Abstract
Jasmonic acid (JA) and its derivatives called jasmonates (JAs) are lipid-derived signalling molecules that are produced by plants and certain fungi. Beside this function, JAs have a great variety of applications in flavours and fragrances production. In addition, they may have a high potential in agriculture. JAs protect plants against infections. Although there is much information on the biosynthesis and function of JA concerning plants, knowledge on these aspects is still scarce for fungi. Taking into account the practical importance of JAs, the objective of this review is to summarize knowledge on the occurrence of JAs from fungal culture media, their biosynthetic pathways and the culture conditions for optimal JA production as an alternative source for the production of these valuable metabolites.
Keywords: Oxylipin, Fungi, Submerged fermentation, Jasmonic acid, Culture medium, Metabolic pathway
Introduction
Jasmonic acid (JA) and its derivatives belong to a group of plant hormones called jasmonates (JAs) (Wasternack & Feussner, 2018). They belong to the large group of oxidized lipid signalling molecules, so-called oxylipins (Gerwick, Moghaddam & Hamberg, 1991). In plants, JAs derive either from α-linolenic acid (18:3(n-3)) or raughanic acid (16:3(n-3)) and their major representatives are the isomers (+)-7-iso-JA and (−)-JA. These compounds are widely distributed in algae (Ueda et al., 1991), angiosperms (Wasternack & Hause, 2013) and certain fungi (Hause et al., 2007; Miersch et al., 1993). They belong to the group of phytohormones playing a role as growth inhibitors and regulating plants defence responses (Pieterse et al., 2009; Wasternack et al., 2006).
Methyl jasmonate (MeJA) was firstly isolated as an odoriferous constituent of the essential oil of Jasminun grandiflorum and other plant species (Crabalona, 1967; Demole, Lederer & Mercier, 1962). It is recognized as an important ingredient in high-grade perfumes, cosmetics and in the preparation of detergents, soaps and food aromas with floral notes (Asamitsu et al., 2006; Dhandhukia & Thakkar, 2007a). JA was first isolated as plant growth inhibitor from cultures of the fungus Lasiodiplodia theobromae (synonym Botryodiplodia theobromae) (Aldridge et al., 1971).
JA and MeJA have attracted the attention of plant physiologists since the development of efficient methods for detecting and quantifying metabolites about 35 years ago. The presence of these compounds in different parts of plants was initially correlated with their strong promotion of senescence and inhibition of growth in angiosperms when applied exogenously (Wasternack & Hause, 2002). Although these compounds act as growth inhibitors or senescence promoters at high concentration, they induce the expression of defensive genes at much lower levels. For instance, they promote the synthesis of proteinase inhibitors, enzymes of phytoalexin synthesis, thionins, defensins and the vegetative storage protein genes (Howe & Jander, 2008).
However, JAs play an important role in agriculture nowadays by regulating the defence of plants against pests and pathogens (Gális et al., 2009; Gavin et al., 2012; Hawkins et al., 2007; Heil et al., 2001; Moreira et al., 2019; Rohwer & Erwin, 2008; Sanches et al., 2017; Stout, Zehnder & Baur, 2002; Wasternack, 2014). Their application seems to be in line with the principles of sustainable agriculture since they may be less aggressive to the environment than pesticides and mineral fertilizers (Secatto, 2013).
Furthermore, it has been observed that adding exogenous of MeJA stimulates the production of many secondary metabolites in cell suspension cultures, such as taxane and derivatives from Taxus sp (Yukimune et al., 1996) and camptothecin production from Ophiorrhiza mungos L. (Deepthi & Satheeshkumar, 2017). These metabolites are very promising anticancer drugs in humans (Miller, Neilan & Sze, 2008; Sriram et al., 2005). Although credible evidence on a mechanism of action was missing until recently (Bömer et al., 2020). Studies have been conducted to optimize the production of these substances; focusing on their metabolic pathways, selecting more productive cell lines, optimizing cell culture processes, product purification, and up scaling of the whole process (Bai et al., 2004; Miller, Neilan & Sze, 2008; Onrubia et al., 2013; Syklowska-Baranek et al., 2009; Tabata, 2006; Wilson & Roberts, 2012).
Currently most of the aroma compounds including JAs may be extracted from natural plant sources. However, recent advances in metabolic engineering have generated a great interest to produce these substances from alternative sources (Gupta, Prakash & Gupta, 2015). An alternative and attractive route for producing JAs could be based on microbial biosynthesis and biotransformation. Microorganisms such as bacteria and yeast can be used at variable scales as safe producers of flavours and fragrances (Gill & Valivety, 1997). Most importantly, these microorganisms can be metabolically and genetically modified to enhance the production of the desired metabolites. Moreover, the production of aroma compounds from microbial cultures or their enzyme preparations offers several advantages over traditional methods. The microbial metabolites can be produced in large quantities by using a fermentation process and can give high yields in very good qualities with better product characteristics along with low economical costs (Gupta, Prakash & Gupta, 2015).
Presently there are numerous projects ongoing for sequencing the genomes of ascomycete fungi (http://mycocosm.jgi.doe.gov/pages/fungi-1000-projects.jsf) and one of them is dealing with the JAs producing fungus L. theobromae. From this project, valuable information will be available in the near future that will help to continue the analysis of fungal JA biosynthesis and other related metabolites using a reverse genetic approach. In fact, the lasiodiplodin biosynthetic gene cluster from the genome of L. theobromae strain NBRC 3,1059 was expressed in Saccharomyces cerevisiae strain BJ5464 to obtain a phytotoxic polyketide that inhibited human blood coagulation factor XIIIa, mineral corticoid receptors and prostaglandin biosynthesis (Xu et al., 2014).
Survey Methodology
Scientific reports and patents dealing with the production and properties of JAs are still steadily increasing (Pirbalouti, Sajjadi & Parang, 2014; Wasternack, 2015). However, there are few reports related to the production of JAs by fungi. Therefore, the aim of this review is to discuss the existing reports related to the fungal production of JAs focusing on the type of fungus, biosynthetic pathways, and culture conditions. By screening the publicly available databases Free Patents Online (http://www.freepatentsonline.com/), Google Patents (https://patents.google.com/), Espacent (https://worldwide.espacenet.com/), Google Scholar (https://scholar.google.de/), PubMed (https://www.ncbi.nlm.nih.gov/) and Web of Science (https://apps.webofknowledge.com/), we aimed to cover the current status of the field and apologize to scientists whose work we overlooked.
JAs from Fungi
Lasiodiplodia theobromae is a common phytopathogenic fungus capable of producing JAs at high level as a result of its primary and secondary metabolism (Alves et al., 2008; Eng et al., 2016; Salvatore, Alves & Andolfi, 2020). Although, JA is produced as the main product, other JAs such as 9,10-didehydro JA (9,10-ddh-JA), 11-hydroxy JA and 12-hydroxy JA sulfate (12-HSO4-JA) were formed to a lesser extent (Fig. 1; Table 1) (Eng, 2012; Miersch et al., 1987). Cucurbic acid (CA) that may also be recognized as a phytohormone and synthesized by a so far unknown pathway has been also detected in trace amounts (Eng, 2012; Miersch et al., 1987).
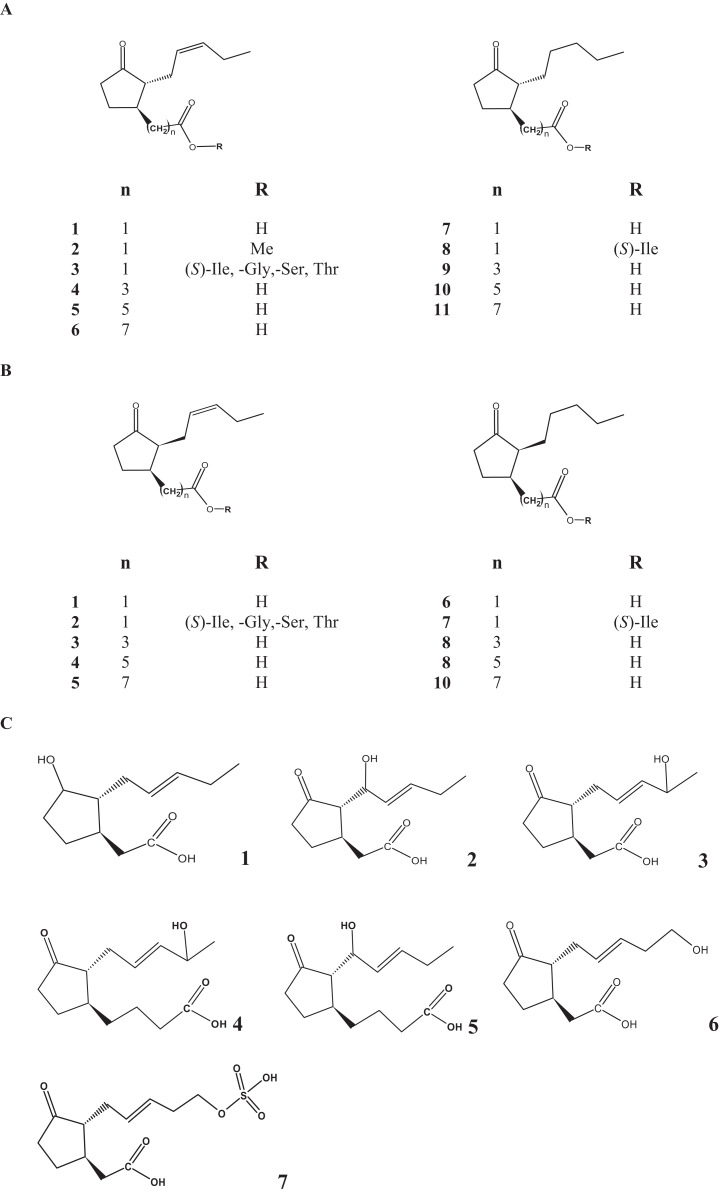
Figure 1. Chemical structure of the most important jasmonates found in fungi.
(A) Trans-compounds: 1, jasmonic acid; 2, jasmonic acid methyl ester; 3, jasmonoyl isoleucine, glycine, serine and threonine conjugates; 4, 3-oxo-2-(2-pentenyl)-1-butyric acid; 5, 3-oxo-2-(2-pentenyl)cyclopentane-1-hexanoic acid; 6, 3-oxo-2-(2-pentenyl)cyclopentane-1-octanoic acid; 7, 9,10-didehydro-JA; 8, 9,10-dihydro-7-iso-jasmonoyl-isoleucine; 9, 3-oxo-2-pentanylcyclopentane-1-butyric acid; 10, 3-oxo-2-pentanylcyclopentane-1-hexanoic acid; 11, 3-oxo-2-pentanyl)cyclopentane-1-octanoic acid. (B) cis-compounds: 1, jasmonic acid; 2, jasmonoyl isoleucine, glycine, serine and threonine conjugates; 3, 3-oxo-2-(2-pentenyl)-1-butyric acid; 4, 3-oxo-2-(2-pentenyl)cyclopentane-1-hexanoic acid; 5, 3-oxo-2-(2-pentenyl)cyclopentane-1-octanoic acid; 6, 9,10-didehydro-JA; 7, 9,10-dihydro-7-iso-jasmonoyl-isoleucine; 8, 3-oxo-2-pentanylcyclopentane-1-butyric acid; 9, 3-oxo-2-pentanylcyclopentane-1-hexanoic acid; 10, 3-oxo-2-pentanyl)cyclopentane-1-octanoic acid (all of them was found with trans- or cis-attached side chains). (C) 1, 2-(-3-hydroxy-2-(-pent-2-en-1-yl)cyclopentyl)acetic acid; 2, 8-hydroxy jasmonic acid; 3, 2-(-2-(-4-hydroxypent-2-en-1-yl)-3-oxocyclopentyl)acetic acid; 4, 4-(2-(4-hydroxypent-2-en-1-yl)-3-oxocyclopentyl)butanoic acid; 5, 4-(-2-(-1-hydroxypent-2-en-1-yl)-3-oxocyclopentyl) butanoic acid; 6: tuberonic acid; 7: 12-hydroxy jasmonic acid sulfate.
