Abstract
Parasitic nematode infections are treated using anthelmintic drugs, some of which target nicotinic acetylcholine receptors (nAChRs) located in different parasite tissues. The limited arsenal of anthelmintic agents and the prevalence of drug resistance imply that future defense against parasitic infections will depend on the discovery of novel targets and therapeutics. Previous studies have suggested that Ascaris suum ACR-16 nAChRs are a suitable target for the development of antinematodal drugs. In this study, we characterized the pharmacology of the Ancylostoma caninum ACR-16 receptor using two-electrode voltage-clamp electrophysiology. This technique allowed us to study the effects of cholinergic agonists and antagonists on the nematode nAChRs expressed in Xenopus laevis oocytes. Aca-ACR-16 was not sensitive to many of the existing cholinomimetic anthelmintics (levamisole, oxantel, pyrantel, and tribendimidine). 3-Bromocytisine was the most potent agonist (> 130% of the control acetylcholine current) on the Aca-ACR-16 nAChR but, unlike Asu-ACR-16, oxantel did not activate the receptor. The mean time constants of desensitization for agonists on Aca-ACR-16 were longer than the rates observed in Asu-ACR-16. In contrast to Asu-ACR-16, the A. caninum receptor was completely inhibited by DHβE and moderately inhibited by α-BTX. In conclusion, we have successfully reconstituted a fully functional homomeric nAChR, ACR-16, from A. caninum, a model for human hookworm infections. The pharmacology of the receptor is distinct from levamisole-sensitive nematode receptors. The ACR-16 homologue also displayed some pharmacological differences from Asu-ACR-16. Hence, A. caninum ACR-16 may be a valid target site for the development of anthelmintics against hookworm infections.
Keywords: nAChR, Hookworms, Aca-ACR-16, Anthelmintic, Xenopus oocyte
Introduction
Infections caused by hookworms (mainly Necator americanus and Ancylostoma duodenale) are one of the leading neglected tropical diseases, affecting approximately 500 million people worldwide, especially in the developing regions of Asia, Africa, Latin America and the Caribbean (Pullan et al. 2014; Loukas et al. 2016; Hotez 2008). These infections account for > 4 million disability adjusted life years (DALYs) lost annually and an estimated global economic loss of over US $100 billion (Loukas et al. 2016; Bartsch et al. 2016). These blood feeding nematodes do not directly account for substantial mortality; instead the major clinical manifestations of hookworm infection are the consequences of chronic intestinal blood loss. Severe infection can result in iron deficiency, anemia, weight loss, abdominal pain, protein loss, and diarrhea (Bethony et al. 2006; Hotez and Pritchard 1995). Hookworm infections pose a major health threat to adolescent girls, women of reproductive age, and children (Menzies et al. 2014; Brooker et al. 2008). Heavy worm burdens can result in retarded physical and cognitive development in children and poor outcomes for pregnant women and their newborns (Guyatt et al. 2000; de Silva et al. 2003; Hotez et al. 2014).
Current hookworm control strategies are limited to deworming of infected people using anthelmintic drugs combined with ancillary strategies such as improvement of water quality, sanitation and hygiene (WASH) in endemic regions (WHO 2015; Campbell et al. 2018; Albonico et al. 2003). At this time, there is no effective vaccine for human use in medical circulation (Hewitson and Maizels 2014; Diemert et al. 2008), and only a limited number of drug options. Unfortunately, there have been failures of mass drug administration in endemic regions due to diminished efficacy and potential resistance to anthelmintic agents (Albonico et al. 2003; Krücken et al. 2017; Flohr et al. 2007; De Clercq et al. 1997a; Reynoldson et al. 1997). The rapid reinfection rate of the worm combined with the ability of adult hookworms to survive up to 7 years in the human gut producing thousands of ova per day further complicates the issue (Albonico et al. 1995; Bennett and Guyatt 2000; Knopp et al. 2012). Due to all of these contributing factors, novel drug targets and drugs are required for efficient control of these parasitic infections.
Research has focused on several different parasite ion channels because they are major target sites of many classes of antinematodal agents (Wolstenholme 2011; Abongwa et al. 2017). Ion channels are essential for fundamental physiological functioning in gastrointestinal worms. Nicotinic acetylcholine receptors (nAChRs) which belong to the cys-loop ligand-gated ion channel family serve as synaptic transmission proteins and mediate fast transduction of signals by opening an intrinsic ion channel (Jones et al. 2007; Thompson et al. 2010). They are pentameric channels which can be homomeric or heteromeric around a central pore. Nicotinic anthelmintics such as pyrantel and levamisole selectively paralyze nematodes by activating cholinergic ion channels (nAChRs) in their body wall muscle (Abongwa et al. 2017; Aceves et al. 1970; Harrow and Gration 1985; Aubry et al. 1970; Martin et al. 2005). The significance of nematode nAChRs as drug targets has been emphasized by the recent development of novel aminoacetonitrile compounds (Kaminsky et al. 2008).
Ancylostoma caninum is the most widespread and pathogenic hookworm of dogs (Nemzek et al. 2015). Infestation typically results in anemia with bloody diarrhea, hemorrhagic enteritis, vomiting, anorexia, dehydration and poor weight gain, sometimes leading to death (Epe 2009; Dias et al. 2013). Zoonotic infection with A. caninum in humans has been associated with eosinophilic enteritis, localized myositis and cutaneous larva migrans (Prociv and Croese 1996; Bowman et al. 2010; Landmann and Prociv 2003; Traversa 2012). Ancylostoma caninum is the most accessible of all hookworms for research and is closely related to human hookworm species, A. duodenale and N. americanus. Therefore, they are used as a model for human hookworm (Nemzek et al. 2015; Prociv and Croese 1996; Blaxter 2000).
In this study, we have cloned and expressed a homologue of ACR-16 from A. caninum, a clade V nematode parasite. The receptors were expressed in Xenopus laevis oocytes, and we used two-electrode voltage-clamp electrophysiology to characterize their pharmacology. ACR-16 has been suggested as a druggable target in the parasitic clade III roundworms, Ascaris suum and Parascaris equorum (Abongwa et al. 2016; Charvet et al. 2018). The focus of this study was to generate a comparative pharmacological analysis of the homomeric channel and establish ACR-16 as a potential target in the hookworm parasites.
Materials and methods
Ethical concerns
No vertebrate animals were used directly in this study. Defolliculated Xenopus laevis oocytes were obtained from Ecocyte Bioscience (Austin, TX, USA).
Parasites
Ancylostoma caninum was obtained opportunistically from a naturally infected dog. Feces containing eggs was mixed with vermiculite and stored in a humidified container for 8 days. The mixture was overlaid with cheesecloth and was placed under a desktop lamp at room temperature for 8 h. L3 larvae were then concentrated and collected by the Baermann method (Baermann 1917).
Sequence analysis
Database searches for A. caninum ACR-16 were performed by BLAST search (WormBase Parasite), using the BLASTP algorithms (Altschul et al. 1997). Signal peptide predictions were done using the SignalP 4.1 server (Petersen et al. 2011), and membrane-spanning regions were identified using TMpred (Hofmann and Stoffel 1993). Alignment of the full-length amino acid sequences with Ascaris suum ACR-16 was carried out using the Clustal Omega program (Sievers et al. 2011).
