kalgutkar A.S., Nguyen H.T., Vaz A.D., Doan A., Dalvie D.K., McLeod D.G., Murray J.C. In vitro metabolism studies on the isoxazole ring scission in the anti-inflammatory agent lefluonomide to its active alpha-cyanoenol metabolite A771726: mechanistic similarities with the cytochrome P450-catalyzed dehydration of aldoximes. Drug Metabol. Disposit. Biolog. Fate Chem. 2003;31(10):1240–1250. doi: 10.1124/dmd.31.10.1240. [DOI] [PubMed] [Google Scholar]
- 41.Sharma P., Gangopadhyay D., Mishra P.C., Mishra H., Singh R.K. Detection of in vitro metabolite formation of leflunomide: a fluorescence dynamics and electronic structure study. J. Med. Chem. 2016;59(7):3418–3426. doi: 10.1021/acs.jmedchem.6b00088. [DOI] [PubMed] [Google Scholar]
- 42.Laub M., Fraser R., Kurche J., Lara A., Kiser T.H., Reynolds P.M. Use of a cholestyramine washout in a patient with septic shock on leflunomide therapy: a case report and review of the literature. J. Intensive Care Med. 2016;31(6):412–414. doi: 10.1177/0885066615610108. [DOI] [PubMed] [Google Scholar]
- 43.Wong S.P., Chu C.M., Kan C.H., Tsui H.S., Ng W.L. Successful treatment of leflunomide-induced acute pneumonitis with cholestyramine wash-out therapy. J. Clin. Rheumatol. Practical Rep. Rheumatic Musculoskeletal Dis. 2009;15(8):389–392. doi: 10.1097/RHU.0b013e3181c3f87e. [DOI] [PubMed] [Google Scholar]
- 44.Dias V.C., Lucien J., LeGatt D.F., Yatscoff R.W. Measurement of the active leflunomide metabolite (A77 1726) by reverse-phase high-performance liquid chromatography. Ther. Drug Monit. 1995;17(1):84–88. doi: 10.1097/00007691-199502000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Lucien J., Dias V.C., LeGatt D.F., Yatscoff R.W. Blood distribution and single-dose pharmacokinetics of leflunomide. Ther. Drug Monit. 1995;17(5):454–459. doi: 10.1097/00007691-199510000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Bertolini G., Aquino M., Biffi M., d'Atri G., Di Pierro F., Ferrario F., Mascagni P., Somenzi F., Zaliani A., Leoni F. A new rational hypothesis for the pharmacophore of the active metabolite of leflunomide, a potent immunosuppressive drug. J. Med. Chem. 1997;40(13):2011–2016. doi: 10.1021/jm970039n. [DOI] [PubMed] [Google Scholar]
- 47.Chan E.C., New L.S. In vitro metabolism of leflunomide by mouse and human liver microsomes. Drug Metab. Lett. 2007;1(4):299–305. doi: 10.2174/187231207783221402. [DOI] [PubMed] [Google Scholar]
- 48.Chong A.S., Gebel H., Finnegan A., Petraitis E.E., Jiang X.L., Sankary H.N., Foster P., Williams J.W. Leflunomide, a novel immunomodulatory agent: in vitro analyses of the mechanism of immunosuppression. Transpl. Proc. 1993;25(1 Pt 1):747–749. [PubMed] [Google Scholar]
- 49.Zhang C., Chu M. Leflunomide: A promising drug with good antitumor potential. Biochem. Biophys. Res. Commun. 2018;496(2):726–730. doi: 10.1016/j.bbrc.2018.01.107. [DOI] [PubMed] [Google Scholar]
- 50.Cherwinski H.M., Cohn R.G., Cheung P., Webster D.J., Xu Y.Z., Caulfield J.P., Young J.M., Nakano G., Ransom J.T. The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. J. Pharmacol. Experim. Therapeut. 1995;275(2):1043–1049. [PubMed] [Google Scholar]
- 51.K. Siemasko, A.S. Chong, H.M. Jack, H. Gong, J.W. Williams, A. Finnegan, Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production, J. Immunol. (Baltimore, Md : 1950) 160(4) (1998) 1581–1588. [PubMed]
- 52.Cao W.W., Kao P.N., Aoki Y., Xu J.C., Shorthouse R.A., Morris R.E. A novel mechanism of action of the immunomodulatory drug, leflunomide: augmentation of the immunosuppressive cytokine, TGF-beta 1, and suppression of the immunostimulatory cytokine, IL-2. Transpl. Proc. 1996;28(6):3079–3080. [PubMed] [Google Scholar]
- 53.Zielinski T., Müller H.J., Bartlett R.R. Effects of leflunomide (HWA 486) on expression of lymphocyte activation markers. Agents Actions. 1993;38 doi: 10.1007/BF01991144. Spec No:C80–82. [DOI] [PubMed] [Google Scholar]
- 54.Xu X., Williams J.W., Bremer E.G., Finnegan A., Chong A.S. Inhibition of protein tyrosine phosphorylation in T cells by a novel immunosuppressive agent, leflunomide. J. Biolog. Chem. 1995;270(21):12398–12403. doi: 10.1074/jbc.270.21.12398. [DOI] [PubMed] [Google Scholar]