Table 1. Occurrence of jasmonic acid and other jasmonates from plants and microorganisms.
| Jasmonates | Plant | Fungi |
|---|---|---|
| Jasmonoyl isoleucine, glycine, serine, threonine, phenylalanine, tyrosine, tryptophan, leucine, isoleucine conjugates | Hamberg & Gardner (1992); Svoboda & Boland (2010) | Cross & Webster (1970); Miersch et al. (1992); Miersch, Bohlmann & Wasternack (1999); Castillo et al. (2014); Cole et al. (2014), Oliw & Hamberg (2017), Oliw & Hamberg (2019) |
| 9,10-didehydro-JA | Hamberg & Gardner (1992) | Eng (2012), Oliw & Hamberg (2019) |
| 9,10-dihydro-7-iso-jasmonoyl-isoleucine | Sembdner, Atzorn & Schneider (1994) | Cross & Webster (1970); Miersch et al. (1992); Miersch, Bohlmann & Wasternack (1999), Oliw & Hamberg (2019) |
| 3-oxo-2-(2-pentenyl)cyclopentane-1-butyric acid, 3-oxo-2-(2-pentenyl)cyclopentane-1-hexanoic acid, 3-oxo-2-(2-pentenyl)cyclopentane-1-octanoic acid | – | Miersch, Bohlmann & Wasternack (1999) |
| Curcurbic acid | Sembdner & Parthier (1993) | Miersch et al. (1987); Eng (2012) |
| 8-hydroxy-jasmonic acid | Hamberg & Gardner (1992) | Miersch, Schneider & Sembdner (1991) |
| 11-hydoxy-jasmonic acid | Wasternack (2006) | Miersch, Schneider & Sembdner (1991) |
| 12-hydoxy-jasmonic acid or tuberonic acid | Hamberg & Gardner (1992); Wasternack (2006) | Miersch, Schneider & Sembdner (1991) |
| 12-hydoxy-jasmonic acid lactone, tuberonic acid-O-β-glucopyranoside, curcubic acid-O-β-glucopyranoside | Hamberg & Gardner (1992) | – |
| 3-oxo-2(1-hydroxy-2’-pentenyl)-cyclopentane-1-butanoic acid, 3-oxo-2(4-hydroxy-2’-pentenyl)-ciclopentane-1-butanoic acid | – | Miersch, Schneider & Sembdner (1991) |
| 12-hydroxy jasmonic acid sulfate | Gidda et al. (2003) | Eng (2012) |
| 4,5 didehydro-7-isojasmonic acid, 3,7-didehydrojasmonic acid, 6-epi-curcubic acid lactone, Homo-7-isojasmonic acid, Dihomo-7-isojasmonic acid, 11-hydroxi-dihomojasmonic acid, 8-hydoxy-dihomojasmonic acid | Hamberg & Gardner (1992); Asamitsu et al. (2006) | – |
| Methyljasmonate | Seo et al. (2001); Cheong & Choi (2003) | Andolfi et al. (2014) |
| cis-Jasmone | Steinegger & Hansel (1988); Koch, Bandemer & Boland (1997) | Matsui et al. (2017) |
Overall 8 hydroxy JAs (11-hydroxy JA, 12-hydroxy JA or tuberonic acid (TA), 8-hydroxy JA, 3-oxo-2(1-hydroxy-2′-pentenyl)-cyclopentane-1-butanoic acid and 3-oxo-2(4-hydroxy-2′-pentenyl)-cyclopentane-1-butanoic acid) were detected in the culture medium and biomass of L. theobromae strain D7/2 growing in a medium containing sucrose, soy flour, corn steep liquor and a mineral salt solution (Miersch, Schneider & Sembdner, 1991). Twenty-two JAs were identified after 8 weeks of culture of Fusarium oxysporum f sp matthiole strain 247.61 grown in liquid potato-dextrose broth under static conditions (Miersch, Bohlmann & Wasternack, 1999). Among the metabolites produced, 9,10-dihydro-7-iso-jasmonoyl-isoleucine, jasmonoyl-isoleucine (JA-Ile), 9,10-dihydro jasmonoyl-isoleucine, 3-oxo-2-(2-pentenyl)cyclopentane-1-butyric acid, 3-oxo-2-(2-pentenyl)cyclopentane-1-hexanoic acid and 3-oxo-2-pentylcyclopentane-1-octanoic acid were identified. The isoleucine conjugates were also produced by the culture of Gibberella fujikuroi (Miersch et al., 1992). Interestingly, F. oxysporum f sp mattiole was unable to accumulate any hydroxylated-JAs as shown for L. theobromae (Miersch et al., 1993).
The occurrence of the JA-serine and JA-threonine conjugates was confirmed in the fermentation broth from Lasiodiplodia sp. strain 2,334 using HPLC-ESI tandem mass spectrometry in negative ionization mode, while JA-glycine and JA-isoleucine conjugates were identified with the same technique but with positive ionization (Castillo et al., 2014). In higher plants, JA amino conjugates are regular constituents accumulating upon sorbitol treatment or wounding (Guranowski et al., 2007; Miersch, Bohlmann & Wasternack, 1999).
While the conjugating enzyme was first isolated form the flowering plant Arabidopsis thaliana (Staswick, Tiryaki & Rowe, 2002), the corresponding peptidase activity was isolated from L. theobromae strain D 7/2 (Hertel et al., 1997). This enzyme was capable of hydrolysing JA-conjugates with α-amino acids. The enzyme was purified by gel filtration, ion exchange and hydrophobic interaction chromatography. It was characterized as glycoprotein with a molecular mass of about 107 kDa and its amidohydrolase activity was very specific with regard to (−)-JA and α-amino acids with (S)-configuration. Therefore, the authors suggested that this fungus may need this enzyme during infection of the host plant for start or modify plant processes, for example, senescence or the release of nutrients, which probably being beneficial for the fungal growth.
JA, MeJA and three JA esters, named lasiojasmonates (botryosphaerilactone A, (3S,4R,5R)-4-hydroxymethyl-3,5-dimethyldihydro-2-furanone and (3R,4S)-botryodiplodin) were detected from culture filtrates of Lasiodiplodia sp. strain BL101 isolated from declining grapevine plants that showed wedge-shaped cankers (Andolfi et al., 2014). However, phytotoxic assays recording necrotic lesions on grapevine and cork oak leaves demonstrated that only JA was found to be active.
The diversity of octadecanoid and jasmonoyl compounds found in the culture filtrate of these fungi raise the question whether the compounds are formed only or at least primarily during the interaction with plants and, if so, what the function of these compounds might be. Evidence suggests that fungal pathogens exploit host oxylipins to facilitate their development via inducing plant lipid metabolism to utilize plant oxylipins in order to promote G-protein-mediated regulation of sporulation and mycotoxin production in the fungus and use of host-ligand mimicry to manipulate plant defence responses from which the fungus benefits (Christensen & Kolomiets, 2011). However, in others cases F. oxysporum colonization remains symptomless or even has beneficial effects on plant growth and/or stress tolerance. Moreover, in pathogenic interactions, a lengthy asymptomatic phase usually precedes disease development. All this suggests for a sophisticated and fine-tuned interaction between F. oxysporum and its host (Di, Takken & Tintor, 2016).
Phytotoxic metabolites were identified in the culture media of six species of Lasiodiplodia isolated in Brazil causing Botryosphaeria dieback of grapevine (Cimmino et al., 2017). It was found by LC-MS, that only four of these strains (L. brasiliense, L. crassispora, L. jatrophicola and L. pseudotheobromae) produced JA. L. brasiliense also synthesized also (3R,4S)-4-hydroxymellein. This was the first report on JA production from these species. Fungal-derived cis-jasmone (CJ) was detected in L. theobromae strain MAFF 306027 (Matsui et al., 2017). These authors carried out studies of the deuterium labelled metabolism of 18:3(n-3)-d5, OPC:4-d6, OPC:6-d6, OPC:8-d6 and cis-OPDA-d5 to MeJA-d5 and/or CJ-d5 in feeding experiments with this strain, revealing that the fungus produced CJ through a single biosynthetic pathway via iso-12-oxo-phytodienoic acid (iso-OPDA). Interestingly, it was suggested that the previously predicted decarboxylation step of 3,7-didehydro JA to afford CJ might be not involved in CJ biosynthesis in L. theobromae (Matsui et al., 2017). However, in plants CJ is synthetized from 18:3(n-3) via two biosynthetic pathways using JA and iso-OPDA as key intermediates (Koch, Bandemer & Boland, 1997).
In spite of the diversity of JAs produced by fungi, JA is the metabolite that has aroused the most interest, due to a higher concentration detected in the culture media, the variety of their applications and their high market values. A large number of fungi JAs with similarities to the JAs of plants could show that probably the biosynthetic pathways and the intermediates involved in fungi and plants are similar.
JA Biosynthetic Pathway
Plants
Many reviews have summarized the developments on the biosynthetic pathway of JA in plants and our knowledge will be briefly summarized in the following section (Agrawal et al., 2004; Creelman & Mullet, 1997; Goepfert & Poirier, 2007; Hamberg & Gardner, 1992; Schaller, Schaller & Stintzi, 2005; Vick & Zimmerman, 1984; Wasternack & Feussner, 2018; Wasternack & Hause, 2002; Wasternack & Hause, 2013).
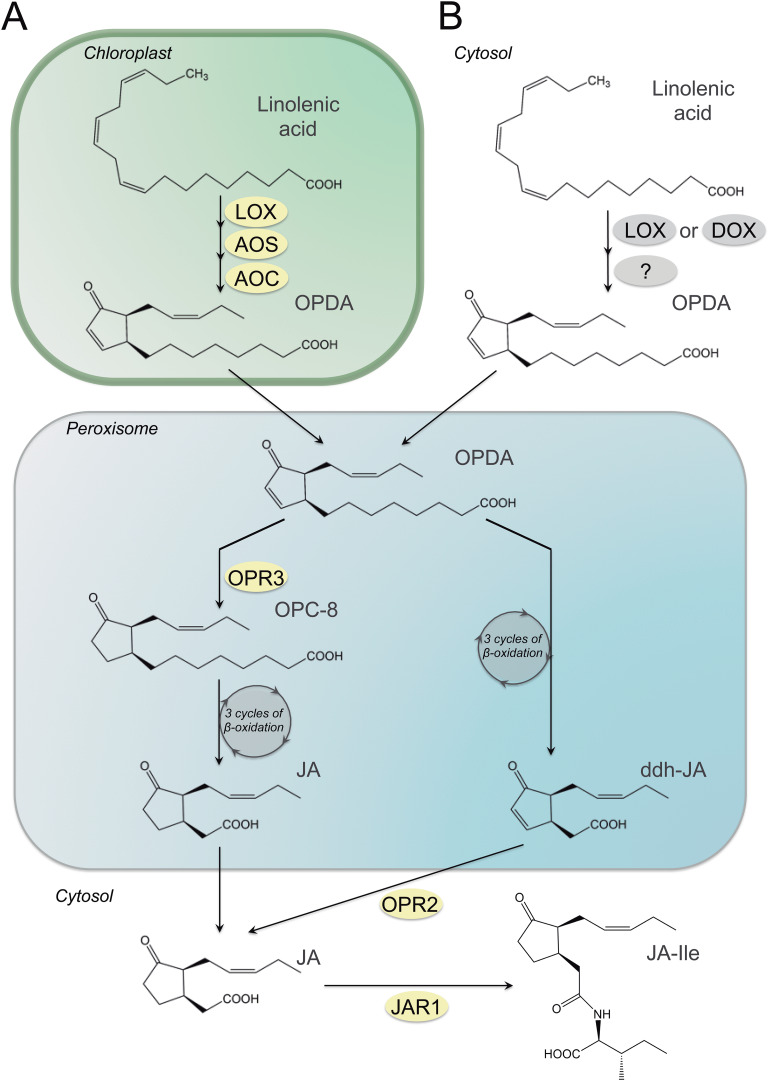
JA biosynthesis in plants starts with the liberation of 18:3(n-3) or 16:3(n-3) from the plastid envelope membranes by lipases (shown in Fig. 2 for 18:3(n-3)). This reaction as well as the next three steps of the pathway are localized in plastid ending with the formation of either cis-(+)-12-oxo-phytodienoic acid (OPDA) or dinor-oxo-phytodienoic acid (dn-OPDA), respectively. This is the result of the sequential action of the enzyme lipoxygenase (LOX), allene oxide synthase (AOS) and allene oxide cyclase (AOC) on 18:3(n-3) or 16:3(n-3). The next steps take place in peroxisomes where OPDA and dn-OPDA are activated and reduced to 10,11-dihydro-12-oxo-phytodienoic acid (OPC-8) and 3-oxo-2(2′-pentenyl)-cyclopentane-1-hexanoic acid (OPC-6) by 12-oxo-phytodienoate reductase isoenzyme 3 (OPR3), respectively. These reactions are followed by two or three rounds of β-oxidation, yielding OPC-6; 3-oxo-2(2′-pentenyl)-cyclopentane-1-butanoic acid (OPC-4) and finally (+)-7-iso-JA that rearranges into the (−)-JA isomer (with an molar ratio of 9:1 for (−)-JA/(+)-7-iso-JA) (Wasternack & Hause, 2013). JA can be further metabolized into its methyl ester (MeJA) by JA carboxyl methyltransferase (JMT) (Cheong & Choi, 2003; Seo et al., 2001), or by conjugation with amino acids (such as leucine and isoleucine) or sugars, respectively (Haroth et al., 2019; Oliw & Hamberg, 2019; Sembdner & Parthier, 1993; Wasternack, 2016).
Figure 2. Synthesis of JA and its amino acid-conjugate JA-Ile in plants and fungi.
Synthesis of JA and its amino acid-conjugate JA-Ile in plants (A) and a scheme for OPDA formation in fungi. (B) Enzymes known only for Arabidopsis thaliana are indicated in yellow circles and those known from fungi are marked by grey circles. Abbreviations: AOC, allene oxide cyclase; AOS, allene oxide synthase; ddh-JA, 4,5-didehydro jasmonic acid; JA, jasmonic acid; JA-Ile, jasmonic acid isoleucine conjugate; JAR1, jasmonoyl amino acid conjugate synthase; LOX, lipoxygenase; OPC:8, 3-oxo-2-(2-pentenyl)-cyclopentane-1-octanoic acid; OPDA, cis-(+)-12-oxo-phytodienoic acid; OPR2,3, 12-oxo-phytodienoic acid reductase.
Recently an alternative pathway was discovered in Arabidopsis thaliana via direct β-oxidation of OPDA leading to formation of 4,5-ddh-JA which is then reduced by OPR2 to JA (Chini et al., 2018). Whether the reactions downstream from OPDA may follow preferentially this pathway or the OPR3-dependent pathway needs to be answered by future research (Han, 2017) (Fig. 2).