Cloning of Aca-ACR-16
TRIzol Reagent™ (Invitrogen™, Carlsbad, CA, USA) was used to extract total RNA from homogenized A. caninum larvae. cDNA was synthesized by using SuperScript VILO Master Mix (Invitrogen™, Carlsbad, CA, USA) according to the manufacturer’s instructions and served as a template for Aca-ACR-16 amplification (WormBase Parasite Gene ID: ANCCAN_01899). We amplified Aca-ACR-16 as two fragments (Fragment 1: amino acid 1–165 and Fragment 2: amino acid 166–498). The amplified fragments were then assembled into the full-length Aca-ACR-16 sequence using the Gibson assembly protocol (Gibson et al. 2009). Full-length product was subcloned into pTB207 expression vector (Boulin et al. 2008) by adding XhoI and ApaI restriction enzyme sites, respectively, to the forward primer (5′ end: TGGCGGCCGctcgagATGCGTTCGTTGGTCGTCTG) and reverse primer (3′ end: ATCAAGCTCgggcccTTAGGCGACGAGATATGGAGC) using In-Fusion cloning (Takara Bio USA, Inc.). Z-competent E. coli JM109 cells (Zymo Research, Irvine, CA) were used for transformation of the ligated product. The final cloned constructs were sequenced with pTB207 vector primers (forward, T7) and (reverse, SP6). Only positive clones were used for cRNA synthesis using in vitro transcription with the mMessage mMachine T7 transcription kit (Invitrogen, CA, USA) and the cRNA was aliquoted and stored at − 80 °C.
Oocyte microinjection and electrophysiology
Xenopus laevis oocyte injections and two-electrode voltage-clamp electrophysiology recordings were performed as previously described in Choudhary et al. (2019). The oocytes were injected with 25–50 ng of Aca-ACR-16 cRNA either alone or in combination with 15–25 ng of each ancillary protein cRNA (Asu-RIC-3, Asu-UNC-50, Asu-UNC-74 and Xle-RIC-3) (Abongwa et al. 2016) in a total volume of 50 nL.
Drug applications
All drugs used, except tribendimidine and derquantel, were purchased from Sigma-Aldrich (St Louis, MO, USA). The drugs were solubilized in recording solution or DMSO (final working concentration did not exceed 0.1%). Derquantel and tribendimidine were a generous gift from Zoetis (Kalamazoo, MI, USA) and Prof Shu Hua Xiao (National Institute of Parasitic Diseases, China), respectively.
Agonists of interest were used at a final concentration of 100 μM except tribendimidine (30 μM) due to solubility issues. In all experiments, 100 μM acetylcholine (ACh) was applied first, and all the responses were normalized to this control response. Each agonist was applied for 10 s followed by 3 min perfusion with recording solution. The sequence for application of agonists for determining the rank-order potency series was random and not predetermined. The concentration-response studies were conducted by application of the drug in ascending order of concentrations in order to minimize any potential desensitization by high concentrations. In each experiment, the drug was applied for 10 s followed by 3 min wash off with the recording solution.
All the antagonists in our study were used at a final concentration of 10 μM. For generating rank-order potency series, a control application of 100 μM ACh (30 s) was first applied followed by 3 min wash off. Thereafter, 100 μM acetylcholine was applied for 10 s, immediately followed by 10 s application of the antagonist in the continued presence of 100 μM ACh and then a final 10 s application of 100 μM ACh. At least 3 min drug wash off interval was allowed between applications in order to minimize desensitization. Note that due to the short time of drug application in this protocol, it is possible to underestimate the potency of an antagonist.
Data and statistical analysis
Clampfit 10.3 (Molecular Devices, Sunnyvale, CA, USA) and GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA, USA) were used to analyze data. The peak currents in response to the applied agonists were measured and normalized to the control current (100 μM acetylcholine). The results were expressed as mean ± SEM. The Hill equation was used to analyze the concentration-response relationships by fitting log concentration-response data points as described in Boulin et al. (2008). Desensitization kinetics in response to the agonists were fitted using a single exponential decay fit:
where n is the number of components, A is the amplitude, t is time, τ is the time constant and C is the constant y-offset for each i component. The mean % inhibition produced by the antagonists on currents elicited by 100 μM acetylcholine was calculated using the equation previously described (Zheng et al. 2016). One-way ANOVA and extra sum of squares F-test were used to test statistical differences between desensitization rate and pEC50, respectively. The significance levels were set to P < 0.05.
Results
Sequence comparison of Aca-ACR-16 with Asu-ACR-16
We were able to identify the putative complete coding sequence of the homologue of ACR-16 in the translated Ancylostoma caninum genome (Gene ID: ANCCAN_01899) by using the Asu-ACR-16 protein sequence (GenBank: KP756901) as a query in a BLASTP search in the nematode protein database, WormBase Parasite. When amplified, the Aca-ACR-16 was shorter than ANCCAN_01899 and lacked 19 amino acids between the cys-loop and loop-B; these amino acids are also missing in the published sequence of Asu-ACR-16. Aca-ACR-16 sequence has been deposited in Genbank with accession number MN232004. The subunit has all the structural characteristics of a nicotinic acetylcholine receptor subunit: a large extracellular NH2-terminal domain of ~ 200 amino acids involved in correct nAChR assembly, a Cys-loop motif separated by 13 intervening amino acids, four transmembrane (TM) domains that form the ion-conducting pore, a cytoplasmic domain inserted between TM3 and TM4, six loops (A–F) and most importantly the presence of vicinal cysteines (a Y–x–C–C motif) in the C-loop making it an alpha subunit. Figure 1 shows the protein sequence alignment of Aca-ACR-16 with Asu-ACR-16. The two worm species belong to different clades of nematode, but their amino acid residues were highly conserved with an identity of 78% (87% similarity). There was a lack of conservation in Aca-ACR-16 loops E and F (involved in agonist binding) which encouraged us to characterize the pharmacology of the ion channel.
Fig. 1.

Amino acid sequence alignment of Aca-ACR-16 and Asu-ACR-16. The signal peptide (light brown), ligand binding loops (A to F; maroon) transmembrane regions TM1–4 (blue) and cys-loop (green) are indicated. The adjacent cysteines (in the Y–x–C–C motif) in loop-C are indicated in the black box. The negatively charged amino acids (E: Glutamic acid and D: Aspartic acid) flanking the TM2 domain are highlighted in orange. Residues involved in binding of α-BTX are highlighted in olive green. Note: The sequence of Aca-ACR-16 amplified from A. caninum larval total RNA is shorter than the WormBase sequence ANCCAN_01899 and lacks 19 amino acids (KVKEPNLFGPWENFHGDLF) between the cys-loop and loop-B. These amino acid residues are also lacking in the A. suum ACR-16 homologue
The ancillary factor RIC-3 is required for the functional expression of Aca-ACR-16
For the heterologous expression of the Aca-ACR-16, we expressed the subunit protein cRNA with different ancillary proteins (RIC-3, UNC-50 and UNC-74 from A. suum and RIC-3 from X. laevis; Fig. 2). None of the combinations, except Aca-ACR-16 with Asu-RIC-3, gave robust responses to control 100 μM ACh. In order to optimize the expressed receptor, we varied the amount of cRNA of Aca-ACR-16 (25–50 ng) and Asu-RIC-3 (15–25 ng). We obtained the largest response from oocytes injected with 50 ng Aca-ACR-16 and 25 ng Asu-RIC-3 and this mix was used for all subsequent recordings.
