Abstract
Objective
To evaluate differences in thromboinflammatory biomarkers between patients with severe coronavirus disease 2019 (COVID-19) infection/death and mild infection.
Patients and Methods
MEDLINE, Cochrane Central Register of Controlled Trials, EMBASE, EBSCO, Web of Science, and CINAHL databases were searched for studies comparing thromboinflammatory biomarkers in COVID-19 among patients with severe COVID-19 disease or death (severe/nonsurvivors) and those with nonsevere disease or survivors (nonsevere/survivors) from January 1, 2020, through July 11, 2020. Inclusion criteria were (1) hospitalized patients 18 years or older comparing severe/nonsurvivors vs nonsevere/survivors and (2) biomarkers of inflammation and/or thrombosis. A random-effects model was used to estimate the weighted mean difference (WMD) between the 2 groups of COVID-19 severity.
Results
We included 75 studies with 17,052 patients. The severe/nonsurvivor group was older, had a greater proportion of men, and had a higher prevalence of hypertension, diabetes, cardiac or cerebrovascular disease, chronic kidney disease, malignancy, and chronic obstructive pulmonary disease. Thromboinflammatory biomarkers were significantly higher in patients with severe disease, including D-dimer (WMD, 0.60; 95% CI, 0.49 to 0.71; I2=83.85%), fibrinogen (WMD, 0.42; 95% CI, 0.18 to 0.67; I2=61.88%; P<.001), C-reactive protein (CRP) (WMD, 35.74; 95% CI, 30.16 to 41.31; I2=85.27%), high-sensitivity CRP (WMD, 62.68; 95% CI, 45.27 to 80.09; I2=0%), interleukin 6 (WMD, 22.81; 95% CI, 17.90 to 27.72; I2=90.42%), and ferritin (WMD, 506.15; 95% CI, 356.24 to 656.06; I2=52.02%). Moderate to significant heterogeneity was observed for all parameters (I2 > 25%). Subanalysis based on disease severity, mortality, and geographic region of the studies revealed similar inferences.
Conclusion
Thromboinflammatory biomarkers (D-dimer, fibrinogen, CRP, high-sensitivity CRP, ferritin, and interleukin 6) and marker of end-organ damage (high-sensitivity troponin I) are associated with increased severity and mortality in COVID-19 infection.
Abbreviations and Acronyms: COVID-19, coronavirus disease 2019; CRP, C-reactive protein; ECMO, extracorporeal membrane oxygenation; hs, high-sensitivity; IL-6, interleukin 6; LDH, lactate dehydrogenase; OR, odds ratio; WMD, weighted mean difference
As coronavirus disease 2019 (COVID-19) continues to spread across the world, there is accumulating evidence supporting the relative contribution of specific comorbidities and laboratory patterns among severely affected patients necessitating intensive care admission or resulting in mortality.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75 The US Food and Drug Administration recently approved remdesivir for the treatment of suspected or laboratory-confirmed COVID-19 in hospitalized patients with severe disease (defined as patients with oxygen saturation of ≤94% while breathing room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation [ECMO]).57 A 10-day course has been approved for COVID-19–infected patients who require invasive mechanical ventilation and/or ECMO and a 5-day course for patients not requiring mechanical ventilation and/or ECMO.56 With the availability of potential treatment, the identification of clinical and laboratory predictors of severe disease is urgently needed to further risk stratify patients and optimize the allocation of medications to improve clinical outcomes. Earlier meta-analyses have evaluated such predictors; however, at the time of their publication, limited data were available, reducing the confidence in their conclusions. Moreover, the data available at the time of prior meta-analyses were exclusively from China, where the COVID-19 infection initially spread. These analyses combined data from multiple studies with overlapping populations and could not account for any racial/ethnic differences in the thromboinflammatory milieu.76, 77, 78 We hypothesized differences in the thromboinflammatory milieu according to disease severity and race/ethnicity. The aim of the current systematic review and meta-analysis was to (1) compare the differences in comorbidities and thromboinflammatory biomarkers between patients with severe COVID-19 infection/death (severe/nonsurvivors) due to COVID-19 infection and mild COVID-19 infection (nonsevere/survivors) and (2) assess the relative contribution of race/ethnicity in the thromboinflammatory milieu by comparing biomarkers between the Chinese population and that of countries other than China.
Patients and Methods
This systematic review was performed according to Cochrane Collaboration guidance and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.79 The study was exempt from institutional review or ethical board review because of no access to patient-level data.
Search Strategy
We searched PubMed, The Cochrane Library, EMBASE, EBSCO, Web of Science, and CINAHL databases from January 1, 2020, through July 11, 2020. We included prospective or retrospective studies that compared severe or fatal COVID-19 infection with mild COVID-19 infection or COVID-19 survivors. The search strategy is included in the Supplementary Appendix (available online at http://mcpiqojournal.org). The reference lists of all the retrieved articles were reviewed for further identification of potentially relevant studies. The identified studies were systematically assessed using the inclusion and exclusion criteria described subsequently.
Eligibility Criteria
Two reviewers (Rahul Chaudhary and J.G.) independently selected the studies and abstracted data on study characteristics, design, reported comorbidities, laboratory parameters, and reported clinical outcomes. Discrepancies between the 2 reviewers were resolved by discussion and consensus. The final results were reviewed by the senior investigators (W.E.W. and R.D.M.) (Figure 1). The eligibility criteria were (1) hospitalized patients 18 years or older comparing severe/nonsurvivor COVID-19–positive patients vs nonsevere/survivor COVID-19–positive patients and (2) reported biomarkers of inflammation and/or thrombosis. Studies of pregnant women (due to inherent changes in markers of thromboinflammation during pregnancy) and reports with incomplete reporting of biomarkers were excluded. Abstracts, case reports, conference presentations, editorials, reviews, expert opinions, and literature not published in English were excluded.
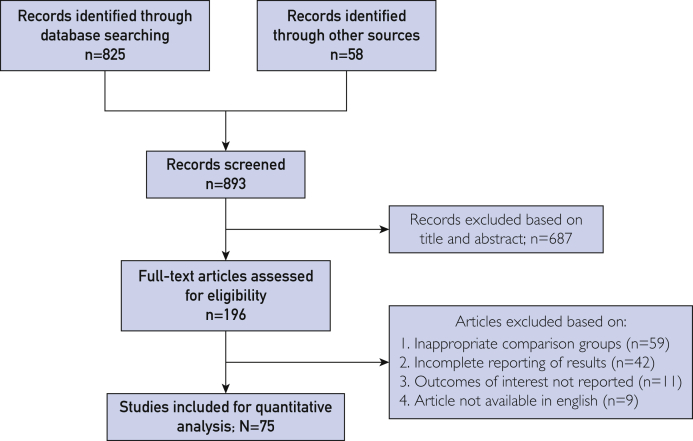
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and study flow.
