Abstract
Objective
Up to one-third of women with ovarian cancer in the United States do not receive surgical care from a gynecologic oncologist specialist despite guideline recommendations. We aim to investigate the impact of rurality on receiving surgical care from a specialist, referral to a specialist, and specialist surgery after referral, and the consequences of specialist care.
Methods
We utilized a retrospective cohort created through extension of standard cancer surveillance in three Midwestern states. Multivariable adjusted logistic regression was utilized to assess gynecologic oncologist treatment of women 18–89 years old, who were diagnosed with primary, histologically confirmed, malignant ovarian cancer in 2010–2012 in Kansas, Missouri and Iowa by rurality.
Results
Rural women were significantly less likely to receive surgical care from a gynecologic oncologist specialist (adjusted odds ratio (OR) 0.37, 95% confidence interval (CI) 0.24–0.58) and referral to a specialist (OR 0.37, 95% CI 0.23–0.59) compared to urban women. There was no significant difference in specialist surgery after a referral (OR 0.56, 95% CI 0.26–1.20). Rural women treated surgically by a gynecologic oncologist versus non-specialist were more likely to receive cytoreduction and more complete tumor removal to ≤ 1cm.
Conclusion
There is a large rural-urban difference in receipt of ovarian cancer surgery from a gynecologic oncologist specialist (versus a non-specialist). Disparities in referral rates contribute to the rural-urban difference. Further research will help define the causes of referral disparities, as well as promising strategies to address them.
Introduction
Ovarian cancer is the fifth leading cause of cancer death and eighth most common incident cancer in women in the United States.1 From 2009 to 2015, women with ovarian cancer had a 5-year relative survival rate of 47.6%.1 Without effective, available screening methods for the population, ovarian cancer is often diagnosed at an advanced stage. Ovarian cancer survival is determined by stage at diagnosis, as well as adherence to guideline recommended care of surgery, chemotherapy, and systemic therapy.2–4
Gynecologic oncologists, or cancer surgeon specialists of the female reproductive tract, achieve surgical results most in line with guideline recommendations.5–10 Consequently, the National Comprehensive Cancer Network, Centers for Disease Control and Prevention (CDC), National Institutes of Health, American College of Obstetrics and Gynecologists, and Society of Gynecologic Oncologists suggest that ovarian cancer patients pursuing surgical treatment receive surgical care from a gynecologic oncologist.11,12 Nevertheless, among ovarian cancer patients in the United States that pursue surgical care as treatment for their ovarian cancer (versus palliative care and non-surgical care) up to one-third do not receive their cancer-directed surgery from gynecologic oncologists.13–16 Instead, they receive care from non-specialists, such as general surgeons and obstetrician-gynecologists. There is a critical need to investigate the contributing barriers to gynecologic oncologist surgical care.13–16 Studies outside and within the United States of women diagnosed in the late 1990s and early 2000s have provided preliminary evidence that rurality may be an important contributing barrier.16–18
Throughout the last two decades, it has been suggested that rurality has grown as a barrier under the Centers of Excellence model, where patients need to travel to receive care from specialists at urban, tertiary medical centers.19 According to a 2011 report from the CDC, over 99% of gynecologic oncologists in the United States work in metropolitan counties, while one-fifth of their ovarian cancer patients live in rural counties.12 Furthermore, over half of the counties in the United States are located greater than 50 miles from a county with a gynecologic oncologist.20 Self-reports from gynecologic oncologists confirm that at least one-third of the ovarian cancer patients travel more than 50 miles for surgery with a specialist.19 Under the Centers of Excellence model, rural patients may face patient-level barriers, such as out-of-network insurance costs and travel difficulties, and system-level barriers, such as rural referral network restrictions and knowledge limitations.21–24
The primary aim of this study was to investigate the impact of rurality on receiving surgical care from a gynecologic oncologist among women that pursue surgical care while controlling for important confounding patient-level and system-level factors, such as the socioeconomic status of a patient’s census tract, in three Midwestern states.20,25 The secondary aim was to assess the impact rurality has on receiving a referral to a surgical specialist and receiving surgery after a surgical referral is made. Finally, our third aim was to investigate consequences of specialist care by rurality, such as time from diagnosis-to-surgery, travel distance to surgery, type of surgery received, and extent of tumor clearance.
Methods
Study Population
We utilized the retrospective cohort addressing Patterns of Ovarian Cancer Care and Survival in the Midwestern Region of the United States—a CDC Investigation.26 This cohort consists of a random population-based sample of 1003 women with ovarian cancer who were residents of Iowa and Missouri at the time of their ovarian cancer diagnosis in 2011–2012, and were residents of Kansas at the time of their ovarian cancer diagnosis in 2010–2012. Kansas cases were abstracted from 2010 due their low number sampling frame. The cohort aimed to include 1000 patient cases with 200–350 coming from each registry; 253 cases (31.5%) were from Iowa, 273 cases (34.0%) from Kansas, and 278 cases (34.6%) from Missouri. Over these time periods, the total number of women diagnosed with ovarian cancer were 441 in Iowa, 549 in Kansas, and 773 in Missouri. Based on this information, the percent of the total women included in this cohort was 57% (253/441) in Iowa, 50% (273/549) in Kansas, and 36% (278/773) in Missouri. We do not know the total number of patients that received cancer-directed surgery in these states. The states included in this CDC investigation were chosen because they have a high prevalence of rural women, and they are located in the Midwestern United States where there is a paucity of specialists, high ovarian cancer mortality, and a prevalence of low access counties located greater than 50 miles from gynecologic oncologists.12,26–28 Thirty-two percent of women in Iowa, Kansas, and Missouri reside in a rural residence.