- 55.Pignataro G., Capone D., Polichetti G., Vinciguerra A., Gentile A., Di Renzo G., Annunziato L. Neuroprotective, immunosuppressant and antineoplastic properties of mTOR inhibitors: current and emerging therapeutic options. Curr. Opin. Pharmacol. 2011;11(4):378–394. doi: 10.1016/j.coph.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Limon J.J., Fruman D.A. Akt and mTOR in B Cell Activation and Differentiation. Front. Immunol. 2012;3:228. doi: 10.3389/fimmu.2012.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doscas M.E., Williamson A.J., Usha L., Bogachkov Y., Rao G.S., Xiao F., Wang Y., Ruby C., Kaufman H., Zhou J., et al. Inhibition of p70 S6 kinase (S6K1) activity by A77 1726 and its effect on cell proliferation and cell cycle progress. Neoplasia (New York, NY) 2014;16(10):824–834. doi: 10.1016/j.neo.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rottem M.M.Y. Mast cells and autoimmunity. Autoimmun. Rev. 2005;4(1):21–27. doi: 10.1016/j.autrev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Nigrovic P.A., Lee D.M. Mast cells in inflammatory arthritis. Arthritis Res. Therapy. 2005;7(1):1–11. doi: 10.1186/ar1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.N. Sawamukai, K. Saito, K. Yamaoka, S. Nakayamada, C. Ra, Y. Tanaka, Leflunomide inhibits PDK1/Akt pathway and induces apoptosis of human mast cells, J. immunol. (Baltimore, Md : 1950) 179(10) (2007) 6479–6484. [DOI] [PubMed]
- 61.Weithmann K.U., Jeske S., Schlotte V. Effect of leflunomide on constitutive and inducible pathways of cellular eicosanoid generation. Agents Actions. 1994;41(3–4):164–170. doi: 10.1007/BF02001911. [DOI] [PubMed] [Google Scholar]
- 62.Kurtz E.S., Bailey S.C., Arshad F., Lee A.A., Przekop P.A. Leflunomide: an active antiinflammatory and antiproliferative agent in models of dermatologic disease. Inflam. Res. Off. J. European Histamine Res. Soc. [et al] 1995;44(Suppl 2):S187–S188. doi: 10.1007/BF01778324. [DOI] [PubMed] [Google Scholar]
- 63.He C., Lu X., Yan Z., Wu M., Liu S., Yu Y., Luo P. Therapeutic effect of leflunomide on the development of experimental lupus nephritis in mice. Rheumatol. Int. 2012;32(3):633–638. doi: 10.1007/s00296-010-1630-z. [DOI] [PubMed] [Google Scholar]
- 64.Kraan M.C., Reece R.J., Barg E.C., Smeets T.J., Farnell J., Rosenburg R., Veale D.J., Breedveld F.C., Emery P., Tak P.P. Modulation of inflammation and metalloproteinase expression in synovial tissue by leflunomide and methotrexate in patients with active rheumatoid arthritis. Findings in a prospective, randomized, double-blind, parallel-design clinical trial in thirty-nine patients at two centers. Arthritis Rheum. 2000;43(8):1820–1830. doi: 10.1002/1529-0131(200008)43:8<1820::AID-ANR18>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 65.S.K. Manna, B.B. Aggarwal, Immunosuppressive leflunomide metabolite (A77 1726) blocks TNF-dependent nuclear factor-kappa B activation and gene expression, J. Immunol. (Baltimore, Md: 1950) 162(4) (1999) 2095–2102. [PubMed]
- 66.Ambrosius B., Faissner S., Guse K., von Lehe M., Grunwald T., Gold R., Grewe B., Chan A. Teriflunomide and monomethylfumarate target HIV-induced neuroinflammation and neurotoxicity. J. Neuroinflam. 2017;14(1):51. doi: 10.1186/s12974-017-0829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Breedveld F.C., Dayer J.M. Leflunomide: mode of action in the treatment of rheumatoid arthritis. Ann. Rheum. Dis. 2000;59(11):841–849. doi: 10.1136/ard.59.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang J.L., Wu S.Y., Xie X.J., Wang M.X., Zhu S., Gu J.R. Inhibiting effects of Leflunomide metabolite on overexpression of CD147, MMP-2 and MMP-9 in PMA differentiated THP-1 cells. Eur. J. Pharmacol. 2011;670(1):304–310. doi: 10.1016/j.ejphar.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 69.Kayhan S., Guzel A., Duran L., Tutuncu S., Guzel A., Gunaydın M., Salis O., Okuyucu A., Selcuk M.Y. Effects of leflunomide on inflamation and fibrosis in bleomycine induced pulmonary fibrosis in wistar albino rats. J. Thoracic Dis. 2013;5(5):641–649. doi: 10.3978/j.issn.2072-1439.2013.09.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cutolo M., Capellino S., Montagna P., Sulli A., Seriolo B., Villaggio B. Anti-inflammatory effects of leflunomide in combination with methotrexate on co-culture of T lymphocytes and synovial macrophages from rheumatoid arthritis patients. Ann. Rheum. Dis. 2006;65(6):728–735. doi: 10.1136/ard.2005.045641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang C., Li J., Lu C., Xie D., Liu J., Zhong C., Wu X., Dai R., Zhang H., Guan D., et al. HIF1alpha inhibition facilitates Leflunomide-AHR-CRP signaling to attenuate bone erosion in CRP-aberrant rheumatoid arthritis. Nat. Commun. 2019;10(1):4579. doi: 10.1038/s41467-019-12163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Donnell E.F., Saili K.S., Koch D.C., Kopparapu P.R., Farrer D., Bisson W.H., Mathew L.K., Sengupta S., Kerkvliet N.I. Tanguay RL et al.: The anti-inflammatory drug leflunomide is an agonist of the aryl hydrocarbon receptor. PLoS ONE. 2010;5(10) doi: 10.1371/journal.pone.0013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.W.W. Cao, P.N. Kao, A.C. Chao, P. Gardner, J. Ng, R.E. Morris, Mechanism of the antiproliferative action of leflunomide. A77 1726, the active metabolite of leflunomide, does not block T-cell receptor-mediated signal transduction but its antiproliferative effects are antagonized by pyrimidine nucleosides, J. Heart Lung Transplant.: Off. Publ. Int. Soc. Heart Transplant. 14(6 Pt 1) (1995) 1016–1030. [PubMed]
