Abstract
Background:
The intradermal route of vaccine administration for pre-exposure rabies prophylaxis, endorsed by the Canadian National Advisory Committee on Immunization, was implemented at a large travel clinic in Montréal in 2008. We evaluated the effect of intradermal vaccination availability on uptake of pre-exposure rabies prophylaxis and rates of seroconversion with intradermal vaccination.
Methods:
We conducted a retrospective cross-sectional study using data from December 2008 to December 2014. The number of travellers who received pre-exposure rabies prophylaxis before and after the introduction of intradermal vaccination was compared. Postvaccination antibody titres were measured in intradermal vaccination recipients. We compared demographic and travel characteristics between vaccinated and unvaccinated travellers and between travellers in the intradermal and intramuscular groups using univariate and multivariate analyses.
Results:
The proportion of travellers who received pre-exposure prophylaxis increased after the introduction of intradermal vaccination (annual average of 300 travellers from December 2009 to December 2014 v. 183 travellers from December 2006 to December 2007). Seroconversion occurred in 99.9% of those in the intradermal group. Travellers who received pre-exposure prophylaxis were older (mean age 35.8 yr v. 32.1 yr) and had longer travel duration than those who did not receive pre-exposure prophylaxis. Travellers to Asia were more likely to receive pre-exposure prophylaxis, and travellers visiting friends and relatives were less likely to receive it. Travellers in the intradermal group were younger than those in the intramuscular group and were more likely to be travelling for tourism.
Interpretation:
The introduction of intradermal vaccination for pre-exposure rabies prophylaxis was associated with an increase in vaccination uptake. Reduced cost may be responsible for the increased coverage among younger travellers and those travelling for tourism. The high seroconversion rate after intradermal vaccination supports the effectiveness of this route of administration for pre-exposure rabies prophylaxis in immunocompetent people.
Rabies virus infection produces encephalitis that is nearly uniformly fatal. It is most commonly transmitted to humans via the bite of an infected animal. The disease causes 60 000 deaths per year in the developing world but remains a rare diagnosis in travellers.1 However, travellers frequently seek medical advice for animal bites or scratches, sometimes requiring rabies postexposure prophylaxis, which disrupts travel plans.2 Pre-exposure prophylaxis greatly facilitates postexposure prophylaxis measures.3 When postexposure prophylaxis is indicated, previously vaccinated people require only 2 doses of vaccine, whereas unvaccinated people require 4 or 5 doses of vaccine in addition to rabies immune globulin. The latter is often not easily available in the region where the injury occurs.3 In fact, only a small proportion of travellers receive rabies immune globulin with postexposure prophylaxis in the country of exposure.4 The Canadian Immunization Guide, produced by the National Advisory Committee on Immunization for the Public Health Agency of Canada, recommends pre-exposure prophylaxis for people at high risk of close contact with potentially rabid animals, including travellers to endemic areas with poor access to medical care and timely postexposure prophylaxis.3
Two rabies vaccines are licensed in Canada: Imovax Rabies (Sanofi Pasteur), a human diploid cell vaccine, and RabAvert (GlaxoSmithKline), a purified chick embryo cell vaccine.3 Both are inactivated virus vaccines and are available only as 1.0-mL doses for intramuscular delivery. However, intradermal administration of these vaccines requires only 0.1 mL per dose, thus reducing cost by increasing the number of doses available from a single vial. Intradermal vaccination for pre-exposure prophylaxis has been shown to be safe and immunogenic in immunocompetent people.5–10 Its use is endorsed by the National Advisory Committee on Immunization for pre-exposure prophylaxis in immunocompetent people not taking chloroquine, which may interfere with the immune response to the vaccine.3 It is recommended that intradermal vaccine be administered by trained staff, that a single vial be used within 6 hours after opening,11 that the “cold chain” always be preserved, and that antibody titres be determined at least 2 weeks after completion of the vaccine series.3 Vaccine costs can be minimized by grouping vaccinee appointments and using needles and syringes with low “dead space” to avoid vaccine wastage.