Outcome Definition
Severe COVID-19 was designated when the patients had one of the following criteria: (1) respiratory distress with respirations of 30 or more per minute, (2) pulse oximeter oxygen saturation of 93% or less at rest, and (3) oxygenation index (arterial partial pressure of oxygen/inspired oxygen fraction) of 300 mm Hg or lower. Nonsevere patients met all the following conditions: (1) epidemiological history, (2) fever or other respiratory symptoms, (3) typical computed tomographic evidence of abnormalities of viral pneumonia, and (4) positive result of the reverse transcription–polymerase chain reaction for COVID-19 RNA. For studies with the categorization of illness in multiple grades of severity, the values from the 2 most extreme groups, eg, critical vs mild illness, were chosen for analysis. The acute cardiac injury was determined if serum levels of cardiac biomarkers (eg, troponin I) were above the 99th percentile upper reference limit or if new abnormalities were detected on electrocardiography and/or echocardiography.
Risk of Bias Appraisal
Assessment of risk of bias for each study was performed using the Newcastle-Ottawa Scale for cohort studies.80 This tool addresses the domains of patient selection, comparability of groups, and outcome assessment.
Statistical Analyses
We used the random-effects model to pool results across studies and estimate the weighted mean difference (WMD) and odds ratio (OR). We evaluated heterogeneity of effects using the Higgins I-squared (I2) statistic with heterogeneity defined as I2<25% as nonsignificant heterogeneity, between 25% and 50% as mild heterogeneity, between 50% and 75% as moderate heterogeneity and greater than 75% as high heterogeneity. We evaluated the assumption of combining data from patients with severe disease with nonsurvivors and combining nonsevere disease data with survivors by doing each analysis separately. We also compared the results of studies with patients from China vs other locations. A 2-tailed P<.05 was considered statistically significant. Meta-analysis was performed using the Comprehensive Meta-Analysis software package, version 3.3.070 (Biostat Solutions, LLC).
Results
A total of 893 studies were identified after the exclusion of duplicate or irrelevant references (Figure 1). After a detailed evaluation, 75 relevant studies were included incorporating a total of 17,052 hospitalized COVID-19–positive patients.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75 There were a total of 3664 patients in the severe/nonsurvivor COVID-19 group and 13,388 patients in the nonsevere/survivor group. Except for 9 prospective cohort studies,9,12,18,23,35,36,46,67,73 all studies were retrospective. Most of the 75 studies were reported from China (80.0% [n=60]), while other studies were from Italy,1,2,33,50 Iran,19,40,44 the United States,17,42 Oman,21 Turkey,43 Mexico,36 Germany,3 Ireland,12 and the Netherlands.35 All studies used reverse transcription–polymerase chain reaction for COVID-19 diagnosis. The overall characteristics of the included studies are described in Table 1 and Supplemental Tables 1 through 4 (available online at http://mcpiqojournal.org).
Table 1.
Characteristics of the 75 Included Studiesa
| Reference, year | Country | Follow-up (d) | Groups | Type of study |
|---|---|---|---|---|
| Bazzan et al,1 2020 | Italy | 11.6 | Nonsurvivor vs survivor | Retrospective |
| Bonetti et al,2 2020 | Italy | NA | Nonsurvivor vs survivor | Retrospective |
| Burian et al,3 2020 | Germany | NA | ICU vs non-ICU | Retrospective |
| Cen et al,4 2020 | China | 28 | Severe vs nonsevere | Retrospective |
| Chen et al (1),5 2020b | China | NA | Severe vs nonsevere | Retrospective |
| Chen et al (2),6 2020b | China | NA | Severe vs nonsevere | Retrospective |
| Chen et al (3),7 2020 | China | NA | Severe/critical vs nonsevere | Retrospective |
| Deng et al,8 2020b | China | NA | Nonsurvivor vs survivor | Retrospective |
| Du et al,9 2020c | China | 33 | Nonsurvivor v. survivor | Prospective |
| Duan et al,10 2020d | China | NA | Severe vs Nonsevere | Retrospective |
| Fan et al,11 2020e | China | NA | Nonsurvivor vs survivor | Retrospective |
| Fogarty et al,12 2020 | Ireland | NA | Severe/critical vs nonsevere | Prospective |
| Fu et al,13 2020 | China | 30 | Severe vs nonsevere | Retrospective |
| Gan et al,14 2020b | China | NA | Nonsurvivor vs survivor | Retrospective |
| Gao et al,15 2020 | China | NA | Severe vs nonsevere | Retrospective |
| Gong et al,16 2020f | China | NA | Severe vs nonsevere | Retrospective |
| Goshua et al,17 2020 | USA | 40 | ICU vs non-ICU | Retrospective |
| Huang et al,18 2020e | China | 10.5 | Critical/ICU vs non-ICU | Prospective |
| Javanian et al,19 2020 | Iran | NA | Nonsurvivor vs survivor | Retrospective |