- 74.Xu X., Shen J., Mall J.W., Myers J.A., Huang W., Blinder L., Saclarides T.J., Williams J.W., Chong A.S. In vitro and in vivo antitumor activity of a novel immunomodulatory drug, leflunomide: mechanisms of action. Biochem. Pharmacol. 1999;58(9):1405–1413. doi: 10.1016/s0006-2952(99)00228-2. [DOI] [PubMed] [Google Scholar]
- 75.Zhu S., Yan X., Xiang Z., Ding H.F., Cui H. Leflunomide reduces proliferation and induces apoptosis in neuroblastoma cells in vitro and in vivo. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu X., Sun J., Song R., Doscas M.E., Williamson A.J., Zhou J., Sun J., Jiao X., Liu X., Li Y. Inhibition of p70 S6 kinase (S6K1) activity by A77 1726, the active metabolite of leflunomide, induces autophagy through TAK1-mediated AMPK and JNK activation. Oncotarget. 2017;8(18):30438–30454. doi: 10.18632/oncotarget.16737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buettner R., Morales C., Wu X., Sanchez J.F., Li H., Melstrom L.G., Rosen S.T. Leflunomide synergizes with gemcitabine in growth inhibition of PC cells and impairs c-Myc signaling through PIM kinase targeting. Mol. Ther. Oncolytics. 2019;14:149–158. doi: 10.1016/j.omto.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buettner R., Morales C., Caserta E., Troadec E., Gunes E.G., Viola D., Khalife J., Li H., Keats J.J., Christofferson A., et al. Leflunomide regulates c-Myc expression in myeloma cells through PIM targeting. Blood Adv. 2019;3(7):1027–1032. doi: 10.1182/bloodadvances.2018027227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu X., Williams J.W., Gong H., Finnegan A., Chong A.S. Two activities of the immunosuppressive metabolite of leflunomide, A77 1726. Inhibition of pyrimidine nucleotide synthesis and protein tyrosine phosphorylation. Biochem. Pharmacol. 1996;52(4):527–534. doi: 10.1016/0006-2952(96)00303-6. [DOI] [PubMed] [Google Scholar]
- 80.Chu M., Zhang C. Inhibition of angiogenesis by leflunomide via targeting the soluble ephrin-A1/EphA2 system in bladder cancer. Sci. Rep. 2018;8(1):1539. doi: 10.1038/s41598-018-19788-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ju D.W., Zheng Q.Y., Wang H.B., Fang J. Leflunomide inhibits cytokine-induced DNA synthesis of rabbit synovial cells in culture. Zhongguo yao li xue bao = Acta Pharmacol. Sin. 1994;15(3):223–226. [PubMed] [Google Scholar]
- 82.Ju D.W., Zheng Q.Y., Wang H.B., Fang J. [Leflunomide inhibits PAF induced DNA synthesis in rabbit synovial cells and PAF production from rat peritoneal macrophages] Yao xue xue bao = Acta Pharm. Sin. 1994;29(2):90–94. [PubMed] [Google Scholar]
- 83.Cherwinski H.M., McCarley D., Schatzman R., Devens B., Ransom J.T. The immunosuppressant leflunomide inhibits lymphocyte progression through cell cycle by a novel mechanism. J. Pharmacol. Experim. Therapeut. 1995;272(1):460–468. [PubMed] [Google Scholar]
- 84.Chacko B., John G.T. Leflunomide for cytomegalovirus: bench to bedside. Transplant Infect. Dis. 2012;14(2):111–120. doi: 10.1111/j.1399-3062.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- 85.Haynes L.D., Waldman W.J., Bushkin Y., Love R.B., Burlingham W.J. CMV-infected allogeneic endothelial cells initiate responder and bystander donor HLA class I release via the metalloproteinase cleavage pathway. Hum. Immunol. 2005;66(3):211–221. doi: 10.1016/j.humimm.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 86.Zeng H., Waldman W.J., Yin D.P., Knight D.A., Shen J., Ma L., Meister G.T., Chong A.S., Williams J.W. Mechanistic study of malononitrileamide FK778 in cardiac transplantation and CMV infection in rats. Transplantation. 2005;79(1):17–22. doi: 10.1097/01.tp.0000137334.46155.94. [DOI] [PubMed] [Google Scholar]
- 87.Dunn M.C., Knight D.A., Waldman W.J. Inhibition of respiratory syncytial virus in vitro and in vivo by the immunosuppressive agent leflunomide. Antiviral Therapy. 2011;16(3):309–317. doi: 10.3851/IMP1763. [DOI] [PubMed] [Google Scholar]
- 88.Maghzi A.H., Houtchens M.K., Preziosa P., Ionete C., Beretich B.D., Stankiewicz J.M., Tauhid S., Cabot A., Berriosmorales I., Schwartz T.H.W., et al. COVID-19 in teriflunomide-treated patients with multiple sclerosis. J. Neurol. 2020;267(10):2790–2796. doi: 10.1007/s00415-020-09944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boschi D., Pippione A.C., Sainas S., Lolli M.L. Dihydroorotate dehydrogenase inhibitors in anti-infective drug research. Eur. J. Med. Chem. 2019;183 doi: 10.1016/j.ejmech.2019.111681. [DOI] [PubMed] [Google Scholar]
