Abstract
Background
Data specifically addressed to whether atrial fibrillation (AF) would contribute to increasing the risk for severe forms of novel coronavirus disease (COVID-19) or worse prognosis remain unclear. Hence, we sought to assess the association of permanent AF with in-hospital outcomes in patients with COVID-19.
Methods
This was a single-centered, retrospective, observational study including consecutive hospitalized patients with COVID-19. The primary outcome for the study was defined as all cause in-hospital mortality. Clinical characteristics and outcomes of patients with AF were compared to patients without AF.
Results
Three hundred and fifty hospitalized COVID-19 patients (median age of 55 years, 55.4% men) were enrolled. Of them 40 (11.4%) had AF. Patients with AF were older; were more likely to have co-morbidities, abnormal chest radiography findings and deteriorated laboratory parameters such as D-dimer, troponin, albumin, urea. In-hospital mortality was higher in patients with AF compared to patients without AF (32.5% vs. 13.5%, log-rank p = 0.002, RR 2.40). The number of patients who needed intensive care unit (55% vs. 31%, p = 0.002) and invasive mechanical ventilation (35% vs 15.2%, p = 0.002) were also higher in the AF group. In addition, length of in-hospital stay was longer in patients with AF (median 8 vs. 7 days, p = 0.008). After adjustment for age and co-morbidities, multivariable analyses revealed that AF (HR: 2.426, 95% CI: 1.089–5.405, p = 0.032) was independently associated with in-hospital death.
Conclusions
AF was seen with together markers of severe COVID-19, and the presence of AF was an independent predictor of in-hospital mortality in patients with COVID-19.
Keywords: Atrial fibrillation, COVID-19, Mortality, Outcomes, Prognosis
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also known as the new coronavirus, infects humans and causes novel coronavirus disease 2019 (COVID-19), a highly transmittable disease [1]. As of 20 Oct 2020, there were more than 40 million patients infected globally with over 1 million deaths [2]. Although the majority of COVID-19 patients present with mild illness, more than 15% have developed severe disease to multi-organ failure [3]. SARS-CoV-2 infection presents with a wide of clinical manifestations including cardiac involvement. A high proportion of severe COVID-19 patients have co-morbidities [4]. COVID-19 is mostly characterized by symptoms in the respiratory tract; however older age, hypertension, coronary artery disease (CAD), diabetes mellitus (DM) and cerebrovascular disease frequently accompany COVID-19 infections increasing morbidity and mortality of these patients [5,6]. In addition, recent findings have highlighted the extra-pulmonary thromboembolic complications of the disease [[7], [8], [9]].
Atrial fibrillation (AF) is one of the most common sustained cardiac arrhythmia encountered in clinical practice. It is usually associated with aging, a variety of cardiovascular co-morbidities and increased thromboembolic risk [10]. AF has been reported to occur in 5–22% of hospitalized patients with SARS-CoV-2 infection [11,12]. COVID-19 patients with other cardiac diseases, such as chronic heart failure (CHF) and hypertension, have been shown that associated with worsening clinical outcomes [13,14]. However, the mechanisms by which cases with AF may be at increased risk are not known [15]. Furthermore, data specifically addressed to whether AF would contribute to increasing the risk for severe forms of COVID-19, worse prognosis, or even higher mortality are scanty. Therefore, in this report, we aimed to investigate the association of permanent AF with in-hospital outcomes in patients with COVID-19.
Methods
Study design and participants
The present retrospective, observational cohort study consisted of all consecutive hospitalized adult patients with confirmed positive nasopharyngeal or oropharyngeal SARS-CoV-2 reverse-transcriptase–polymerase-chain-reaction assay who were admitted to Manisa-Merkezefendi State Hospital between April 1 and July 1, 2020. The study received approval by the Institutional Review Board at the Manisa Celal Bayar University (Approval No: 24/08/2020–96). Approval was also obtained from The Ministry of Health of Turkish Republic. Informed consent was waived because of the retrospective nature of the study.
Data collection
All demographic characteristics (age and sex), symptoms, physical examination findings, pre-existing co-morbidities, laboratory parameters, medications and outcome data were extracted from the in-hospital medical records. All patients underwent chest computed tomography (CT), and tomographic findings were abstracted from the radiology reports and categorized based on the most abnormal findings. Complications including intensive care unit (ICU) admission and respiratory failure requiring mechanical ventilation were noted. After the patients were admitted to the hospital, their myocardial troponin I (cTnI), D-dimer, C reactive protein (CRP), albumin, ferritin, serum electrolyte, lymphocyte concentrations were recorded. We evaluated the medical therapy during hospitalization. The electrocardiogram (ECG) recording device was MAC 2000, GE Medical Systems Information Technologies, Inc., Milwaukee, USA. ECGs were recorded at 25 mm/s and 1 mV/cm according to standard protocol. The first ECG done at the time of presentation was considered the admission ECG. All standard 12‑lead ECGs recorded during hospitalization were reviewed. ECG analysis was independently performed by two experienced cardiologists. The diagnosis of AF was based on a 12‑lead standard ECG performed.
The exclusion criteria were: uncertain COVID-19 diagnosis or unavailability of 12‑lead ECG.
Definitions
Fever was defined as axillary temperature of at least 37.5 °C. Body mass index (BMI) was calculated as weight divided by height2 and expressed as kg/m2. DM was defined as a fasting glucose of ≥126 mg/dL, random glucose of ≥200 mg/dL, or the use of hypoglycemic medications. Hypertension was defined based on current guidelines. Anemia was defined as haemoglobin <13 g/dL in men, and <12 g/dL in women. CAD was determined systematically using a combination of self-report (a history of myocardial infarction, coronary revascularization, or angiographic evidence of stenosis in one or more coronary arteries of >50% of the luminal diameter), ECG results, review of all available prior medical records. Renal failure (RF) was defined by the presence of an estimated glomerular filtration rate, calculated by the CKD-EPI equation, of ≤60 mL/min/m2. Other comorbid conditions were abstracted from electronic health records.