Fig. 2.
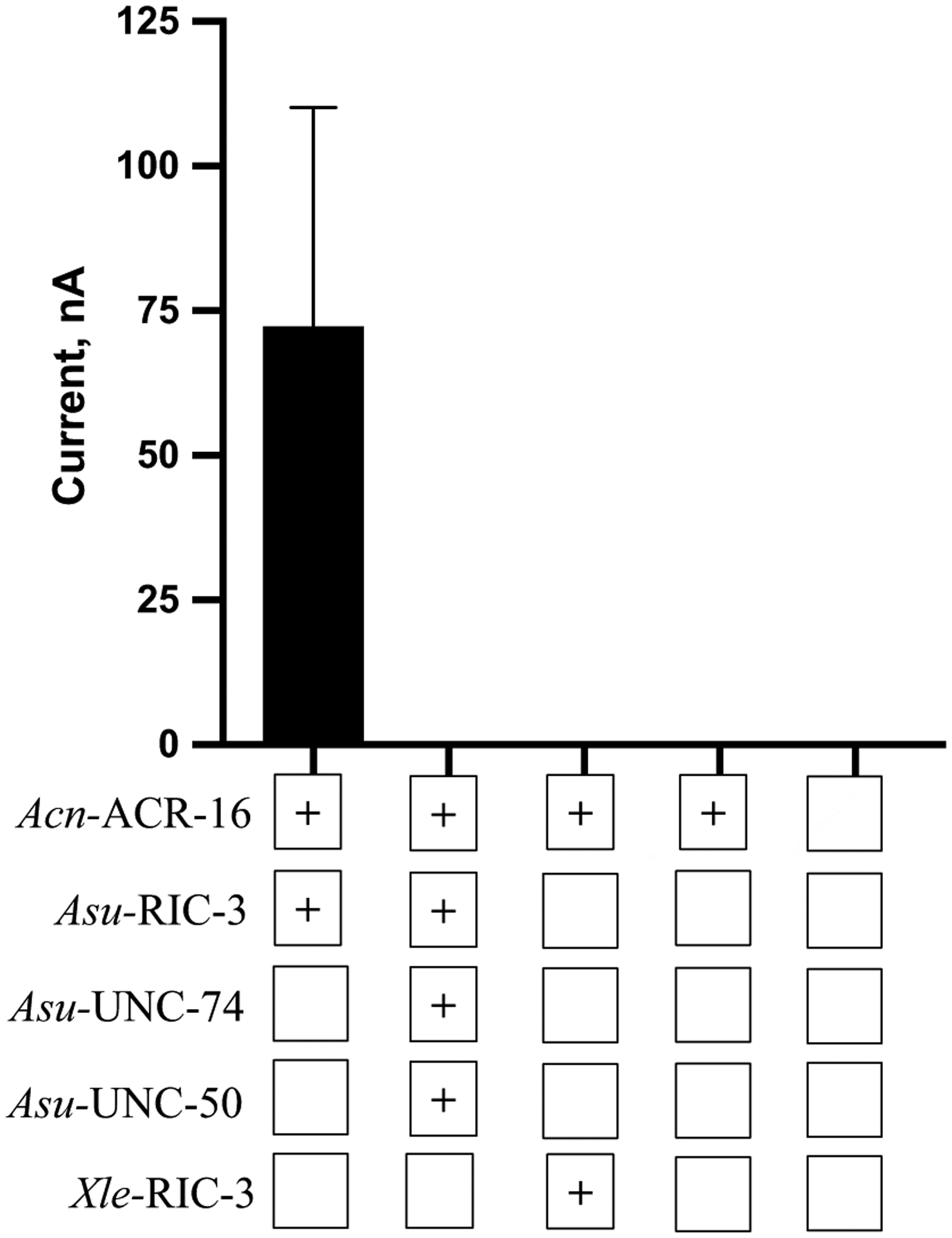
Bar chart showing the effects of ancillary proteins on the expression of Aca-ACR-16 nAChR (n ≥ 6). The receptor was able to express functionally only when co-injected with Asu-RIC-3. Final column represents un-injected control oocytes
Aca-ACR-16 forms 3-bromocytisine sensitive nAChR
We tested a selection of nicotinic agonists including cholinergic anthelmintics on the expressed A. caninum ACR-16 ligand-gated ion channel. Figure 3a shows the rank-order potency series for the agonists on the expressed Aca-ACR-16 receptor. 3-Bromocytisine was the most potent agonist (> 130% of the acetylcholine current). Epibatidine, cytisine, nicotine and DMPP also activated the receptor. Interestingly, the cholinergic anthelmintics including levamisole, oxantel, pyrantel, morantel, bephenium and tribendimidine were not active on the expressed nAChR. The rank-order potency series on Aca-ACR-16 when normalized to the control 100 μM acetylcholine current was: 3-bromocytisine > ACh > epibatidine > cytisine > nicotine > DMPP ≫ levamisole = oxantel = pyrantel = morantel = choline = bephenium = tribendimidine. None of cholinomimetic anthelmintics currently used in the field activated the homomeric receptor which shows that this channel is distinct from the other somatic nAChRs of nematodes.
Fig. 3.