In December 2008, intradermal vaccination for pre-exposure rabies prophylaxis was implemented at the Clinique Santé-voyage de la Fondation du Centre hospitalier de l’Université de Montréal. We reviewed data from 6 years of experience with intradermal pre-exposure rabies vaccination, with 4 objectives: 1) to evaluate the impact of the introduction of intradermal vaccine administration on the proportion of travellers who accepted pre-exposure prophylaxis, 2) to document the seroconversion rate among travellers who received intradermal vaccination for pre-exposure prophylaxis, 3) to describe and compare the characteristics of travellers who received any pre-exposure prophylaxis to those who did not and 4) to compare the characteristics of travellers who chose intradermal delivery to those who received the vaccine intramuscularly.
Methods
Setting and sources of data
The Clinique Santé-voyage de la Fondation du Centre hospitalier de l’Université de Montréal is one of the largest travel clinics in Quebec. It received about 20 000 visits per year for pretravel assessment between 2007 and 2017. In December 2008, intradermal vaccination for pre-exposure rabies prophylaxis was introduced by offering a weekly clinic staffed by nurses trained and experienced in intradermal administration. People who presented for pretravel assessment were evaluated by the travel clinic practitioner for an indication for pre-exposure rabies prophylaxis based on characteristics of the proposed travel, such as destination, duration and type of activity. Those with an indication for pre-exposure rabies prophylaxis were offered the options of intradermal and intramuscular routes of administration. Factors associated with intradermal vaccination, such as decreased cost, potential increased local injection reaction and need for postvaccination serologic testing to verify response, were explained to travellers. Although Canada experienced a shortage of rabies vaccine in 2008–2009, stocks were sufficient to continue offering a free choice of intramuscular or intradermal administration. All vaccinations were given as a 3-dose vaccine series on days 0, 7, and 21 or 28. Groups of 3 or more travellers were booked per intradermal vaccination clinic to minimize vaccine wastage. Postvaccination antibody titres were measured 2 to 4 weeks after the last dose for all those who received intradermal injections.3 Serum samples were sent to the National Microbiology Laboratory, Winnipeg, and tested for rabies antibody levels by means of a modified fluorescent antibody virus neutralization assay. An adequate response after vaccination was defined as a titre of 0.5 IU/mL or greater.
Design
This was a retrospective cross-sectional study using data from December 2008 through December 2014. We retrieved data for all travellers who presented for pretravel assessment from a computerized database, with only 1 pretravel assessment included per traveller. Variables collected were age, sex, receipt of any pre-exposure prophylaxis, type and route of administration where applicable, country and continent of travel, reason for travel, duration of travel, and whether travel was alone, in a couple or in a group. We reviewed the number of travellers who received any pre-exposure prophylaxis for a 1-year period before implementation of intradermal vaccination for pre-exposure rabies prophylaxis (December 2006 to December 2007) and compared it to the number of travellers who received pre-exposure prophylaxis during the study period.
Statistical analysis
To compare characteristics between travellers who received and did not receive pre-exposure prophylaxis, and between travellers who received intradermal injections and those who received intramuscular injections, we expressed categorical variables as frequencies and percentages, and continuous variables as means and standard deviations. We compared continuous and categorical variables using standardized differences with 95% confidence intervals (CIs) for means and proportions, respectively. We performed the analyses using Stata version 14.2 (StataCorp). We performed univariate and multivariate analyses with logistic regression modelling using SPSS version 20.
Ethics approval
The study was approved by the Centre hospitalier de l’Université de Montréal.