Clinical outcomes
All participants were followed-up during hospitalization. The primary outcome for the study was defined as all cause in-hospital mortality. Secondary endpoints included length of in-hospital stay, need for ICU, and receiving invasive mechanical ventilation. Clinical characteristics and outcomes of patients with AF were compared to patients without AF. Also, survivors were compared with non-survivors.
Statistical analysis
Categorical data were expressed as absolute values and proportions. Distribution of continuous data was tested with the Kolmogorov–Smirnov and the Shapiro-Wilk test. Normally distributed variables were expressed as mean ± standard deviation, whereas non-normal distributed ones as median and interquartile range (IQR). Variables were compared between patients with and without AF as well as between survivors and non-survivors by using the Fisher exact test or Chi-square test for categorical variables, and the t-test or the Mann–Whitney U test, as appropriate, for continuous variables. We analyzed predictors of in-hospital mortality. This was performed by creating multivariable logistic regression models by including age and comorbid conditions that had univariate significance. Survival curves were plotted using the Kaplan–Meier method and compared between patients with and without AF by the log-rank test. All analyses were performed with IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA). A 2-sided p value of <0.05 was considered statistically significant.
Results
Three hundred and fifty-eight hospitalized COVID-19 patients retrospectively were reviewed. Eight patients were excluded from the study because of unavailability of 12‑lead ECG. The present analysis included consecutive 350 hospitalized patients with COVID-19. Of them, 40 (11.4%) patients had permanent AF. New-onset or paroxysmal AF was not detected during hospitalization. The median length of follow-up was 7 (IQR: 5–10) days. In whole cohort, 55 (15.7%) of patients died during the follow-up.
Clinical characteristics of patients with and without AF
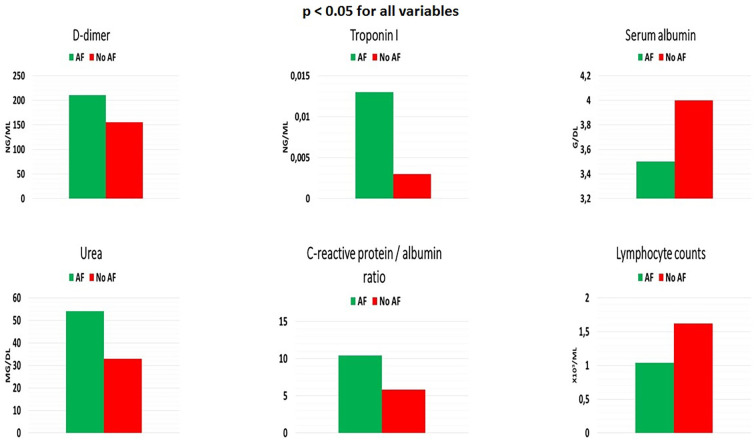
Comparison of clinical characteristics classified by presence of AF are given in Table 1 . Compared to patients without AF, those with AF were older (76 vs. 51 years, p < 0.001), more likely to be female (62.5% vs. 42.3%, p = 0.015), and more likely to have higher heart rate. There were no significant differences in smoking, alcohol use, BMI or blood pressure between the two groups. Prevalence of the symptoms on admission such as fever, cough, headache, diarrhea, fatigue, muscle ache or chest pain were also similar between the two groups. However, shortness of breath was more likely to occur in patients with AF. There were some differences in terms of comorbid conditions between patients with and without AF. Hypertension, CAD, CHF (with reduced ejection fraction), hyperlipidemia, anemia, cerebrovascular accident (CVA), and malignancy were more frequent in patients with AF compared to those without AF. Moreover, patients with AF were more likely to have pleural effusion in chest CT findings. With regard to laboratory parameters, higher urea, creatinine, D-dimer, cTnI levels and higher CRP/albumin ratio were measured at the time of hospitalization in patients with AF than those without AF. On the other hand, serum albumin, blood uric acid levels and total lymphocyte counts were lower in AF group (Fig. 1 ). Anticoagulant drugs use rate was 90% in patients with AF. Finally, a higher proportion of patients with AF were on medications such as angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, β-blockers, aldosterone antagonists, antiarrhythmics, and statins. Of note, hydroxychloroquine, azithromycin, and favipiravir treatment regimens were similar between the two groups.
Table 1.
Baseline characteristics of patients with and without atrial fibrillation.