- 90.Li E.K., Tam L.S., Tomlinson B. Leflunomide in the treatment of rheumatoid arthritis. Clin. Ther. 2004;26(4):447–459. doi: 10.1016/s0149-2918(04)90048-3. [DOI] [PubMed] [Google Scholar]
- 91.Affleck A.G., Williams H. Is leflunomide effective in the treatment of psoriasis in a patient who is unable to benefit from standard first- and second-line therapies and needs an affordable treatment option? Arch. Dermatol. 2008;144(12):1642–1643. doi: 10.1001/archdermatol.2008.502. [DOI] [PubMed] [Google Scholar]
- 92.Prakash A., Jarvis B. Leflunomide: a review of its use in active rheumatoid arthritis. Drugs. 1999;58(6):1137–1164. doi: 10.2165/00003495-199958060-00010. [DOI] [PubMed] [Google Scholar]
- 93.Tam L.S., Li E.K., Wong C.K., Lam C.W., Szeto C.C. Double-blind, randomized, placebo-controlled pilot study of leflunomide in systemic lupus erythematosus. Lupus. 2004;13(8):601–604. doi: 10.1191/0961203304lu1067sr. [DOI] [PubMed] [Google Scholar]
- 94.Pego-Reigosa J.M., Cobo-Ibáñez T., Calvo-Alén J., Loza-Santamaría E., Rahman A., Muñoz-Fernández S., Rúa-Figueroa Í. Efficacy and safety of nonbiologic immunosuppressants in the treatment of nonrenal systemic lupus erythematosus: a systematic review. Arthritis Care Res. 2013;65(11):1775–1785. doi: 10.1002/acr.22035. [DOI] [PubMed] [Google Scholar]
- 95.Aly L., Hemmer B., Korn T. From Leflunomide to Teriflunomide: Drug Development and Immunosuppressive Oral Drugs in the Treatment of Multiple Sclerosis. Curr. Neuropharmacol. 2017;15(6):874–891. doi: 10.2174/1570159X14666161208151525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bar-Or A. Teriflunomide (Aubagio®) for the treatment of multiple sclerosis. Exp. Neurol. 2014 doi: 10.1016/j.expneurol.2014.06.005. 262 Pt A:57–65. [DOI] [PubMed] [Google Scholar]
- 97.sanofi-aventis: Teriflunomide (AUBAGIO). Multiple sclerosis: just a metabolite of leflunomide. Prescrire Int. 24(158) (2015) 61–64. [PubMed]
- 98.Akkoc N., van der Linden S., Khan M.A. Ankylosing spondylitis and symptom-modifying vs disease-modifying therapy. Best Pract. Res. Clin. Rheumatol. 2006;20(3):539–557. doi: 10.1016/j.berh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 99.Morris R.E., Huang X., Cao W., Zheng B., Shorthouse R.A. Leflunomide (HWA 486) and its analog suppress T- and B-cell proliferation in vitro, acute rejection, ongoing rejection, and antidonor antibody synthesis in mouse, rat, and cynomolgus monkey transplant recipients as well as arterial intimal thickening after balloon catheter injury. Transpl. Proc. 1995;27(1):445–447. [PubMed] [Google Scholar]
- 100.Xiao F., Chong A., Foster P., Sankary H., McChesney L., Williams J.M., Frieders D., Williams J.W. Effect of leflunomide in control of acute rejection in hamster-to-rat cardiac xenografts. Transpl. Proc. 1994;26(3):1263–1265. [PubMed] [Google Scholar]
- 101.Foster P.F., Xiao F., Kociss K., Chong A.S., Sankary H.N., McChesney L., Cohn S., Williams J.W. Leflunomide immunosuppression in rat small intestinal transplantation. Transpl. Proc. 1994;26(3):1599–1600. [PubMed] [Google Scholar]
- 102.McChesney L.P., Xiao F., Sankary H.N., Foster P.F., Sharma S., Haklin M., Williams J.W. An evaluation of leflunomide in the canine renal transplantation model. Transplantation. 1994;57(12):1717–1722. [PubMed] [Google Scholar]
- 103.Niederkorn J.Y., Lang L.S., Ross J., Mellon J., Robertson S.M. Promotion of corneal allograft survival with leflunomide. Invest. Ophthalmol. Vis. Sci. 1994;35(10):3783–3785. [PubMed] [Google Scholar]
- 104.Wright J.R., Jr., Kearns H., McDonald A.S. Leflunomide and cyclosporin-A prolong fish-to-mouse islet xenograft survival in BALB/C mice. Transpl. Proc. 1994;26(3):1310. [PubMed] [Google Scholar]
- 105.Punatar S., Mohite A., Gokarn A., Nayak L., Bonda A., Shanmugam K., Vijaysekharan K., Khattry N. Leflunomide for chronic musculoskeletal graft versus host disease following allogeneic hematopoietic stem cell transplant. Bone Marrow Transplant. 2020;55(2):467–469. doi: 10.1038/s41409-019-0545-x. [DOI] [PubMed] [Google Scholar]
- 106.Cherwinski H.M., Byars N., Ballaron S.J., Nakano G.M., Young J.M., Ransom J.T. Leflunomide interferes with pyrimidine nucleotide biosynthesis. Inflam. Res. Off. J. Eur. Histamine Res. Soc. et al] 1995;44(8):317–322. doi: 10.1007/BF01796261. [DOI] [PubMed] [Google Scholar]
- 107.M.H. Mabry, Mitoxantrone and Prednisone With or Without Leflunomide in Treating Patients With Stage IV Prostate Cancer. In.: https://ClinicalTrials.gov/show/NCT00004071, 2007.