Results
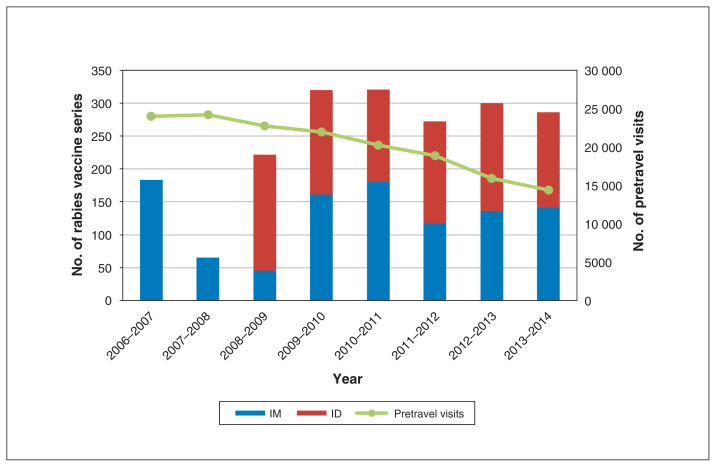
From December 2009 to December 2014, an average of 300 travellers were vaccinated annually (Figure 1). In comparison, from December 2006 to December 2007 (before the implementation of intradermal vaccination for pre-exposure prophylaxis), 183 vaccine series were given. The number of pretravel visits decreased from 24 022 in 2006–2007 to 14 336 in 2013–2014 (Figure 1).
Figure 1:
Number of series of pre-exposure rabies prophylaxis vaccine administered, by type, and number of pretravel visits, 2006–2014. Note: ID = intradermal, IM = intramuscular.
A total of 37 032 people presented for pretravel assessment between December 2008 and December 2014. Of the 37 032, 1721 (4.6%) received pre-exposure prophylaxis, and 35 311 (95.4%) did not. Of the 1721 travellers who received pre-exposure prophylaxis, 941 (54.7%) received intradermal injections, and 780 (45.3%) received intramuscular injections.
Of the 941 people who received intradermal injections, 940 (99.9%) seroconverted, with an antibody titre greater than 0.5 IU/mL when measured 2–4 weeks after completion of the vaccine series. One traveller had not seroconverted by 2 weeks and could not be tested at 4 weeks to assess for delayed seroconversion because of imminent travel. A single booster dose was given intramuscularly to this person before travel.
Travellers who received pre-exposure prophylaxis were older than those who did not (mean age 35.8 yr v. 32.1 yr), although the effect size was small (standardized difference 0.205, 95% CI 0.156 to 0.253) (Table 1). There was no significant difference in sex between the 2 groups. Travellers who received pre-exposure prophylaxis had longer travel duration than those who did not (mean 16.7 wk v. 5.2 wk, standardized difference 0.944, 95% CI 0.893 to 0.996). Fewer vaccinated travellers than unvaccinated travellers had travel duration of less than 4 weeks (602/1560 [38.6%] v. 24 641/32 516 [75.8%]). Travellers to Asia were most likely to have received pre-exposure prophylaxis, and travellers to the Americas were least likely to have received pre-exposure prophylaxis (standardized difference 0.449, 95% CI 0.398 to 0.499). People travelling for work/business, education/research or volunteer/aid work were more likely to be vaccinated, and those visiting friends and relatives were less likely to be vaccinated, but the effect size was small (standardized difference 0.027, 95% CI −0.023 to 0.077). Those travelling in groups were less likely to be vaccinated than other travellers, although effect size was small (standardized difference 0.092, 95% CI 0.041 to 0.142). These results were confirmed on univariate and multivariate analyses (Table 2) with the exception of the finding that people travelling for work/business were more likely to be vaccinated than those travelling for other reasons, which did not reach statistical significance in multivariate analysis.