| Patients with atrial fibrillation (n = 40) | Patients without atrial fibrillation (n = 310) | p value | |
|---|---|---|---|
| Age, years | 76 (64–82) | 51 (37–65) | <0.001 |
| Female sex, n (%) | 25 (62.5) | 131 (42.3) | 0.015 |
| Smoking, n (%) | 11 (27.5) | 117 (37.7) | 0.206 |
| Alcohol use, n (%) | 4 (10) | 34 (11) | 0.853 |
| Body mass index, kg/m2 | 27 (24–29) | 28 (24–31) | 0.190 |
| Systolic blood pressure, mmHg | 130 (110–150) | 120 (110–145) | 0.677 |
| Diastolic blood pressure, mmHg | 77.5 (60–90) | 80 (70–90) | 0.514 |
| Heart rate, bpm | 99.5 (78–120) | 80 (69–98) | <0.001 |
| Symptoms at admission, n (%) | |||
| Fever | 14 (35) | 152 (49) | 0.094 |
| Cough | 16 (40) | 148 (47.7) | 0.356 |
| Shortness of breath | 26 (65) | 92 (29.7) | <0.001 |
| Headache | 0 (0) | 12 (3.9) | 0.205 |
| Diarrhea | 0 (0) | 3 (1) | 0.532 |
| Fatigue, tiredness | 2 (5) | 38 (12.3) | 0.175 |
| Muscle ache | 1 (2.5) | 21 (6.8) | 0.295 |
| Sore throat | 0 (0) | 28 (9) | 0.048 |
| Chest pain | 0 (0) | 11 (3.5) | 0.226 |
| Comorbidities, n (%) | |||
| Hypertension | 27 (67.5) | 104 (33.5) | <0.001 |
| Diabetes mellitus | 10 (25) | 54 (17.4) | 0.243 |
| Anemia | 13 (32.5) | 41 (13.2) | 0.001 |
| Renal failure | 8 (20) | 39 (12.6) | 0.195 |
| Dialysis | 4 (10) | 20 (6.5) | 0.403 |
| Coronary artery disease | 13 (32.5) | 44 (14.2) | 0.003 |
| PCI/CABG | 8 (20) | 25 (8.1) | 0.015 |
| Peripheral vascular disease | 1 (2.5) | 3 (1) | 0.391 |
| Chronic heart failure (HFrEF) | 14 (35) | 16 (5.2) | <0.001 |
| Hyperlipidemia | 7 (17.5) | 14 (4.5) | 0.001 |
| Chronic obstructive pulmonary disease | 5 (12.5) | 37 (11.9) | 0.917 |
| Malignancy | 4 (10) | 6 (1.9) | 0.004 |
| CVA/TIA | 6 (15) | 4 (1.3) | <0.001 |
| Chest CT findings, n (%) | |||
| No significant finding | 3 (7.5) | 51 (16.5) | 0.140 |
| Ground glass opacity | 34 (85) | 219 (70.6) | 0.056 |
| Pneumonic consolidation | 3 (7.5) | 45 (14.5) | 0.225 |
| Pleural effusion | 5 (12.5) | 9 (2.9) | 0.004 |
| Laboratory parameters | |||
| Urea, mg/dL | 54 (35–75) | 34 (26–52) | <0.001 |
| Serum creatinine, mg/dL | 1 (0.8–1.3) | 0.9 (0.7–1) | 0.014 |
| Serum potassium, mEq/L | 4.2 (3.7–4.7) | 4.2 (3.8–4.4) | 0.874 |
| Serum calcium, mEq/L | 8.5 (8–9.1) | 8.8 (8.3–9.2) | 0.122 |
| Uric acid, mg/dL | 5.1 (4.2–6.2) | 6.7 (4.6–8.2) | <0.001 |
| Albumin, g/dL | 3.5 (2.7–4) | 4 (3.6–4.3) | <0.001 |
| Aspartate transaminase, U/L | 26.5 (22–41) | 24.5 (18–35) | 0.122 |
| Alanine transaminase, U/L | 21.5 (16–36) | 24 (15–36) | 0.782 |
| D-dimer, ng/mL | 210.5 (150–686) | 155.5 (150–283) | 0.014 |
| Troponin I, ng/mL | 0.013 (0.002–0.074) | 0.003 (0.002–0.006) | <0.001 |
| Haemoglobin, g/dL | 11.4 (10.1–12.9) | 13 (11.2–14.7) | <0.001 |
| Leukocyte, x103/μL | 7.8 (5.4–10.1) | 7.8 (5.5–10.5) | 0.654 |
| Lymphocyte, x103/μL | 1.04 (0.7–1.4) | 1.62 (1.1–2.4) | <0.001 |
| C-reactive protein, mg/dL | 35.7 (13.1–117) | 24.3 (10–79.4) | 0.075 |
| C-reactive protein/albumin ratio | 10.4 (4.9–29.2) | 5.8 (2.8–18.5) | 0.025 |
| Ferritin, ng/mL | 63.8 (17.7–306.8) | 53.1 (25.8–138.6) | 0.874 |
| Medications, n (%) | |||
| Antiplatelet | 6 (15) | 58 (18.7) | 0.568 |
| Anticoagulant | 36 (90) | 1 (0.3) | <0.001 |
| ACE-i/ARB | 17 (42.5) | 68 (21.9) | 0.004 |
| Beta-blocker | 30 (75) | 43 (13.9) | <0.001 |
| Aldosterone antagonists | 9 (22.5) | 5 (1.6) | <0.001 |
| Antiarrhythmic | 1 (2.5) | 0 (0) | 0.005 |
| Statin | 7 (17.5) | 14 (4.5) | 0.001 |
| Hydroxychloroquine | 40 (100) | 310 (100) | – |
| Azithromycin | 27 (67.5) | 225 (72.6) | 0.501 |
| Favipiravir | 4 (10) | 40 (12.9) | 0.602 |
| Immunosuppressive agent | 0 (0) | 0 (0) | – |
Abbreviations: ACE-i, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery by-pass graft; CT, computed tomography; CVA, cerebrovascular accident; HFrEF, heart failure with reduced ejection fraction; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack.
Fig. 1.
Laboratory parameters in patients with and without atrial fibrillation (AF).