- 108.Pfizer: SU-101 Compared With Procarbazine in Treating Patients With Glioblastoma Multiforme. In.: https://ClinicalTrials.gov/show/NCT00003293, 1998.
- 109.M.A. Rosenzweig, Leflunomide for the Treatment of High-Risk Smoldering Multiple Myeloma. In.: https://ClinicalTrials.gov/show/NCT04370483. June 2020, 2020b.
- 110.Rosenzweig M., Palmer J., Tsai N.C., Synold T., Wu X., Tao S., Hammond S.N., Buettner R., Duarte L., Htut M., et al. Repurposing leflunomide for relapsed/refractory multiple myeloma: a phase 1 study. Leukemia Lymphoma. 2020:1–9. doi: 10.1080/10428194.2020.1742900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Imanishi S., Takahashi R., Katagiri S., Kobayashi C., Umezu T., Ohyashiki K., Ohyashiki J.H. Teriflunomide restores 5-azacytidine sensitivity via activation of pyrimidine salvage in 5-azacytidine-resistant leukemia cells. Oncotarget. 2017;8(41):69906–69915. doi: 10.18632/oncotarget.19436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sykes D.B., Kfoury Y.S., Mercier F.E., Wawer M.J., Law J.M., Haynes M.K., Lewis T.A., Schajnovitz A., Jain E., Lee D., et al. Inhibition of dihydroorotate dehydrogenase overcomes differentiation blockade in acute myeloid leukemia. Cell. 2016;167(1):171–186 e115. doi: 10.1016/j.cell.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Landais A., Alhendi R., Gouverneur A., Teron-Aboud B. A case of lymphoma in a patient on teriflunomide treatment for relapsing multiple sclerosis. Multiple Sclerosis Related Dis. 2017;17:92–94. doi: 10.1016/j.msard.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 114.A. Muehler, N. Ahuja, A study to evaluate the efficacy, safety and tolerability of IMU-838 as addition to investigator's choice of standard of care therapy, in Patients With Coronavirus Disease 19 (COVID-19). clinicaltrial.gov: https://ClinicalTrials.gov/show/NCT04379271, 2020.
- 115.Aithal G.P. Hepatotoxicity related to antirheumatic drugs. Nat. Rev. Rheumatol. 2011;7(3):139–150. doi: 10.1038/nrrheum.2010.214. [DOI] [PubMed] [Google Scholar]
- 116.Subramanian S., Yanamandra U. Monitoring adverse effects of leflunomide: role of clinical audit. Indian J. Rheumatol. 2011;6(1):1–2. [Google Scholar]
- 117.Turkoski B.B. Rheumatoid arthritis and the Arava (leflunomide) controversy. Orthopedic Nursing. 2003;22(1):48–51. doi: 10.1097/00006416-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 118.EMEA: EMEA Public Statement On Leflunomide (ARAVA)-Severe And Serious Hepatic Reactions-24 Oct 2020, in: EMEA, 2001.
- 119.publiccitizin: Arava Linked to Liver Complications and Deaths, Public Citizen Tells FDA. In.; 2002.