Table 1:
Demographic and travel characteristics of people who presented for pretravel assessment between December 2008 and December 2014, according to pre-exposure rabies prophylaxis coverage
| Characteristic | No. of travellers (% of category)* | Standardized difference (95% CI) | |
|---|---|---|---|
| Pre-exposure prophylaxis n = 1721 |
No pre-exposure prophylaxis n = 35 311 |
||
| Age, mean ± SD; yr | 35.77 ± 15.14 | 32.07 ± 18.20 | 0.205 (0.156 to 0.253) |
| Age, yr | 0.190 (0.142 to 0.238) | ||
| < 18 | 94 (1.5) | 6286 (98.5) | |
| 18–40 | 1044 (5.3) | 18 673 (94.7) | |
| 41–60 | 470 (5.9) | 7502 (94.1) | |
| > 60 | 113 (3.8) | 2835 (96.2) | |
| Missing | 0 (0.0) | 15 (0.04) | |
| Sex | −0.023 (−0.071 to 0.026) | ||
| Female | 941 (4.7) | 18 898 (95.2) | |
| Male | 780 (4.5) | 16 392 (95.4) | |
| Missing | 0 (0.0) | 21 (0.1) | |
| Travel duration, mean ± SD; wk | 16.67 ± 22.81 | 5.18 ± 11.41 | 0.944 (0.893 to 0.996) |
| Travel duration, wk | 1.118 (1.066 to 1.169) | ||
| ≤ 4 | 602 (2.4) | 24 641 (97.6) | |
| 5–12 | 385 (6.8) | 5261 (93.2) | |
| 13–24 | 253 (14.2) | 1525 (85.8) | |
| 25–52 | 248 (21.7) | 894 (78.3) | |
| > 52 | 72 (27.0) | 195 (73.0) | |
| Missing | 161 (9.4) | 2795 (7.9) | |
| Continent | 0.449 (0.398 to 0.499) | ||
| Africa | 274 (3.9) | 6684 (96.1) | |
| Americas | 328 (2.0) | 15 696 (98.0) | |
| Asia | 981 (8.9) | 10 041 (91.1) | |
| Europe | 14 (3.2) | 428 (96.8) | |
| Missing | 124 (7.2) | 2462 (7.0) | |
| Reason for travel | 0.027 (−0.023 to 0.077) | ||
| Tourism | 1103 (4.2) | 25 391 (95.8) | |
| Work/business | 183 (6.4) | 2665 (93.6) | |
| Education/research | 56 (5.5) | 964 (94.5) | |
| Volunteer/aid work | 255 (10.1) | 2268 (89.9) | |
| Visiting friends and relatives | 2 (0.2) | 992 (99.8) | |
| Adoption | 0 (0.0) | 93 (100.0) | |
| Missing | 122 (7.1) | 2938 (8.3) | |
| No. of travellers in group | 0.092 (0.041 to 0.142) | ||
| 1 | 412 (7.4) | 5158 (92.6) | |
| 2 | 636 (5.1) | 11 828 (94.9) | |
| ≥ 3 | 552 (3.4) | 15 763 (96.6) | |
| Missing | 121 (7.0) | 2562 (7.2) | |
Note: CI = confidence interval, SD = standard deviation.
Except where noted otherwise.
Table 2:
Univariate and multivariate analyses of demographic and travel characteristics of travellers who received pre-exposure rabies prophylaxis compared to those who did not
| Variable | OR (95% CI) | |
|---|---|---|
| Univariate analysis | Multivariate analysis | |
| Age, per year of age | 1.011 (1.008 to 1.014) | 1.015 (1.012 to 1.018) |
| Female sex | 1.046 (0.950 to 1.153) | 1.082 (0.969 to 1.209) |
| Travel duration, per week of travel | 1.032 (1.030 to 1.035) | 1.028 (1.026 to 1.031) |
| Continent | ||
| Africa | 1.000 | 1.000 |
| Americas | 0.510 (0.433 to 0.600) | 0.613 (0.512 to 0.734) |
| Asia | 2.383 (2.077 to 2.734) | 3.029 (2.585 to 3.549) |
| Europe | 0.798 (0.462 to 1.377) | 0.867 (0.487 to 1.542) |
| Reason for travel | ||
| Tourism | 1.000 | 1.000 |
| Work/business | 1.581 (1.345 to 1.858) | 1.061 (0.877 to 1.282) |
| Education/research | 1.337 (1.015 to 1.762) | 1.386 (1.015 to 1.894) |
| Volunteer/aid work | 2.588 (2.244 to 2.986) | 3.906 (3.263 to 4.675) |
| Adoption* | – | – |
| Visiting friends and relatives | 0.046 (0.120 to 0.186) | 0.030 (0.004 to 0.213) |
| No. of travellers in group | ||
| 1 | 1.000 | 1.000 |
| 2 | 0.673 (0.592 to 0.765) | 0.994 (0.857 to 1.153) |
| ≥ 3 | 0.438 (0.384 to 0.500) | 0.641 (0.552 to 0.745) |
Note: CI = confidence interval, OR = odds ratio.