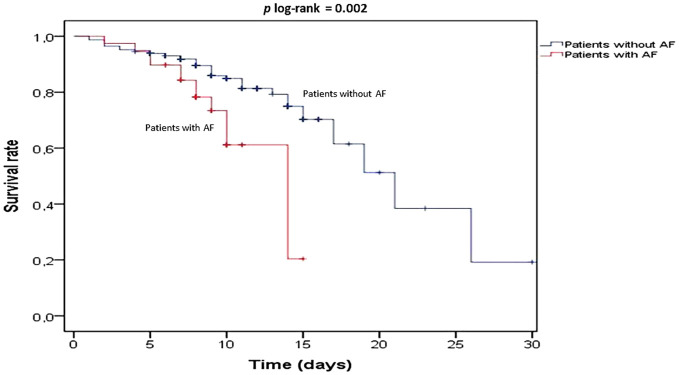
Clinical outcomes of patients with and without AF are presented in Table 2 . In-hospital mortality was significantly higher in patients with AF compared to patients without AF (32.5% vs. 13.5%, log-rank p = 0.002, relative risk 2.40, 95% confidence interval [CI] 1.28–5.89) (Fig. 2 ). In addition, median length of in-hospital stay was significantly longer in patients with AF than in those without AF (8 [7–10] vs. 7 [5–10] days, p = 0.008). Compared to patients without AF, the number of patients who needed ICU (55% vs. 31%, p = 0.002) and invasive mechanical ventilation (35% vs 15.2%, p = 0.002) were also higher in the AF group.
Table 2.
Clinical outcomes of patients with and without atrial fibrillation.
| Atrial fibrillation | No atrial fibrillation | p value | |
|---|---|---|---|
| Length of in-hospital stay, [median (IQR), days] | 8 (7–10) | 7 (5–10) | 0.008 |
| Need for intensive care unit, n (%) | 22 (55) | 96 (31) | 0.002 |
| Invasive mechanical ventilation, n (%) | 14 (35) | 47 (15.2) | 0.002 |
| In-hospital mortality, n (%) | 13 (32.5) | 42 (13.5) | 0.002 |
Abbreviations: IQR, interquartile range.
Fig. 2.
Kaplan-Meier 30-day survival rates for the patients with and without atrial fibrillation (AF).
Clinical characteristics of survivors and non-survivors
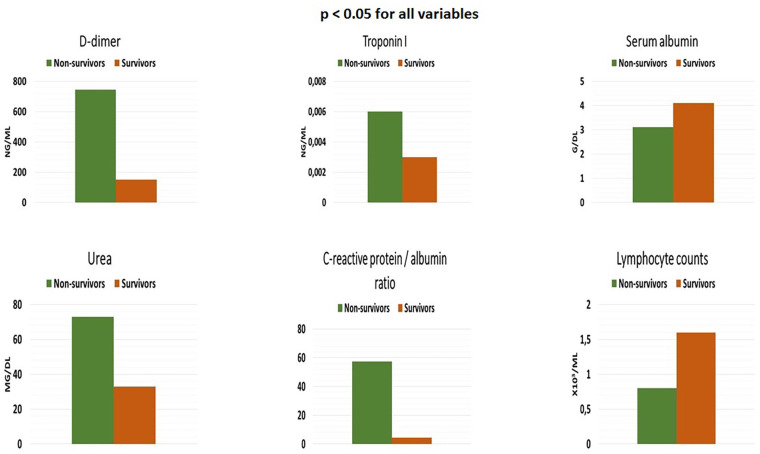
Baseline characteristics of patients who were alive and deceased are shown in Table 3 . Compared with patients who were alive, those who died were older (75 vs. 49 years, p < 0.001), had lower BMI, lower blood pressure, and higher heart rate. Although cough was more frequently in survivors on admission, dyspnea was more common in those who died. With regard to comorbid diseases, patients who deceased were more likely to have AF (23.6% vs. 9.2%, p = 0.002), hypertension, DM, CHF, CAD, RF, anemia, chronic obstructive pulmonary disease, and CVA compared to survivors. According to the chest CT reports, pleural effusion was more prevalent in patients who died. However, lung involvement at CT was less prevalent in survivors. In terms of the biomarker profile, patients who deceased were more likely to have an elevated urea, creatinine, aspartate transaminase, D-dimer, cTnI, leukocyte, CRP concentrations and higher CRP/albumin ratio on admission than those who were alive. In contrast, serum albumin levels and total lymphocyte counts were significantly lower in non-survivors (Fig. 3 ). As expected, patients who died were more likely to be administered antiplatelet, anticoagulant, β-blockers, azithromycin, and favipiravir than those survivors.
Table 3.
Comparison of clinical characteristics of survivor and non-survivor patients.
