Abstract
Background
Coronavirus Disease 2019 (COVID-19) is rapidly transmitted from person to person, causing global pandemic since December 2019. Instantly detecting COVID-19 is crucial for epidemic prevention. In this study, olfactory dysfunction is a significant symptom in mild to moderate COVID-19 patients but relatively rare in other respiratory viral infections. The Taiwan smell identification test (TWSIT) is a speedy and inexpensive option for accurately distinguishing anosmia that also quantifies the degree of anosmia. Using TWSIT in the outpatient clinic for early identifying the patients with mild to moderate COVID-19 can be promising.
Methods
Nineteen patients confirmed COVID-19 in central Taiwan were collected and divided into two groups: olfactory dysfunction and non-olfactory dysfunction. Demographic characteristics, laboratory findings, and the results of the olfactory test were compared between these two groups.
Findings
Thirteen (68.4%) of the 19 patients had olfactory dysfunction. The patients with olfactory dysfunction were younger than those without this symptom. The statistical difference in age distribution was significant between these two groups (IQR: 25.5–35.5 vs. IQR: 32.5–60.3; p-value: 0.012). There was no significant difference in gender, smoking history, comorbidities, travel history, respiratory tract infection symptoms, and laboratory findings between these two groups.
Conclusion
This study demonstrated that young adults were prone to develop olfactory dysfunctions. In the flu season, olfactory dysfunction is considered a specific screening criterion for early detecting COVID-19 in the community. TWSIT can serve as a decent test for quantifying and qualifying olfactory dysfunction.
Keywords: Olfactory dysfunction, Taiwan smell identification test (TWSIT), Mild-to-moderate, COVID-19 patients
Introduction
Coronavirus Disease 2019 (COVID-19) is a pandemic disease that began since December 2019, and its pathogen, known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can transmit from person to person by exhaled droplets.1 Owing to pre-symptomatic and asymptomatic transmission, COVID-19 spreads rapidly in the community. Pre-symptomatic transmission of COVID-19 is probably caused by the shorter duration of serial interval than that of the incubation period.2 , 3 Therefore, early detection of COVID-19 patients is essential for the prevention and control of this endemic infection.
In this pandemic period, those presenting with mild to moderate symptoms occupy the majority of COVID-19 patients, nearly 80% of patients reported from the study of China.4 , 5 Flu-like syndrome includes fever, dry or productive cough, rhinorrhea, stuffy nose, myalgia, sore throat, headache, chills, diarrhea, and nausea; these are the major presentations of COVID-19. The oxygen saturation of these patients often maintained above 94% without an additional oxygen supply, and lymphopenia, and elevations of C-reactive protein, d-dimer, lactate dehydrogenase, and ferritin may be present.2 , 6, 7, 8
In the 2019–2020 flu season, many countries were flooded with COVID-19 patients. Additional respiratory viruses, including Influenza A/B, Coronavirus 229E/OC43, and Adenovirus9 were circulating at the same time, and their yielded symptoms and signs were similar to those of SARS-Cov-2-infected patients. Recent reports demonstrated that quite a few patients suffered from a loss of taste or smell as their initial presentation.10 , 11 Post-respiratory non-SARS-CoV-2 infection, like Influenza or other pathogens, may induce olfactory dysfunction, the rate of which has been less than 20%.12 The prevalence of olfactory dysfunction was high in COVID-19 patients of western countries. One study revealed the prevalence rate of subjective olfactory dysfunction reached 85.6%, and another study employed the University of Pennsylvania Smell Identification Test (UPSIT) which objectively disclosed an even higher prevalence rate.11 , 13
Examination of olfactory dysfunction could be valuable as initial screen method to differentiate COVID-19 from other viral respiratory infections, however, the supportive data was limited and none from Asian population. Quantifying the degree of anosmia, along with subjective description of smell loss, could be essential for clinical identification of COVID-19 patients. UPSIT has been one of the most widely used tests to evaluate the olfactory function, and Traditional Chinese version of the UPSIT (UPSIT-TC) was modified according to the cultural differences.14 Pearson correlation revealed a significant positive correlation between the two tests.15 As it was revised from UPSIT and UPSIT-TC and its test items were chosen for Taiwanese according to their culture, Taiwan smell identification test (TWSIT) was designed for Taiwanese. Since the correlation between TWSIT and UPSIT-TC was high, TWSIT has been widely used to discover olfactory dysfunction caused by infection, head trauma, surgery, age, toxin or medication.16 It evaluates the grade of anosmia or hyposmia more reliably than a subjective loss of smell and is a faster, convenient, and low expense. Most standard tests for the diagnosis of COVID-19 patients are confined to specific microbiologic laboratories and time-consuming. TWSIT is likely useful, in the community setting, to recognize SARS-CoV-2-infected patients on an outpatient basis. As loss of smell is one of the major symptoms in COVID-19 patients, we conducted a innovative study and applied TWSIT to assess the smell sense of COVID-19 patients via grading their olfactory function. As well, the relating factors of olfactory dysfunction such as demographic characteristics, symptoms, and laboratory tests were explicated.