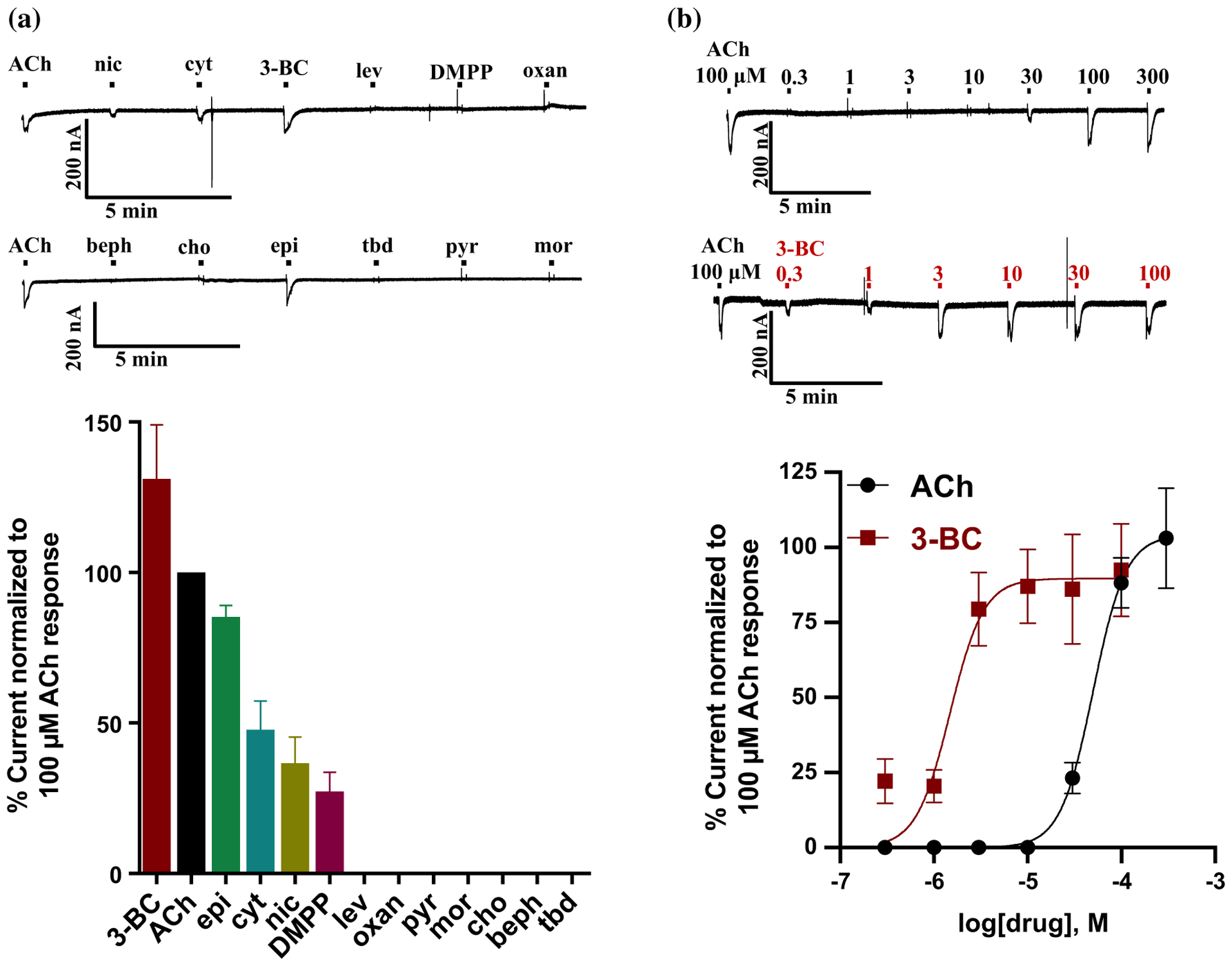
Effects of nAChR agonists and antiparasitic drugs on the Aca-ACR-16 receptor. a Bar graph (mean ± SEM, %, n = 4) along with sample traces showing the effect of agonists and anthelmintics on the nAChR. The rank-order potency series when normalized to the control 100 μM ACh current was as follows: 3-bromocytisine (3-BC; 131.0 ± 18.0) > ACh (100.0 ± 0.0) > epibatidine (epi; 85.0 ± 4.0) > cytisine (cyt; 48.0 ± 9.5) > nicotine (nic; 37.0 ± 8.7) > DMPP (dimethyl-4-phenylpiperazinium; 27.0 ± 6.4) ⋙ levamisole (lev; 0.0 ± 0.0) = oxantel (oxan; 0.0 ± 0.0) = pyrantel (pyr; 0.0 ± 0.0) = morantel (mor; 0.0 ± 0.0) = choline (cho; 0.0 ± 0.0) = bephenium (beph; 0.0 ± 0.0) = tribendimidine (tbd; 0.0 ± 0.0). b Sample traces and concentration-response relationships of 3-bromocytisine and ACh for Ancylostoma caninum ACR-16. The pEC50 and hill slope (nH) values, expressed as mean ± SEM, were, respectively, 4.3 ± 0.0 and 2.5 ± 0.3 for ACh (n = 6); 5.0 ± 0.1 and 2.4 ± 0.7 for 3-BC (n = 6)
Comparative pharmacology of acetylcholine and 3-bromocytisine
Figure 3b shows the concentration-response relationships of acetylcholine and 3-BC for the A. caninum homomeric channel. The sigmoidal plots were constructed by application of drugs in ascending order (0.3–300 μM depending on the agonist). 3-Bromocytisine (EC50 = 1.5 μM) was ~ 33 times more potent than acetylcholine (EC50 = 50.0 μM) on the receptor. The curves for both the nicotinic agonists were steep with the hillslope (nH) values greater than 1. This suggests that the ligands are binding to more than one site in the receptor and exhibit positive cooperativity as expected of a homomeric ligand-gated ion channel.
Aca-ACR-16 desensitization
Desensitization is defined as decrease or loss of biological response following prolonged or repetitive stimulation. It is a common feature of many nAChRs including α−7 homomeric nAChRs (Giniatullin et al. 2005; Picciotto et al. 2008; Quick and Lester 2002). In the case of A. suum ACR-16, all the potent agonists exhibited desensitization (Abongwa et al. 2016). We observed a similar trend characterized by peak and then waning current responses observed during maintained (10 s) agonist applications with Aca-ACR-16 as shown in Fig. 4. The time constant for desensitization was the highest for epibatidine and lowest for 3-bromocytisine. The mean time constants for desensitization rates ranged between 1.5 and 4.8 s for Aca-ACR-16 and were less than the rates observed in the Asu-ACR-16 which ranged between 6.2 and 12.6 s (Abongwa et al. 2016).
Fig. 4.
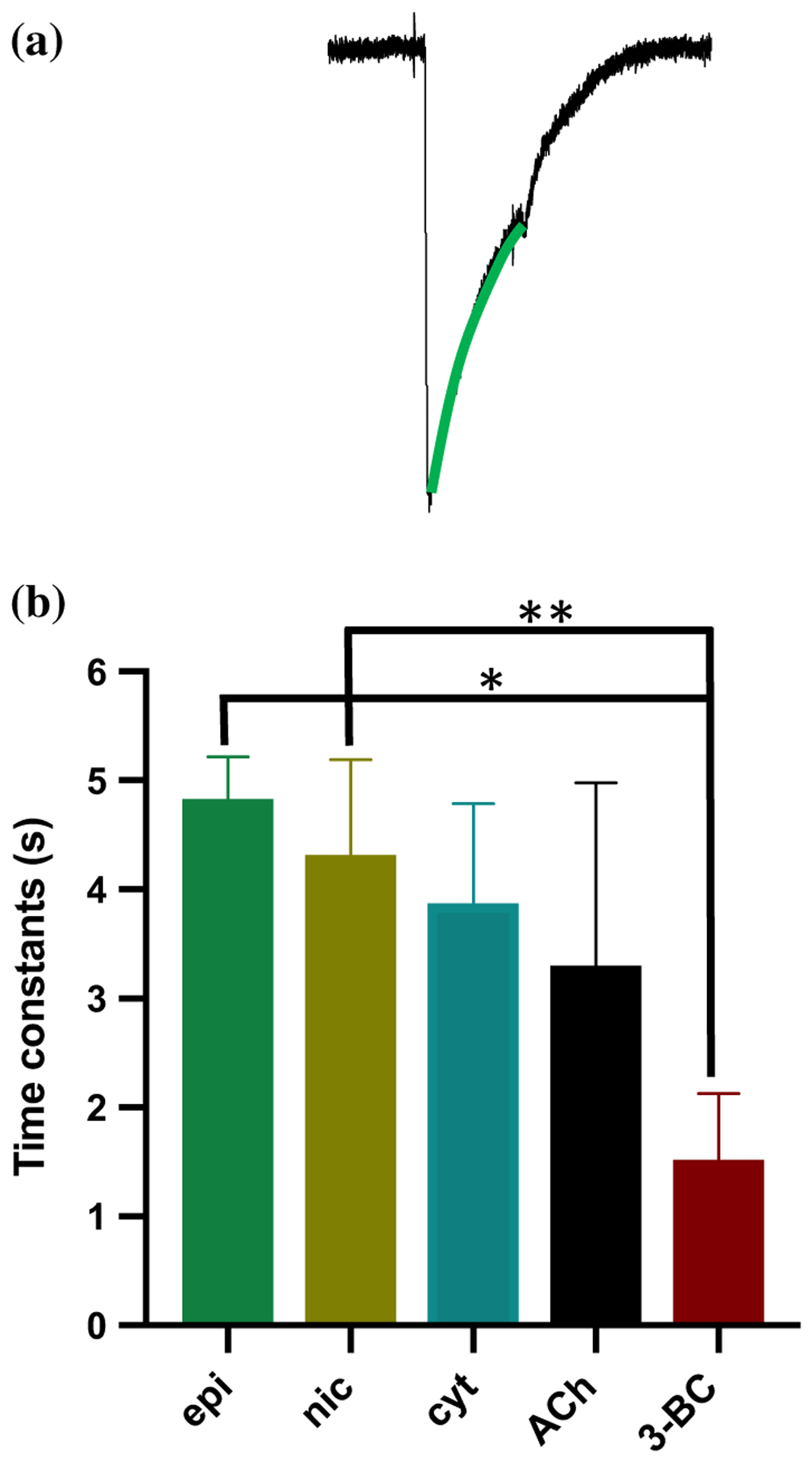
Aca-ACR-16 desensitization rate constant fit. a Sample trace of response to agonist with green line signifying the desensitization fit. b Bar graph showing desensitization time constants of the Ancylostoma caninum ACR-16 nAChR in response to agonists (100 μM, n = 4). The rank order of time constants of desensitization (mean ± SEM, s) was as follows: epi (4.8 ± 0.2) > nic (4.3 ± 0.5) > cyt (3.9 ± 0.5) > ACh (3.3 ± 1.0) > 3-BC (1.5 ± 0.3). *P < 0.05, **P < 0.01; significantly different as indicated; Tukey’s multiple comparison test
Antagonist pharmacology
Six nAChR antagonists (10 μM each) were tested on the expressed cation selective Aca-ACR-16 channel. The antagonists were d-tubocurarine (d-TC), mecamylamine, dihydro-β-erythroidine (DHβE), derquantel, hexamethonium and α-bungarotoxin (α-BTX). α-BTX produced least inhibition of the acetylcholine-mediated current, while d-TC and mecamylamine produced ~ 100% inhibition of the control current. DHβE, a selective antagonist for α4β2 receptors (Levin 2002), interestingly also produced almost complete inhibition of acetylcholine currents. The complete rank-order potency for antagonists (Fig. 5) was: d-TC ≈ mecamylamine ≈ DHβE > derquantel > hexamethonium > α-BTX.