| Ji et al,20 2020g | China | NA | Severe vs nonsevere | Retrospective |
| Khamis et al,21 2020 | Oman | NA | ICU vs non-ICU | Retrospective |
| Li et al (1),22 2020 | China | NA | Severe vs nonsevere | Retrospective |
| Li et al (2),23 2020 | China | NA | Severe vs nonsevere | Prospective |
| Li et al (3),24 2020b | China | 30 | Nonsurvivor vs survivor | Retrospective |
| Li et al (4),25 2020h | China | NA | Nonsurvivor vs survivor | Retrospective |
| Li et al (5),26 2020b | China | NA | Nonsurvivor vs survivor | Retrospective |
| Liu et al (1),27 2020 | China | NA | Severe vs nonsevere | Retrospective |
| Liu et al (2),28 2020 | China | NA | Severe vs nonsevere | Retrospective |
| Liu et al (3),29 2020b | China | NA | Nonsurvivor vs survivor | Retrospective |
| Lu et al,30 2020 | China | 14 | Severe vs nonsevere | Retrospective |
| Lv et al,31 2020g | China | NA | Severe vs nonsevere | Retrospective |
| Ma et al,32 2020 | China | NA | Severe vs nonsevere | Retrospective |
| Masetti et al,33 2020 | Italy | NA | Nonsurvivor vs survivor | Retrospective |
| Mao et al,34 2020b | China | NA | Severe vs nonsevere | Retrospective |
| Middeldorp et al,35 2020 | Netherlands | 15 | Critical/ICU vs non-ICU | Prospective |
| Ortiz-Brizuela et al,36 2020 | Mexico | 13 | ICU vs non-ICU | Prospective |
| Pan et al,37 2020b | China | NA | Severe vs nonsevere | Retrospective |
| Qian et al,38 2020 | China | NA | Severe vs nonsevere | Retrospective |
| Qin et al,39 2020b | China | NA | Severe vs nonsevere | Retrospective |
| Rastad et al,40 2020 | Iran | NA | Nonsurvivor vs survivor | Retrospective |
| Ruan et al,41 2020b,e | China | 22 | Nonsurvivor vs survivor | Retrospective |
| Salacup et al,42 2021 | USA | NA | Nonsurvivor vs survivor | Retrospective |
| Satici et al,43 2020 | Turkey | NA | Severe vs nonsevere | Retrospective |
| Shahriarirad et al,44 2020 | Iran | NA | Nonsurvivor vs survivor | Retrospective |
| Shi et al,45 2020g | China | NA | Nonsurvivor vs survivor | Retrospective |
| Sun et al,46 2020 | China | NA | Severe vs nonsevere | Prospective |
| Tang et al (1),47 2020b | China | NA | Nonsurvivor vs survivor | Retrospective |
| Tang et al (2),48 2020b | China | 28 | Nonsurvivor vs survivor | Retrospective |
| Tian et al,49 2020b,c,e | China | 30 | Severe vs nonsevere | Retrospective |
| Vultaggio et al,50 2020 | Italy | 21 | Severe vs nonsevere | Retrospective |
| Wan et al,51 2020d | China | NA | Severe vs nonsevere | Retrospective |
| Wang et al (1),52 2020f | China | 34 | Critical/ICU vs non-ICU | Retrospective |
| Wang et al (2),53 2020f | China | 21 | Nonsurvivor vs survivor | Retrospective |
| Wang et al (3),54 2020 | China | NA | Severe vs nonsevere | Retrospective |
| Wang et al (4),55 2020 | China | NA | Severe vs nonsevere | Retrospective |
| Wang et al (5),56 2020b | China | NA | Critical/ICU vs non-ICU | Retrospective |
| Wang et al (6),57 2020b | China | NA | Severe vs nonsevere | Retrospective |
| Wu et al (1),58 2020e | China | 50 | ARDS vs non-ARDS | Retrospective |
| Yan et al,59 2020b | China | NA | Nonsurvivor vs survivor | Retrospective |
| Yang et al (1),60 2020e | China | 28 | Nonsurvivor vs survivor | Retrospective |
| Yang et al (2),61 2020 | China | NA | Severe vs nonsevere | Retrospective |
| Yang et al (3),62 2020 | China | NA | Severe vs nonsevere | Retrospective |
| Yang et al (4),63 2020 | China | NA | Nonsurvivor vs survivor | Retrospective |
| Ye et al,64 2020c | China | NA | Nonsurvivor vs survivor | Retrospective |
| Zeng et al,65 2021 | China | 30 | ICU vs non-ICU | Retrospective |
| Zhang et al (1),66 2020 | China | NA | Severe vs nonsevere | Retrospective |
| Zhang et al (2),67 2020 | China | NA | Severe vs nonsevere | Prospective |
| Zhang et al (3),68 2020b | China | NA | Severe vs nonsevere | Retrospective |
| Zhang et al (4),69 2020b | China | 36 | Nonsurvivor vs survivor | Retrospective |
| Zhang et al (5),70 2020f | China | NA | Severe vs nonsevere | Retrospective |
| Zheng et al,71 2020 | China | NA | Severe vs nonsevere | Retrospective |
| Zhou et al (1),72 2020c,e | China | 21 | Nonsurvivor vs survivor | Retrospective |
| Zhou et al (2),73 2020 | China | NA | Severe vs nonsevere | Prospective |
| Zhu et al (1),74 2020 | China | NA | Severe vs nonsevere | Retrospective |
| Zhu et al (2),75 2020 | China | NA | Nonsurvivor vs survivor | Retrospective |
ARDS = acute respiratory distress syndrome; ICU = intensive care unit; NA = not available; USA = United States.
Data from the same hospital—Tongji Hospital, China (n=18 exclusive; n=2 shared).
Data from the same hospital—Wuhan Pulmonary Hospital, China (n=2 exclusive; n=2 shared).
Data from the same hospital—Chongqing Three Gorges Hospital, China (n=2 exclusive).