| Overall (n = 350) | Deceased (n = 55) | Surviving (n = 295) | p value | |
|---|---|---|---|---|
| Age, years | 55 (39–70) | 75 (62–81) | 49 (37–64) | <0.001 |
| Female sex, n (%) | 156 (44.6) | 26 (47.3) | 130 (44.1) | 0.661 |
| Smoking, n (%) | 128 (36.6) | 20 (36.4) | 108 (36.6) | 0.972 |
| Body mass index, kg/m2 | 28 (24–31) | 26 (24–29) | 28 (25–31) | <0.001 |
| Systolic blood pressure, mmHg | 125 (110–145) | 110 (105–150) | 125 (110–140) | 0.002 |
| Diastolic blood pressure, mmHg | 80 (70–90) | 70 (60–90) | 80 (70–90) | 0.003 |
| Heart rate, bpm | 81 (70–99) | 99 (78–120) | 79 (69–97) | <0.001 |
| Symptoms at admission, n (%) | ||||
| Fever | 166 (47.4) | 29 (52.7) | 137 (46.4) | 0.391 |
| Cough | 164 (46.9) | 19 (34.5) | 145 (49.2) | 0.046 |
| Shortness of breath | 118 (33.7) | 40 (72.7) | 78 (26.4) | <0.001 |
| Headache | 12 (3.4) | 0 (0) | 12 (4.1) | 0.128 |
| Diarrhea | 3 (0.9) | 0 (0) | 3 (1) | 0.453 |
| Fatigue, tiredness | 40 (11.4) | 0 (0) | 40 (13.6) | 0.004 |
| Muscle ache | 22 (6.3) | 2 (3.6) | 20 (6.8) | 0.378 |
| Sore throat | 28 (8) | 1 (1.8) | 27 (9.2) | 0.066 |
| Chest pain | 11 (3.1) | 0 (0) | 11 (3.7) | 0.146 |
| Comorbidities, n (%) | ||||
| Atrial fibrillation | 40 (11.4) | 13 (23.6) | 27 (9.2) | 0.002 |
| Hypertension | 131 (37.4) | 33 (60) | 98 (33.2) | <0.001 |
| Diabetes mellitus | 64 (18.3) | 17 (30.9) | 47 (15.9) | 0.008 |
| Anemia | 54 (15.4) | 15 (27.2) | 39 (13.2) | 0.008 |
| Renal failure | 47 (13.4) | 21 (38.2) | 26 (8.8) | <0.001 |
| Dialysis | 24 (6.9) | 8 (14.5) | 16 (5.4) | 0.014 |
| Coronary artery disease | 57 (16.3) | 14 (25.5) | 43 (14.6) | 0.045 |
| PCI/CABG | 33 (9.4) | 8 (14.5) | 25 (8.5) | 0.157 |
| Peripheral vascular disease | 4 (1.1) | 1 (1.8) | 3 (1) | 0.608 |
| Chronic heart failure (HFrEF) | 30 (8.6) | 11 (20) | 19 (6.4) | 0.001 |
| Hyperlipidemia | 21 (6.0) | 3 (5.5) | 18 (6.1) | 0.853 |
| COPD | 42 (12.0) | 12 (21.8) | 30 (10.1) | 0.015 |
| Malignancy | 10 (2.9) | 2 (3.6) | 8 (2.7) | 0.706 |
| CVA/TIA | 10 (2.9) | 5 (9.1) | 5 (1.7) | 0.003 |
| Chest CT findings, n (%) | ||||
| No significant finding | 54 (15.4) | 2 (3.6) | 52 (17.6) | 0.008 |
| Ground glass opacity | 253 (72.3) | 43 (78.2) | 210 (71.2) | 0.287 |
| Pneumonic consolidation | 48 (13.7) | 11 (20) | 37 (12.5) | 0.140 |
| Pleural effusion | 14 (4) | 6 (10.9) | 8 (2.7) | 0.004 |
| Laboratory parameters | ||||
| Urea, mg/dL | 35 (27–54) | 73 (32–92) | 33 (23–49) | <0.001 |
| Serum creatinine, mg/dL | 0.9 (0.7–1.1) | 1.3 (0.8–1.6) | 0.9 (0.7–1) | <0.001 |
| Albumin, g/dL | 4 (3.5–4.3) | 3.1 (2.7–3.9) | 4.1 (3.7–4.3) | <0.001 |
| Aspartate transaminase, U/L | 25 (19–35) | 40 (22–59) | 23 (18–34) | <0.001 |
| Alanine transaminase, U/L | 23 (15–36) | 25 (15–36) | 23 (15–36) | 0.293 |
| D-dimer, ng/mL | 164 (150–291) | 745 (207–1078) | 150 (150–271) | <0.001 |
| Troponin I, ng/mL | 0.003 (0.002–0.007) | 0.006 (0.002–0.029) | 0.003 (0.002–0.006) | <0.001 |
| Haemoglobin, g/dL | 12.8 (11–14.6) | 11 (10–12.8) | 13.1 (11.2–14.7) | <0.001 |
| Leukocyte, x103/μL | 7.8 (5.5–10.3) | 10.6 (5.5–14.7) | 7.4 (5.5–10.1) | <0.001 |
| Lymphocyte, x103/μL | 1.5 (1–2.3) | 0.8 (0.5–1.3) | 1.6 (1.1–2.4) | <0.001 |
| CRP, mg/dL | 25 (10.7–94) | 94.9 (36.6–140) | 18.2 (7.8–45.2) | <0.001 |
| CRP/albumin ratio | 6.1 (3.1–21.9) | 57.5 (26.4–72.6) | 4.4 (2.6–15.1) | <0.001 |
| Medications, n (%) | ||||
| Antiplatelet | 64 (18.3) | 19 (34.5) | 45 (15.3) | 0.001 |
| Anticoagulant | 37 (10.6) | 13 (23.6) | 24 (8.1) | 0.001 |
| ACE-i/ARB | 85 (24.3) | 19 (34.5) | 66 (22.4) | 0.053 |
| Beta-blocker | 73 (20.9) | 18 (32.7) | 55 (18.6) | 0.018 |
| Aldosterone antagonists | 14 (4) | 5 (9.1) | 9 (3.1) | 0.480 |
| Antiarrhythmic | 1 (0.3) | 0 (0) | 1 (0.3) | 0.665 |
| Statin | 21 (6) | 3 (5.5) | 18 (6.1) | 0.853 |
| Hydroxychloroquine | 350 (100) | 55 (100) | 295 (100) | – |
| Azithromycin | 252 (72) | 48 (87.3) | 204 (69.2) | 0.006 |
| Favipiravir | 44 (12.6) | 20 (36.4) | 24 (8.1) | <0.001 |
| Immunosuppressive agent | 0 (0) | 0 (0) | 0 (0) | – |
Abbreviations: ACE-i, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery by-pass graft; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CT, computed tomography; CVA, cerebrovascular accident; HFrEF, heart failure with reduced ejection fraction; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack.
Fig. 3.
Laboratory parameters in non-survivors and survivors.