- 120.Charatan F. Arthritis drug should be removed from market, says consumer group. BMJ (Clin. Res. ed) 2002;324(7342):869. doi: 10.1136/bmj.324.7342.869/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thong B.Y., Koh E.T., Chng H.H., Chow W.C. Outcomes of chronic hepatitis B infection in Oriental patients with rheumatic diseases. Ann. Acad. Med. Singapore. 2007;36(2):100–105. [PubMed] [Google Scholar]
- 122.Zheng X.Y., Wei R.B., Tang L., Li P., Zheng X.D. Meta-analysis of combined therapy for adult hepatitis B virus-associated glomerulonephritis. World J. Gastroenterol. 2012;18(8):821–832. doi: 10.3748/wjg.v18.i8.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Üsküdar Cansu D., Öztaş E., Yilmaz E., Korkmaz C. Cyclophosphamide-induced severe acute hepatitis in a rheumatic disease: case-based review. Rheumatol. Int. 2019;39(2):377–385. doi: 10.1007/s00296-018-4189-8. [DOI] [PubMed] [Google Scholar]
- 124.Yuyuan L., Xuqing Z. Leflunomide-induced acute liver failure: a case report. J. Med. Colleges PLA. 2010;25:62–64. [Google Scholar]
- 125.Yao H.W., Li J., Jin Y., Zhang Y.F., Li C.Y., Xu S.Y. Effect of leflunomide on immunological liver injury in mice. World J. Gastroenterol. 2003;9(2):320–323. doi: 10.3748/wjg.v9.i2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alves J.A., Fialho S.C., Morato E.F., Castro G.R., Zimmermann A.F., Ribeiro G.G., Neves F.S., Pereira I.A. Liver toxicity is rare in rheumatoid arthritis patients using combination therapy with leflunomide and methotrexate. Revista brasileira de reumatologia. 2011;51(2):141–144. [PubMed] [Google Scholar]
- 127.Bilasy S.E., Essawy S.S., Mandour M.F., Ali E.A., Zaitone S.A. Myelosuppressive and hepatotoxic potential of leflunomide and methotrexate combination in a rat model of rheumatoid arthritis. Pharmacolog. Rep. PR. 2015;67(1):102–114. doi: 10.1016/j.pharep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 128.Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat. Rev. Drug Discov. 2005;4(6):489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- 129.Elshaer R.E., Tawfik M.K., Nosseir N., El-Ghaiesh S.H., Toraih E.A., Elsherbiny N.M., Zaitone S.A. Leflunomide-induced liver injury in mice: Involvement of TLR4 mediated activation of PI3K/mTOR/NFκB pathway. Life Sci. 2019;235 doi: 10.1016/j.lfs.2019.116824. [DOI] [PubMed] [Google Scholar]
- 130.Ren Z., Chen S., Qing T., Xuan J., Couch L., Yu D., Ning B., Shi L., Guo L. Endoplasmic reticulum stress and MAPK signaling pathway activation underlie leflunomide-induced toxicity in HepG2 Cells. Toxicology. 2017;392:11–21. doi: 10.1016/j.tox.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xuan J., Ren Z., Qing T., Couch L., Shi L., Tolleson W.H., Guo L. Mitochondrial dysfunction induced by leflunomide and its active metabolite. Toxicology. 2018;396–397:33–45. doi: 10.1016/j.tox.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lodhi L. Evaluation of mechanism of hepatotoxicity of leflunomide using albino wistar rats. African J. Pharm. Pharmacol. 2013;7:1625–1631. [Google Scholar]
- 133.Spodnik J.H., Wozniak M., Budzko D., Teranishi M.A., Karbowski M., Nishizawa Y., Usukura J., Wakabayashi T. Mechanism of leflunomide-induced proliferation of mitochondria in mammalian cells. Mitochondrion. 2002;2(3):163–179. doi: 10.1016/s1567-7249(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 134.Latchoumycandane C., Seah Q.M., Tan R.C., Sattabongkot J., Beerheide W., Boelsterli U.A. Leflunomide or A77 1726 protect from acetaminophen-induced cell injury through inhibition of JNK-mediated mitochondrial permeability transition in immortalized human hepatocytes. Toxicol. Appl. Pharmacol. 2006;217(1):125–133. doi: 10.1016/j.taap.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 135.Imose M., Nagaki M., Kimura K., Takai S., Imao M., Naiki T., Osawa Y., Asano T., Hayashi H., Moriwaki H. Leflunomide protects from T-cell-mediated liver injury in mice through inhibition of nuclear factor kappaB. Hepatology (Baltimore, MD) 2004;40(5):1160–1169. doi: 10.1002/hep.20438. [DOI] [PubMed] [Google Scholar]
- 136.Tarantino G., Di Minno M.N., Capone D. Drug-induced liver injury: is it somehow foreseeable? World J. Gastroenterol. 2009;15(23):2817–2833. doi: 10.3748/wjg.15.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bohanec Grabar P., Rozman B., Tomšič M., Šuput D., Logar D., Dolžan V. Genetic polymorphism of CYP1A2 and the toxicity of leflunomide treatment in rheumatoid arthritis patients. Eur. J. Clin. Pharmacol. 2008;64:871–876. doi: 10.1007/s00228-008-0498-2. [DOI] [PubMed] [Google Scholar]
- 138.Legras A., Bergemer-Fouquet A.M., Jonville-Bera A.P. Fatal hepatitis with leflunomide and itraconazole. Am. J. Med. 2002;113(4):352–353. doi: 10.1016/s0002-9343(02)01177-4. [DOI] [PubMed] [Google Scholar]
- 139.Zhang R.Z., Qiu H., Wang N., Long F.L., Mao D.W. Effect of Rheum palmatum L. on NF-kappaB signaling pathway of mice with acute liver failure. Asian Pac J. Trop Med. 2015;8(10):841–847. doi: 10.1016/j.apjtm.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 140.Hashem A., Taha N., Mandour A., Lebda M., Balbaa M., Morshedy A. Hepatoprotective Effect of Silymarin and Propolis in Chemically Induced Chronic Liver Injury in Rats. Alexandria J. Veterinary Sci. 2016;49(1):35. [Google Scholar]