Fungi
Knowledge about metabolic pathways leading to the production of JAs by fungi is scarce (Han, 2017). Therefore, more physiological and biochemical studies are required and the existing data will be summarized throughout the next paragraphs.
Starting with the products formed, the same ratio of isomers (−)-JA:(+)-7-iso-JA that was found in plants was measured in the culture filtrate of F. oxysporum strain 247.61 (Miersch, Bohlmann & Wasternack, 1999). By contrast, only the (+)-7-iso-JA isomer was found in a culture of L. theobromae strain D7/2 (Miersch et al., 1987), but later both isomers, with a ratio of ~15:1 and 1:1 in two different experiments in the culture medium filtrate from Lasiodiplodia sp. strain 2,334, were described (Jernerén et al., 2012).
For L. theobromae, it was shown that JA production derived from 18:3(n-3) by using a culture medium that was supplemented either with 13C-sodium acetate or (2H6)-18:3(n-3) (Tsukada, Takahashi & Nabeta, 2010). Appreciable amounts of (13C)-JA and (2H5)-JA were detected in the culture supernatants, and the MeJA of OPDA was detected in mycelium extracts.
The studies in the JAs biosynthesis from F. oxysporum f. sp. tulipae led also the detection of some key intermediates involved in this pathway (Oliw & Hamberg, 2017; Oliw & Hamberg, 2019). This strain released over 230 mg L−1 of (+)-JA-Ile and with about 10 times less 9,10-dihydro-(+)-7-iso-JA-Ile from potato dextrose broth cultures when shaking at 100 rpm and 28 °C after 15 days. Incubation of mycelium of F. oxysporum f. sp. tulipae with radiolabelled d5-18:3(n-3) was able to detected an allene oxide and 12-OPDA derivative. They concluded that allene oxide was formed by a cytochrome P450 or catalase-related hydroperoxidase and 12-OPDA by an allene oxide cyclase (AOC) and, as the plants; this fungus forms JAs with an allene oxide and 12-OPDA as intermediates (Oliw & Hamberg, 2017). However, incubation of mycelia from this fungus with radiolabelled d5-18:2(n-6) was oxidized to 12-oxo-10-phytoenoic acid and finally converted to 9,10-dihydro-JA-Ile in analogy with the reactions of 18:3(n-3) via 12-OPDA into JA-Ile in fungi and plants by LOX, AOS and AOC. Therefore, the fungal AOC has a broader substrate specificity than the plant AOC, but it may form these intermediates from allene oxides by the same reactions (Oliw & Hamberg, 2019). Then, these results suggest that JA is synthesised by these strains from 18:3(n-3) via OPDA and that the enzymes involved may be similar to those governing JA biosynthesis in higher plants. However, there are probably also some differences in the genes and enzymes of the JA pathway between plants and fungi. For example, although higher plants and the fungus G. fujikuroi produce structurally identical gibberellins (GAs) using similar steps, there are important differences in pathways and enzymes involved (Hedden, 2008). These profound differences suggest that higher plants and fungi have evolved their complex biosynthetic pathways to GAs independently and not by horizontal gene transfer.
In fact, the fatty acid composition in Lasiodiplodia sp. strain 2,334 showed that the mycelium contained polyunsaturated C18 fatty acids, including 18:3(n-3) as probable substrate for JA biosynthesis (Eng, 2012; Eng et al., 2016; Jernerén et al., 2012). However, polyunsaturated C16 fatty acids were not detected (Jernerén et al., 2012). OPDA and OPC:4 were also detected in culture filtrates from this fungus as probable intermediates on the JA pathway (Eng, 2012; Eng et al., 2016). In addition, the JA precursors 3-oxo-2-pentylcyclopentane-1-butyric acid, 3-oxo-2-(2-pentenyl)cyclopentane-1-hexanoic and 3-oxo-2-(2-pentenyl)cyclopentane-1-octanoic acid were detected in a culture filtrate from F. oxysporum f sp matthiole strain 247.61 (Miersch, Bohlmann & Wasternack, 1999; Miersch et al., 1993, 1989). These data suggest that an OPR3-dependent JA biosynthesis pathway exists in this fungus (Fig. 2, left peroxisomal pathway).
Studies aiming at identifying single steps in fungal JA biosynthesis have been reported using different exogenously applied substrates (Jernerén et al., 2012), a reverse genetic approach (Brodhun et al., 2013) and enzyme purification (Patel, Patel & Thakkar, 2015). In the first case, a fatty acid dioxygenase activity from three strains of Lasiodiplodia was described (Jernerén et al., 2012). Two of the strains revealed low secretion of JA (~0.2 mg L−1). These strains oxygenated 18:3(n-3) to 5,8-dihydroxy linolenic acid as well as to 9R-hydroperoxy linolenic acid, which was further metabolized by an AOS activity into 9-hydroxy-10-oxo-12Z,15Z-octadecadienoic acid. Analogous conversions were observed with linoleic acid (18:2(n-6)) as a substrate. Studies using (11S-2H)18:2 revealed that the putative 9R-dioxygenase catalysed the stereospecific removal of the 11R hydrogen followed by a suprafacial attack of dioxygen at C-9. Mycelia from these strains contained 18:2 as the major polyunsaturated fatty acid but lacked 18:3(n-3). The third strain however secreted high amounts of JA (~200 mg L−1). It contained 18:3(n-3) as major fatty acid and produced 5,8-dihydroxy linolenic acid from exogenously added 18:3(n-3). From these three strains together no enzyme activity pointing to a JA pathway and being similar to that of higher plants could be identified.
As no sequence information on the L. theobromae genome is yet available, a reverse genetic strategy focused on a 13-LOX from F. oxysporum that may initiate JA production was used as second approach. It was based on using sequences similar to those found from enzymes being part of the JA biosynthetic pathway of plants (Brodhun et al., 2013). One of the sequences called FoxLOX was cloned and expressed in E. coli. FoxLOX was found to be the only non-heme Fe-LOX in the genome, which oxidizes polyunsatured C18 fatty acids to 13S-hydroperoxy derivatives by an antarafacial reaction mechanism where the bis-allylic hydrogen abstraction is the rate-limiting step. Having 18:3(n-3) as substrate, FoxLOX was found to exhibit a multifunctional activity, because the hydroperoxy derivatives formed were further converted to dihydroxy-, keto- and epoxy alcohol derivatives. The identification of FoxLOX as a specific linoleate 13S-LOX could suggest a JA biosynthetic pathway in F. oxysporum, which is analogous to that in plants.
A LOX enzyme was purified from the mycelium of L. theobromae strain MTCC 3,068 by chromatography (Patel, Patel & Thakkar, 2015). It was found that this fungus contains two LOXs isoenzymes, one of 93 kDa (LOX1) and another of 45 kDa (LOX2). The latter being most likely a degradation product of LOX1. Both LOX isozymes oxidized linoleic acid to produce a mixture of 9- and 13-hydroperoxy linoleic acid, optimum pH at 6 and temperature in the range of 40–50 °C. Therefore, both or one these LOX may be another candidate enzyme involved in fungal JA production.
It could be expected that fungal LOX involved in JA pathway contains Fe or Mn as the catalytic metal, whereas animals, plants and prokaryotes express LOX with only Fe (Wennman et al., 2016). Moreover, fungal LOX could have Ile- or the Val-group in the amino acid sequence. The Ile-group has a conserved WRYAK sequence that appears to be characteristics for these enzymes and has a C-terminal amino acid Ile. While the Val-group has a highly conserved WL-L/F-AK sequence that is also found in LOXs of plant and animal origin. Some LOXs have signal sequences implying these LOXs will be expressed extracellular (Heshof et al., 2014).
Therefore, these first data suggest, that JA may be synthesised from 18:3(n-3) via OPDA in fungi (Fig. 2) as was mentioned previously. Since fungi do not have plastids, the reactions leading to the formation of OPDA most likely take place in the cytosol or associated to a membrane leaflet facing the cytosol by LOX, AOS and AOC (Oliw & Hamberg, 2017). Whether this pathway may be initiated by LOX enzymes or other dioxygenases (DOX) is still unclear, just like the identity of the following enzymatic activities. However, the reactions downstream from OPDA may follow a discovered pathway in Arabidopsis thaliana via direct β-oxidation of OPDA leading to formation of 4,5-ddh-JA, which could be reduced by a fungal OPR2 homologue to JA (Chini et al., 2018) or/and the OPR3-dependent pathway needs to be addressed by future research (Han, 2017) (Fig. 2).
Chemical Synthesis of JAs
Chemical synthesis and isolation of JAs from microorganisms and plants started in the 70s of the last century (Aldridge et al., 1971). JA is traditionally isolated from plants; mainly from jasmine and tea flowers. A large number of flowers produce small amounts of essential oils. For instance, it takes about 500 Kg of petals to obtain approximately 1 Kg of rose oil and this is a very expensive and time-consuming process that accounts for the high price of these oils (Dhandhukia & Thakkar, 2007a). Therefore, this is a very expensive and time-consuming process that accounts for the high price of these oils (Dhandhukia & Thakkar, 2007b). Consequently, numerous and different chemical synthesis strategies for obtaining JA, MeJA and other derivatives have been developed. In that way, some of these strategies are summarized below. The synthesis of MeJA and methyl curcubate (MeCA) have been reported by using 2-allylcyclohexan-1,3-dione as starting compound and hydroboration-oxidation followed of seven or eight steps for the first and second product, respectively (Kitahara et al., 1987). Moreover, the same authors improved the total yield for MeJA to up to 20% in twelve reaction steps by improving the stereoselectivity of the hydroboration-oxidation by using 3-hydroxy methylcyclopentanone as starting compound (Kitahara et al., 1991).
Shortly after these reports, racemic 7-substituted derivatives of MeJA have been synthesized (Taapken et al., 1994). 7-Methyl MeJA was also synthesized in enantiomerically pure form in seven steps from the Hajos-Wichert ketone. In addition, the biological activity of the prepared compounds has been investigated for the induction of tendril coiling in Bryonia dioica and the elicitation of the phytoalexin production in Eschscholtzia californica. However, beside 7-methyl MeJA all synthesized compounds showed poor activity in the bioassays (Taapken et al., 1994).
An interesting methodology leading only to racemic MeJA was proposed by Dos Santos et al. (2001) based on the synthon equivalent to a carboxymethyl anion to enones and nitroalkenes, through a 1,4-addition reaction of 2,4,4-trimethyl-2-oxazoline cyanocuprate 3; afforded the (±)-MeJA in 32% overall yield from 2-cyclopenten-1-one.
Suzuki, Inomata & Endo (2004) developed a new method of MeJA and MeCA synthesis using a chiral tricyclic lactone as starting compound via a new type of tandem retro-Diels-Alder-ene reaction activated by a trimethysilyl substituent as the key step, followed at seven reaction steps.
Other authors have dedicated their efforts to the synthesis of β-oxidation intermediates of JA, such as 10,11-dihydro-12-OPDA (OPC:8) and cis-(+)-OPDA by chemical or enzymatic means with good yields (Löwe, Dietz & Gröger, 2020; Nonaka et al., 2010; Takayuki, Michitaka & Yuichi, 2003; Zerbe, Weiler & Schaller, 2007).
JA and TA (Fig. 1C, free fatty acid is shown as compound 6) were synthesized from the key aldehyde, all cis-2-(2-hydroxy-5-vinylcyclopentyl)acetaldehyde, which was in turn prepared stereoselectively from the (1R)-acetate of 4-cyclopentene-1,3-diol through a SN2-type allylic substitution with CH2-CHMgBr followed by Mitsunobu inversion, Eschenmoser–Claisen rearrangement, and regioselective Swern oxidation of the corresponding bis-TES ether. A Wittig reaction of the aldehyde with (PH3P(CH2)Me)+Br- followed by oxidation afforded JA stereoselectivity over the trans isomer (Nonaka et al., 2010). Similarly, TA was synthesized.
Secatto proposed a racemic synthesis of JA involving additional steps to obtain higher yield. This would envisage an application at industrial scale (Secatto, 2013). This synthetic route consisted of seven steps with an overall yield of 30%. The improvement of this route is due to the use of a starting compound without hygroscopic characteristics and no requirement for any pretreatment and easy handling. Moreover, the starting materials (adipic acid and cyclohexane and ethanol as solvents) are not expensive, leading overall to low production costs.
Two macrolactones (JA-Ile-lactones) derived from 12-OH-JA-Ile were synthesized in seven steps with an overall yield of 33% from commercially available MeJA (Jimenez-Aleman et al., 2015a). The biological activity of macrolactones was tested for their ability to elicit nicotine production, a well-known jasmonate dependent secondary metabolite. Both macrolactones showed biological activity, inducing nicotine accumulation to a similar extent as MeJA does in Nicotiana attenuata leaves. Surprisingly, the highest nicotine contents were found in plants treated with the JA-Ile-lactone, which has (3S,7S) configuration at the cyclopentanone ring and is yet not known from natural JAs.
A new synthetic route to JA-Ile-lactones was developed recently using the Z-selective cross-metathesis of (±)-MeJA and 3-butenyl acetate (both compounds are commercially available and inexpensive) resulting in the (±)-1-acetate derivative in excellent yield (>80%) and Z-selectivity (>90%) (Jimenez-Aleman et al., 2015b). Saponification of the (±)-1-acetate derivative (>85% yield) and conjugation to L-Ile resulted in the 1-hydroxy-12-L-Ile derivative. Finally, this derivative was exposed to macrolactonization resulting in enantiomerically pure macrolactones in only three steps. In agreement with the previous studies (Jimenez-Aleman et al., 2015a), these macrolactones also induced the accumulation of nicotine suggesting that these compounds open the possibility of uncoupling defence and growth in plants by using small molecules.