No person travelling for adoption received pre-exposure rabies prophylaxis.
Travellers who received the vaccine intradermally were significantly younger than those with intramuscular delivery (mean age 34.6 yr v. 37.2 yr), although the effect size was small (standardized difference −0.171, 95% CI −0.266 to −0.076) (Table 3, Table 4). There was no significant difference in sex or mean duration of travel between the 2 groups in multivariate analysis. More travellers to Asia than to other continents received intradermal injections (standardized difference 0.274, 95% CI 0.174 to 0.373), but this difference was not significant in multivariate analysis. People travelling for work/business and volunteer/aid work more often received intramuscular vaccination, whereas those travelling for tourism more often received intradermal vaccination, with a small to medium effect size (standardized difference −0.361, 95% CI −0.264 to −0.067).
Table 3:
Demographic and travel characteristics according to route of vaccine administration
| Variable | Route; no. of travellers (% of category)* | Standardized difference (95% CI) | |
|---|---|---|---|
| Intradermal n = 941 |
Intramuscular n = 780 |
||
| Age, mean ± SD; yr | 34.60 ± 15.15 | 37.18 ± 15.02 | −0.171 (−0.266 to −0.076) |
| Age, yr | −0.121 (−0.216 to −0.026) | ||
| < 18 | 60 (63.8) | 34 (36.2) | |
| 18–40 | 581 (55.6) | 463 (44.3) | |
| 41–60 | 246 (52.3) | 224 (47.6) | |
| > 60 | 54 (47.8) | 59 (52.2) | |
| Sex | −0.097 (−0.191 to −0.002) | ||
| Female | 535 (56.8) | 406 (43.1) | |
| Male | 406 (52.0) | 374 (47.9) | |
| Travel duration, mean ± SD; wk | 16.85 ± 19.70 | 16.44 ± 26.20 | 0.018 (−0.082 to 0.117) |
| Travel duration, wk | 0.153 (0.053 to 0.253) | ||
| ≤ 4 | 302 (50.2) | 300 (49.8) | |
| 5–12 | 208 (54.0) | 177 (46.0) | |
| 13–24 | 169 (66.8) | 84 (33.2) | |
| 25–52 | 156 (62.9) | 92 (37.1) | |
| > 52 | 32 (44.4) | 40 (55.6) | |
| Missing | 74 (7.9) | 87 (11.2) | |
| Continent | 0.274 (0.174 to 0.373) | ||
| Africa | 129 (47.1) | 145 (52.9) | |
| Americas | 145 (44.2) | 183 (55.8) | |
| Asia | 610 (62.2) | 371 (37.8) | |
| Europe | 6 (42.8) | 8 (57.1) | |
| Missing | 51 (5.4) | 73 (9.4) | |
| Reason for travel | −0.361 (−0.460 to −0.262) | ||
| Tourism | 696 (63.1) | 407 (36.9) | |
| Work/business | 40 (21.8) | 143 (78.1) | |
| Education/research | 38 (67.8) | 18 (32.1) | |
| Volunteer/aid work | 101 (39.6) | 154 (60.4) | |
| Visiting friends and relatives | 0 (0.0) | 2 (100.0) | |
| Missing | 66 (7.0) | 56 (7.2) | |
| No. of travellers in group | −0.166 (−0.264 to −0.067) | ||
| 1 | 212 (51.4) | 200 (48.5) | |
| 2 | 388 (61.0) | 248 (39.0) | |
| ≥ 3 | 285 (51.6) | 267 (48.4) | |
| Missing | 56 (6.0) | 65 (8.3) | |
Note: CI = confidence interval, SD = standard deviation.