Associated factors with in-hospital mortality
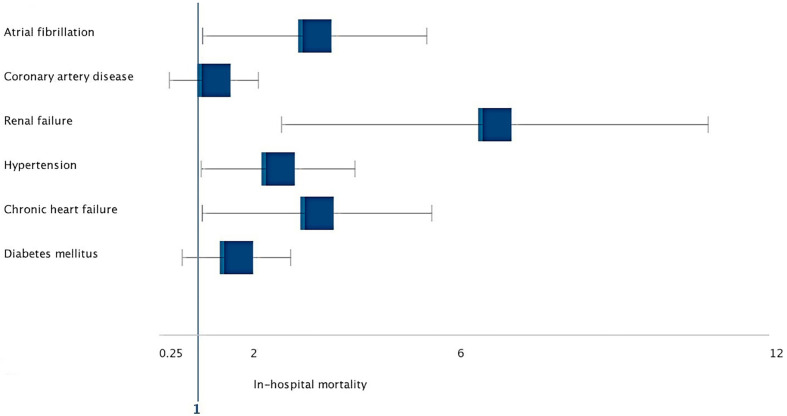
Multivariable logistic regression model was created for age and comorbid diseases to analyze independently associated factors with in-hospital mortality. All variables with p values less than or equal to 0.05 were included in a multivariable logistic regression model. After adjustment for age and co-morbidities, multivariable analyses showed that older age (hazard ratio [HR]: 5.67, 95% CI: 3.210–7.879, p < 0.001), AF (HR: 2.426, 95% CI: 1.089–5.405, p = 0.032), hypertension (HR: 2.064, 95% CI: 1.059–4.022, p = 0.035), CHF (HR: 2.451, 95% CI: 1.078–5.501, p = 0.030), and RF (HR: 5.312, 95% CI: 2.607–10.823, p = 0.001) were independently associated with in-hospital death (Fig. 4 ).
Fig. 4.
Forest plot of adjusted hazard ratios for all cause in-hospital mortality.
Discussion
We analyzed 350 consecutive hospitalized COVID-19 patients with and without AF in the present study. The principal findings of this study include: (1) permanent AF was observed in 11.4% of patients; (2) patients with AF were older and had more comorbid diseases; (3) in-hospital mortality rate was 17.7% in whole cohort; (4) comorbid conditions and abnormal chest CT findings were more common in patients who died; (5) AF was seen with together markers of disease severity such as abnormal chest radiographs, deteriorated laboratory parameters, higher mortality, longer in-hospital stay, need for ICU admission and invasive mechanical ventilation; and (6) after adjustment for age and co-morbidities the presence of AF was independently associated with in-hospital death.
In recent epidemiological and observational studies, several cardiovascular complications in COVID-19 have been reported including thromboembolism, ventricular arrhythmia, myocardial injury, and cardiomyopathy which associate with poorer outcomes [16]. Arrhythmia recognised as the second most common complication after acute respiratory distress syndrome [17], and their prevalence varies significantly between study populations. Ventricular arrhythmias are relatively well defined in SARS-CoV-2 infection. However, the incidence and nature of atrial arrhythmias among patients with SARS-CoV-2 was poorly documented. Newly published studies suggest that the most frequent incident sustained arrhythmia is AF in the hospitalized COVID-19 population [18]. Abrams et al. retrospectively described clinical characteristics and compared factors contributing toward arrhythmic versus non-arrhythmic death in patients with COVID-19 [19]. In this study, 133 patients who died were enrolled, and AF was present on 14.3% of admission ECGs. During hospitalization, 13 more patients were diagnosed with new-onset AF. At the end of follow-up, 31 (23.3%) patients had AF in those who died. The authors conclude that AF or atrial flutter (AFL) was one of the most common ECG abnormalities on admission and during hospitalization [19]. Another study described the proportion of AF or AFL as 19% (8/43) in COVID-19 patients who deceased [11]. According to the results of multicentric Italian study, AF or AFL was detected in 22% (96/431) of the patients [12]. Consistent with these findings, our results showed that, AF was present in 11.4% (40/350) of whole cohort on admission, and the prevalence of AF was 23.6% (13/55) in non-survivors. On the other hand, another large scale study has recently identified that, AF or AFL was observed in 15.7% (166/1053) of patients with infected SARS-CoV-2. Compared to the previous studies, AF or AFL rate among non-survivors were relatively higher as 35.3% (65/184) in this analysis [20].
The pathophysiology of COVID-19 related AF is not well understood. Some hypotheses have been proposed on the mechanism of new-onset AF in COVID-19. Hypoxemia precipitated by COVID-19 may cause AF, and this condition could be permanent when accompanied by impaired pulmonary function [21]. On the other hand, the incidence of new-onset or paroxysmal AF in COVID-19 is not well known yet. While the rate of new-onset AF/AFL in all AF/AFL cases was above 40% in some studies [19,20], new-onset AF/AFL was not reported among all AF/AFL cases in some other studies [11,22]. New-onset AF was not observed in our cohort during in-hospital follow-up. These conflicting results can be associated with different patient profile, sample size of AF population, follow-up time and inclusion criteria.