So far, JAs are only accessible today in industry through very tedious multistep synthesis (Chapuis, 2012). Together, the improvements introduced, including enantiodivergent routes that prevent the formation of all possible isomers (Nájera et al., 2020), and with total yields of around 33% are promising. However, further improvements of these multistep synthesis pathways are still necessary in order to increase the overall yield even from cheap starting materials.
Fungi as Producers of JAs
The first report on JA production by microbes was published already 50 years ago (Broadbent, Hemming & Turner, 1968). These authors obtained JA from a culture of L. theobromae in a culture medium containing glucose, glycerol or a mixture of both as carbon source, as well as sodium nitrate, potassium nitrate or ammonium nitrate as nitrogen source. JA reached a concentration of 475 mg L−1 and a productivity of 37 mg L−1 d−1. In order to purify the produced JA, biomass was removed by filtration and the filtrate was acidified and further extracted with ethyl acetate. Three years later, JA biosynthesis was reported in a concentration of 500 mg L−1 and a productivity of 38 mg L−1 d−1 from L. theobromae, using a surface culture in 1 L ceramic vessels with Czapek medium (Aldridge et al., 1971). These authors also observed that the culture supernatant inhibited the growth of higher plants and that the active component was JA. Similar results were obtained by L. theobromae strain D7/2 isolated from orange and cacao residues (Miersch et al., 1987). This strain was grown in a liquid medium based on sucrose, soybean meal, corn steep liquor and salt solution with a JA concentration and productivity of 500 mg L−1 and 71 mg L−1 d−1, respectively.
The same authors performed a screening for JA production using 46 species of Ascomycetes and Basiodimicetes belonging to 23 different genera (Agrocybe, Aspergillus, Collybia, Coprinus, Cunninghamella, Daedalea, Fomes, Fusarium, Gleooporus, Homoconis, Marasmius, Mucor, Mycena, Paecilomyces, Phellinus, Penicillium, Pleurotus, Polyporus, Rhizoctonia, Stropharia, Talaromyces, Trametes and Trichoderma) that were grown under the same conditions as L. theobromae. In this screening trial, Collibya, Coprinus and Mycena were the best producers of JA. However, the JA concentrations were four to eight times lower than those found with L. theobromae cultures (Miersch et al., 1993). In addition, some mutants of G. fujikuroi were also able to produce JA in culture supernatants with a maximum amount of 2.5 mg L−1 (Miersch et al., 1992). Similarly, mycorrhizal fungi such as Laccaria laccata and Pisolithus tinctorius were identified as JA producers but again only in trace amounts (Miersch, Regvar & Wasternack, 1999).
A mutant approach was applied to obtain better JA producers of L. theobromae (Patel & Thakkar, 2015). The mutants were generated using ethylmethanesulfonate and two mutants were isolated having the capacity to produce JA with 70 mg L−1 and 78 mg L−1 compared to wild type 32 mg L−1.
However, the highest rates for JA production were described however for Diplodia gossypina strain ATCC 10936 (Farbood et al., 2001). Under optimal culture conditions, the JA concentration and productivity were 1,200 mg L−1 and 171 mg L−1 d−1, respectively. This study even included the up scaling of JA production up to a volume of 150 L. Therefore, the microorganisms that provide the highest potential for JA production are the ascomycete fungi from strains of Diplodia and Lasiodiplodia genera.
Culture Conditions for JA Production
Although the annual demand for JA increases primarily for applications in perfume production and flavourings (Dhandhukia & Thakkar, 2007a), there are still only a few reports published related to the practical aspects of the commercial production of JA as shown in Table 2 and most of the information and strains is still only published in patents.
Table 2. Studies of jasmonic acid production by microbial way reported in the literature.
| Microorganism | Experimental Procedure | Yield (mg L−1); Productivity (mg L−1 d−1) | References |
|---|---|---|---|
| Lasiodiplodia theobromae strain S22L | Surface culture, ceramic vessels (1L) | 475; 37 | Broadbent, Hemming & Turner (1968) |
| L. theobromae | Surface culture, ceramic vessels (1L) | 500; 38 | Aldridge et al. (1971) |
| L. theobromae strain D7/2 | Surface culture (static), Erlenmeyer 400 mL | 500; 71 | Miersch et al. (1984, 1987) |
| L. theobromae strain 715 | Surface culture (static), Erlenmeyer 250 mL | 1,200; 120 | Altuna et al. (1996) |
| Lasiodiplodia sp. strain 2334 | Surface culture (static), Erlenmeyer 100 mL | 900; 90 | Eng, Gutiérrez-Rojas & Favela-Torres (1998) |
| Fusarium oxysporum f sp matthiolae strain 247.61 | Surface culture (static), Bottle (2,000 mL) | 0.5; 0.01 | Miersch, Bohlmann & Wasternack, 1999 |
| L. theobromae strain 715 | Surface culture (static), Erlenmeyer 500 mL | 1,000; 83 | Almeida et al. (1999) |
| Diplodia gossypina strain ATCC 10936 | Erlenmeyer 500 mL, agitation velocity 200 rpm | 1,200; 171 | Farbood et al. (2001) |
| Reactor 150 L, agitation velocity 150 rpm | 120; 17 | ||
| D. gossypina strain ATCC 10936 | Erlenmeyer 500 mL, agitation velocity 200 rpm | 600; 86 | Inho, Kyoungju & Yonghwi (2006) |
| L. theobromae strain MTCC 3068 | Surface culture (static), Erlenmeyer 250 mL | 299; 43 | Dhandhukia & Thakkar (2007a) |
| L. theobromae strain RC1 | Surface culture (static), Erlenmeyer 250 mL | 550; 56 | Eng, Gutierrez-Rojas & Favela-Torres (2008) |
| Lasiodiplodia sp. strain 2334 | Surface culture (static), Erlenmeyer 250 mL | 1,270; 127 | Eng (2012) |
| Botryosphaeria rhodina | Surface culture (static) Erlenmeyer 250 mL | 352; 25 | Dos Santos et al. (2014) |
| L. theobromae | Surface culture (static), Erlenmeyer 250 mL | 784; 56 | Laredo-Alcalá et al. (2016) |
| Solid state fermentation, Erlenmeyer 125 mL | 23 mg g−1; 2 mg g−1 d−1 | ||
| L. theobromae strain 2334 | Surface culture (static), Erlenmeyer 500 mL | 1,250; 139 | Eng et al. (2016) |
| F. oxysporum f. sp. tulipae | Shaking culture (100rpm) Erlenmeyer 250–500 mL | 230; 15 | Oliw & Hamberg, 2017 |
| L. theobromae strain 3C | Surface culture (static), Erlenmeyer 250 mL | 565; 38 | Laredo-Alcalá et al. (2016) |
The ability of fungi to produce JA varies between strains from 1 mg L−1 to 1,300 mg L−1 of JA, even of the same species (Dhandhukia & Thakkar, 2007a; Eng, Gutiérrez-Rojas & Favela-Torres, 1998; Farbood et al., 2001). Therefore, the first strains of L. theobromae or D. gossypina were screened for JA production in order to select strains with higher productivity (Altuna et al., 1996; Eng, 2012; Farbood et al., 2001).
Batch fermentation in static conditions using a stationary Fernbach flask culture, an aseptic stationary tray culture or Erlenmeyers flasks were tested between 5 and 10 days at temperatures between 27 °C and 30 °C and slightly acidic initial pH values between 5 and 6 of the culture medium (Altuna et al., 1996; Farbood et al., 2001; Miersch et al., 1987). As a carbon source for producing JA, soybean meal, citrus pulp, corn steep liquor and milk serum were used and supplemented with oilseed meal, which can supply sources of protein, minerals and water soluble vitamins (Miersch et al., 1987). However, using more complex media had the drawback of needing more complicated processes for purifying JA for some applications such as in perfumery, a removal of malodorous compounds and allergens is required. Another drawback is that the composition thereof is not constant and therefore results may be difficult to reproduce. Primarily synthetic media were used that are based on sucrose or glucose as carbon source and mineral salts such as potassium nitrate as nitrogen source, with the addition of monobasic potassium phosphate, ammonium molybdate, and the sulfates of magnesium, iron, zinc and copper, respectively (Almeida et al., 1999; Miersch et al., 1987). Also only one type of carbon source (glucose or sucrose) can be used for JA production (Eng, Gutiérrez-Rojas & Favela-Torres, 1998). Then, catabolic repression is not evident as it occurs in other biosynthic routes of secondary metabolites in fungi. However, Farbood et al. used glucose or a mixture of glucose and xylose in their studies of strain selection and JA production with D. gossypina strain ATCC 10936 (Farbood et al., 2001). Broadbent, Hemming & Turner (1968) reported that sucrose, glucose, glycerol or mixtures of these carbon sources allowed a higher production of JA with L. theobromae strain S22L than the use of a single carbon source. Therefore, it is possible to use either a single or a mixed carbon source depending on the strain used.
The same was true in case of the nitrogen source when ammonium salts were replaced by nitrate salts (Eng, Gutiérrez-Rojas & Favela-Torres, 1998; Günther et al., 1989). The consumption of ammonium ions by the fungus during its growth very likely generates an acid pH in the culture medium, which could be responsible for the slow growth and therefore to the low JA production.
An early study showed that the addition of an inductor is not required to produce JA in synthetic culture medium (Miersch et al., 1987). In fact, the addition of 18:3(n-3) (1 µM) (Dos Santos et al., 2014) or edible oil (1 g L−1) (Eng, 1996) as substrate and fatty acid source for JA synthesis to the culture media of Botryosphaeria rhodina strain Kinf 3.1 or Lasiodiplodia sp. strain 2,334, respectively, was not significantly favoured in the JA production. However, the addition of yeast extract and/or soy peptone as a source of vitamins and cofactors to the culture medium stimulated the rate of JA biosynthesis (Dhandhukia & Thakkar, 2008; Eng, 2012; Eng, Gutierrez-Rojas & Favela-Torres, 2008; Farbood et al., 2001). In fact, the addition of these nutrients could cause a positive effect on the growth of these fungi and may promote an early onset of JA synthesis by decreasing the time at which the maximal production and stationary phase is reached.
Under these culture conditions JA production took place at the late exponential growth phase or stationary phase showing a behaviour similar to the accumulation of secondary metabolites (Eng et al., 2016) and may only partially be associated with the growth phase of the culture (Dhandhukia & Thakkar, 2007a). Using these optimized culture conditions JA production levels reached 500–1,300 mg L−1 and productivities of 28–170 mg L−1 d−1 (Table 2).
During static conditions, some Lasiodiplodia strains formed a mat on the surface of the culture medium (Eng, 1996). Therefore, the effect of the available surface area by increasing the vessel diameter may be another critical aspect for JA production. This was confirmed by a study on JA production by L. theobromae strain MTCC 3068 using the same amount of culture medium with Erlenmeyer flasks of 250, 500 and 1,000 mL in which the authors could show, that increasing the surface area of the culture lead to an increase of the JA yield (Dhandhukia & Thakkar, 2007a). In another study, the surface of the culture (100–500 mL) was simultaneously increased with the volume of the culture medium (25–100 mL). Here, JA production was highest at the largest surface area in combination with the highest volume of culture medium (Eng et al., 2016). However, an increase of the flask volume to 5 or even to 50 L and for the culture medium volume up to 10 L did not lead to further increases in JA yield (Eng, 2012), because the fungal mycelium grows on the surface of the medium. An increase in its volume limits only the diffusion of the nutrients from the culture medium to the fungal mycelium thereby limiting growth and JA production.
However, scaling up JA production in a fermenter or in a shaking incubator at 190 rpm and 30 °C with a dissolved oxygen saturation in the culture medium of up to 150 L (Farbood et al., 2001) as well as using a fixed inoculation ratio of 0.5 g L−1 of dry biomass of culture medium was shown to further improve JA yield (Miersch et al., 1987). In addition, it was of advantage to use homogenized mycelium and not spores (Almeida, Altuna & Michelena, 2001; Dos Santos et al., 2014; Dos Santos, Innocentini & Lourenco, 2014). Agitation turned out to be another critical aspect for JA production, because shaking speeds above 200 rpm lead to increased synthesis of extracellular polysaccharides that visibly increased the viscosity of the culture medium (Selbmann, Crognale & Petruccioli, 2004), which had a negative effect on JA production (Eng, Gutiérrez-Rojas & Favela-Torres, 1998; Miersch et al., 1987). These results suggest that stronger agitation of the culture lead to a higher concentration of dissolved oxygen in the medium and as a consequence, this microorganism produced more extracellular polysaccharides instead of JAs. In fact, Selbmann et al. confirmed these results by selecting Botryosphaeria rhodina DABAC-P82 for the production of exopolysaccharides using this strategy (Selbmann, Crognale & Petruccioli, 2004; Selbmann, Stingele & Petruccioli, 2003). This fungal strain was capable of producing up to 17.7 ± 0.8 g L−1 of an exopolysaccharide after only 24 h. The production of this exopolysaccharide was even further increased in stirred fermenters or with propeller turbines by increasing the stirring speed from 300 to 500 rpm. These extracellular polysaccharides may be used by these fungi to form capsules, which may protect them against the stress caused by the agitation of the culture medium.