Fig. 5.
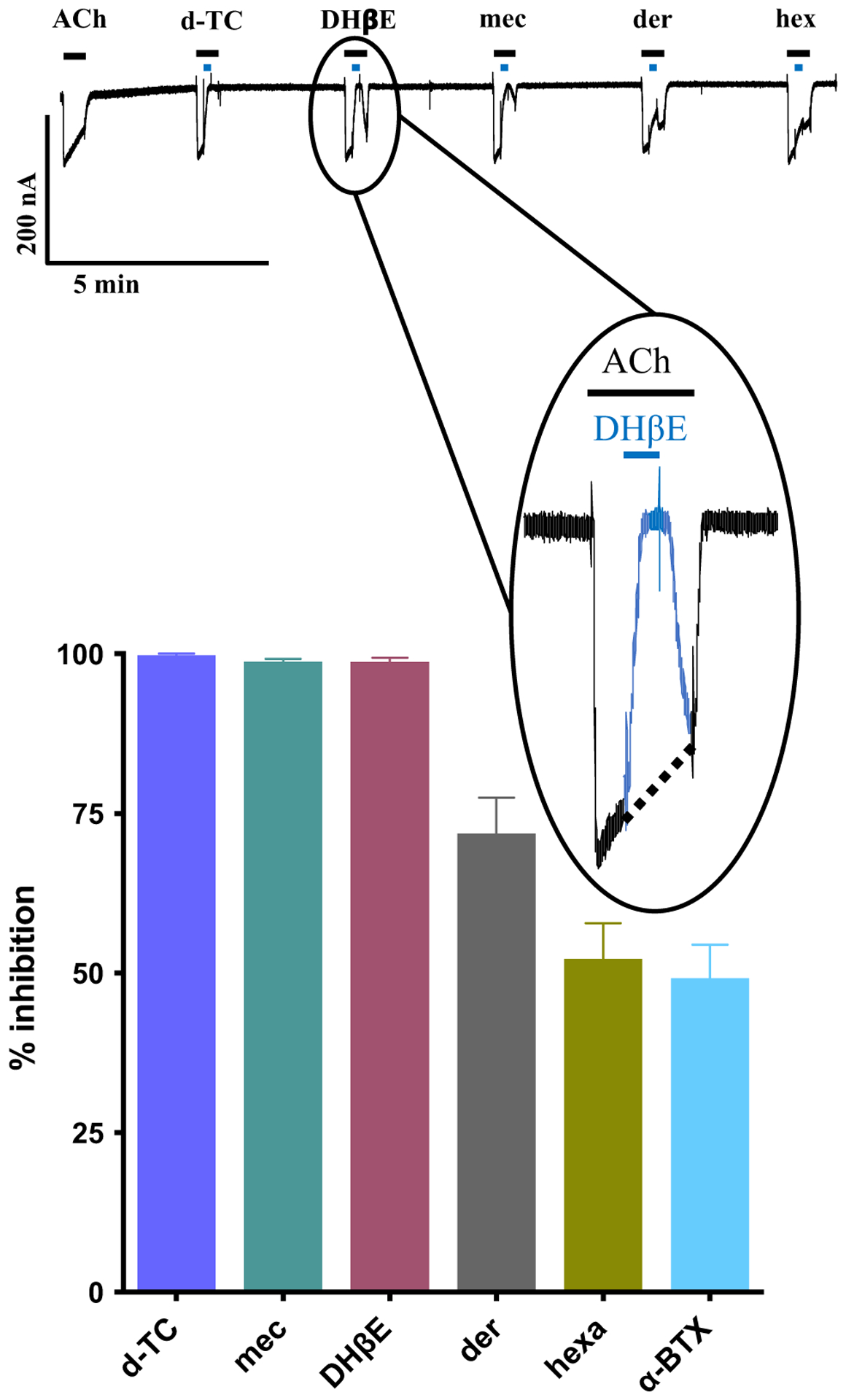
Effects of selected nAChR antagonists on the Aca-ACR-16. Sample trace and bar chart showing inhibition (mean ± SEM, %; n = 6) of acetylcholine-mediated currents by selected antagonists (10 μM). d-tubocurarine (d-TC), mecamylamine (mec) and dihydro-β-erythroidine (DHβE) produced almost complete inhibition of ACh mediated responses. Derquantel (der) and hexamethonium (hexa) produced moderate blockade of Aca-ACR-16 mediated ACh responses and α-BTX was the least potent antagonist. The rank-order potency series for nAChR antagonists is as follows: d-TC (100.0 ± 0.1) ≈ mec (98.8 ± 0.6) ≈ DHβE (98.8 ± 0.4) > der (72.0 ± 5.6) > hexa (52.2 ± 5.6) ≈ α-BTX (49.3 ± 5.2). Inset: magnified view of current trace showing predicted acetylcholine response in the absence of DHβE (dotted line) and inhibition of acetylcholine-mediated response in the presence of DHβE (highlighted in blue)
Discussion
Comparison of pharmacology of Aca-ACR-16 with homologues from other nematodes
In this study, we have shown that ACR-16 from Ancylostoma caninum, a Clade V nematode and model for human hookworm infections, expresses as a homomeric channel in Xenopus oocytes. Abongwa et al. (2016) and Charvet et al. (2018) successfully recapitulated and characterized the ACR-16 homologue from clade III gastrointestinal parasites, Ascaris suum and Parascaris equorum, respectively. Ballivet et al. (1996) and Raymond et al. (2000) characterized the pharmacology of ACR-16 nAChR from Caenorhabditis elegans, a clade V free-living nematode. Similar to the A. suum and C. elegans channel, Aca-ACR-16 was not sensitive to many of the currently used cholinomimetic anthelmintics including levamisole, pyrantel and tribendimidine. However, the A. caninum nAChR was most sensitive to 3-bromocytisine, while nicotine was the most potent agonist for the A. suum, P. equorum and C. elegans ACR-16 receptors. The acetylcholine concentration-response curve for Aca-ACR-16 (nH = 2.5 ± 0.3) had a comparable slope factor to Cel-ACR-16 (nH = 2.1) but was shallower in comparison with the Asu-ACR-16 (nH = 3.9 ± 0.3). This may account for the higher sensitivity to acetylcholine for the A. suum α nAChR (EC50 = 5.9 μM) and the similar sensitivity to the agonist for A. caninum (EC50 = 50.0 μM) and C. elegans (EC50 = 55.4 μM) ACR-16 receptors. Oxantel produced weak agonist activity on the Asu-ACR-16 nAChRs (< 10% of control acetylcholine current) but failed to activate the Cel-ACR-16 and Aca-ACR-16 receptor. In terms of antagonist pharmacology, the A. caninum cation channel was moderately inhibited by α-BTX (49.3 ± 5.2%), while Asu-ACR-16 (5.5 ± 0.8%) and Cel-ACR-16 nAChRs were nearly insensitive. DHβE produced complete inhibition of acetylcholine-mediated responses on the Aca-ACR-16 and Cel-ACR-16 nAChRs, while the A. suum homologue was only moderately inhibited. Similar to Asu-ACR-16, the Aca-ACR-16 receptor was highly sensitive to mecamylamine and d-TC; moderately sensitive to derquantel and hexamethonium. The protein sequence of the ACR-16 homologues from all the nematode parasites is highly conserved; Aca-ACR-16 shares 78%, 78% and 77% identity with C. elegans, A. suum and P. equorum homologues, respectively. There are variable amino acids residues in the loops E and F which can account for differences in the pharmacological properties (Corringer et al. 2000). Previous studies have shown that Cel-ACR-16 (Ballivet et al. 1996; Raymond et al. 2000) expresses without ancillary proteins, while Ascaris suum (Abongwa et al. 2016) and Parascaris equorum ACR-16 (Charvet et al. 2018) both require at least RIC-3 for functional expression. In our study, we also found RIC-3 was required for functional expression but interestingly further addition of ancillary proteins (UNC-50 and UNC-74) actually abolished expression. This observation is somewhat similar to Asu-ACR-16 where addition of UNC-50 and UNC-74 actually reduced expression cf RIC-3 alone. The reason for the differences in ancillary protein requirements for functional expression of ACR-16 from different species remains unclear.