- 141.S.M.A. El-Sheikh, Abd El-Alim AE-AF, A.A.A. Galal, R.G. El-Sayed, N.I. El-naseery, Anti-arthritic effect of β-caryophyllene and its ameliorative role on methotrexate and/or leflunomide-induced side effects in arthritic rats. Life Sci. 233 (2019) 116750. [DOI] [PubMed]
- 142.Ai G., Huang Z.M., Liu Q.C., Han Y.Q., Chen X. The protective effect of total phenolics from Oenanthe Javanica on acute liver failure induced by D-galactosamine. J. Ethnopharmacol. 2016;186:53–60. doi: 10.1016/j.jep.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 143.Kang S.W., Haydar G., Taniane C., Farrell G., Arias I.M., Lippincott-Schwartz J., Fu D. AMPK activation prevents and reverses drug-induced mitochondrial and hepatocyte injury by promoting mitochondrial fusion and function. PLoS ONE. 2016;11(10) doi: 10.1371/journal.pone.0165638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang H., Zhang Q., Chai Y., Liu Y., Li F., Wang B., Zhu C., Cui J., Qu H., Zhu M. 1,25(OH)2D3 downregulates the Toll-like receptor 4-mediated inflammatory pathway and ameliorates liver injury in diabetic rats. J. Endocrinol. Invest. 2015;38(10):1083–1091. doi: 10.1007/s40618-015-0287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luo J.X., Zhang Y., Hu X.Y., Chen G., Liu X.Y., Nie H.M., Liu J.L., Wen D.C. Aqueous extract from Aconitum carmichaelii Debeaux reduces liver injury in rats via regulation of HMGB1/TLR4/NF-KappaB/caspase-3 and PCNA signaling pathways. J. Ethnopharmacol. 2016;183:187–192. doi: 10.1016/j.jep.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 146.Huang Y., Wang H., Chen Z., Wang Y., Qin K., Huang Y., Ba X., Lin W., Tu S. Efficacy and safety of total glucosides of paeony combined with methotrexate and leflunomide for active rheumatoid arthritis: a meta-analysis. Drug Des. Develop. Therapy. 2019;13:1969–1984. doi: 10.2147/DDDT.S207226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cai Y., Yuan Q., Xu K., Zhu J., Li Y., Wu X., Yang L., Qiu Y., Xu P. Assessment of the therapeutic effect of total glucosides of peony for juvenile idiopathic arthritis: a systematic review and meta-analysis. Evid. Based Complement. Alternative Med. eCAM. 2016;2016:8292486. doi: 10.1155/2016/8292486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ogawa T., Inazu M., Gotoh K., Inoue T., Hayashi S. Therapeutic effects of leflunomide, a new antirheumatic drug, on glomerulonephritis induced by the antibasement membrane antibody in rats. Clin. Immunol. Immunopathol. 1991;61(1):103–118. doi: 10.1016/s0090-1229(06)80011-5. [DOI] [PubMed] [Google Scholar]
- 149.Popovic S., Bartlett R.R. The use of the murine chronic graft vs host (CGVH) disease, a model for systemic lupus erythematosus (SLE), for drug discovery. Agents Actions. 1987;21(3–4):284–286. doi: 10.1007/BF01966492. [DOI] [PubMed] [Google Scholar]
- 150.Hoskin D.W., Taylor R.M., Makrigiannis A.P., James H., Lee T.D. Dose-dependent enhancing and inhibitory effects of A77 1726 (leflunomide) on cytotoxic T lymphocyte induction. Int. J. Immunopharmacol. 1998;20(9):505–513. doi: 10.1016/s0192-0561(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 151.Rückemann K., Fairbanks L.D., Carrey E.A., Hawrylowicz C.M., Richards D.F., Kirschbaum B., Simmonds H.A. Leflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humans. J. Biolog. Chem. 1998;273(34):21682–21691. doi: 10.1074/jbc.273.34.21682. [DOI] [PubMed] [Google Scholar]
- 152.Eber E., Uhlig T., McMenamin C., Sly P.D. Leflunomide, a novel immunomodulating agent, prevents the development of allergic sensitization in an animal model of allergic asthma. Clin. Experim. Allergy J. British Soc. Allergy Clin. Immunol. 1998;28(3):376–384. doi: 10.1046/j.1365-2222.1998.00240.x. [DOI] [PubMed] [Google Scholar]
- 153.Küchle C.C., Thoenes G.H., Langer K.H., Schorlemmer H.U., Bartlett R.R., Schleyerbach R. Prevention of kidney and skin graft rejection in rats by leflunomide, a new immunomodulating agent. Transpl. Proc. 1991;23(1 Pt 2):1083–1086. [PubMed] [Google Scholar]
- 154.Schorlemmer H.U., Seiler F.R., Bartlett R.R. Prolongation of allogeneic transplanted skin grafts and induction of tolerance by leflunomide, a new immunosuppressive isoxazol derivative. Transpl. Proc. 1993;25(1 Pt 1):763–767. [PubMed] [Google Scholar]
- 155.He G., McAlister V.C., Lee T.D., Bitter-Suermann H., Theal M., Wright J., MacDonald A.S. Oral leflunomide prevents small bowel allograft rejection in the rat. Transpl. Proc. 1994;26(3):1613. [PubMed] [Google Scholar]
- 156.Jiao Z.X., Leng Y., Xia J.J., Wu H.Q., Jin N., Fu J.Z., Cheng L.N., Wang J.H., Ni S.B., Qi Z.Q. As2 O3 combined with leflunomide prolongs heart xenograft survival via suppressing the response of Th1, Th2, and B cells in a rat model. Xenotransplantation. 2016;23(3):237–248. doi: 10.1111/xen.12238. [DOI] [PubMed] [Google Scholar]