AF is one of the most important burden upon healthcare resources. In general population, patients with AF have an increased risk of mortality and morbidity compared those without AF. These patients have a higher risk of death due to cardiovascular disease [23]. Increased cardiovascular mortality risk due to AF varies between 2 and 12 times. Of note, AF contributes to 15–25% of all strokes and these contribute to a significant proportion of AF-related mortality. AF-related strokes tend to be associated with higher mortality, and more severe disability [24]. Moreover, AF was related to a worsen prognosis in some infectious diseases in pre-COVID era. Large scale studies suggest that the presence of AF is associated with poor prognosis and increased mortality in sepsis-related hospitalizations [25]. COVID-19 is a global pandemic that appeared about 10 months ago and spread worldwide rapidly. Therefore, sufficient data about the implications of AF on clinical outcomes in the COVID-19 population are not yet available, and recently published a few data is conflictive in this regard. A small scale Italian study found that a high rate of in-hospital mortality and complications in cardiac patients compared with those without a history of cardiac disease. However, AF rate was similar in survivors and non-survivors in this study [26]. In a series of 414 patients hospitalized with COVID-19, Russo et al. studied potential association of sustained tachyarrhythmias with disease severity and in-hospital death. The results of this study suggest that AF was not associated with mortality [18]. Elias et al. analyzed 1258 patients and identified only 42 incident AF or AFL events. They reported that the combination of abnormal respiratory vital signs and ECG findings of AF or AFL, right ventricular strain, or ST segment abnormalities accurately prognosticates early deterioration in patients with COVID-19. On multivariable analysis, the presence of only AF or AFL was not associated with poor outcomes in this study, which may be related to the relatively small number of patients with AF in this cohort [11]. An observational, multicenter study from New York included 1053 patients with SARS-CoV-2 infection. Similar to the our findings, this study found an independent association between AF or AFL and all-cause mortality [20]. In addition, another cohort study conclude that AF was the independent risk factor of in-hospital death and ventilator use in patients with COVID-19 [22].
A limited number of studies revealed that COVID-19 patients with AF were older and most of them had at least one preexisting risk factor, including hypertension, CAD, CHF, renal disease, prior stroke or pulmonary disease [18,20,27], while some did not report any illness [28]. COVID-19 patients with comorbid diseases, such as CHF, hypertension, CAD, DM and RF, have an extremely poor prognosis, compared with subjects without co-morbidities [13,26]. Our findings support that patients who have poor outcomes more likely to have these comorbid conditions. Remarkebly, compared to patients without AF, some of these co-morbidities such as hypertension, CAD and CHF were more common in patients with AF in our cohort. One result of our study that needs to be explained is that the patients who died received more medication than those survivors. These medications do not increase the risk of death but are markers of more ill patients. Furthermore, these associations were diminished in multivariable analyses.
Several clinical and laboratory parameters have been identified that correlate with the severity of COVID-19. Zheng et al. showed a significant positive association of shortness of breath or dyspnea with COVID-19 progression to severe illness [29]. In our entire cohort, shortness of breath was more common in patients with AF on admission. Moreover, compared to survivors, patients who died suffered more from dyspnea in our study. Abnormal chest CT findings are significant predictor for the severity of SARS-CoV-2 infection [30]. Our results revealed that abnormal CT findings were more prominent in patients with AF. Recently studies have shown that elevated troponin, D-dimer, urea, CRP concentrations, lower albumin levels and lower lymphocyte counts were associated with severe complications of COVID-19 [[31], [32], [33]]. The results of our study have confirmed previous findings on these biochemical markers. Importantly, we observed that there was a similar trend between non-survivors and patients with AF compared to survivors and patients without AF in terms of these markers (Fig. 1, Fig. 3). Furthermore, patients with AF had longer in-hospital stay, were more likely to need ICU and invasive mechanical ventilation in our study. In the light of all these findings, it seems to possible to say that COVID-19 patients with AF have a higher risk of severe illness. Similar to the our results, two cohort studies showed that the incidence of AF or AFL among patients with SARS-CoV-2 infection corresponds to the severity of disease [34,35].
Study limitations
As a retrospective study during an ongoing pandemic, this study has several limitations. Some laboratory data (such as N-terminal pro-B-type natriuretic peptide) and echocardiographic data were not collected. Our cohort did not include patients with new-onset or paroxysmal AF. Data were extracted from the medical records, and it is probable that some co-morbidities were incompletely characterized. In addition, we did not adjudicate cause of death in our patients who experienced mortality. Data from larger cardiovascular populations and multiple centres are more valuable. However, our sample size is relatively small and data are from single center. As such, our findings may not be generalizable to patients with COVID-19 from across the world. Larger, multicenter, prospective studies are required to confirm our preliminary findings. Finally, our analysis was restricted to inpatient follow-up only.
Conclusions
The proportion of AF is substantial in hospitalized patients with COVID-19. Permanent AF is seen with together markers of severe illness such as abnormal chest CT findings, deteriorated laboratory parameters, longer in-hospital stay, need for ICU admission and invasive mechanical ventilation. Moreover, the presence of AF is independently associated with in-hospital death. Hence, permanent AF portends poor outcomes in hospitalized patients with SARS-CoV-2 infection. Identification of AF with ECG or anamnesis on admission may facilitate risk stratification and optimal clinical management in COVID-19. Further multicenter, large-scale and prospective studies are needed to elucidate the exact effects of AF on COVID-19.
Funding
None.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of Competing Interest
None.
Acknowledgements
We are grateful to Dr. Emre Başer for his assistance in statistical analysis.