Material and methods
The inclusion criteria of COVID-19 patients
From March 25 to May 5, 2020, a total of 19 SARS-CoV-2-infected patients in five hospitals of central Taiwan were included in this study. The patients all belonged to mild to moderate COVID-19 cases,2 who were diagnosed by a positive result of sputum, nasopharyngeal, or oropharyngeal specimens for SARS-CoV-2 real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR). All of them were hospitalized in a single isolation room. TWSIT was performed during the above mentioned study period for further evaluation of the olfactory function. These participants were communicable and well understood the tests. The answer sheets were pictured and sent to the investigators by e-mail. Some of the patients did TWSIT soon after they were admitted, and others did TWSIT days or up to 4 weeks after they were hospitalized.
Clinical presentation and data collection
Demographic characteristics, comorbidities, travel or contact history, clinical symptoms, and signs were retrospectively collected from medical records. All the patients were subjected to blood tests and chest-X-ray during their admission in negative pressure isolation rooms. The criterion for patients to be discharged was fulfilling three successive results of negative SARS-CoV-2 RT-PCR tests. TWSIT was given either in a negative pressure isolation room or in the outpatient clinic during the illness Day 5–60. The ENT doctors were consulted for the prescription and the evaluation of the result of TWSIT.
Olfactory dysfunction
In this study, olfactory dysfunction was defined as having subjective symptoms including anosmia, hyposmia, or smell decay detected by unbiased TWSIT. Anosmia and hyposmia denote loss and decreased sense of smell, respectively. These subjective sensations may occur at first and then improved by the time receiving TWSIT, therefore few patients had smell dysfunction symptom and acceptable TWSIT score. The patients possessing no smell symptom and satisfactory TWSIT score were identified as no olfactory problem.
Taiwan smell identification test (TWSIT)
The odorants used for smell tests are culturally customized. A total of eight odorants, including honey peach, passion fruit, cantaloupe, lemon, smoked plum, coffee, jasmine, and garlic, were used for TWSIT. Two multiple-choice questions about the smell and sensory evaluation tested twice in different orders were prearranged for each odorant. The answer for each of the questions was scored based on the standardized scale. Based on the sum score of the total 16 questions, the olfactory function of a patient was divided into three groups: anosmia with the score 0–11; hyposmia with the score 12–43 for 0–35 years (yr) age group, 12–42 for 36–55 yr, and 12–39 for 56–80 yr or more; normosmia with the score 44–48 for 0–35 yr, 43–48 for 36–55 yr, and 40–48 for 56–80 yr or more.17 The flavors included in TWSIT were microencapsulated odorants and thinned by odorless propylene glycol. These commercially standardized and stable odorants of TWSIT (produced by Wei Teh Flavor and Fragrance Biotechnology Corp., Changhua, Taiwan) have been approved by Taiwan Food and Drug Administration.18 The odorants were protected by plastic cover and needed to scratch the paper with a pencil to produce an odor immediately prior to use.
Statistical analysis
The continuous variables were presented as median (IQR), and categorical variables were presented as n/N (%). The differences between olfactory dysfunction and non-olfactory dysfunction group were compared by the Mann–Whitney U test for continuous variables and Fisher's exact test for categorical variables. The P-value of less than 0.05 was considered significant. Simple linear regression was performed to evaluate the correlation between age and olfactory score. SPSS ver. 20 was employed for all analyses.