Data from the same hospital—Wuhan Jin Yin-tan Hospital, China (n=4 exclusive; n=2 shared).
Data from the same hospital—Zhongnan Hospital of Wuhan University, China (n=4 exclusive).
Data from the same hospital—Wuhan University Renmin Hospital, China (n=3 exclusive).
Data compiled from >1 hospital noted above.
Risk of Bias
We deemed all the studies to be at a high risk of bias because of unadjusted analyses and variability in groups with comorbidities and prognostic factors.
Meta-analysis in the Combined Group of Disease Severity and Mortality
Among demographics, patients in the severe/nonsurvivor group were older, a greater proportion were men, and had a higher prevalence of hypertension, diabetes, cardiac or cerebrovascular disease, chronic kidney disease, chronic liver disease, malignancy, and chronic obstructive pulmonary disease compared to the nonsevere/survivor group (Supplemental Table 1).
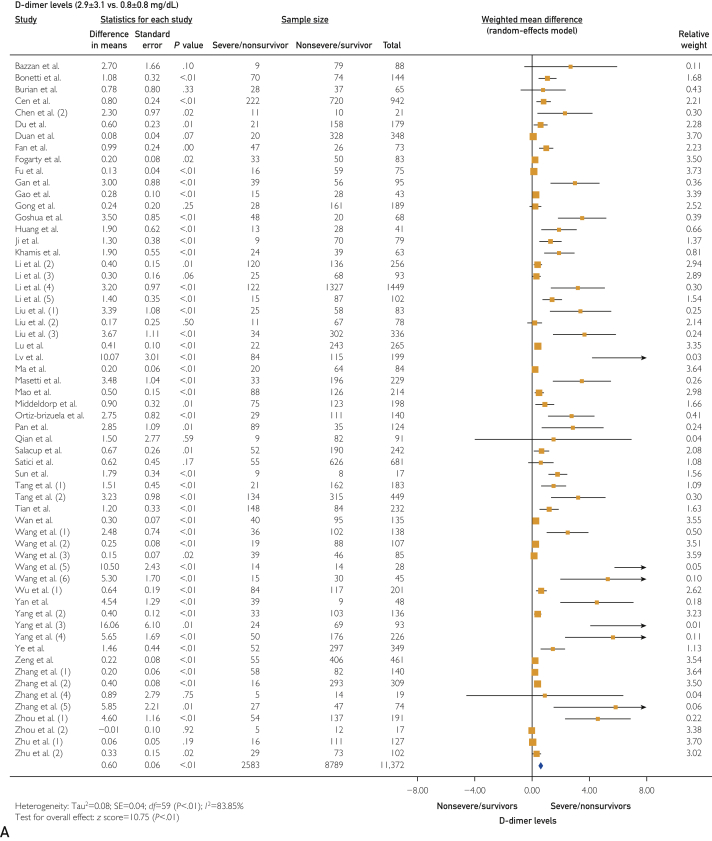
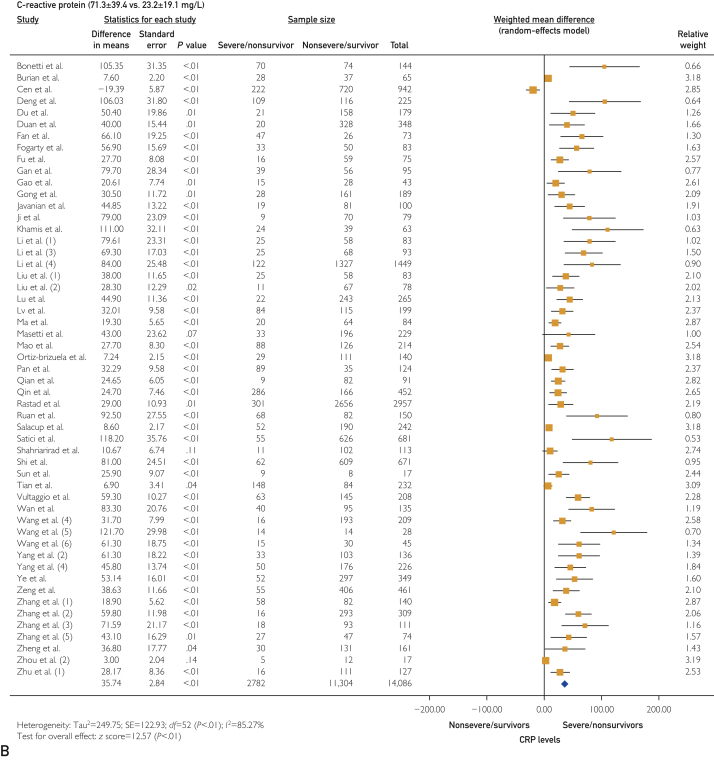
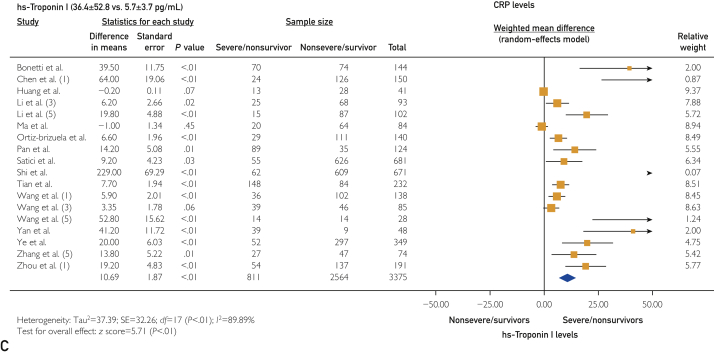
The platelet count was statistically lower in the severe/nonsurvivor COVID-19 group (171±34 vs 197±30 ×109/L; WMD, −11.75 [95% CI, −16.10 to −7.39]; I2=76.32%; P<.001). Thromboinflammatory biomarkers were elevated in the severe/nonsurvivor group compared with the nonsevere/survivor group, including D-dimer levels (2.9±3.1 vs 0.8±0.8 mg/dL [to convert values to nmol/L, multiply by 5.476]; WMD, 0.60 [95% CI, 0.49 to 0.71]; I2=83.85%; P<.001) (Figure 2A), prothrombin time (13.9±2.0 vs 12.7±1.3 s; WMD, 0.75 [95% CI, 0.57 to 0.78]; I2=37.01%; P<.001), activated partial thromboplastin time (36.6±8.7 vs 35.1±5 s; WMD, 0.81 [95% CI, 0.03 to 1.59]; I2=70.84%; P=.04), fibrinogen (4.4±1.1 vs 4.0±1.1 g/L; WMD, 0.42 [95% CI, 0.18 to 0.67]; I2=61.88%; P<.001), C-reactive protein (CRP) (71.3±39.4 vs 23.2±19.1 mg/L; WMD, 35.74 [95% CI, 30.16 to 41.31]; I2=85.27%; P<.001) (Figure 2B), high-sensitivity (hs)–CRP (96.6±24.9 vs 22.9±6.5 mg/L; WMD, 62.68 [95% CI, 45.27 to 80.09]; I2=0%; P<.001), interleukin 6 (IL-6) (49.3±35.7 vs 12.5±12.3 pg/L; WMD, 22.81 [95% CI, 17.90 to 27.72]; I2=90.42%; P<.001), ferritin (1367.0±744.5 vs 635.1±323.0 ng/mL [to convert values to μg/L, multiply by 1]; WMD, 506.15 [95% CI, 356.24 to 656.06]; I2=52.02%; P<.001), hs-troponin I (36.4±52.8 vs 5.7±3.7 pg/mL [to convert values to μg/L, multiply by 1]; WMD, 10.69 [95% CI, 7.02 to 14.36]; I2=89.89%; P<.001) (Figure 2C), and lactate dehydrogenase (LDH) (448.6±147.1 vs 267.5±67.3 U/L [to convert values to μkat/L, multiply by 0.0167]; WMD, 155.40 [95% CI, 114.41 to 196.40]; I2=88.07%; P<.001).
Figure 2.
Forest plots showing differences in thromboinflammatory biomarkers between severe/nonsurvivor and nonsevere/survivor groups for D-dimer levels (2.9±3.1 vs 0.8±0.8 mg/dL) (A), C-reactive protein (CRP) levels (71.3±39.4 vs 23.2±19.1 mg/L) (B), and high-sensitivity (hs) troponin I levels (36.4±52.8 vs 5.7±3.7 pg/mL).
As expected, the severe/nonsurvivor group had higher mortality (OR, 28.14 [95% CI, 14.99 to 52.83]; I2=0%; P<.001), higher incidence of acute cardiac injury (OR, 12.86 [95% CI, 5.11 to 32.41]; I2=75.12%; P<.001), and higher incidence of acute respiratory distress syndrome (OR, 59.83 [95% CI, 30.40 to 117.76]; I2=73.41%; P<.001) compared with the nonsevere/survivor group.
Sensitivity Analyses
Sensitivity analysis was performed by separating disease severity from survivorship. Thus, a separate analysis was done comparing severe vs nonsevere disease, and another analysis compared survivors to nonsurvivors. In general, both analyses provided similar conclusions (Table 2). Additionally, the WMDs in thromboinflammatory biomarkers were compared between studies conducted in China (n=60) and other countries (n=15) to address the overlap of the study population in the published studies from China (Table 1). The non-Chinese population had a higher comorbidity burden, including hypertension, diabetes, cardiac or cerebrovascular disease, chronic kidney disease, and chronic obstructive pulmonary disease. Otherwise, results were similar in the 2 populations (Supplemental Table 5, available online at http://mcpiqojournal.org). Also, there were significant differences between the groups in the WMD for platelet count, fibrinogen level, and hs-troponin I level. The difference in D-dimer levels between the severe/nonsurvivor and the nonsevere/survivor groups was more pronounced in the non-Chinese population. In contrast, the difference between the 2 groups in the CRP levels was more pronounced in the Chinese population (Supplemental Table 5). Similar results were noted when studies were stratified between China and Europe/United States to determine racial/ethnic differences in thromboinflammatory profile (Supplemental Table 5).
Table 2.