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.https://covid19.who.int
- 3.Hu Y.F., Cheng W.H., Hung Y., Lin W.Y., Chao T.F., Liao J.N., et al. Management of atrial fibrillation in COVID-19 pandemic. Circ J. 2020;84(10):1679–1685. doi: 10.1253/circj.CJ-20-0566. [DOI] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwenandar F., Japar K.V., Damay V., Hariyanto T.I., Tanaka M., Lugito N.P.H., et al. Coronavirus disease 2019 and cardiovascular system: a narrative review. Int J Cardiol Heart Vasc. 2020;29:100557. doi: 10.1016/j.ijcha.2020.100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han H., Yang L., Liu R., Liu F., Wu K.L., Li J., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 9.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020 Apr 3:1–4. doi: 10.1007/s11239-020-02105-8. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2020:ehaa612. doi: 10.1093/eurheartj/ehaa612. 2020 Aug 29. Online ahead of print. [DOI] [Google Scholar]
- 11.Elias P., Poterucha T.J., Jain S.S., Sayer G., Raikhelkar J., Fried J., et al. The prognostic value of electrocardiogram at presentation to emergency department in patients with COVID-19. Mayo Clin Proc. 2020;95(10):2099–2109. doi: 10.1016/j.mayocp.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertini M., Ferrari R., Guardigli G., Malagù M., Vitali F., Zucchetti O., et al. Electrocardiographic features of 431 consecutive, critically ill COVID-19 patients: an insight into the mechanisms of cardiac involvement. Europace. 2020 Sep 18:euaa258. doi: 10.1093/europace/euaa258. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao C., Cai Y., Zhang K., Zhou L., Zhang Y., Zhang X., et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41(22):2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li G., Saguner A.M., An J., Ning Y., Day J.D., Ding L., et al. Cardiovascular disease during the COVID-19 pandemic: think ahead, protect hearts, reduce mortality. Cardiol J. 2020 Aug 13 doi: 10.5603/CJ.a2020.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inciardi R.M., Adamo M., Lupi L., Metra M. Atrial fibrillation in the COVID-19 era: simple bystander or marker of increased risk? Eur Heart J. 2020;41(32):3094. doi: 10.1093/eurheartj/ehaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malaty M., Kayes T., Amarasekera A.T., Kodsi M., MacIntyre C.R., Tan T.C. Incidence and treatment of arrhythmias secondary to coronavirus infection in humans: a systematic review. Eur J Clin Invest. 2020 Oct 12 doi: 10.1111/eci.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo V., Di Maio M., Mottola F.F., Pagnano G., Attena E., Verde N., et al. Clinical characteristics and prognosis of hospitalized COVID-19 patients with incident sustained tachyarrhythmias: a multicenter observational study. Eur J Clin Invest. 2020 Aug;19 doi: 10.1111/eci.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrams M.P., Wan E.Y., Waase M.P., Morrow J.P., Dizon J.M., Yarmohammadi H., et al. Clinical and cardiac characteristics of COVID-19 mortalities in a diverse New York City Cohort. J Cardiovasc Electrophysiol. 2020 Oct 6 doi: 10.1111/jce.14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peltzer B., Manocha K.K., Ying X., Kirzner J., Ip J.E., Thomas G., et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J Cardiovasc Electrophysiol. 2020 Oct 5 doi: 10.1111/jce.14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haseeb S., Gul E.E., Çinier G., Bazoukis G., Alvarez-Garcia J., Garcia-Zamora S., et al. Value of electrocardiography in coronavirus disease 2019 (COVID-19) J Electrocardiol. 2020;62:39–45. doi: 10.1016/j.jelectrocard.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Chen L., Wang J., He X., Huang F., Chen J., et al. Electrocardiogram analysis of patients with different types of COVID-19. Ann Noninvasive Electrocardiol. 2020 Sep;20 doi: 10.1111/anec.12806. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee E., Choi E.K., Han K.D., Lee H., Choe W.S., Lee S.R., et al. Mortality and causes of death in patients with atrial fibrillation: a nationwide population-based study. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0209687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sankaranarayanan R., Kirkwood G., Visweswariah R., Fox D.J. How does chronic atrial fibrillation influence mortality in the modern treatment era? Curr Cardiol Rev. 2015;11(3):190–198. doi: 10.2174/1573403x10666140902143020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai R., Hanna B., Singh S., Omar A., Deshmukh A., Kumar G., et al. Trends and outcomes in sepsis hospitalizations with and without atrial fibrillation: a nationwide inpatient analysis. Crit Care Med. 2019;47(8):e630–e638. doi: 10.1097/CCM.0000000000003806. [DOI] [PubMed] [Google Scholar]
- 26.Inciardi R.M., Adamo M., Lupi L., Cani D.S., Di Pasquale M., Tomasoni D., et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in northern Italy. Eur Heart J. 2020;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gawałko M., Kapłon-Cieślicka A., Hohl M., Dobrev D., Linz D. COVID-19 associated atrial fibrillation: incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc. 2020;30:100631. doi: 10.1016/j.ijcha.2020.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kochav S.M., Coromilas E., Nalbandian A., Ranard L.S., Gupta A., Chung M.K., et al. Cardiac arrhythmias in COVID-19 infection. Circ Arrhythm Electrophysiol. 2020;13(6) doi: 10.1161/CIRCEP.120.008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghweil A.A., Hassan M.H., Khodeary A., Mohamed A.O., Mohammed H.M., Abdelazez A.A., et al. Characteristics, outcomes and indicators of severity for COVID-19 among sample of ESNA quarantine hospital’s patients, Egypt: a retrospective study. Infect Drug Resist. 2020;13:2375–2383. doi: 10.2147/IDR.S263489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moutchia J., Pokharel P., Kerri A., McGaw K., Uchai S., Nji M., et al. Clinical laboratory parameters associated with severe or critical novel coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0239802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y.M., Xie J., Chen M.M., Zhang X., Cheng X., Li H., et al. Kidney function indicators predict adverse outcomes of COVID-19. Med (N Y) 2020 Oct 2 doi: 10.1016/j.medj.2020.09.001. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kermali M., Khalsa R.K., Pillai K., Ismail Z., Harky A. The role of biomarkers in diagnosis of COVID-19 - a systematic review. Life Sci. 2020;254:117788. doi: 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colon C.M., Barrios J.G., Chiles J.W., McElwee S.K., Russell D.W., Maddox W.R., et al. Atrial arrhythmias in COVID-19 patients. JACC Clin Electrophysiol. 2020;6(9):1189–1190. doi: 10.1016/j.jacep.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatla A., Mayer M.M., Adusumalli S., Hyman M.C., Oh E., Tierney A., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.