JA was also obtained by solid-state fermentation (SSF) from Lasiodiplodia sp. strain 2334 using columns with sugar cane bagasse impregnated as support, at 30 °C and with a similar culture medium that was used in liquid fermentation. JA productivity was two times higher in SSF probably due to growth conditions that were more similar to the natural environment of this fungus (Eng, 1996) and monosaccharides released by the fungal cellulolytic activity on the substrate in the stationary late phase. Using similar conditions, JA productivity of a strain of B. theobromae isolated from cacao tissue was reported to be three times higher by SSF as with submerged fermentation (Laredo-Alcalá et al., 2016). Therefore, the most promising approach is to continue studying this fermentation method taking into account the benefits of SSF with low costs and due to the absence of free water, small fermenters can be used and therefore less effort is required for the separation processes. The main drawback so far is its control and dissipating the metabolic heat produced in the reactors, which potentially could reduce the fungal activity.
Finally, it should be noted that JA production was possible with D. gossypina strain ATCC 10936 in stirred fermenters of 150 L at an agitation velocity of 450 rpm, but productivity decreased at about two times with respect to the production in 500 mL Erlenmeyer flasks agitated at a speed of 200 rpm (Farbood et al., 2001).
The progress made in the production of JA by fungi is undoubted. However, the development of new studies starting with the selection and mutagenesis of the producer strains, culture media and conditions to scale up production, the biosynthesis pathway and the genes involved and the evaluation of effectiveness of their applications are essential and should continue to be explored.
The broader use of JAs has been limited by the high costs of commercial production so far. The production of JAs from fungi, via fermentation, has emerged as a promising alternative to reduce production costs. The use of simple and relatively inexpensive culture conditions is an attractive strategy to bring JA production with these strains to industrial levels. It may allow to significantly reduce the production costs which makes the development of future bioproducts more attractive.
Patents
There is a growing number of patents describing the production and application of JAs since the 60’s and their quantity have increased during the last decades showing a growing interest in this substance class (Pirbalouti, Sajjadi & Parang, 2014). In the beginning patents dealing with the isolation, detection and culture conditions for production of JAs in microorganisms such as L. theobromae were published (Aldridge et al., 1971; Broadbent, Hemming & Turner, 1968; Farbood et al., 2001; Günther et al., 1989; Miersch et al., 1984; Yoshihara, 2007).
Other topics deal with agricultural applications of JAs in order to improve plant yield most likely by inducing plants defence against herbivores and pathogens, including the cultivation of algae and edible fungi (Beibei et al., 2019; Beibei et al., 2018; Dathe et al., 1990; Guo et al., 2019; Ryan & Farmer, 1991; Wang et al., 2020b; Zhang et al., 2020). Recently these effects were combined with new formulations for JAs in water in combination with herbicides, pesticides, bioactive or biological seed treatments and semiochemicals (Deng, Lan & Zhao, 2020; Lulai, Orr & Glynn, 1995; Marks, 2012; Pirbalouti, Sajjadi & Parang, 2014; Wang et al., 2020a, 2020c). The application of MeJA to grapes in order to improve the quality of machine-harvested raisin grapes allowed the harvest without damaging the fruit or plants associated with traditional mechanical harvesting and thereby eliminating the need for expensive hand picking (Pirbalouti, Sajjadi & Parang, 2014). Meanwhile a method was also reported for improving the turf grass quality (Mcelroy, 2011) and others to applicate in plant leaf blade flavonoids that permit the accumulation of terpene lactones using MeJA (Wang, Guo & Feng, 2019). There is an only one patent claiming the use of a JA extract to inhibit the growth of the bacterium Leuconostoc sp. and thereby dextran production during the juice processing of the sugar cane industry (Michelena et al., 2010).
Nowadays, patents about JAs have expanded to medicinal, cosmetic and flavouring applications (Pirbalouti, Sajjadi & Parang, 2014). During the last 20 years, the vast majority of studies and inventions claim that JA, MeJA and dihydro-MeJA have anticancer activity against various forms of cancer (Fleischer & Fingrut, 2007; Fleischer, Herzberg & Kashman, 2012; Fleischer et al., 2010; Guzel et al., 2019; Herzberg, Harel & Mang, 2006; Martinez et al., 2010; Pirbalouti, Sajjadi & Parang, 2014). However, convincing evidence that supports these claims is still missing. Additional patents focus on improving the convenience and safety of their administration and on expanding the applications for the treatment, for example, the use of nanocarriers in order to increase the solubility of JAs, because these compounds are poorly water-soluble, not allowing an application by an intravenous route without an efficient nanostructured carrier system. A major problem is still that they are not easily delivered to cancerous cells, because they often degraded before they reach the tumour cells (Da Silva et al., 2014; Katona, Vempati & Savir, 2015; Lopes, 2014). JAs are also used as skin care and hair care products, for example, for treating hair, the scalp, dry and greasy skin (Bababunmi, 2005; Broady, 2012; Dalko, 2006; Deyong, Mengjiao & Naixing, 2019; Malik, 2006; Pirbalouti, Sajjadi & Parang, 2014) and also in Bladder dysfunction (Pirbalouti, Sajjadi & Parang, 2014). Jasmone, MeJA, CJ and γ-jasmolactone are considered as the main odorous substances in the essential oil of jasmine flowers (jasmine oil) used in perfumes (Smets et al., 2020; Steinegger & Hansel, 1988). Other authors described the use of dihydro-MeJA as enhancer or imparter fragrances in or to a perfume composition, perfumed articles and colognes (Boden, Fujioka & Hanna, 1993). In addition, JAs are also used to flavour fruit beverages, confectionery like sweets and candy, cigarette, food products like cocoa and tooth cleansing products like toothpaste (Hurst et al., 2015; Hurst et al., 2011; Johnson, Paul & Favara, 1977; Mookherjee et al., 1981).
Conclusions
Beside plants, fungi are additional producers of JAs, and those providing the highest yields for JA production are Ascomycetes from the genus Lasiodiplodia and Diplodia.
There is a great of diversity of JAs that are produced by fungi, but JA, MeJA and dihydro-MeJA have studied the most because of their numerous applications. In the fungus L. theobromae, plant-type jasmonate derivatives such as hydroxy and amino acids conjugates, as methyl and sulfate ester occur. In addition, derivatives being specific for fungi such as hydroxy-lactones, didehydro or dihomo-JAs are found. However, until today the function of JAs being produced by these fungi is not known. However, it can be assumed that they are involved regulating the interaction between plants and microorganisms.
Strategies to produce JAs via microbial chemical synthesis suffer still from low yields. In case of fungal production strategies, a the number of promising strains from the genus Lasiodiplodia and Diplodia have been selected, but they suffer from producing jasmonate mixtures and strategies for purifying the elaborated product are needed to develop an industrial processes for JA production.
The knowledge gained so far provides a promising basis for additional research on the interaction of these fungi with plants, the chemical nature of JA biosynthesis in fungi, mechanisms that regulate this pathway in fungi and design simpler and viable technological strategies to produce JAs in these fungi in order to satisfy the high demand for these products will be the next challenges in this field of research.
It is very likely that applications for JAs continue to increase in the biomedical, cosmetic, food and in agricultural sector, as soon as a better understanding of their biosynthesis and mode of action and their molecular interactions with biological targets will became available.
Abbreviations
- 12-HSO4-JA
12-hydroxy jasmonic acid sulfate
- 16:3 (=16:3(n-3))
raughanic acid
- 18:2 (=18:2(n-6))
linoleic acid, hexadecatrienoic acid
- 18:3 (=18:3(n-3))
α-linolenic acid
- ABA
abscisic acid
- AOC
allene oxide cyclase
- AOS
allene oxide synthase
- CA
curcurbic acid
- MeCA
methyl curcurbate
- CJ
cis-jasmone
- ddh-JA
didehydro jasmonic acid
- dn-OPDA
dinor-12-oxo-phytodienoic acid
- GA3
gibberellic acid
- JA
jasmonic acid
- JAs
Jasmonates
- JA-Leu
jasmonoyl leucine
- JMT
JA carboxyl methyltransferase
- MeJA
methyl jasmonate
- LOX
lipoxygenase
- OPC-4
3-oxo-2(2′-pentenyl)-cyclopentane-1-butanoic acid
- OPC-6
3-oxo-2(2′-pentenyl)-cyclopentane-1-hexanoic acid
- OPC-8
10,11-dihydro-12-oxo-phytodienoic acid
- OPDA
12-oxo-phytodienoic acid
- SSF
solid state fermentation
- TA
tuberonic acid
Funding Statement
This work was supported by Deutscher Akademischer Austauschdienst (DAAD) through a short stay scholarship to Felipe Eng. Studies in the lab of Ivo Feussner were financially supported by the Deutsche Forschungsgemeinschaft (DFG) in frame of the GRK-1422. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Ivo Feussner is an Academic Editor for PeerJ.
Author Contributions
Felipe Eng analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Jorge Erick Marin analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Krzysztof Zienkiewicz analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Mariano Gutiérrez-Rojas analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Ernesto Favela-Torres analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Ivo Feussner analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Patent Disclosures
The following patent dependencies were disclosed by the authors:
All patents are cited in the reference section.
Data Availability
The following information was supplied regarding data availability:
The research in this article did not generate any data or code; this is a literature review.
References
- Agrawal et al. (2004).Agrawal GK, Tamogami S, Han O, Iwahashi H, Rakwal R. Rice octadecanoid pathway. Biochemical and Biophysical Research Communications. 2004;317(1):1–15. doi: 10.1016/j.bbrc.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Aldridge et al. (1971).Aldridge DC, Galt S, Giles D, Turner WB. Metabolites of Lasiodiplodia theobromae. Journal of the Chemical Society C: Organic. 1971;1971:1623–1627. doi: 10.1039/j39710001623. [DOI] [Google Scholar]
- Almeida, Altuna & Michelena (2001).Almeida G, Altuna B, Michelena G. Espoluración en Botryodiplodia theobromae—influencia de la luz en la producción de picnidios. Laboract Acta. 2001;13:89–92. [Google Scholar]
- Almeida et al. (1999).Almeida G, Klibansky M, Altuna B, Eng F, Legrá S, Armenteros S. Some considerations of the carbon sources used in jasmonic acid production. Revista Iberoamericana de Micología. 1999;16:146–148. [PubMed] [Google Scholar]
- Altuna et al. (1996).Altuna B, Klibansky M, Almeida G, Eng F, Legra S, Armenteros S. Jasmonic acid detection as a new plant growth regulator in strains of Botryodiplodia theobromae. Revista Sobre los Derivados de la Cana de Azucar. 1996;30:16–21. [Google Scholar]
- Alves et al. (2008).Alves A, Crous PW, Correia A, Phillips AJL. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Diversity. 2008;28:1–13. [Google Scholar]
- Andolfi et al. (2014).Andolfi A, Maddau L, Cimmino A, Linaldeddu BT, Basso S, Deidda A, Serra S, Evidente A. Lasiojasmonates A–C, three jasmonic acid esters produced by Lasiodiplodia sp., a grapevine pathogen. Phytochemistry. 2014;103:145–153. doi: 10.1016/j.phytochem.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Asamitsu et al. (2006).Asamitsu Y, Nakamura Y, Ueda M, Kuwahara S, Kiyota H. Synthesis and odor description of both enantiomers of methyl 4,5-didehydrojasmonate, a component of jasmin absolute. Chemistry & Biodiversity. 2006;3(6):654–659. doi: 10.1002/cbdv.200690068. [DOI] [PubMed] [Google Scholar]
- Bababunmi (2005).Bababunmi E. Formulation and method for treating skeletal muscle degeneration caused by malnutrition and disease. International Journal of Scientific Reports. 2005;3(2):54. doi: 10.18203/issn.2454-2156.IntJSciRep20170358. [DOI] [Google Scholar]
- Bai et al. (2004).Bai J, Kitanabe M, Toyoizumi K, Fu L, Zhang S, Dai J, Sakai J, Hiroe K, Yamori T, Turuo T, Ando M. Production of biologically active taxoids by a callus culture of Taxus cuspidata. Journal of Natural Products. 2004;67(1):58–63. doi: 10.1021/np0301083. [DOI] [PubMed] [Google Scholar]
- Beibei et al. (2019).Beibei L, Xueming T, Xiao W, Wei J, Lan B, Guoqian W, Yanna H, Lili S, Jinbin W, Hua L, Peng L. A kind of cultural method and its culture medium of Morchella esculenta (L.) Pers mycelium. 2019. Patent CN109504612A.
- Beibei et al. (2018).Beibei L, Xueming T, Xiaojiang W, Wang W, Bin J, Hailong Y. A kind of methyl jasmonate culture medium and preparation method thereof suitable for straw mushroom. 2018. Patent CN108849245A.
- Boden, Fujioka & Hanna (1993).Boden R, Fujioka F, Hanna M. Fragrance use of hydromethyl jasmonic acid. 1993. Patent US 5300489.
- Broadbent, Hemming & Turner (1968).Broadbent D, Hemming HG, Turner WB. Preparation of jasmonic acid. 1968. Patent GB 1286266.
- Broady (2012).Broady B. Use of jasmone for modulating melatonin production and calcification of the pineal gland. 2012. Patent US13/467606A1.