Consideration of the Aca-ACR-16 as a drug target
Hookworm infections affect approximately 500 million people globally, with 5.1 billion at risk for acquiring infections (Global Burden of Disease Study 2015; Pullan and Brooker 2012). Despite decades of strong research efforts and identification of promising candidate antigens, there are still no commercially available vaccines for human hookworm infections. Consequently, identification of novel drug targets and development of associated therapeutic agents is a logical approach for future defense against these infections. In parasitic nematodes, nicotinic acetylcholine receptors are required for various physiological functions. These ligand-gated ion channels are targets of important cholinergic antinematodal drugs such as levamisole and pyrantel. Recently introduced “novel” anthelmintics including tribendimidine and derquantel also target nematode nAChRs (Abongwa et al. 2017; Wolstenholme 2011). acr-16 encodes for nicotine-sensitive nAChRs that are expressed in body wall muscles in C. elegans and contribute to the fast synaptic cholinergic neurotransmission in these muscles (Francis et al. 2005; Touroutine et al. 2005; Richmond and Jorgensen 1999). The unc-63/acr-16 double mutants in C. elegans exhibit locomotor defects which are more severe than either unc-63 or acr-16 alone (Touroutine et al. 2005). Similarly, unc-29/acr-16 double mutants also display far greater movement impairment than either unc-29 or acr-16 single mutants (Li et al. 2014). This demonstrates that ACR-16 in combination with UNC-63 and UNC-29, components of levamisole-sensitive nAChR, contributes to locomotor behavior in the worms. In Brugia malayi, a clade V worm, knockdown of acr-16/acr-26 had no effect on motility (Verma et al. 2017) possibly suggesting a different physiological function of the ACR-16 homologue in the filarial worm. In A. suum, the ACR-16 homologue was detected in the ovijector and digestive tract tissues in addition to the body wall muscles. It is plausible that the ACR-16 nAChRs not only regulates neurotransmission in A. suum but also serve other tissue-related functions including reproduction and digestion. The ACR-16 homologue from A. suum has been suggested as a drug target (Abongwa et al. 2016). We have successfully reconstituted a fully functional homomeric nAChR, ACR-16, in the Xenopus oocyte expression system from A. caninum, a model for human hookworm infections. The pharmacology of the receptor is distinct from the levamisole-sensitive nematode receptors (Martin et al. 2012; Boulin et al. 2008; Richmond and Jorgensen 1999). The A. caninum ACR-16 homologue also displayed some pharmacological differences from Asu-ACR-16. Benzimidazoles are the commonly used antiparasitic drugs for treatment of hookworm infections but there have been multiple reports of resistance in veterinary medicine (Kaplan 2004; Wolstenholme et al. 2004) and decreased cure rates in humans (Geerts and Gryseels 2000; De Clercq et al. 1997b; Keiser and Utzinger 2008; Conder and Campbell 1995). There is a need for new drugs against hookworms, and ACR-16 may be a valid target site with the potential to circumvent existing drug resistance.
Acknowledgements
This research was funded by NIH R21AI092185 to APR, NIH RO1AI047194 and the E. A. Benbrook Foundation for Pathology and Parasitology to RJM. We would like to thank Dr. Claude L. Charvet, INRA, Université Tours, Nouzilly, France for the generous gift of pTB207 vector.
Footnotes
Conflict of interest The authors declare that this work has no conflict of interest.