Except where noted otherwise.
Table 4:
Univariate and multivariate analyses of demographic and travel characteristics of travellers who received intradermal vaccination compared to those who received intramuscular vaccination
| Variable | OR (95% CI) | |
|---|---|---|
| Univariate analysis | Multivariate analysis | |
| Age, per year of age | 0.989 (0.983 to 0.995) | 0.986 (0.979 to 0.993) |
| Female sex | 1.214 (1.003 to 1.469) | 1.069 (0.857 to 1.577) |
| Travel duration, per week of travel | 1.001 (0.996 to 1.005) | 1.004 (0.999 to 1.009) |
| Continent | ||
| Africa | 1.000 | 1.000 |
| Americas | 0.891 (0.645 to 1.229) | 0.834 (0.584 to 1.193) |
| Asia | 1.848 (1.411 to 2.421) | 1.258 (0.907 to 1.743) |
| Europe | 0.843 (0.285 to 2.494) | 0.396 (0.123 to 1.274) |
| Reason for travel | ||
| Tourism | 1.000 | 1.000 |
| Work/business | 0.164 (0.113 to 0.237) | 0.185 (0.123 to 0.279) |
| Education/research | 1.235 (0.695 to 2.192) | 0.909 (0.496 to 1.663) |
| Volunteer/aid work | 0.384 (0.290 to 0.507) | 0.444 (0.313 to 0.629) |
| Visiting friends and relatives* | – | – |
| No. of travellers in group | ||
| 1 | 1.000 | 1.000 |
| 2 | 1.476 (1.149 to 1.896) | 1.183 (0.887 to 1.577) |
| ≥ 3 | 1.007 (0.780 to 1.300) | 1.281 (0.953 to 1.723) |
Note: CI = confidence interval, OR = odds ratio.
Only 2 people in this category received pre-exposure prophylaxis, both intramuscularly.
Interpretation
Overall, a small proportion (4.6%) of travellers seen at our travel clinic received pre-exposure rabies prophylaxis, even among travellers to highly endemic areas where proper postexposure prophylaxis is often difficult to obtain. The proportion of travellers who received pre-exposure rabies prophylaxis increased substantially after the introduction of intradermal vaccination. During the study period, the number of visits decreased, anecdotally attributed to the worldwide economic recession at the time. Another potential explanation for the decrease is the increasing number of competing private pretravel clinics. The seroconversion rate among travellers who received intradermal vaccination was 99.9%, in keeping with previously reported rates (above 95%).12 No serious adverse events related to vaccination were reported to the clinic, although there was no active surveillance for such complications. Travellers who received pre-exposure prophylaxis were older and had longer travel duration than travellers who did not receive pre-exposure prophylaxis, presumably owing to the increased perception of risk in these groups; financial resources may also have been a factor. Travellers to Asia were more likely to receive pre-exposure prophylaxis. Travellers visiting friends and relatives were infrequently vaccinated. Travellers who chose to receive the vaccine intradermally were younger than those who received it intramuscularly and were more likely to be travelling for tourism.