- Brodhun et al. (2013).Brodhun F, Cristobal-Sarramian A, Zabel S, Newie J, Hamberg M, Feussner I. An iron 13S-lipoxygenase with an α-linolenic acid specific hydroperoxidase activity from Fusarium oxysporum. PLOS ONE. 2013;8(5):e64919. doi: 10.1371/journal.pone.0064919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bömer et al. (2020).Bömer M, Pérez-Salamó I, Florance HV, Salmon D, Dudenhoffer J-H, Finch P, Cinar A, Smirnoff N, Harvey A, Devoto A. Jasmonates induce Arabidopsis bioactivities selectively inhibiting the growth of breast cancer cells through CDC6 and mTOR. New Phytologist. 2020;229(4):2120–2134. doi: 10.1111/nph.17031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo et al. (2014).Castillo G, Torrecillas A, Nogueiras C, Michelena G, Sanchez-Bravo J, Acosta M. Simultaneous quantification of phytohormones in fermentation extracts of Botryodiplodia theobromae by liquid chromatography-electrospray tandem mass spectrometry. World Journal of Microbiology and Biotechnology. 2014;30(7):1937–1946. doi: 10.1007/s11274-014-1612-5. [DOI] [PubMed] [Google Scholar]
- Chapuis (2012).Chapuis C. The Jubilee of Methyl Jasmonate and Hedione®. Helvetica Chimica Acta. 2012;95(9):1479–1511. doi: 10.1002/hlca.201200070. [DOI] [Google Scholar]
- Cheong & Choi (2003).Cheong JJ, Choi YD. Methyl jasmonate as a vital substance in plants. Trends in Genetics. 2003;19(7):409–413. doi: 10.1016/S0168-9525(03)00138-0. [DOI] [PubMed] [Google Scholar]
- Chini et al. (2018).Chini A, Monte I, Zamarreño AM, Hamberg M, Lassueur S, Reymond P, Weiss S, Stintzi A, Schaller A, Porzel A, García-Mina JM, Solano R. An OPR3-independent pathway uses 4,5-didehydrojasmonate for jasmonate synthesis. Nature Chemical Biology. 2018;14(2):171–178. doi: 10.1038/nchembio.2540. [DOI] [PubMed] [Google Scholar]
- Christensen & Kolomiets (2011).Christensen SA, Kolomiets MV. The lipid language of plant–fungal interactions. Fungal Genetics and Biology. 2011;48(1):4–14. doi: 10.1016/j.fgb.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Cimmino et al. (2017).Cimmino A, Cinelli T, Masi M, Reveglia P, da Silva MA, Mugnai L, Michereff SJ, Surico G, Evidente A. Phytotoxic lipophilic metabolites produced by grapevine strains of Lasiodiplodia species in Brazil. Journal of Agricultural and Food Chemistry. 2017;65(6):1102–1107. doi: 10.1021/acs.jafc.6b04906. [DOI] [PubMed] [Google Scholar]
- Cole et al. (2014).Cole SJ, Yoon AJ, Faull KF, Diener AC. Host perception of jasmonates promotes infection by Fusarium oxysporum formae speciales that produce isoleucine- and leucine-conjugated jasmonates. Molecular Plant Pathology. 2014;15:589–600. doi: 10.1111/mpp.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabalona (1967).Crabalona L. Presence of levorotatory methyl jasmonate, methyl cis-2(2-penten1-yl)-3-oxocyclopentenyl acetate in the essential oil of Tunisian rosemary. Comptes Rendus de l’Académie des Sciences. 1967;264:2074–2076. [Google Scholar]
- Creelman & Mullet (1997).Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48(1):355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Cross & Webster (1970).Cross BE, Webster GRB. New metabolites of Gibberella fujikuroi. Part XV. N-jasmonoyl-isoleucine and N-dihydrojasmonoyl-isoleucine. Journal of the Chemical Society. 1970:1839–1942. doi: 10.1039/j39700001839. [DOI] [PubMed] [Google Scholar]
- Da Silva et al. (2014).Da Silva IBRF, Scarpa MV, Rossanezi G, Do Egito EST, De Oliveira AG. Development and characterization of biocompatible isotropic and anisotropic oil-in-water colloidal dispersions as a new delivery system for methyl dihydrojasmonate antitumor drug. International Journal of Nanomedicine. 2014;9:867–876. doi: 10.2147/IJN.S46055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalko (2006).Dalko M. Use of a (dihydro)jasmonic acid derivative for dry skin treatment. 2006. Patent EP1442737.
- Dathe et al. (1990).Dathe W, Miersch O, Sembdner G, Kochmann W, Kunchnel F, Reinhardt P. Mittel zur ertragssteigerung bei erdbeeren. 1990. Patent DD 276025 A1.
- Deepthi & Satheeshkumar (2017).Deepthi S, Satheeshkumar K. Cell line selection combined with jasmonic acid elicitation enhance camptothecin production in cell suspension cultures of Ophiorrhiza mungos L. Applied Microbiology and Biotechnology. 2017;101(2):545–558. doi: 10.1007/s00253-016-7808-x. [DOI] [PubMed] [Google Scholar]
- Demole, Lederer & Mercier (1962).Demole E, Lederer E, Mercier D. Isolement et détermination de la structure du jasmonate de méthyle, constituant odorant caractéristique de l’essence de jasmin. Helvetica Chimica Acta. 1962;45(2):675–685. doi: 10.1002/hlca.19620450233. [DOI] [Google Scholar]
- Deng, Lan & Zhao (2020).Deng X, Lan Y, Zhao H. Pesticide composition containing vanilthioketal and plant growth regulator. 2020. Patent CN111165491A.
- Deyong, Mengjiao & Naixing (2019).Deyong Z, Mengjiao D, Naixing Y. Airflow circulating fat-based absorbs the method that Jasmine aromatic substance prepares face cream and fragrant dew. 2019. Patent CN109628224A.
- Dhandhukia & Thakkar (2007a).Dhandhukia PC, Thakkar VR. Significant medium components for enhanced jasmonic acid production by Lasiodiplodia theobromae using plackett-burman design. Current Trends in Biotechnology & Pharmacy. 2007a;1:79–86. [Google Scholar]
- Dhandhukia & Thakkar (2007b).Dhandhukia PC, Thakkar VR. Standardization of growth and fermentation criteria of Lasiodiplodia theobromae for production of jasmonic acid. African Journal of Biotechnology. 2007b;6:707–712. [Google Scholar]
- Dhandhukia & Thakkar (2008).Dhandhukia PC, Thakkar VR. Response surface methodology to optimize the nutritional parameters for enhanced production of jasmonic acid by Lasiodiplodia theobromae. Journal of Applied Microbiology. 2008;105(3):636–643. doi: 10.1111/j.1365-2672.2008.03803.x. [DOI] [PubMed] [Google Scholar]
- Di, Takken & Tintor (2016).Di X, Takken FLW, Tintor N. How phytohormones shape interactions between plants and the soil-borne fungus Fusarium oxysporum. Frontiers in Plant Science. 2016;7(1248):170. doi: 10.3389/fpls.2016.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos et al. (2014).Dos Santos AZ, Araujo Leite VL, De Mello Innocentini MD, Lourenco MV. Evaluation of process parameters in the production of jasmonic acid. BMC Proceedings. 2014;8(Suppl. 4):244. doi: 10.1186/1753-6561-8-S4-P244. [DOI] [Google Scholar]
- Dos Santos, Innocentini & Lourenco (2014).Dos Santos AZ, Innocentini MDM, Lourenco MV. Optimization of jasmonates bioproduction. BMC Proceedings. 2014;8(S4):208. doi: 10.1186/1753-6561-8-S4-P208. [DOI] [Google Scholar]
- Dos Santos et al. (2001).Dos Santos AA, Clososki GC, Simonelli F, De Oliveira ARM, Marques FA, Zarbin PHG. A new approach to the synthesis of (±)-methyl jasmonate and (±)-baclofen via conjugated addition of oxazoline cyanocuprate to Michael acceptors. Journal of the Brazilian Chemical Society. 2001;12(5):673–679. doi: 10.1590/S0103-50532001000500011. [DOI] [Google Scholar]
- Eng (1996).Eng F. Jasmonic acid production from Botryodiplodia theobromae by liquid and solid state fermentation. 1996. M.Sc. Thesis. University of Autonomous Metropolitan, Campus Iztapalapa, Mexico.
- Eng (2012).Eng F. Characterization of a new strain of fungus Laslodiplodia sp 2334 for obtaining jasmonic acid and its application in agriculture. 2012. PhD Dissertation. University of Havana, Cuba.
- Eng, Gutierrez-Rojas & Favela-Torres (2008).Eng F, Gutierrez-Rojas M, Favela-Torres E. Studies of the effects of carbon: nitrogen ratio inoculum type and yeast extract addition on jasmonic acid production by Botryodiplodia theobromae Pat. strain RC1. Revista Iberoamericana de Micología. 2008;25(3):188–192. doi: 10.1016/S1130-1406(08)70045-7. [DOI] [PubMed] [Google Scholar]
- Eng, Gutiérrez-Rojas & Favela-Torres (1998).Eng F, Gutiérrez-Rojas M, Favela-Torres E. Culture conditions for jasmonic acid and biomass production by Botryodiplodia theobromae in submerged fermentation. Process Biochemistry. 1998;33(7):715–720. doi: 10.1016/S0032-9592(98)00035-1. [DOI] [Google Scholar]
- Eng et al. (2016).Eng F, Haroth S, Feussner K, Meldau D, Rekhter D, Ischebeck T, Brodhun F, Feussner I. Optimized jasmonic acid production by Lasiodiplodia theobromae reveals formation of valuable plant secondary metabolites. PLOS ONE. 2016;11(12):e0167627. doi: 10.1371/journal.pone.0167627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbood et al. (2001).Farbood MI, Blocker RW, McLean LB, Sprecker MA, McLean MP, Kossiakoff N, Kim AY, Hagedorn M. Bioprocess for the high-yield production of food flavor-acceptable jasmonic acid and methyl jasmonate, United States. 2001. Patent US6333180B.
- Fleischer & Fingrut (2007).Fleischer E, Fingrut O. A jasmonate pharmaceutical composition for treatment of cancer. 2007. Patent EP1379229.
- Fleischer, Herzberg & Kashman (2012).Fleischer E, Herzberg M, Kashman Y. Chemical derivatives of jasmonate, pharmaceutical compositions and methods of use thereof. 2012. Patent EP2402321.
- Fleischer et al. (2010).Fleischer F, Kashman Y, Reischer D, Shimony S. Jasmonate derivative compounds pharmaceutical compounds and methods of use thereof. 2010. Patent US7683211B2.
- Gavin et al. (2012).Gavin AS, Faggion SA, Hernandes C, Lourenco MV, Franca SC, Beleboni RO. Nematocidal effects of natural phytoregulators jasmonic acid and methyl-jasmonate against Pratylenchus zeae and Helicotylenchus spp. Natural Product Research. 2012;10:1–8. doi: 10.1080/14786419.2012.686910. [DOI] [PubMed] [Google Scholar]
- Gerwick, Moghaddam & Hamberg (1991).Gerwick WH, Moghaddam M, Hamberg M. Oxylipin metabolism in the red alga Gracilariopsis lemaneiformis: Mechanism of formation of vicinal dihydroxy fatty acids. Archives of Biochemistry and Biophysics. 1991;290(2):436–444. doi: 10.1016/0003-9861(91)90563-X. [DOI] [PubMed] [Google Scholar]
- Gidda et al. (2003).Gidda SK, Miersch O, Levitin A, Schmidt J, Wasternack C, Varin L. Biochemical and molecular characterization of a hydroxyjasmonate sulfotransferase from Arabidopsis thaliana. Journal of Biological Chemistry. 2003;278:17895–17900. doi: 10.1074/jbc.M211943200. [DOI] [PubMed] [Google Scholar]
- Gill & Valivety (1997).Gill I, Valivety R. Polyunsaturated fatty acids, part 2: biotransformations and biotechnological applications. Trends in Biotechnology. 1997;15(11):470–478. doi: 10.1016/S0167-7799(97)01077-9. [DOI] [PubMed] [Google Scholar]
- Goepfert & Poirier (2007).Goepfert S, Poirier Y. ß-Oxidation in fatty acid degradation and beyond. Current Opinion in Plant Biology. 2007;10(3):245–251. doi: 10.1016/j.pbi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2019).Guo L, Hou X, Wang F, Dalong W, Zhen Z, Hong X. Method for improving drought resistance of oil peony by using jasmonic acid. 2019. Patent CN110972798A.
- Gupta, Prakash & Gupta (2015).Gupta C, Prakash D, Gupta S. A biotechnological approach to microbial based perfumes and flavours. Journal of Microbiology & Experimentation. 2015;2:00034. doi: 10.15406/jmen.2015.01.00034. [DOI] [Google Scholar]
- Guranowski et al. (2007).Guranowski A, Miersch O, Staswick PE, Suza W, Wasternack C. Substrate specificity and products of side-reactions catalyzed by jasmonate: amino acid synthetase (JAR1) FEBS Letters. 2007;581(5):815–820. doi: 10.1016/j.febslet.2007.01.049. [DOI] [PubMed] [Google Scholar]
- Guzel et al. (2019).Guzel M, Ozdatli S, Savlug O, Sucu BO. Methyl jasmonate derivatives as possible drug candidates for use in treatment of cancer. 2019. Patent WO2019182527.