- 157.Sun Y., Chen X., Zhao J., Zou X., Li G., Li X., Shen B., Sun S. Combined use of rapamycin and leflunomide in prevention of acute cardiac allografts rejection in rats. Transpl. Immunol. 2012;27(1):19–24. doi: 10.1016/j.trim.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 158.R.T. Elder, X. Xu, J.W. Williams, H. Gong, A. Finnegan, A.S. Chong, The immunosuppressive metabolite of leflunomide, A77 1726, affects murine T cells through two biochemical mechanisms, J. immunol. (Baltimore, Md: 1950) 159(1) (1997) 22–27. [PubMed]
- 159.Fukushima R., Kanamori S., Hirashiba M., Hishikawa A., Muranaka R.I., Kaneto M., Nakamura K., Kato I. Teratogenicity study of the dihydroorotate-dehydrogenase inhibitor and protein tyrosine kinase inhibitor Leflunomide in mice. Reprod. Toxicol. (Elmsford, NY) 2007;24(3–4):310–316. doi: 10.1016/j.reprotox.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 160.Curnock A.P., Robson P.A., Yea C.M., Moss D., Gadher S., Thomson T.A., Westwood R., Ruuth E., Williamson R.A. Potencies of leflunomide and HR325 as inhibitors of prostaglandin endoperoxide H synthase-1 and -2: comparison with nonsteroidal anti-inflammatory drugs. J. Pharmacol. Experim. Therapeut. 1997;282(1):339–347. [PubMed] [Google Scholar]
- 161.Bahr H.I., Toraih E.A., Mohammed E.A., Mohammad H.M., Ali E.A., Zaitone S.A. Chemopreventive effect of leflunomide against Ehrlich's solid tumor grown in mice: effect on EGF and EGFR expression and tumor proliferation. Life Sci. 2015;141:193–201. doi: 10.1016/j.lfs.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 162.Carazo A., Dusek J., Holas O., Skoda J., Hyrsova L., Smutny T., Soukup T., Dosedel M., Pávek P. Teriflunomide Is an Indirect Human Constitutive Androstane Receptor (CAR) Activator Interacting With Epidermal Growth Factor (EGF) Signaling. Front. Pharmacol. 2018;9:993. doi: 10.3389/fphar.2018.00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen Y., Huang Q., Zhou H., Wang Y., Hu X., Li T. Inhibition of canonical WNT/β-catenin signaling is involved in leflunomide (LEF)-mediated cytotoxic effects on renal carcinoma cells. Oncotarget. 2016;7(31):50401–50416. doi: 10.18632/oncotarget.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bilger A., Plowshay J., Ma S., Nawandar D., Barlow E.A., Romero-Masters J.C., Bristol J.A., Li Z., Tsai M.H., Delecluse H.J., et al. Leflunomide/teriflunomide inhibit Epstein-Barr virus (EBV)- induced lymphoproliferative disease and lytic viral replication. Oncotarget. 2017;8(27):44266–44280. doi: 10.18632/oncotarget.17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Waldman W.J., Knight D.A., Blinder L., Shen J., Lurain N.S., Miller D.M., Sedmak D.D., Williams J.W., Chong A.S. Inhibition of cytomegalovirus in vitro and in vivo by the experimental immunosuppressive agent leflunomide. Intervirology. 1999;42(5–6):412–418. doi: 10.1159/000053979. [DOI] [PubMed] [Google Scholar]
- 166.Knight D.A., Hejmanowski A.Q., Dierksheide J.E., Williams J.W., Chong A.S., Waldman W.J. Inhibition of herpes simplex virus type 1 by the experimental immunosuppressive agent leflunomide. Transplantation. 2001;71(1):170–174. doi: 10.1097/00007890-200101150-00031. [DOI] [PubMed] [Google Scholar]
- 167.Read S.W., DeGrezia M., Ciccone E.J., DerSimonian R., Higgins J., Adelsberger J.W., Starling J.M., Rehm C., Sereti I. The effect of leflunomide on cycling and activation of T-cells in HIV-1-infected participants. PLoS ONE. 2010;5(8) doi: 10.1371/journal.pone.0011937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nesselhauf N., Strutt J., Bastani B. Evaluation of leflunomide for the treatment of BK viremia and biopsy proven BK nephropathy; a single center experience. J. Nephropathol. 2016;5(1):34–37. doi: 10.15171/jnp.2016.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.L. Wennberg, B.K. Viremia, After Renal Transplantation: Screening, Early Diagnosis, Early Reduction in Immunosuppression and Treatment With Leflunomide (Arava), https://ClinicalTrials.gov/show/NCT00684372. June 2020, 2009.
- 170.K. Flaherty, Leflunomide+Vemurafenib in V600 Mutant Met. Melanoma. https://ClinicalTrials.gov/show/NCT01611675. June 2020, 2017.
- 171.C.L. Shapiro, Leflunomide in Previously Treated Metastatic Triple Negative Cancers https://ClinicalTrials.gov/show/NCT03709446. June 2020, 2021.
- 172.Wang Y., Zhao Z., Gao D., Wang H., Liao S., Dong C., Luo G., Ji X., Li Y., Wang X., et al. Additive effect of leflunomide and glucocorticoids compared with glucocorticoids monotherapy in preventing relapse of IgG4-related disease: a randomized clinical trial. Semin. Arthritis Rheum. 2020 doi: 10.1016/j.semarthrit.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 173.Zhang M., Qi C., Zha Y., Chen J., Luo P., Wang L., Sun Z., Wan J., Xing C., Wang S., et al. Leflunomide versus cyclophosphamide in the induction treatment of proliferative lupus nephritis in Chinese patients: a randomized trial. Clin. Rheumatol. 2019;38(3):859–867. doi: 10.1007/s10067-018-4348-z. [DOI] [PubMed] [Google Scholar]