Result
Demographic characteristics
From March 25 to May 5, 2020, a total of 19 COVID-19 patients were enrolled in this study (Table 1 ). Thirteen of them had olfactory dysfunction. The median age of olfactory dysfunction group (n = 13) was 26.0 yr, ranging from 5 to 58 yr old (IQR: 25.5–35.5), whereas the six patients who develop no objective olfactory dysfunction had a median age of 43.0 yr (28–64 yr; IQR: 32.5–60.3). The ratio of male to female was 1.72. In the olfactory dysfunction group (n = 13), three patients had smoking history and one patient had allergic rhinitis. In the olfactory non-dysfunction group (n = 6), there was one patient with diabetes and another patient with heart disease and hepatitis. Comorbidities, including hypertension, airway disease, sinusitis, autoimmune, and malignancy, and septal deviation, nasal polyposis, and previous sinonasal surgery were absent among all patients.
Table 1.
Demographic data, comorbidities and travel history of 19 SARS-CoV-2-infected patients with or without olfactory dysfunction.
| Total (n = 19) | 0lfactory dysfunction (n = 13) | No olfactory dysfunction (n = 6) | P value | |
|---|---|---|---|---|
| Age, median (IQR), y | 28.0 (24.0–48.0) | 26.0 (22.5–35.5) | 43.0 (32.5–60.3) | 0.012 |
| Male, No. (%) | 12 (63.2) | 9 (69.2) | 3 (50.0) | 0.617 |
| Comorbiditiesa, No. (%) | 3 (15.8) | 1 (7.7) | 2 (33.3) | 0.222 |
| Travel to other country | 16 (84.2) | 12 (92.3) | 4 (66.7) | 0.222 |
| Europe | 6 (31.6) | 5 (38.5) | 1 (16.7) | |
| United States | 5 (26.3) | 3 (23.1) | 2 (33.3) | |
| Asia | 4 (21.1) | 3 (23.1) | 1 (16.7) | |
| Warship to Palau | 1 (5.3) | 1 (7.7) | 0 (0) |
Comorbidities are including diabetes, heart disease and hepatitis in the patient without olfactory dysfunction patient and allergic rhinitis in the patient with olfactory dysfunction; there were no comorbidities of hypertension, airway disease, sinusitis, autoimmunity disease, or malignancy.
Sixteen of the COVID-19 patients (16/19; 84.2%) had an overseas travel history: 6 to Europe (31.6%), 5 to the United States (26.3%), 4 to Asia (21.1%), including 3 to Turkey in the Middle East (15.8%), 1 to Indonesia (5.3%) and 1 boarded warship to Palau (5.3%). Of the patients without any travel history, two had contact with confirmed COVID-19 cases, and one was a healthcare worker. Twelve of the 13 (92.3%) patients who had olfactory dysfunction acquired COVID-19 upon their overseas travel, on the other hand, only 4 of 6 patients (66.7%) without olfactory dysfunction possessed a recent travel history (no significant difference; P-value = 0.222; Table 1).
Statistical analyses demonstrated that the age distribution was significantly different between the olfactory dysfunction and non-dysfunction groups (IQR: 25.5–35.5 vs. IQR: 32.5–60.3; P-value = 0.012). There was no significant difference in gender, smoking history, and comorbidities between these two groups. Collectively, patients with olfactory dysfunction were younger than those without this symptom.
Symptoms and laboratory findings
All the 19 patients had mild to moderate manifestations and did not need intensive care. Statistical comparison between groups was examined based on six categories of clinical presentations, which included fever, upper respiratory infection (URI) symptoms, lower respiratory infection (LRI) symptoms, loss of smell, loss of taste, and other symptoms containing headache, myalgia, and diarrhea (Table 2 ). For specific symptoms, rhinorrhea, stuff nose and sore throat were categorized as URI symptoms, while cough, short of breath and chest tightness were LRI symptoms. The common symptoms of the 19 patients were fever (11/19, 57.9%), loss of smell (9/19, 47.7%), and cough (8/19, 42.1%). Between the patients with and without olfactory dysfunction, these symptoms were not presented differently, except for loss of smell (69.2% vs. 0%, P-value = 0.011; Table 2).
Table 2.