References
- Abongwa M, Buxton SK, Courtot E, Charvet CL, Neveu C, McCoy CJ et al. (2016) Pharmacological profile of Ascaris suum ACR-16, a new homomeric nicotinic acetylcholine receptor widely distributed in Ascaris tissues. Br J Pharmacol 173(16):2463–2477. 10.1111/bph.13524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abongwa M, Martin RJ, Robertson AP (2017) A brief review on the mode of action of antinematodal drugs. Acta Vet (Beogr) 67(2):137–152. 10.1515/acve-2017-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceves J, Erlij D, Martínez-Marañón R (1970) The mechanism of the paralysing action of tetramisole on Ascaris somatic muscle. Br J Pharmacol 38(3):602–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albonico M, Smith PG, Ercole E, Hall A, Chwaya HM, Alawi KS et al. (1995) Rate of reinfection with intestinal nematodes after treatment of children with mebendazole or albendazole in a highly endemic area. Trans R Soc Trop Med Hyg 89(5):538–541 [DOI] [PubMed] [Google Scholar]
- Albonico M, Bickle Q, Ramsan M, Montresor A, Savioli L, Taylor M (2003) Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bull World Health Organ 81(5):343–352 [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25(17):3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry ML, Cowell P, Davey MJ, Shevde S (1970) Aspects of the pharmacology of a new anthelmintic: pyrantel. Br J Pharmacol 38(2):332–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baermann G (1917) A simple method for the detection of Ankylostomum (nematode) larvae in soil tests. Javasche Boekhandel & Drukkerij, Batavia [Google Scholar]
- Ballivet M, Alliod C, Bertrand S, Bertrand D (1996) Nicotinic acetylcholine receptors in the NematodeCaenorhabditis elegans. J Mol Biol 258(2):261–269. 10.1006/jmbi.1996.0248 [DOI] [PubMed] [Google Scholar]
- Bartsch SM, Hotez PJ, Asti L, Zapf KM, Bottazzi ME, Diemert DJ et al. (2016) The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis 10(9):e0004922 10.1371/journal.pntd.0004922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A, Guyatt H (2000) Reducing intestinal nematode infection: efficacy of albendazole and mebendazole. Parasitol Today 16(2):71–74 [DOI] [PubMed] [Google Scholar]
- Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D et al. (2006) Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367(9521):1521–1532. 10.1016/S0140-6736(06)68653-4 [DOI] [PubMed] [Google Scholar]
- Blaxter M (2000) Genes and genomes of Necator americanus and related hookworms. Int J Parasitol 30(4):347–355. 10.1016/S0020-7519(99)00198-8 [DOI] [PubMed] [Google Scholar]
- Boulin T, Gielen M, Richmond JE, Williams DC, Paoletti P, Bessereau JL (2008) Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc Natl Acad Sci USA 105(47):18590–18595. 10.1073/pnas.0806933105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman DD, Montgomery SP, Zajac AM, Eberhard ML, Kazacos KR (2010) Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol 26(4):162–167. 10.1016/j.pt.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Brooker S, Hotez PJ, Bundy DA (2008) Hookworm-related anaemia among pregnant women: a systematic review. PLoS Negl Trop Dis 2(9):e291 10.1371/journal.pntd.0000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SJ, Biritwum NK, Woods G, Velleman Y, Fleming F, Stothard JR (2018) Tailoring water, sanitation, and hygiene (WASH) targets for soil-transmitted helminthiasis and schistosomiasis control. Trends Parasitol 34(1):53–63. 10.1016/j.pt.2017.09.004 [DOI] [PubMed] [Google Scholar]
- Charvet CL, Guégnard F, Courtot E, Cortet J, Neveu C (2018) Nicotine-sensitive acetylcholine receptors are relevant pharmacological targets for the control of multidrug resistant parasitic nematodes. Int J Parasitol Drugs Drug Resist 8(3):540–549. 10.1016/j.ijpddr.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S, Marjianović DS, Wong CR, Zhang X, Abongwa M, Coats JR et al. (2019) Menthol acts as a positive allosteric modulator on nematode levamisole sensitive nicotinic acetylcholine receptors. Int J Parasitol Drugs Drug Resist 9:44–53. 10.1016/j.ijpddr.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conder GA, Campbell WC (1995) Chemotherapy of nematode infections of veterinary importance, with special reference to drug resistance. Adv Parasitol 35:1–84 [DOI] [PubMed] [Google Scholar]
- Corringer PJ, Le Novere N, Changeux JP (2000) Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol 40:431–458. 10.1146/annurev.pharmtox.40.1.431 [DOI] [PubMed] [Google Scholar]
- De Clercq D, Sacko M, Behnke J, Gilbert F, Dorny P, Vercruysse J (1997a) Failure of mebendazole in treatment of human hookworm infections in the Southern Region of Mali. Am J Trop Med Hyg 57(1):25–30. 10.4269/ajtmh.1997.57.25 [DOI] [PubMed] [Google Scholar]
- De Clercq D, Sacko M, Behnke J, Gilbert F, Dorny P, Vercruysse J (1997b) Failure of mebendazole in treatment of human hookworm infections in the southern region of Mali. Am J Trop Med Hyg 57(1):25–30 [DOI] [PubMed] [Google Scholar]
- de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L (2003) Soil-transmitted helminth infections: updating the global picture. Trends Parasitol 19(12):547–551 [DOI] [PubMed] [Google Scholar]
- Dias SRC, Cunha DES, da Silva SM, dos Santos HA, Fujiwara RT, Rabelo ÉML (2013) Evaluation of parasitological and immunological aspects of acute infection by Ancylostoma caninum and Ancylostoma braziliense in mixed-breed dogs. Parasitol Res 112(6):2151–2157. 10.1007/s00436-013-3370-y [DOI] [PubMed] [Google Scholar]
- Diemert DJ, Bethony JM, Hotez PJ (2008) Hookworm vaccines. Clin Infect Dis 46(2):282–288. 10.1086/524070 [DOI] [PubMed] [Google Scholar]
- Epe C (2009) Intestinal nematodes: biology and control. Vet Clin North Am Small Anim Pract 39(6):1091–1107. 10.1016/j.cvsm.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Flohr C, Tuyen LN, Lewis S, Minh TT, Campbell J, Britton J et al. (2007) Low efficacy of mebendazole against hookworm in Vietnam: two randomized controlled trials. Am J Trop Med Hyg 76(4):732–736 [PubMed] [Google Scholar]
- Francis MM, Evans SP, Jensen M, Madsen DM, Mancuso J, Norman KR et al. (2005) The Ror receptor tyrosine kinase CAM-1 is required for ACR-16-mediated synaptic transmission at the C. elegans neuromuscular junction. Neuron 46(4):581–594. 10.1016/j.neuron.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Geerts S, Gryseels B (2000) Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev 13(2):207–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, Smith HO (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6(5):343–345. 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL (2005) Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci 28(7):371–378. 10.1016/j.tins.2005.04.009 [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease Study, C (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386(9995):743–800. 10.1016/S0140-6736(15)60692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt HL, Brooker S, Peshu N, Shulman CE (2000) Hookworm and anaemia prevalence. Lancet 356(9247):2101. [DOI] [PubMed] [Google Scholar]
- Harrow ID, Gration KAF (1985) Mode of action of the anthelmintics morantel, pyrantel and levamisole on muscle cell membrane of the nematode Ascaris suum. Pestic Sci 16(6):662–672. 10.1002/ps.2780160612 [DOI] [Google Scholar]