Weighted Mean Differences and Odds Ratios for Biomarkers and Outcomes for the 2 Comparisons of Severe vs Nonsevere (47 Studies, 7388 Patients) and Nonsurvivor vs Survivor (28 Studies, 9664 Patients)a,b
| Parameter | Severe vs nonsevere |
Nonsurvivor vs survivor |
||
|---|---|---|---|---|
| Mean±SD | WMD/OR (95% CI) | Mean±SD | WMD/OR (95% CI) | |
| Platelet count (×109/L) | 179±33 vs 195±32 (n=5135) | WMD: −8.01 (−14.51 to −1.51); I2=63.76%; P<.001 | 159±33 vs 201±28 (n=4518) | WMD: −26.33 (−35.99 to −16.66); I2=84.75%; P<.001 |
| D-dimer (mg/dL) | 2.9±3.7 vs 0.8±0.9 (n=5863) | WMD: 0.43 (0.32 to 0.54); I2=83.08%; P<.001 | 3±1.8 vs 0.9±0.7 (n=5509) | WMD: 1.35 (0.99 to 1.71); I2=85.58%; P<.001 |
| Prothrombin time (s) | 13.5±2.3 vs 12.4±1.2 (n=2533) | WMD: 0.53 (0.39 to 0.66); I2=0%; P<.001 | 14.3±1.6 vs 13.1±1.2 (n=3951) | WMD: 1.01 (0.77 to 1.26); I2=35.39%; P<.001 |
| aPTT (s) | 33.5±5 vs 33.6±5 (n=2559) | WMD: 0.38 (−0.84 to 1.61); I2=76.51%; P=.54 | 41.1±11 vs 37.1±4.6 (n=2797) | WMD: 1.14 (0.12 to 2.16); I2=59.94%; P=.03 |
| Fibrinogen (g/L) | 4.3±1.5 vs 3.5±1.2 (n=1100) | WMD: 0.62 (0.26 to 0.99); I2=59.14%; P<.001 | 4.6±0.6 vs 4.4±0.7 (n=3520) | WMD: 0.23 (−0.09 to 0.56); I2=58.32%; P=.16 |
| CRP (mg/L) | 59.2±34.8 vs 19.1±16.3 (n=6099) | WMD: 30.42 (24.31 to 36.53); I2=85.74%; P<.001 | 97±37.1 vs 31.7±22 (n=7987) | WMD: 58.58 (41.23 to 75.93); I2=84.39%; P<.001 |
| hs-CRP (mg/L) | 102.4±32 vs 25.4±4.8 (n=486) | WMD: 62.72 (37.97 to 87.46); I2=13.07%; P<.001 | Not enough data | Not enough data |
| Interleukin 6 (pg/L) | 49.2±32.1 vs 12.6±13.1 (n=2385) | WMD: 28.14 (19.93 to 36.35); I2=91.41%; P<.001 | 49.4±46.7 vs 12.2±10.6 (n=1958) | WMD: 15.30 (7.06 to 25.53); I2=86.71%; P<.001 |
| Ferritin (ng/mL) | 1109±371 vs 584±319 (n=1154) | WMD: 320.92 (1197.54 to 444.30); I2=12.06%; P<.001 | 1626±947 vs 687±341 (n=3179) | WMD: 700.21 (497.52 to 902.90); I2=27.06%; P<.001 |
| hs-Troponin I (pg/mL) | 22.5±23.5 vs 5.5±4.5 (n=972) | WMD: 5.39 (1.84 to 8.94); I2=88.81%; P<.001 | 50.2±70.3 vs 6±3 (n=2403) | WMD: 18.68 (10.92 to 26.44); I2=75.69%; P<.001 |
| LDH (U/L) | 377±94 vs 242±54 (n=3371) | WMD: 124.04 (75.42 to 172.66); I2=90.08%; P<.001 | 561±134 vs 303±70 (n=5784) | WMD: 188.77 (153.07 to 224.47); I2=12.57%; P<.001 |
| Mortality | 30.1% (115 of 383) vs. 1.3% (11 of 862) (n=1319) | OR: 28.14 (14.99 to 52.83); I2=0%; P<.001 | NA | NA |
| Acute cardiac injury | 24.8% (38 of 153) vs. 9.0% (36 of 402) (n=555) | OR: 4.73 (1.64 to 13.67); I2=57.83%; P<.001 | 56.6% (172 of 304) vs. 3.8% (64 of 1,668) (n=1972) | OR: 43.83 (15.54 to 123.65); I2=59.33%; P<.001 |
| ARDS | 67.2% (76 of 133) vs. 3.6% (12 of 338) (n=471) | OR: 33.49 (16.75 to 66.98); I2=17.30%; P<.001 | 81.9% (334 of 408) vs. 4.4% (94 of 2,155) (n=2563) | OR: 73.80 (29.66 to 1183.61); I2=83.21%; P<.001 |
aPTT = activated partial thromboplastin time; ARDS = acute respiratory distress syndrome; CRP = C-reactive protein; hs = high-sensitivity; LDH = lactate dehydrogenase; NA = not applicable; OR = odds ratio; WMD = weighted mean difference.
SI conversion factors: To convert D-dimer values to nmol/L, multiply by 5.476; to convert ferritin values to μg/L, multiply by 1; to convert hs-troponin I values to μg/L, multiply by 1; to convert LDH values to μkat/L, multiply by 0.0167.
Discussion
This systematic review and meta-analysis of 75 published articles and 17,052 COVID-19–positive patients is the largest meta-analysis on the topic and provides a comprehensive analysis of demographic factors and thromboinflammatory biomarkers associated with COVID-19 severity and mortality. In our article, we summarize all the available evidence on the biomarkers of both thrombosis and inflammation in patients with COVID-19 and further analyze the published literature on the differential impact of region and race/ethnicity in the COVID-19 thromboinflammatory milieu. Major findings of our study were (1) severe COVID-19 infection involved older patients with a high proportion of men; (2) comorbidities associated with disease severity and COVID-19–associated mortality included hypertension, diabetes, chronic kidney disease, cardiac or cerebrovascular disease, malignancy, and chronic obstructive pulmonary disease; (3) patients with severe COVID-19 had lower platelet counts compared with patients with nonsevere COVID-19; and (4) the severe/nonsurvivor COVID-19 group had elevated markers of thrombosis, inflammation, and cardiac injury: elevated D-dimer, fibrinogen, CRP, hs-CRP, IL-6, ferritin, hs-troponin I, and LDH levels.
COVID-19 has been described as a thromboinflammatory syndrome.81,82 Among patients with severe disease and mortality, diffuse endothelial dysfunction, widespread coagulopathy, and complement-induced thrombosis have been noted to result in the development of systemic microangiopathy and thromboembolism.83 The diffuse endothelial dysfunction, coupled with a hyperinflammatory response to the COVID-19 infection, is the harbinger of cytokine storm associated with poor clinical outcomes.84 Inflammation and vascular endothelial dysfunction predominantly affect the lungs in the early stages, resulting in diffuse alveolar damage and formation of pulmonary microthrombi affecting both ventilation and perfusion (termed pulmonary intravascular coagulopathy), which is distinct from disseminated intravascular coagulation.85, 86, 87, 88 Our findings resonate with those of prior analyses.77,78,89, 90, 91, 92, 93, 94 With incremental evidence, the thromboinflammatory biomarkers continue to hold their importance in predicting poor prognosis and severity of COVID-19 infection, especially D-dimer, CRP, and LDH.48,58,72,95,96
We observed that a substantial proportion of patients with severe COVID-19 infection had comorbidities of hypertension, diabetes, chronic kidney disease, cardiac or cerebrovascular disease, and chronic obstructive pulmonary disease. All these disorders are associated with endothelial dysfunction manifested by reduced nitric oxide bioavailability as an early event in their pathogenesis.97, 98, 99, 100, 101 Coronaviruses have a unique affinity to the host angiotensin-converting enzyme 2 receptors, which are expressed in the vascular endothelium.102,103 The enhanced endothelial dysfunction due to COVID-19 among patients with preexisting endothelial dysfunction (due to comorbidities) promotes the likelihood of a cytokine storm leading to adverse clinical outcomes and death.