Symptoms, laboratory findings and TWSIT score of 19 SARS-CoV-2-infected patients with or without olfactory dysfunction.
| Total (n = 19) | Olfactory dysfunction (n = 13) | No olfactory dysfunction (n = 6) | P value | |
|---|---|---|---|---|
| Symptoms, No. (%) | ||||
| Fever | 11 (57.9) | 7 (53.8) | 4 (66.7) | 1.000 |
| URI symptomsa | 11 (57.9) | 8 (61.5) | 3 (50.0) | 1.000 |
| LRI symptomsb | 8 (42.1) | 6 (46.2) | 2 (33.3) | 1.000 |
| Loss of taste | 4 (21.1) | 4 (30.8) | 0 (0) | 0.255 |
| Other symptomsc | 9 (47.4) | 7 (53.8) | 2 (33.3) | 0.629 |
| No loss of smell | 10 (52.6) | 4 (30.8) | 6 (100) | 0.011 |
| Laboratory test, median (IQR) | ||||
| White blood count | 5400 (4110–6700) | 5500 (4100–7275) | 5050 (3900–6100) | 0.467 |
| Neutrophil | 3197 (2459–4670) | 3492 (2294–4996) | 2849 (2449–4108) | 0.639 |
| Lymphocyte | 1161 (995–1534) | 1161 (1005–1459) | 1215 (855–1705) | 1.000 |
| N/L ratio | 3.00 (1.51–4.42) | 3.00 (1.45–3.99) | 2.91 (1.58–4.46) | 0.765 |
| Creatinine | 0.82 (0.69–0.96) | 0.82 (0.73–0.95) | 0.82 (0.64–0.97) | 0.966 |
| AST | 23.0 (17.0–32.0) | 27.0 (18.5–35.5) | 19.0 (15.3–27.8) | 0.210 |
| ALT | 21.0 (14.0–41.0) | 21.0 (14.0–47.0) | 20.5 (14.5–30.0) | 0.639 |
| Bilirubin | 0.46 (0.35–0.60) | 0.48 (0.34–0.60) | 0.44 (0.35–0.64) | 0.820 |
| CRP | 0.50 (0.24–1.11) | 0.50 (0.26–1.16) | 0.46 (0.11–1.42) | 0.639 |
| CK | 76.0 (66.0–120.0) | 79.5 (72.0–122.8) | 73.0 (45.5–136.0) | 0.513 |
| LDH | 183.0 (141.3–219.0) | 184.0 (163.0–225.0) | 148.0 (123.0–234.0) | 0.441 |
| TWSIT score | 41 (36–46) | 38 (31–43) | 46 (44–46) | 0.022 |
Abbreviation: URI = upper respiratory infection, LRI = lower respiratory infection, N/L ratio = neutrophil to lymphocytes ratio, AST = aspartate aminotransferase, ALT = alanine aminotransferase, CK = creatine kinase, LDH = lactate dehydrogenase, TWSIT = Taiwan smell identification test.
Upper respiratory infection symptoms comprised rhinorrhea, stuff nose and sore throat.
Lower respiratory infection symptoms included cough, short of breath and chest tightness.
Other symptoms contained headache, myalgia, and diarrhea but there were no abdominal pain, nausea/vomiting, or skin rash.
Similarly, there were no significant differences in the laboratory values, including white blood count, neutrophil, lymphocyte, neutrophil to lymphocytes ratio, creatinine, aspartate aminotransferase, alanine aminotransferase, bilirubin, C-reactive protein, creatine kinase, and lactate dehydrogenase, between the olfactory dysfunction and non-dysfunction groups (Table 2).
Olfactory function tests
To better evaluate the olfactory function objectively, TWSIT was introduced in this study. According to the age-adjusted TWSIT score, the olfactory function of a patient was divided into three groups, that is anosmia, hyposmia, and normosmia. The median of TWSIT scores of the olfactory dysfunction group (n = 13) was 38 (IQR: 31–43), which was significantly lower than that of the non-dysfunction group (n = 6, TWIST score 46; IQR: 44–46). Based on the scoring scheme, ten of the patients of the olfactory dysfunction group were considered hyposmia, and their TWSIT scores ranged from 13 to 42. Of the ten hyposmia patients, six suffered from subjective anosmia and four without noticing this symptom. For the patient who got the lowest TWSIT score (13 points), a paired TWSIT was performed 2 weeks after first testing, and the score increased to 40 points, indicating that olfactory dysfunction had been improved significantly.
As presented in Table 2, thirteen of the COVID-19 patients (13/19, 68.4%) were confirmed olfactory dysfunction, either subjectively or objectively detected. Nine patients had an initial history of subjective olfactory dysfunction, and 6 of them were confirmed olfactory dysfunction objectively by TWSIT. Upon objective TWSIT, 3 of 9 subjective-dysfunction patients had improved olfactory sense and obtained normal scores. It is likely that these patients recovered from initial olfactory dysfunction. Finally, a total of six patients presented no symptom of olfactory dysfunction and had had their TWSIT scores in the normal range during the whole course of COVID-19 infection. Four of the tested olfactory dysfunction patients who did not complain of smell problems were validated by TWSIT.