- Hewitson JP, Maizels RM (2014) Vaccination against helminth parasite infections. Expert Rev Vaccines 13(4):473–487. 10.1586/14760584.2014.893195 [DOI] [PubMed] [Google Scholar]
- Hofmann K, Stoffel W (1993) TMbase—a database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler 374:166 [Google Scholar]
- Hotez P (2008) Hookworm and poverty. Ann N Y Acad Sci 1136:38–44. 10.1196/annals.1425.000 [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Pritchard DI (1995) Hookworm infection. Sci Am 272(6):68 10.1038/scientificamerican0695-68 [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Alvarado M, Basanez MG, Bolliger I, Bourne R, Boussinesq M et al. (2014) The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis 8(7):e2865 10.1371/journal.pntd.0002865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AK, Davis P, Hodgkin J, Sattelle DB (2007) The nicotinic acetylcholine receptor gene family of the nematode Caenorhabditis elegans: an update on nomenclature. Invertebr Neurosci 7(2):129–131. 10.1007/s10158-007-0049-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky R, Ducray P, Jung M, Clover R, Rufener L, Bouvier J et al. (2008) A new class of anthelmintics effective against drug-resistant nematodes. Nature 452(7184):176–180. 10.1038/nature06722 [DOI] [PubMed] [Google Scholar]
- Kaplan RM (2004) Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol 20(10):477–481. 10.1016/j.pt.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Keiser J, Utzinger J (2008) Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA 299(16):1937–1948. 10.1001/jama.299.16.1937 [DOI] [PubMed] [Google Scholar]
- Knopp S, Steinmann P, Keiser J, Utzinger J (2012) Nematode infections: soil-transmitted helminths and trichinella. Infect Dis Clin North Am 26(2):341–358. 10.1016/j.idc.2012.02.006 [DOI] [PubMed] [Google Scholar]
- Krücken J, Fraundorfer K, Mugisha JC, Ramünke S, Sifft KC, Geus D et al. (2017) Reduced efficacy of albendazole against Ascaris lumbricoides in Rwandan schoolchildren. Int J Parasitol Drugs Drug Resist 7(3):262–271. 10.1016/j.ijpddr.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann JK, Prociv P (2003) Experimental human infection with the dog hookworm, Ancylostoma caninum. Med J Aust 178(2):69–71 [DOI] [PubMed] [Google Scholar]
- Levin ED (2002) Nicotinic receptor subtypes and cognitive function. J Neurobiol 53(4):633–640. 10.1002/neu.10151 [DOI] [PubMed] [Google Scholar]
- Li Z, Liu J, Zheng M, Xu XZS (2014) Encoding of both analog- and digital-like behavioral outputs by one C. elegans interneuron. Cell 159(4):751–765. 10.1016/j.cell.2014.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukas A, Hotez PJ, Diemert D, Yazdanbakhsh M, McCarthy JS, Correa-Oliveira R et al. (2016) Hookworm infection. Nat Rev Dis Primers 2:16088 10.1038/nrdp.2016.88 [DOI] [PubMed] [Google Scholar]
- Martin RJ, Verma S, Levandoski M, Clark CL, Qian H, Stewart M et al. (2005) Drug resistance and neurotransmitter receptors of nematodes: recent studies on the mode of action of levamisole. Parasitology 131(Suppl):S71–S84. 10.1017/s0031182005008668 [DOI] [PubMed] [Google Scholar]
- Martin RJ, Robertson AP, Buxton SK, Beech RN, Charvet CL, Neveu C (2012) Levamisole receptors: a second awakening. Trends Parasitol 28(7):289–296. 10.1016/j.pt.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies SK, Rodriguez A, Chico M, Sandoval C, Broncano N, Guadalupe I et al. (2014) Risk factors for soil-transmitted helminth infections during the first 3 years of life in the tropics; findings from a birth cohort. PLoS Negl Trop Dis 8(2):e2718 10.1371/journal.pntd.0002718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemzek JA, Lester PA, Wolfe AM, Dysko RC, Myers DD (2015) Chapter 12—Biology and diseases of dogs In: Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT(eds) Laboratory animal medicine, 3rd edn. Academic Press, Boston, pp 511–554 [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8(10):785–786. 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH (2008) It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84(4):329–342. 10.1016/j.pneurobio.2007.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prociv P, Croese J (1996) Human enteric infection with Ancylostoma caninum: hookworms reappraised in the light of a “new” zoonosis. Acta Trop 62(1):23–44 [DOI] [PubMed] [Google Scholar]
- Pullan RL, Brooker SJ (2012) The global limits and population at risk of soil-transmitted helminth infections in 2010. Parasit Vectors 5:81 10.1186/1756-3305-5-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan RL, Smith JL, Jasrasaria R, Brooker SJ (2014) Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors 7:37 10.1186/1756-3305-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Lester RA (2002) Desensitization of neuronal nicotinic receptors. J Neurobiol 53(4):457–478. 10.1002/neu.10109 [DOI] [PubMed] [Google Scholar]
- Raymond V, Mongan NP, Sattelle DB (2000) Anthelmintic actions on homomer-forming nicotinic acetylcholine receptor subunits: chicken α7 and ACR-16 from the nematode Caenorhabditis elegans. Neuroscience 101(3):785–791. 10.1016/S0306-4522(00)00279-7 [DOI] [PubMed] [Google Scholar]
- Reynoldson JA, Behnke JM, Pallant LJ, Macnish MG, Gilbert F, Giles S et al. (1997) Failure of pyrantel in treatment of human hookworm infections (Ancylostoma duodenale) in the Kimberley region of North West Australia. Acta Trop 68(3):301–312. 10.1016/S0001-706X(97)00106-X [DOI] [PubMed] [Google Scholar]
- Richmond JE, Jorgensen EM (1999) One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci 2(9):791–797. 10.1038/12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W et al. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Lester HA, Lummis SC (2010) The structural basis of function in Cys-loop receptors. Q Rev Biophys 43(4):449–499. 10.1017/S0033583510000168 [DOI] [PubMed] [Google Scholar]
- Touroutine D, Fox RM, Von Stetina SE, Burdina A, Miller DM, Richmond JE (2005) acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J Biol Chem 280(29):27013–27021 [DOI] [PubMed] [Google Scholar]
- Traversa D (2012) Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors 5:91 10.1186/1756-3305-5-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Kashyap SS, Robertson AP, Martin RJ (2017) Functional genomics in Brugia malayi reveal diverse muscle nAChRs and differences between cholinergic anthelmintics. Proc Natl Acad Sci USA 114(21):5539–5544. 10.1073/pnas.1619820114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2015) Investing to overcome the global impact of neglected tropical diseases: third WHO report on neglected diseases. World Health Organisation, Geneva [Google Scholar]
- Wolstenholme AJ (2011) Ion channels and receptor as targets for the control of parasitic nematodes. Int J Parasitol Drugs Drug Resist 1(1):2–13. 10.1016/j.ijpddr.2011.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme AJ, Fairweather I, Prichard R, von Samson-Himmelstjerna G, Sangster NC (2004) Drug resistance in veterinary helminths. Trends Parasitol 20(10):469–476. 10.1016/j.pt.2004.07.010 [DOI] [PubMed] [Google Scholar]
- Zheng F, Robertson AP, Abongwa M, Yu EW, Martin RJ (2016) The Ascaris suum nicotinic receptor, ACR-16, as a drug target: four novel negative allosteric modulators from virtual screening. Int J Parasitol Drugs Drug Resist 6(1):60–73. 10.1016/j.ijpddr.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