The observed low rate of pretravel vaccination is consistent with other studies.13,14 Factors contributing to the low uptake of pre-exposure prophylaxis reported in the literature include low perception of benefit, concern about adverse reactions and belief that vaccination is not necessary,14 but the main barrier to vaccination cited by travellers was cost.13
Recognized risk factors for animal-associated rabies exposure in travellers include travel to Southeast Asia, India and North Africa, young age and travelling for tourism.15 Many cases of rabies exposure have occurred early on in the travel period in the setting of short travel duration.1,15 However, Dolan and colleagues reported that travellers seeking pretravel vaccination were more likely to be travelling for longer periods.14 In our study, younger people were less likely to receive pre-exposure prophylaxis. They were more likely to receive intradermal than intramuscular vaccination. Review of confirmed rabies cases among travellers reveals that the population of travellers visiting friends and relatives is at heightened risk.15,16 This confirms previously described problems with acceptance of preventive measures in this group, despite attendance at a travel clinic.17 Tourists, another at-risk group, were also more likely to receive intradermal vaccination in our study. Those travelling for work/business and volunteer/aid work more often received intramuscular vaccination, possibly because vaccine-related costs were frequently assumed by third parties. Our results support the hypothesis that the reduced cost associated with intradermal vaccination for pre-exposure rabies prophylaxis may allow vaccination of younger travellers and tourists, 2 groups known to be at heightened risk for rabies exposure.
Strengths and limitations
A strength of our study is the large number of cases from a single clinic analyzed, which minimized the problem of demographic variability. Limitations include its retrospective nature and absence of information on the reasons for not receiving pre-exposure prophylaxis (e.g., prior immunity, vaccination not indicated, patient preference, medical contraindication or insufficient time before travel). Other covariates of interest that were not available included occupation, income level, comorbidities (e.g., immunocompromising conditions), previous travel to rabies-endemic countries and type of destination (rural v. urban). However, our study included several covariates, such as age, reason for travel, duration and destination of travel, that are recognized as key factors in rabies vaccination uptake.
Conclusion
We found an increase in rates of pre-exposure rabies prophylaxis after the implementation of intradermal vaccination at a large travel clinic. Provision of a weekly clinic where many travellers can be vaccinated by trained nurses during a 6-hour period has provided a lower-cost alternative for pre-exposure prophylaxis in our setting. Moreover, intradermal injection appeared to improve the acceptance of pre-exposure prophylaxis among younger travellers and those travelling for tourism, possibly because of reduced cost. With a seroconversion rate of 99.9% in our series, intradermal vaccination for pre-exposure prophylaxis is a reliable alternative to intramuscular vaccination. Its use should continue to be promoted in an attempt to increase pre-exposure rabies prophylaxis coverage among at-risk travellers.
Supplementary Material
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Sapha Barkati and Jean Vincelette conceived the study, and Jean Vincelette, Gaétan Laplante, Jo-Anne Duchesne and Sapha Barkati designed it. Jean Vincelette, Gaétan Laplante, Jo-Anne Duchesne and Sapha Barkati acquired the data. Sapha Barkati analyzed the data, and Ling-Yuan Kong, Jean Vincelette, Michael Libman and Sapha Barkati contributed to data interpretation. Ling-Yuan Kong drafted the manuscript, overseen by Sapha Barkati. Jean Vincelette, Gaétan Laplante, Jo-Anne Duchesne, Michael Libman and Sapha Barkati critically revised the manuscript for important intellectual content. All of the authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/6/2/E168/suppl/DC1.