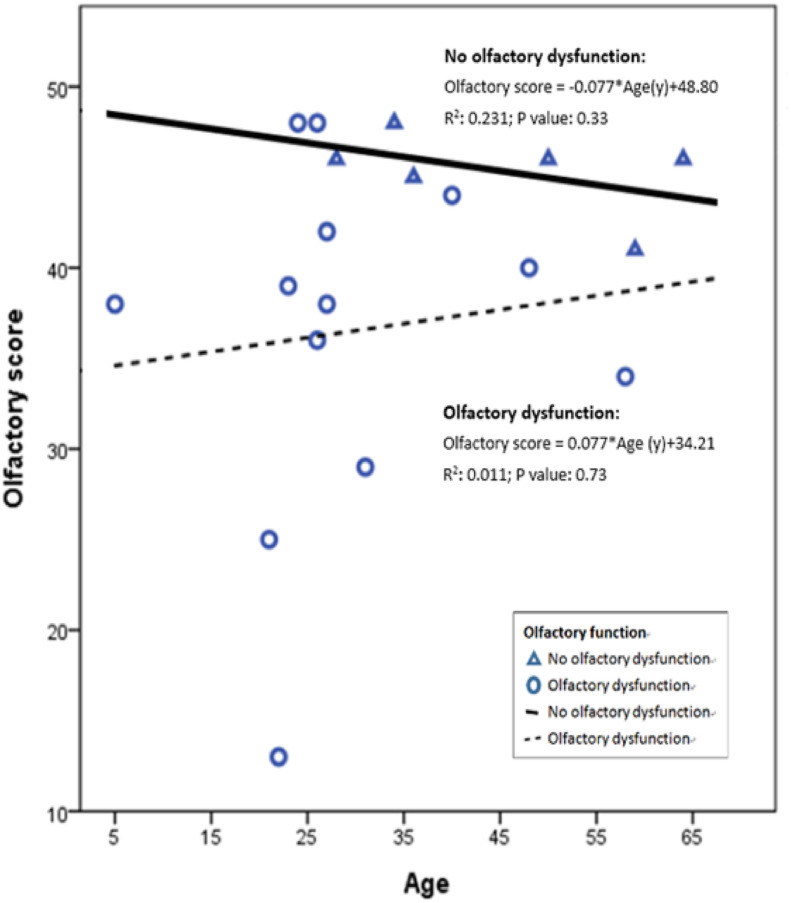
COVID-19 patients who developed olfactory dysfunction were statistically younger than those without this finding (Table 1). In general, older people had degeneration of olfactory function. The TWSIT scores from patients in the olfactory non-dysfunction group decreased with age (Fig. 1 ; solid line). However, when the loss of olfactory function was associated with SARS-CoV infections, younger patients were likely to have a lower TWSIT score (Fig. 1; dotted line).
Figure 1.
The correlation between age and olfactory score of SARS-CoV-2-infected patients with or without olfactory dysfunction. The TWSIT scores from patients in the olfactory non-dysfunction group likely decreased with age (solid line). For of olfactory dysfunction group, younger patients seemed to have a lower TWSIT score (Fig. 1; dotted line).
Discussion
It is challenging to distinguish COVID-19 from seasonal flu at the initial stage of illness. The frequent finding of olfactory dysfunction in COVID-19 patients, in contrast to the uncommon presentation of that in other flu-like patients, can be a valuable clue for early diagnosis of COVID-19, particularly in young patients. In this study, the TWSIT scoring scheme was employed to grade the severity of olfactory dysfunction in COVID-19 Taiwanese patients. For infected patients at the ill stage, the TWSIT-based diagnosis correlated well with both subjective and objective dysfunction of smell sense.
The infection of patients by SARS-CoV-2, a novel respiratory pathogen, often brings about fever and respiratory symptoms, including dry or productive cough, rhinorrhea, stuffy nose, and sore throat. Common symptoms of viral infections, such as myalgia, headache, chills, diarrhea, and nausea, are frequently observed. Although some reports demonstrated that olfactory dysfunction is one of the major presentations of COVID-19 in the western population, its prevalence in the Taiwanese population had not been characterized.13 In this study, the prevalence of olfactory dysfunction (68.4%) in COVID-19 patients was higher than that of fever (57.9%) and cough (42.1%). Seeing its sensitivity to detect COVID-19, it is suggested that the inclusion of olfactory dysfunction in the screening criterion is feasible in the Taiwanese population.