Our analysis further revealed that patients with severe COVID-19 infection and mortality with COVID-19 had higher levels of D-dimer and fibrinogen. Increased D-dimer levels support the notion of pulmonary intravascular coagulopathy as an early form of disseminated intravascular coagulation and support secondary fibrinolytic conditions in these patients. Several prior studies have reported the association of elevated D-dimer levels with poor prognosis of patients.78,104 However, D-dimer levels need to be interpreted with caution in COVID-19–infected patients. The major issues identified with measuring D-dimer levels include the following. First, D-dimer has poor specificity, and elevated levels are often seen with advanced age, African American race, female sex, active malignancy, surgery, pregnancy, immobility, cocaine use, connective tissue disorders, end-stage renal disease, and prior thromboembolic disease. Second, D-dimer reflects a later stage in the hemostatic process and is released when a clot is degraded by the fibrinolytic processes. Third, the studies reporting D-dimer levels had considerable variation in the units for D-dimer levels, making the pooling of the uncorrected levels unreliable. Finally, D-dimer levels do not capture the dynamic effects of functional interactions among platelets, endothelium, and fibrinolytic processes.105
The elevation in the inflammatory biomarkers, including CRP, hs-CRP, ferritin, and IL-6 among severe COVID-19 infections noted in our analysis, is in agreement with findings reported in previous publications.90,106 In a study by Herold et al106 with 89 COVID-19–positive patients, biomarkers of inflammation, including IL-6 and CRP, were highly predictive of the need for mechanical ventilation, and LDH was highly predictive of respiratory failure.
Prior studies have found racial/ethnic differences in the baseline levels of thromboinflammatory biomarkers, including D-dimer levels and CRP.107 Because the inherent differences in the thromboinflammatory milieu across races could theoretically affect clinical outcomes, especially in COVID-19 infection, we evaluated the differences in a subgroup analysis. Most reported studies included only the East Asian population (80% of studies with Chinese patients) with only 15 studies from other countries. Among the included studies, the non-Chinese study participants had a higher prevalence of comorbidities, including hypertension, diabetes, cardiac or cerebrovascular disease, chronic kidney disease, chronic liver disease, and chronic obstructive pulmonary disease. Also, the difference in the D-dimer levels between the severe/nonsurvivor and the nonsevere/survivor groups was more pronounced in the non-Chinese population. In contrast, the difference between CRP levels was more pronounced in the Chinese population (Supplemental Table 5). It can be hypothesized that a difference in the comorbidity burden and thromboinflammatory milieu between the East Asians, Whites, and African Americans could be contributory to the higher case fatality rate noted in Europe and the United States. However, because of the limited published literature from other countries, our confidence in these estimates is low. It remains to be determined whether racial differences in the thromboinflammatory milieu affect COVID-19 outcomes.
Our study has several limitations. In our analysis, we combined the subgroups of severe COVID-19 with nonsurvivors, which could lead to potential confounders. We addressed the confounders by performing a subgroup analysis comparing severe vs nonsevere COVID-19 and nonsurvivors vs survivors, and the results were consistent with the main analysis (Table 2). Additionally, the included studies had heterogeneous populations with differing burdens of comorbidities and not all outcomes were available in all included studies. This issue was reflected in the Higgins I2 statistic with 57% reflecting significant heterogeneity and 29% reflecting moderate heterogeneity in the analyzed biomarkers. Another confounder was that most of the studies were Chinese with potential overlapping populations artificially amplifying the effect of certain comorbidities and biomarkers (multiple studies reported from the same hospital, Table 1). To address this limitation, WMDs among thromboinflammatory biomarkers were compared according to the country of origin of the study, ie, Chinese vs non-Chinese (Supplemental Table 5). However, because data from non-Chinese countries was lacking, a definite conclusion could not be drawn about the differential weightage of comorbidities and biomarkers among racial/ethnic groups. As the literature continues to increase, it would be imperative to identify the potential role of genetics in the prevalence of poor clinical outcomes among African Americans and Whites compared with East Asians. Another problem with the available data was that the values for D-dimer levels (concerning units of measurement) varied considerably among the studies, and several studies misreported the measuring unit, making the values 1000 times smaller or higher.105 While performing our analysis, these values were adjusted to reflect appropriate differences between the 2 groups. Additionally, substantial heterogeneity among studies coupled with the high risk of bias (due to unadjusted analyses and unbalanced groups) reduces confidence in the interpretation of the results. Publication bias is also highly likely in a field that primarily consists of small unregistered observational studies.
Conclusion
Thromboinflammatory biomarkers (D-dimer, fibrinogen, CRP, hs-CRP, ferritin, and IL-6) and indicators of cardiac damage (hs-troponin I) on admission were associated with the severity and mortality of COVID-19 infection. Comorbidities conferring higher risk coupled with thromboinflammatory biomarkers might assist in the development of risk prediction models for the severity and prognosis of COVID-19. Such models could potentially aid in the selection of patients to receive early therapeutic strategies, eg, remdesivir therapy, and improve clinical outcomes.
Footnotes
Potential Competing Interests: The authors report no competing interests.
Supplemental material can be found online at http://mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Bazzan M., Montaruli B., Sciascia S., Cosseddu D., Norbiato C., Roccatello D. Low ADAMTS 13 plasma levels are predictors of mortality in COVID-19 patients. Intern Emerg Med. 2020;15(5):861–863. doi: 10.1007/s11739-020-02394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonetti G., Manelli F., Patroni A., et al. Laboratory predictors of death from coronavirus disease 2019 (COVID-19) in the area of Valcamonica, Italy. Clin Chem Lab Med. 2020;58(7):1100–1105. doi: 10.1515/cclm-2020-0459. [DOI] [PubMed] [Google Scholar]
- 3.Burian E., Jungmann F., Kaissis G.A., et al. Intensive care risk estimation in COVID-19 pneumonia based on clinical and imaging parameters: experiences from the Munich cohort. J Clin Med. 2020;9(5):1514. doi: 10.3390/jcm9051514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cen Y., Chen X., Shen Y., et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019—a multi-centre observational study. Clin Microbiol Infect. 2020;26(9):1242–1247. doi: 10.1016/j.cmi.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C., Chen C., Yan J.T., Zhou N., Zhao J.P., Wang D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19 [in Chinese] Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(7):567–571. doi: 10.3760/cma.j.cn112148-20200225-00123. [DOI] [PubMed] [Google Scholar]