- Gális et al. (2009).Gális I, Gaquerel E, Pandey SP, Baldwin IT. Molecular mechanisms underlying plant memory in JA-mediated defence responses. Plant, Cell & Environment. 2009;32(6):617–627. doi: 10.1111/j.1365-3040.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- Günther et al. (1989).Günther T, Miersch O, Fritsche W, Sembdner G. Synthetic medium for manufacture of 7-iso-jasmonic acid with Botryodiplodia theobromae. 1989. Patent DD 272869 A1.
- Hamberg & Gardner (1992).Hamberg M, Gardner HW. Oxylipin pathway to jasmonates: biochemistry and biological significance. Biochimica et Biophysica Acta. 1992;1165(1):1–18. doi: 10.1016/0005-2760(92)90069-8. [DOI] [PubMed] [Google Scholar]
- Han (2017).Han G-Z. Evolution of jasmonate biosynthesis and signaling mechanisms. Journal of Experimental Botany. 2017;68:1323–1331. doi: 10.1093/jxb/erw470. [DOI] [PubMed] [Google Scholar]
- Haroth et al. (2019).Haroth S, Feussner K, Kelly AA, Zienkiewicz K, Shaikhqasem A, Herrfurth C, Feussner I. The glycosyltransferase UGT76E1 significantly contributes to 12-O-glucopyranosyl-jasmonic acid formation in wounded Arabidopsis thaliana leaves. Journal of Biological Chemistry. 2019;294(25):9858–9872. doi: 10.1074/jbc.RA119.007600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hause et al. (2007).Hause B, Mrosk C, Isayenkov S, Strack D. Jasmonates in arbuscular mycorrhizal interactions. Phytochemistry. 2007;68(1):101–110. doi: 10.1016/j.phytochem.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Hawkins et al. (2007).Hawkins LK, Luthe DS, Windham GL, Williams WP. Effect of exogenous jasmonic acid application on Aspergillus flavus kernel infection and aflatoxin production in two maize hybrids (Zea mays L.). Proceedings of the 2006 Multicrop Aflatoxin/Fumonisin Elimination & Fungal Genomics Workshop, Ft Worth (Texas); 2007. pp. 45–48. [Google Scholar]
- Hedden (2008).Hedden P. Plant biology: gibberellins close the lid. Nature. 2008;456(7221):455–456. doi: 10.1038/456455a. [DOI] [PubMed] [Google Scholar]
- Heil et al. (2001).Heil M, Koch T, Hilpert A, Fiala B, Boland W, Linsenmair KE. Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect, defense elicited by jasmonic acid. Proceedings of the National Academy of Sciences USA. 2001;98(3):1083–1088. doi: 10.1073/pnas.98.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel et al. (1997).Hertel SC, Knöfel H-D, Kramell R, Miersch O. Partial purification and characterization of a jasmonic acid conjugate cleaving amidohydrolase from the fungus Botryodiplodia theobromae. FEBS Letters. 1997;407(1):105–110. doi: 10.1016/S0014-5793(97)00307-4. [DOI] [PubMed] [Google Scholar]
- Herzberg, Harel & Mang (2006).Herzberg M, Harel A, Mang C. Jasmonate derivatives, pharmaceutical compositions and methods of use thereof. 2006. Patent US8247439.
- Heshof et al. (2014).Heshof R, Van Schayck J, Tamayo-Ramos J, De Graaff L. Heterologous expression of Gaeumannomyces graminis lipoxygenase in Aspergillus nidulans. AMB Express. 2014;4(1):65. doi: 10.1186/s13568-014-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe & Jander (2008).Howe GA, Jander G. Plant immunity to insect herbivores. Annual Review of Plant Biology. 2008;59(1):41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- Hurst et al. (2015).Hurst W, Stuart D, Calderon A, Van Breemen R. Jasmonic acid compounds in cocoa products. 2015. Patent US9040096.
- Hurst et al. (2011).Hurst W, Stuart D, Justavino A, Van Breemen R. Jasmonic acid compounds in cocoa products. 2011. Patent US9040096.
- Inho, Kyoungju & Yonghwi (2006).Inho G, Kyoungju K, Yonghwi K. Optimal conditions for the production of (+)-jasmonic acid by Diplodia gossypina ATCC 10936. The Korean Journal of Microbiology. 2006;42:210–215. [Google Scholar]
- Jernerén et al. (2012).Jernerén F, Eng F, Hamberg M, Oliw E. Linolenate 9R-dioxygenase and allene oxide synthase activities of Lasiodiplodia theobromae. Lipids. 2012;47(1):65–73. doi: 10.1007/s11745-011-3622-5. [DOI] [PubMed] [Google Scholar]
- Jimenez-Aleman et al. (2015a).Jimenez-Aleman GH, Machado RAR, Gorls H, Baldwin IT, Boland W. Synthesis, structural characterization and biological activity of two diastereomeric JA-Ile macrolactones. Organic & Biomolecular Chemistry. 2015a;13(21):5885–5893. doi: 10.1039/C5OB00362H. [DOI] [PubMed] [Google Scholar]
- Jimenez-Aleman et al. (2015b).Jimenez-Aleman GH, Scholz SS, Heyer M, Reichelt M, Mithöfer A, Boland W. Synthesis, metabolism and systemic transport of a fluorinated mimic of the endogenous jasmonate precursor OPC-8: 0. Biochimica et Biophysica Acta. 2015b;1851(12):1545–1553. doi: 10.1016/j.bbalip.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Johnson, Paul & Favara (1977).Johnson F, Paul K, Favara D. Process for preparing methyl jasmonate and related compounds. 1977. Patent US0257263.
- Katona, Vempati & Savir (2015).Katona D, Vempati S, Savir O. Brazilian scientist is part of elite group of researchers fighting cancer, obtains an unprecedented patent in the United States. Brazilian Journal of Biology. 2015;75(1):256–258. doi: 10.1590/1519-6984.25014. [DOI] [PubMed] [Google Scholar]
- Kitahara et al. (1987).Kitahara T, Miura K, Warita Y, Takagi Y, Mori K. Synthesis of (±)-methyl epijasmonate and (±)-methyl cucurbate. Agricultural and Biological Chemistry. 1987;51(4):1129–1133. doi: 10.1271/bbb1961.51.1129. [DOI] [Google Scholar]
- Kitahara et al. (1991).Kitahara T, Warita Y, Abe M, Seya M, Takagi Y, Mori K. Stereoselective synthesis of methyl epijasmonate. Agricultural and Biological Chemistry. 1991;55(4):1013–1017. doi: 10.1271/bbb1961.55.1013. [DOI] [Google Scholar]
- Koch, Bandemer & Boland (1997).Koch T, Bandemer K, Boland W. Biosynthesis of cis-Jasmone: a pathway for the inactivation and the disposal of the plant stress hormone jasmonic acid to the gas phase? Helvetica Chimica Acta. 1997;80(3):838–850. doi: 10.1002/hlca.19970800318. [DOI] [Google Scholar]
- Laredo-Alcalá et al. (2016).Laredo-Alcalá E, Michelena-Álvarez G, Iliná A, Borjas-Ramos J, Hernández-Castillo F, Martínez-Hernández J. Uso de Botryodiplodia theobromae como productor de ácido jasmónico en dos sistemas de fermentación. Revista Mexicana de Micología. 2016;44:1–9. [Google Scholar]
- Lopes (2014).Lopes J. Compositions of jasmonate compounds. 2014. Patent US 8883220.
- Lulai, Orr & Glynn (1995).Lulai EC, Orr PH, Glynn MT. Natural suppression of sprouting in stored potatoes using jasmonates. 1995. Patent WO001995012311A1.
- Löwe, Dietz & Gröger (2020).Löwe J, Dietz K-J, Gröger H. From a biosynthetic pathway toward a biocatalytic process and chemocatalytic modifications: three-step enzymatic cascade to the plant metabolite cis-(+)-12-OPDA and metathesis-derived products. Advanced Science. 2020;7(13):1902973. doi: 10.1002/advs.201902973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik (2006).Malik S. Wound and skin care compositions. 2006. Patent US7098189.
- Marks (2012).Marks M. Agricultural compositions containing a jasmonic acid or dihydrojasmonic acid salt. 2012. Patent EP1740045.
- Martinez et al. (2010).Martinez R, Pedreño G, Navarro S, Romero L. Combined use of methyl jasmonate and cyclodextrins for the production of resveratrol. 2010. Patent EP2256209.
- Matsui et al. (2017).Matsui R, Amano N, Takahashi K, Taguchi Y, Saburi W, Mori H, Kondo N, Matsuda K, Matsuura H. Elucidation of the biosynthetic pathway of cis-jasmone in Lasiodiplodia theobromae. Scientific Reports. 2017;7(1):6688. doi: 10.1038/s41598-017-05851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcelroy (2011).Mcelroy S. Improved turfgrass quality. 2011. Patent EP2352372.
- Michelena et al. (2010).Michelena G, Bell A, Martínez A, Carrera E, Cerrutti G, López-Munguia A, Olvera C. Biocida compuesto por una sal de jasmonato y otros aditivos de uso en la industria azucarera. 2010. Patent CU 23555 A1.
- Miersch, Bohlmann & Wasternack (1999).Miersch O, Bohlmann H, Wasternack C. Jasmonates and related compounds from Fusarium oxysporum. Phytochemistry. 1999;50(4):517–523. doi: 10.1016/S0031-9422(98)00596-2. [DOI] [Google Scholar]
- Miersch et al. (1992).Miersch O, Brückner B, Schmidt J, Sembdner G. Cyclopentane fatty acids from Gibberella fujikuroi. Phytochemistry. 1992;31(11):3835–3837. doi: 10.1016/S0031-9422(00)97537-X. [DOI] [Google Scholar]
- Miersch et al. (1993).Miersch O, Günther T, Fritsche W, Sembdner G. Jasmonates from different fungal species. Natural Product Letters. 1993;2(4):293–299. doi: 10.1080/10575639308043825. [DOI] [Google Scholar]
- Miersch et al. (1987).Miersch O, Preiss A, Sembdner G, Schreiber K. (+)-7-iso-jasmonic acid and related compounds from Botryodiplodia theobromae. Phytochemistry. 1987;26(4):1037–1039. doi: 10.1016/S0031-9422(00)82345-6. [DOI] [Google Scholar]
- Miersch, Regvar & Wasternack (1999).Miersch O, Regvar M, Wasternack C. Metabolism of jasmonic acid in Pisolithus tinctorius cultures. Phyton—Annales Rei Botanicae. 1999;39:243–247. [Google Scholar]
- Miersch et al. (1989).Miersch O, Schmidt J, Sembdner G, Schreiber K. Jasmonic acid-like substances from the culture filtrate of Botryodiplodia theobromae. Phytochemistry. 1989;28(5):1303–1305. doi: 10.1016/S0031-9422(00)97735-5. [DOI] [Google Scholar]
- Miersch, Schneider & Sembdner (1991).Miersch O, Schneider G, Sembdner G. Hydroxylated jasmonic acid and related compounds from Botryodiplodia theobromae. Phytochemistry. 1991;30(12):4049–4051. doi: 10.1016/0031-9422(91)83464-V. [DOI] [Google Scholar]
- Miersch et al. (1984).Miersch O, Sembdner G, Schreiber K, Richter K, Kochmann W, Fritche W. Verfahren zur Herstellung von Jasmonsäureanaloga. 1984. Patent DD 216734A1.
- Miller, Neilan & Sze (2008).Miller K, Neilan BA, Sze DM. Development of taxol and other endophyte produced anticancer agents. Recent Patents in Anticancer Drug Discovery. 2008;3(1):14–19. doi: 10.2174/157489208783478685. [DOI] [PubMed] [Google Scholar]
- Mookherjee et al. (1981).Mookherjee B, Wilson R, Schmitt F, Vock M. Flavoring with mixture of nor-methyl jasmonate and dihydro-nor-methyl jasmonate. 1981. Patent US4294863.