Furthermore, the TWSIT provides an easy-to-use platform, and can therefore be applied in the outpatient clinics. It is particularly useful for the identification of mild to moderate COVID-19 patients. The odorants used for smell tests vary from nation to nation. The familiarity of the odorants is influenced by the food, culture, and religious habits of people. The UPSIT is regarded as a valid and accurate tool to identify olfactory dysfunction in the western countries11 however, it had an increased false-positive rate when applied in Iran.18 As a result, the odorant list of UPSIT needs to be modified and validated before it can be used for the tested population.
We are the first to demonstrate that olfactory dysfunction was more frequently present in the younger patients infected by COVID-19 (Table 2 and Fig. 1). Yet the underlying mechanism is not clarified.19 It is known that, when activated by a specific odorant, the neuronal receptor in the olfactory epithelium at the roof of the nasal cavity transmits a signal along the axon of the olfactory nerve. Through the convergence of axons in the olfactory bulbs connecting the primary sensory cortex and the amygdala, the odor is produced. Aging is a factor of olfactory degeneration. When the patient age increases, the integrity of olfactory epithelium and the size of the olfactory bulb naturally decrease.20 Nasal inflammation and obstruction induced by viral infections, allergic rhinitis, nasal obstruction, nasal surgery, asthma, or head trauma probably contribute to olfactory dysfunction.21 Lechien JR et al. (2020) unveiled the presence of SARS-CoV antigens in the olfactory bulb, basal ganglia, and midbrain.11 In-depth investigation is required to establish the molecular pathogenesis of SARS-CoV-2, which possibly attach to olfactory nerve and deteriorate olfactory function. As well, several studies showed that typical viral infections could damage the olfactory epithelium and bulb.16 , 21 , 22
When the central nervous system (CNS) is affected by SARS-CoV-2 infection, patients present the symptoms of dizziness, headache, conscious disturbance, ataxia, or seizure. The SARS-CoV-2 infections of CNS, such as encephalitis, toxic encephalopathy, acute cerebrovascular disease, and acute demyelinating lesions, are often life-threatening and might destroy CNS through several mechanisms, including direct infection via blood circulation, neurologic transmission, systemic immune response, and the impairment of blood–brain barrier via binding of angiotensin-converting enzyme 2 receptor.23 When the peripheral nervous system is affected, patients present loss of smell, loss of taste, vision disturbance, or nerve pain.24 All the patients in this study had characteristics of mild to moderate COVID-19 cases and they did not have severe neurologic problems except for anosmia.
COVID-19-associated olfactory dysfunction is likely reversible, and one study demonstrated that the recovery rate reached 80%.25 In this current study, the patient with worst olfactory function had a TWSIT score of 13 initially and improved to a score of 40 two weeks later. Repeated stimuli have been recommended to help the regenerative capacity of olfactory function16 and through the anti-inflammatory effects on olfactory receptors, corticosteroids have been reported to improve the olfactory dysfunction.21 Nevertheless, no standard regimen so far is formulated for the treatment of post-infection olfactory dysfunction. All the COVID-19 patients with olfactory dysfunction in this study received no specific treatment, and their hyposmia symptom partially or entirely resolved two or more weeks later.
Limitation
There were several limitations to this study. First, during March 25 to May 5, 2020, the number of confirmed COVID-19 patients in central Taiwan was limited. Although the tested patients is small, the yielded data is important to apply for clinical identification of COVID-19. Accompanying with the subjective smell complaints, this portable olfactory test is easy-to-interpret via telecommunication and is likely to serve as a screening test for early recognizing COVID-19 patients. Second, there was no unified timing for preforming the TWSIT; some patients did this test early in the disease process, and some in the recovery phase. Third, TWSIT is solitarily used in Chinese speaking regions and less internationally recognized.
Conclusion
In the study, anosmia or hyposmia is the most common symptom of COVID-19 patients. The patients with olfactory dysfunction were statistically younger than those without such finding. In the flu season, olfactory dysfunction is presumably a potent screening criterion for early detection of COVID-19 in the community and can be quantified and qualified by TWSIT. By using the TWSIT, we could quantify the severity score of COVID-19 patients, and demonstrate the improvement of anosmia in their recovery stage. In conclusion, TWSIT could be a valuable test for the identification of COVID-19 patients in Taiwan community, and the recognized patients with olfactory dysfunction should be subjected to testing by real Time RT-PCR of SARS-CoV-2 to confirm infection.