References
- 1.Gautret P, Harvey K, Pandey P, et al. GeoSentinel Surveillance Network. Animal-associated exposure to rabies virus among travelers, 1997–2012. Emerg Infect Dis. 2015;21:569–77. doi: 10.3201/eid2104.141479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leder K, Torresi J, Libman MD, et al. GeoSentinel Surveillance Network. GeoSentinel surveillance of illness in returned travelers, 2007–2011. Ann Intern Med. 2013;158:456–68. doi: 10.7326/0003-4819-158-6-201303190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabies vaccine. Ottawa: Public Health Agency of Canada; [accessed 2017 Aug 11]. Page 18 Canadian Immunization Guide: Part 4 — active vaccines. [updated January 2015]. Available: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-18-rabies-vaccine.html. [Google Scholar]
- 4.Christiansen AH, Rodriguez AB, Nielsen J, et al. Should travellers to rabies-endemic countries be pre-exposure vaccinated? An assessment of post-exposure prophylaxis and pre-exposure prophylaxis given to Danes travelling to rabies-endemic countries 2000–12. J Travel Med. 2016;23 doi: 10.1093/jtm/taw022. [DOI] [PubMed] [Google Scholar]
- 5.Madhusudana SN, Mani RS. Intradermal vaccination for rabies prophylaxis: conceptualization, evolution, present status and future. Expert Rev Vaccines. 2014;13:641–55. doi: 10.1586/14760584.2014.901893. [DOI] [PubMed] [Google Scholar]
- 6.Mills DJ, Lau CL, Fearnley EJ, et al. The immunogenicity of a modified intradermal pre-exposure rabies vaccination schedule — a case series of 420 travelers. J Travel Med. 2011;18:327–32. doi: 10.1111/j.1708-8305.2011.00540.x. [DOI] [PubMed] [Google Scholar]
- 7.Warrell MJ. Intradermal rabies vaccination: the evolution and future of pre-and post-exposure prophylaxis. Curr Top Microbiol Immunol. 2012;351:139–57. doi: 10.1007/82_2010_121. [DOI] [PubMed] [Google Scholar]
- 8.Recuenco S, Warnock E, Osinubi MOV, et al. A single center, open label study of intradermal administration of an inactivated purified chick embryo cell culture rabies virus vaccine in adults. Vaccine. 2017;35:4315–20. doi: 10.1016/j.vaccine.2017.06.083. [DOI] [PubMed] [Google Scholar]
- 9.Wieten RW, Leenstra T, van Thiel PP, et al. Rabies vaccinations: Are abbreviated intradermal schedules the future? Clin Infect Dis. 2013;56:414–9. doi: 10.1093/cid/cis853. [DOI] [PubMed] [Google Scholar]
- 10.Khawplod P, Jaijaroensup W, Sawangvaree A, et al. One clinic visit for pre-exposure rabies vaccination (a preliminary one year study) Vaccine. 2012;30:2918–20. doi: 10.1016/j.vaccine.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Protocole d’immunisation du Québec, édition mai 2013. Section 10.7 — Rage : vaccin contre la rage. Québec: ministère de la Santé et des services sociaux; 2013. p. 407. [Google Scholar]
- 12.Lau C, Sisson J. The effectiveness of intradermal pre-exposure rabies vaccination in an Australian travel medicine clinic. J Travel Med. 2002;9:285–8. doi: 10.2310/7060.2002.30088. [DOI] [PubMed] [Google Scholar]
- 13.Piyaphanee W, Shantavasinkul P, Phumratanaprapin W, et al. Rabies exposure risk among foreign backpackers in Southeast Asia. Am J Trop Med Hyg. 2010;82:1168–71. doi: 10.4269/ajtmh.2010.09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolan SB, Jentes ES, Sotir MJ, et al. Pre-exposure rabies vaccination among US international travelers: findings from the global TravEpiNet consortium. Vector Borne Zoonotic Dis. 2014;14:160–7. doi: 10.1089/vbz.2013.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautret P, Parola P. Rabies in travelers. Curr Infect Dis Rep. 2014;16:394. doi: 10.1007/s11908-014-0394-0. [DOI] [PubMed] [Google Scholar]
- 16.Carrara P, Parola P, Brouqui P, et al. Imported human rabies cases worldwide, 1990–2012. PLoS Negl Trop Dis. 2013;7:e2209. doi: 10.1371/journal.pntd.0002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leder K, Tong S, Weld L, et al. GeoSentinel Surveillance Network. Illness in travelers visiting friends and relatives: a review of the GeoSentinel Surveillance Network. Clin Infect Dis. 2006;43:1185–93. doi: 10.1086/507893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.