- 6.Chen G., Wu D., Guo W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X., Zhao B., Qu Y., et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;71(8):1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng Y., Liu W., Liu K., et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020;133(11):1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du R.-H., Liang L.-R., Yang C.-Q., et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study [published correction appears in Eur Respir J. 2020;56(3):2050524] Eur Respir J. 2020;55(5):2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan J., Wang X., Chi J., et al. Correlation between the variables collected at admission and progression to severe cases during hospitalization among patients with COVID-19 in Chongqing. J Med Virol. 2020;92(11):2616–2622. doi: 10.1002/jmv.26082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan H., Zhang L., Huang B., et al. Cardiac injuries in patients with coronavirus disease 2019: not to be ignored. Int J Infect Dis. 2020;96:294–297. doi: 10.1016/j.ijid.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogarty H., Townsend L., Ni Cheallaigh C., et al. COVID19 coagulopathy in Caucasian patients. Br J Haematol. 2020;189(6):1044–1049. doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu J., Kong J., Wang W., et al. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: a retrospective study in Suzhou China. Thromb Res. 2020;192:3–8. doi: 10.1016/j.thromres.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gan J., Li J., Li S., Yang C. Leucocyte subsets effectively predict the clinical outcome of patients with COVID-19 pneumonia: a retrospective case-control study. Front Public Health. 2020;8:299. doi: 10.3389/fpubh.2020.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Y., Li T., Han M., et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92(7):791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong J., Ou J., Qiu X., et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71(15):833–840. doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020;395(10223):496] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Javanian M., Bayani M., Shokri M., et al. Clinical and laboratory findings from patients with COVID-19 pneumonia in Babol North of Iran: a retrospective cohort study. Rom J Intern Med. 2020;58(3):161–167. doi: 10.2478/rjim-2020-0013. [DOI] [PubMed] [Google Scholar]
- 20.Ji M., Yuan L., Shen W., et al. Characteristics of disease progress in patients with coronavirus disease 2019 in Wuhan, China. Epidemiol Infect. 2020;148:e94. doi: 10.1017/S0950268820000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khamis F., Al-Zakwani I., Al Naamani H., et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J Infect Public Health. 2020;13(7):906–913. doi: 10.1016/j.jiph.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li K., Wu J., Wu F., et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55(6):327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Zhao K., Wei H., et al. Dynamic relationship between D-dimer and COVID-19 severity. Br J Haematol. 2020;190(1):e24–e27. doi: 10.1111/bjh.16811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L., Yang L., Gui S., et al. Association of clinical and radiographic findings with the outcomes of 93 patients with COVID-19 in Wuhan, China. Theranostics. 2020;10(14):6113–6121. doi: 10.7150/thno.46569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q., Cao Y., Chen L., et al. Hematological features of persons with COVID-19. Leukemia. 2020;34(8):2163–2172. doi: 10.1038/s41375-020-0910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li K., Chen D., Chen S., et al. Predictors of fatality including radiographic findings in adults with COVID-19. Respir Res. 2020;21(1):146. doi: 10.1186/s12931-020-01411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X., Li Z., Liu S., et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm Sin B. 2020;10(7):1205–1215. doi: 10.1016/j.apsb.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W., Tao Z.-W., Wang L., et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q., Song N.C., Zheng Z.K., Li J.S., Li S.K. Laboratory findings and a combined multifactorial approach to predict death in critically ill patients with COVID-19: a retrospective study. Epidemiol Infect. 2020;148:e129. doi: 10.1017/S0950268820001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu H., Ai J., Shen Y., et al. A descriptive study of the impact of diseases control and prevention on the epidemics dynamics and clinical features of SARS-CoV-2 outbreak in Shanghai, lessons learned for metropolis epidemics prevention. medRxiv. 2020 doi: 10.1101/2020.02.19.20025031. Preprint posted online February 23, 2020. [DOI] [Google Scholar]
- 31.Lv Z., Cheng S., Le J., et al. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: a retrospective cohort study. Microbes Infect. 2020;22(4-5):195–199. doi: 10.1016/j.micinf.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma K.-L., Liu Z.-H., Cao C.-F., et al. COVID-19 myocarditis and severity factors: an adult cohort study. medRxiv. 2020 doi: 10.1101/2020.03.19.20034124. Preprint posted online March 23, 2020. [DOI] [Google Scholar]
- 33.Masetti C., Generali E., Colapietro F., et al. Humanitas Covid-19 Task Force High mortality in COVID-19 patients with mild respiratory disease. Eur J Clin Invest. 2020;50(9):e13314. doi: 10.1111/eci.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortiz-Brizuela E., Villanueva-Reza M., González-Lara M.F., et al. Clinical and epidemiological characteristics of patients diagnosed with COVID-19 in a tertiary care center in Mexico City: a prospective cohort study [published correction appears in Rev Invest Clin. 2020;72(4):252-258] Rev Invest Clin. 2020;72(3):165–177. doi: 10.24875/RIC.20000211. [DOI] [PubMed] [Google Scholar]
- 37.Pan F., Yang L., Li Y., et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int J Med Sci. 2020;17(9):1281–1292. doi: 10.7150/ijms.46614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian G.-Q., Yang N.-B., Ding F., et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. 2020;113(7):474–481. doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin C., Zhou L., Hu Z., et al. Dysregulation of immune response in patients with coronavirus 19 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rastad H., Karim H., Ejtahed H.-S., et al. Risk and predictors of in-hospital mortality from COVID-19 in patients with diabetes and cardiovascular disease. Diabetol Metab Syndr. 2020;12:57. doi: 10.1186/s13098-020-00565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China [published correction appears in Intensive Care Med. 2020;46(6):1294-1297] Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salacup G., Lo K.B., Gul F., et al. Characteristics and clinical outcomes of COVID-19 patients in an underserved-inner city population: a single tertiary center cohort. J Med Virol. 2021;93(1):416–423. doi: 10.1002/jmv.26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satici C., Demirkol M.A., Altunok E.S., et al. Performance of pneumonia severity index and CURB-65 in predicting 30-day mortality in patients with COVID-19. Int J Infect Dis. 2020;98:84–89. doi: 10.1016/j.ijid.2020.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shahriarirad R., Khodamoradi Z., Erfani A., et al. Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID-19) in the South of Iran. BMC Infect Dis. 2020;20(1):427. doi: 10.1186/s12879-020-05128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi S., Qin M., Cai Y., et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y., Dong Y., Wang L., et al. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. J Autoimmun. 2020;112:102473. doi: 10.1016/j.jaut.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian J., Yuan X., Xiao J., et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vultaggio A., Vivarelli E., Virgili G., et al. Prompt predicting of early clinical deterioration of moderate-to-severe COVID-19 patients: usefulness of a combined score using IL-6 in a preliminary study. J Allergy Clin Immunol Pract. 2020;8(8):2575–2581.e2. doi: 10.1016/j.jaip.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan S., Xiang Y., Fang W., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang D., Yin Y., Hu C., et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24(1):188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C.-Z., Hu S.-L., Wang L., Li M., Li H.-T. Early risk factors of the exacerbation of coronavirus disease 2019 pneumonia. J Med Virol. 2020;92(11):2593–2599. doi: 10.1002/jmv.26071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang G., Wu C., Zhang Q., et al. C-reactive protein level may predict the risk of COVID-19 aggravation. Open Forum Infect Dis. 2020;7(5):ofaa153. doi: 10.1093/ofid/ofaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang F., Yang Y., Dong K., et al. Clinical characteristics of 28 patients with diabetes and COVID-19 in Wuhan, China. Endocr Pract. 2020;26(6):668–674. doi: 10.4158/EP-2020-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang F., Hou H., Luo Y., et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020;5(10):e137799. doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan Y., Yang Y., Wang F., et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001343. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Q., Xie L., Zhang W., et al. Analysis of the clinical characteristics, drug treatments and prognoses of 136 patients with coronavirus disease 2019. J Clin Pharm Ther. 2020;45(4):609–616. doi: 10.1111/jcpt.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang A.-P., Li H.-M., Tao W.-Q., et al. Infection with SARS-CoV-2 causes abnormal laboratory results of multiple organs in patients. Aging (Albany NY) 2020;12(11):10059–10069. doi: 10.18632/aging.103255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Q., Zhou Y., Wang X., et al. Effect of hypertension on outcomes of adult inpatients with COVID-19 in Wuhan, China: a propensity score-matching analysis. Respir Res. 2020;21(1):172. doi: 10.1186/s12931-020-01435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye W., Chen G., Li X., et al. Dynamic changes of D-dimer and neutrophil-lymphocyte count ratio as prognostic biomarkers in COVID-19. Respir Res. 2020;21(1):169. doi: 10.1186/s12931-020-01428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeng Z., Ma Y., Zeng H., et al. Simple nomogram based on initial laboratory data for predicting the probability of ICU transfer of COVID-19 patients: multicenter retrospective study. J Med Virol. 2021;93(1):434–440. doi: 10.1002/jmv.26244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J.-J., Dong X., Cao Y.-Y., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 67.Zhang X., Tan Y., Ling Y., et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- 68.Zhang J., Yu M., Tong S., Liu L.-Y., Tang L.-V. Predictive factors for disease progression in hospitalized patients with coronavirus disease 2019 in Wuhan, China. J Clin Virol. 2020;127:104392. doi: 10.1016/j.jcv.2020.104392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y., Cao W., Jiang W., et al. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J Thromb Thrombolysis. 2020;50(3):580–586. doi: 10.1007/s11239-020-02182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q., Wei Y., Chen M., Wan Q., Chen X. Clinical analysis of risk factors for severe COVID-19 patients with type 2 diabetes. J Diabetes Complications. 2020;34(10):107666. doi: 10.1016/j.jdiacomp.2020.107666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng F., Tang W., Li H., Huang Y.-X., Xie Y.-L., Zhou Z.-G. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24(6):3404–3410. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- 72.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published corrections appear in Lancet. 2020;395(10229):1038] Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Y., Zhang Z., Tian J., Xiong S. Risk factors associated with disease progression in a cohort of patients infected with the 2019 novel coronavirus. Ann Palliat Med. 2020;9(2):428–436. doi: 10.21037/apm.2020.03.26. [DOI] [PubMed] [Google Scholar]
- 74.Zhu Z., Cai T., Fan L., et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu Y., Du Z., Zhu Y., Li W., Miao H., Li Z. Evaluation of organ function in patients with severe COVID-19 infections. Med Clin (Barc) 2020;155(5):191–196. doi: 10.1016/j.medcli.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu J., Zhong Z., Ji P., et al. Clinicopathological characteristics of 8697 patients with COVID-19 in China: a meta-analysis [published correction appears in Fam Med Community Health. 2020;8(2):e000406corr1] Fam Med Community Health. 2020;8(2):e000406. doi: 10.1136/fmch-2020-000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fu L., Wang B., Yuan T., et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80(6):656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shah S., Shah K., Patel S.B., et al. Elevated D-dimer levels are associated with increased risk of mortality in COVID-19: a systematic review and meta-analysis. medRxiv. 2020 doi: 10.1101/2020.04.29.20085407. Preprint posted online May 5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wells G.A., Shea B., O’Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 81.Ciceri F., Beretta L., Scandroglio A.M., et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henry B.M., Vikse J., Benoit S., Favaloro E.J., Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:167–173. doi: 10.1016/j.cca.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perico L., Benigni A., Casiraghi F., Ng L.F.P., Renia L., Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2021;17(1):46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zeng Y., Zhang B., Zhang X., Yi C. Clinical characteristics of 9 cancer patients with SARS-CoV-2 infection. Chin Med. 2020;15:47. doi: 10.1186/s13020-020-00328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McGonagle D., Sharif K., O'Regan A., Bridgewood C. Interleukin-6 use in COVID-19 pneumonia related macrophage activation syndrome. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Menter T., Haslbauer J.D., Nienhold R., et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lax S.F., Skok K., Zechner P., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiong M., Liang X., Wei Y.-D. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Br J Haematol. 2020;189(6):1050–1052. doi: 10.1111/bjh.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeng F., Huang Y., Guo Y., et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tian W., Jiang W., Yao J., et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020;92(10):1875–1883. doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Z.-L., Hou Y.-L., Li D.-T., Li F.-Z. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand J Clin Lab Invest. 2020;80(6):441–447. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 94.Zheng Z., Peng F., Xu B., et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu F., Li L., Xu M., et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fan B.E., Chong V.C.L., Chan S.S.W., et al. Hematologic parameters in patients with COVID-19 infection [published correction appears in Am J Hematol. 2020;95(11):1442] Am J Hematol. 2020;95(6):E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 97.Hermann M., Flammer A., Lüscher T.F. Nitric oxide in hypertension. J Clin Hypertens (Greenwich) 2006;8(12, suppl 4):17–29. doi: 10.1111/j.1524-6175.2006.06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Honing M.L., Morrison P.J., Banga J.D., Stroes E.S., Rabelink T.J. Nitric oxide availability in diabetes mellitus. Diabetes Metab Rev. 1998;14(3):241–249. doi: 10.1002/(sici)1099-0895(1998090)14:3<241::aid-dmr216>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 99.Kinlay S., Ganz P. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am J Cardiol. 1997;80(9A):11I–16I. doi: 10.1016/s0002-9149(97)00793-5. [DOI] [PubMed] [Google Scholar]
- 100.Malhotra R., Hess D., Lewis G.D., Bloch K.D., Waxman A.B., Semigran M.J. Vasoreactivity to inhaled nitric oxide with oxygen predicts long-term survival in pulmonary arterial hypertension. Pulm Circ. 2011;1(2):250–258. doi: 10.4103/2045-8932.83449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marrazzo F., Spina S., Zadek F., et al. Protocol of a randomised controlled trial in cardiac surgical patients with endothelial dysfunction aimed to prevent postoperative acute kidney injury by administering nitric oxide gas. BMJ Open. 2019;9(7):e026848. doi: 10.1136/bmjopen-2018-026848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gheblawi M., Wang K., Viveiros A., et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bavishi C., Maddox T.M., Messerli F.H. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiol. 2020;5(7):745–747. doi: 10.1001/jamacardio.2020.1282. [DOI] [PubMed] [Google Scholar]
- 104.Lippi G., Favaloro E.J. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis [letter] Thromb Haemost. 2020;120(5):876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chaudhary R., Kreutz R.P., Bliden K.P., Tantry U.S., Gurbel P.A. Personalizing antithrombotic therapy in COVID-19: role of thromboelastography and thromboelastometry [letter] Thromb Haemost. 2020;120(11):1594–1596. doi: 10.1055/s-0040-1714217. [DOI] [PubMed] [Google Scholar]
- 106.Herold T., Jurinovic V., Arnreich C., et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(1):128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaudhary R., Bliden K.P., Kreutz R.P., et al. Race-related disparities in COVID-19 thrombotic outcomes: beyond social and economic explanations. EClinicalMedicine. 2020;29:100647. doi: 10.1016/j.eclinm.2020.100647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.