Acknowledgements
This study was supported by a grant, CMUH DMR-109-214, from China Medical University Hospital, Taichung, Taiwan.
References
- 1.World Health Organization Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available from:
- 2.Gandhi R.T., Lynch J.B., del Rio C. Mild or moderate COVID-19. NEJM. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. https://www.nejm.org/doi/full/10.1056/NEJMcp2009249 [DOI] [PubMed] [Google Scholar]
- 3.Nishiura H., Linton N.M., Akhmetzhanov A.R. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan A., Liu L., Wang C., Guo H., Hao X., Wang Q. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. J Am Med Assoc. 2020;323:1915–1923. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARSCoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53(3):404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. NEJM. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsih W.H., Cheng M.Y., Ho M.W., Chou C.H., Lin P.C., Chi C.Y. Featuring COVID-19 cases via screening symptomatic patients with epidemiologic link during flu season in a medical center of central Taiwan. J Microbiol Immunol Infect. 2020;53(3):459–466. doi: 10.1016/j.jmii.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention Coronavirus (COVID-19) https://www.cdc.gov/coronavirus/2019-nCoV/index.html Available from:
- 11.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol. 2020:1–11. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malhotra P., Luka A., McWilliams C.S., Poeth K.G., Schwartz R., Elfekey M. Clinical features of respiratory viral infections among inpatients at a major US tertiary care hospital. South Med J. 2016;109(8):481–486. doi: 10.14423/SMJ.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 13.Sedaghat A.R., Gengler I., Speth M.M. Olfactory Dysfunction: a highly prevalent symptom of COVID-19 with public health significance. Otolaryngol Head Neck Surg. 2020;163(1):12–15. doi: 10.1177/0194599820926464. [DOI] [PubMed] [Google Scholar]
- 14.Doty R.L., Shaman P., Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 15.Jiang R.S., Su M.C., Liang K.L., Shiao J.Y., Wu S.H., Hsin C.H. A pilot study of a traditional Chinese version of the University of Pennsylvania Smell Identification Test for application in Taiwan. Am J Rhinol Allergy. 2010;24:45–50. doi: 10.2500/ajra.2010.24.3388. [DOI] [PubMed] [Google Scholar]
- 16.Daramola O.O., Becker S.S. An algorithmic approach to the evaluation and treatment of olfactory disorders. Curr Opin Otolaryngol Head Neck Surg. 2015;23:8–14. doi: 10.1097/MOO.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 17.Hsu N.I., Lai J.T., Shen P.H. Development of Taiwan Smell Identification Test: a quick office-based smell screening test for Taiwanese. Am J Rhinol Allergy. 2015;29:e50–e54. doi: 10.2500/ajra.2015.29.4174. [DOI] [PubMed] [Google Scholar]
- 18.Moein S.T., Hashemian S.M., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10(8):944–950. doi: 10.1002/alr.22587. Wiley Online Library. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soler Z.M., Patel Z.M., Turner J.H., Holbrook E.H. A primer on viral-associated olfactory loss in the era of COVID-19. Int Forum Allergy Rhinol. 2020;10(7):814–820. doi: 10.1002/alr.22578. Wiley Online Library. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doty R.L., Kamath V. The influences of age on olfaction: a review. Front Psychol. 2014;5:20. doi: 10.3389/fpsyg.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D.H., Kim S.W., Hwang S.H., Kim B.G., Kang J.M., Cho J.H. Prognosis of olfactory dysfunction according to etiology and timing of treatment. Otolaryngology–Otolaryngol Head Neck Surg. 2017;156(2):371–377. doi: 10.1177/0194599816679952. [DOI] [PubMed] [Google Scholar]
- 22.Holbrook E.H., Leopold D.A. Anosmia: diagnosis and management. Curr Opin Otolaryngol Head Neck Surg. 2003;11:54–60. doi: 10.1097/00020840-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopkins C., Surda P., Whitehead E., Kumar B.N. Early recovery following new onset anosmia during the COVID-19 pandemic–an observational cohort study. J Otolaryngol Head Neck Surg. 2020;49:1–6. doi: 10.1186/s40463-020-00423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]