- Moreira et al. (2019).Moreira B, Viana R, Lisboa P, Lopes P, Figueiredo P, Ramos S, Bonini C, Trindade V, Andrade M, May A. Jasmonic acid and K-phosphite enhance productivity and technological quality of sugarcane crop. Journal of Agricultural Science. 2019;11(14):254–264. doi: 10.5539/jas.v11n14p254. [DOI] [Google Scholar]
- Nonaka et al. (2010).Nonaka H, Ogawa N, Maeda N, Yong-Gang W, Kobayashi Y. Stereoselective synthesis of epi-jasmonic acid, tuberonic acid, and 12-oxo-PDA. Organic & Biomolecular Chemistry. 2010;8(22):5212–5223. doi: 10.1039/c0ob00218f. [DOI] [PubMed] [Google Scholar]
- Nájera et al. (2020).Nájera C, Foubelo F, Sansano JM, Yus M. Stereodivergent routes in organic synthesis: marine natural products, lactones, other natural products, heterocycles and unnatural compounds. Organic & Biomolecular Chemistry. 2020;18(7):1279–1336. doi: 10.1039/C9OB02597A. [DOI] [PubMed] [Google Scholar]
- Oliw & Hamberg (2017).Oliw EH, Hamberg M. An allene oxide and 12-oxophytodienoic acid are key intermediates in jasmonic acid biosynthesis by Fusarium oxysporum. Journal of Lipid Research. 2017;58(8):1670–1680. doi: 10.1194/jlr.M077305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliw & Hamberg (2019).Oliw EH, Hamberg M. Biosynthesis of jasmonates from linoleic acid by the fungus Fusarium oxysporum—evidence for a novel allene oxide cyclase. Lipids. 2019;54(9):543–556. doi: 10.1002/lipd.12180. [DOI] [PubMed] [Google Scholar]
- Onrubia et al. (2013).Onrubia M, Cusidó RM, Ramirez K, Hernandez-Vazquez L, Moyano E, Bonfill M, Palazon J. Bioprocessing of plant in vitro systems for the mass production of pharmaceutically important metabolites: paclitaxel and its derivatives. Current Medicinal Chemistry. 2013;20(7):880–891. doi: 10.2174/0929867311320070004. [DOI] [PubMed] [Google Scholar]
- Patel, Patel & Thakkar (2015).Patel DD, Patel RR, Thakkar VR. Purification, characterization and application of lipoxygenase isoenzymes from Lasiodiplodia theobromae. Applied Biochemistry and Biotechnology. 2015;175(1):513–525. doi: 10.1007/s12010-014-1278-3. [DOI] [PubMed] [Google Scholar]
- Patel & Thakkar (2015).Patel DD, Thakkar VR. Screening and characterization of hyper jasmonic acid producing mutant of Lasiodiplodia theobromae. FASEB Journal. 2015;29(S1):884.22. doi: 10.1096/fasebj.29.1_supplement.884.22. [DOI] [Google Scholar]
- Pieterse et al. (2009).Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. Networking by small-molecule hormones in plant immunity. Nature Chemical Biology. 2009;5(5):308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- Pirbalouti, Sajjadi & Parang (2014).Pirbalouti AG, Sajjadi SE, Parang K. A review (research and patents) on jasmonic acid and its derivatives. Archiv der Pharmazie. 2014;347(4):229–239. doi: 10.1002/ardp.201300287. [DOI] [PubMed] [Google Scholar]
- Rohwer & Erwin (2008).Rohwer CL, Erwin JE. Horticultural applications of jasmonates: a review. Journal of Horticultural Science & Biotechnology. 2008;83(3):283–304. doi: 10.1080/14620316.2008.11512381. [DOI] [Google Scholar]
- Ryan & Farmer (1991).Ryan CA, Farmer EE. Method of inducing plant defense mechanisms. 1991. Patent US91/03685.
- Salvatore, Alves & Andolfi (2020).Salvatore MM, Alves A, Andolfi A. Secondary metabolites of Lasiodiplodia theobromae: distribution, chemical diversity, bioactivity, and implications of their occurrence. Toxins. 2020;12(7):457. doi: 10.3390/toxins12070457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches et al. (2017).Sanches PA, Santos F, Peñaflor MFGV, Bento JMS. Direct and indirect resistance of sugarcane to Diatraea saccharalis induced by jasmonic acid. Bulletin of Entomological Research. 2017;107(6):828–838. doi: 10.1017/S0007485317000372. [DOI] [PubMed] [Google Scholar]
- Sembdner, Atzorn & Schneider (1994).Sembdner G, Atzorn R, Schneider G. Plant hormone conjugation. Plant Molecular Biology. 1994;26:1459–1481. doi: 10.1007/BF00016485. [DOI] [PubMed] [Google Scholar]
- Svoboda & Boland (2010).Svoboda J, Boland W. Plant defense elicitors: analogues of jasmonoyl-isoleucine conjugate. Phytochemistry. 2010;71:1445–1449. doi: 10.1016/j.phytochem.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Schaller, Schaller & Stintzi (2005).Schaller F, Schaller A, Stintzi A. Biosynthesis and metabolism of jasmonates. Journal of Plant Growth Regulation. 2005;23(3):179–199. doi: 10.1007/s00344-004-0047-x. [DOI] [Google Scholar]
- Secatto (2013).Secatto M. Desenvolvimiento da síntese total do (±)-ácido jasmónico visando aplicaçao en escala industrial. 2013. https://repositorio.ufscar.br/handle/ufscar/6650 https://repositorio.ufscar.br/handle/ufscar/6650
- Selbmann, Crognale & Petruccioli (2004).Selbmann L, Crognale C, Petruccioli M. Beta-glucan production by Botryosphaeria rhodina in different bench-top bioreactors. Journal of Applied Microbiology. 2004;95(5):1074–1081. doi: 10.1111/j.1365-2672.2004.02241.x. [DOI] [PubMed] [Google Scholar]
- Selbmann, Stingele & Petruccioli (2003).Selbmann L, Stingele F, Petruccioli M. Exopolysaccharide production by filamentous fungi: the example of Botryosphaeria rhodina. Antonie Van Leeuwenhoek. 2003;84(2):135–145. doi: 10.1023/A:1025421401536. [DOI] [PubMed] [Google Scholar]
- Sembdner & Parthier (1993).Sembdner G, Parthier B. The biochemistry and the physiological and molecular actions of jasmonates. Annual Review of Plant Physiology and Plant Molecular Biology. 1993;44(1):569–589. doi: 10.1146/annurev.pp.44.060193.003033. [DOI] [Google Scholar]
- Seo et al. (2001).Seo HS, Song JT, Cheong JJ, Lee YH, Lee YW, Hwang I, Lee JS, Choi YD. Jasmonic acid carboxyl methyltransferase: a key enzyme for jasmonate- regulated plant responses. Proceedings of the Nationall Academy of Sciences USA. 2001;98(8):4788–4793. doi: 10.1073/pnas.081557298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets et al. (2020).Smets JL, Trujillo RM, Jukes AKWSP, Pintens AB. Compositions with perfume encapsulates. 2020. Patent 20200283704.
- Sriram et al. (2005).Sriram D, Yogeeswari P, Thirumurugan R, Ratan Bal T. Camptothecin and its analogues: a review on their chemotherapeutic potential. Natural Product Research. 2005;19(4):393–412. doi: 10.1080/14786410412331299005. [DOI] [PubMed] [Google Scholar]
- Staswick, Tiryaki & Rowe (2002).Staswick PE, Tiryaki I, Rowe ML. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002;14(6):1405–1415. doi: 10.1105/tpc.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinegger & Hansel (1988).Steinegger E, Hansel R. Lehrbuch der Pharmakognosie und Phytopharmazie. Berlin: Springer; 1988. [Google Scholar]
- Stout, Zehnder & Baur (2002).Stout MJ, Zehnder GW, Baur ME. Potential for the use of elicitors of plant resistance in arthropod management programs. Archives of Insect Biochemistry and Physiology. 2002;51(4):222–235. doi: 10.1002/arch.10066. [DOI] [PubMed] [Google Scholar]
- Suzuki, Inomata & Endo (2004).Suzuki K, Inomata K, Endo Y. Enantiocontrolled synthesis of jasmonates via tandem retro-Diels−Alder−ene reaction activated by a silyl substituent. Organic Letters. 2004;6(3):409–411. doi: 10.1021/ol036253p. [DOI] [PubMed] [Google Scholar]
- Syklowska-Baranek et al. (2009).Syklowska-Baranek K, Pietrosiuk A, Kokoszka A, Furmanowa M. Enhancement of taxane production in hairy root culture of Taxus media var. Hicksii. Journal of Plant Physiology. 2009;166(17):1950–1954. doi: 10.1016/j.jplph.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Taapken et al. (1994).Taapken T, Blechert S, Weiler EW, Zenk MH. Stereoselective synthesis of 7-substituted jasmonic acid derivatives and investigation of their biological activity. Journal of the Chemical Society, Perkin Transactions 1. 1994;11(11):1439–1442. doi: 10.1039/p19940001439. [DOI] [Google Scholar]
- Tabata (2006).Tabata H. Production of paclitaxel and the related taxanes by cell suspension cultural of Taxus species. Current Drug Target. 2006;7(4):453–461. doi: 10.2174/138945006776359368. [DOI] [PubMed] [Google Scholar]
- Takayuki, Michitaka & Yuichi (2003).Takayuki A, Michitaka M, Yuichi K. Efficient total synthesis of 12-oxo-PDA and OPC-8:0. Journal of Organic Chemistry. 2003;68(20):7825–7832. doi: 10.1021/jo0348571. [DOI] [PubMed] [Google Scholar]
- Tsukada, Takahashi & Nabeta (2010).Tsukada K, Takahashi K, Nabeta K. Biosynthesis of jasmonic acid in a plant pathogenic fungus, Lasiodiplodia theobromae. Phytochemistry. 2010;71(17–18):2019–2023. doi: 10.1016/j.phytochem.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Ueda et al. (1991).Ueda J, Miyamoto K, Aoki M, Hirata T, Sato T, Momotani Y. Identification of jasmonic acid in Chlorella and Spirulina. Bulletin of the University of Osaka Prefecture Ser B. 1991;43:103–108. [Google Scholar]
- Vick & Zimmerman (1984).Vick BA, Zimmerman DC. Biosynthesis of jasmonic acid by several plant species. Plant Physiology. 1984;75(2):458–461. doi: 10.1104/pp.75.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2020a).Wang Y, Bai Y, Pan S, Pang Y, Shi Q. Preparation for inducing plant to resist aphids and preparation method thereof. 2020a. Patent CN111149526A.
- Wang, Guo & Feng (2019).Wang G, Guo Y, Feng Y. The methods and applications of plant leaf blade flavonoids and the accumulation of terpene lactones compound are induced using methyl jasmonate. 2019. Patent CN110024787A.
- Wang et al. (2020b).Wang C, Shen Q, Liu J, Lu J. Method for improving cuttage rooting of chinese roses. 2020b. Patent CN111201999A.
- Wang et al. (2020c).Wang X, Wu Y, Zhang L, Meng Q, Chen X. Method for promoting growth of free filaments and formation of sporangial branches of Porphyra haitanensis. 2020c. Patent CN 111357774A.
- Wasternack (2006).Wasternack C. Oxylipins: biosynthesis, signal traduction and action. In: Hedden P, Thomas S, editors. Annual Plant Reviews. Oxford: Blackwell Publishing Ltd; 2006. pp. 185–222. [DOI] [Google Scholar]
- Wasternack (2014).Wasternack C. Action of jasmonates in plant stress responses and development—applied aspects. Biotechnology Advances. 2014;32(1):31–39. doi: 10.1016/j.biotechadv.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Wasternack (2015).Wasternack C. How jasmonates earned their laurels: past and present. Journal of Plant Growth Regulation. 2015;34(4):1–34. doi: 10.1007/s00344-015-9526-5. [DOI] [Google Scholar]
- Wasternack (2016).Wasternack C. eLS. Chichester: John Wiley & Sons, Ltd; 2016. Jasmonates: synthesis, metabolism, signal transduction and action; pp. 1–9. [Google Scholar]
- Wasternack & Feussner (2018).Wasternack C, Feussner I. The oxylipin pathways: biochemistry and function. Annual Review of Plant Biology. 2018;69(1):363–386. doi: 10.1146/annurev-arplant-042817-040440. [DOI] [PubMed] [Google Scholar]
- Wasternack & Hause (2002).Wasternack C, Hause B. Jasmonates and octadecanoids: signals in plant stress responses and development. Progress in Nucleic Acid Research and Molecular Biology. 2002;72:165–221. doi: 10.1016/s0079-6603(02)72070-9. [DOI] [PubMed] [Google Scholar]
- Wasternack & Hause (2013).Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development—an update to the 2007 review in Annals of Botany. Annals of Botany. 2013;111(6):1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack et al. (2006).Wasternack C, Stenzel I, Hause B, Hause G, Kutter C, Maucher H, Neumerkel J, Feussner I, Miersch O. The wound response in tomato—role of jasmonic acid. Journal of Plant Physiology. 2006;163(3):297–306. doi: 10.1016/j.jplph.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Wennman et al. (2016).Wennman A, Oliw EH, Karkehabadi S, Chen Y. Crystal structure of manganese lipoxygenase of the rice blast fungus Magnaporthe oryzae. Journal of Biological Chemistry. 2016;291(15):8130–8139. doi: 10.1074/jbc.M115.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson & Roberts (2012).Wilson SA, Roberts SC. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnology Journal. 2012;10(3):249–268. doi: 10.1111/j.1467-7652.2011.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2014).Xu Y, Zhou T, Espinosa-Artiles P, Tang Y, Zhan J, Molnar I. Insights into the biosynthesis of 12-membered resorcylic acid lactones from heterologous production in Saccharomyces cerevisiae. ACS Chemical Biology. 2014;9(5):1119–1127. doi: 10.1021/cb500043g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara (2007).Yoshihara T. Method for producing jasmonic acids and theobroxide using microorganisms. 2007. Patent JP3916144.
- Yukimune et al. (1996).Yukimune Y, Tabata H, Higashi Y, Hara Y. Methyl jasmonate-induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nature Biotechnology. 1996;14(9):1129–1132. doi: 10.1038/nbt0996-1129. [DOI] [PubMed] [Google Scholar]
- Zerbe, Weiler & Schaller (2007).Zerbe P, Weiler EW, Schaller F. Preparative enzymatic solid phase synthesis of cis(+)-12-oxo-phytodienoic acid—physical interaction of AOS and AOC is not necessary. Phytochemistry. 2007;68(2):229–236. doi: 10.1016/j.phytochem.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2020).Zhang R, Song X, Xia A, Cheng W, Li G, Wei J, Li C, Hu R. Method for enhancing weeding effect of fluroxypyridine ester and relieving pesticide damage function. 2020. Patent CN111248205A.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
The research in this article did not generate any data or code; this is a literature review.