The women included in the cohort were diagnosed with a primary, histologically confirmed epithelial, sex-cord or germ cell (International Classification of Diseases for -Oncology [ICD-O]-3 8000–8576, 8930–9110) malignant tumor of the ovary, fallopian tube or primary peritoneum (ICD-O-3 C56.9, C57.0, C48.1, C48.2 and C48.8) between the ages of 18 and 89 years. Women with low malignant potential histology (ICD-O-3 codes 8442, 8451, 8462, 8472, 8473), a diagnosis at autopsy or by death certificate, or a synchronous tumor within six months of their ovarian cancer diagnosis were excluded from the cohort.
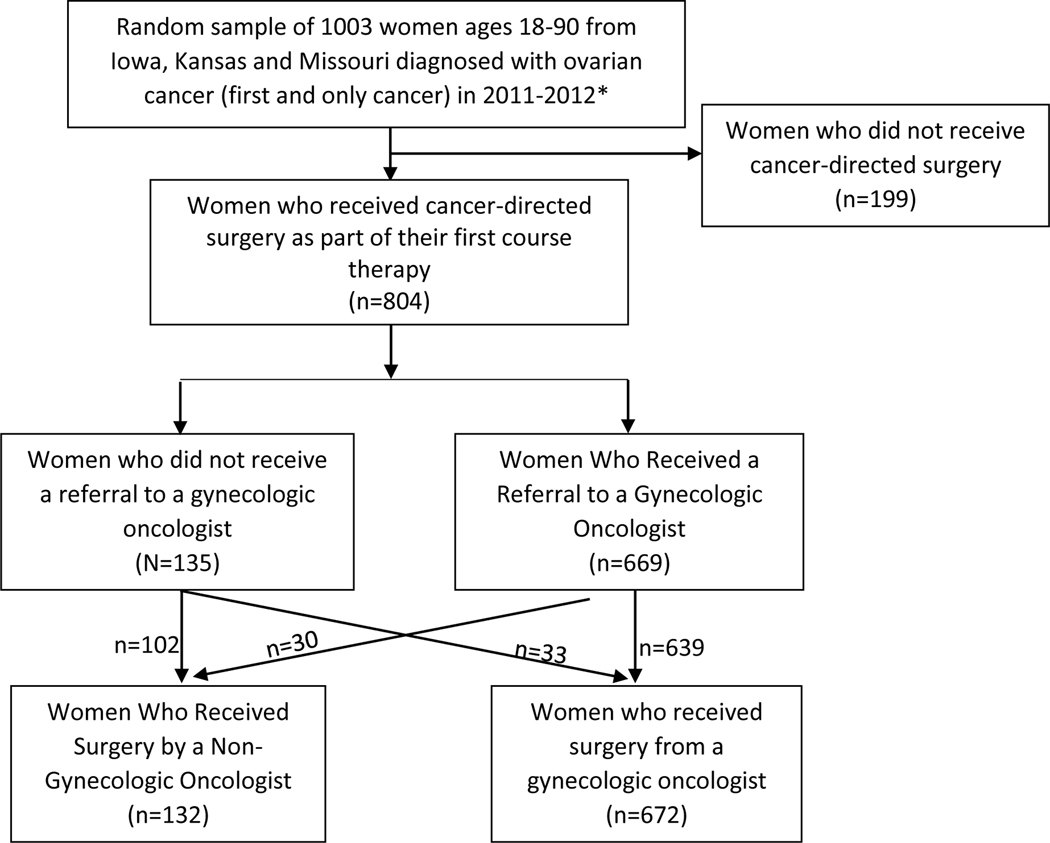
We analyzed the subset of women who received surgical care in Patterns of Ovarian Cancer Care and Survival in the Midwestern Region of the United States—a CDC Investigation. Our analysis included only women who had cancer-directed surgery for their ovarian cancer, and excluded women who did not receive cancer-directed surgery (Image 1).
Image 1.
Flow Chart of Inclusion and Exclusion
*In order to meet desired sample size, Kansas included 178 cases diagnosed in 2010.
Data Sources
All the variables in our analysis were obtained through an extension of standard surveillance protocols for state cancer registries. Trained cancer registrars used standardized definitions and abstraction manuals to abstract data from the medical record of each participant. Alternative options, such as follow-up with medical providers, were pursued when data were not available in the medical record. Abstraction and dataset creation lasted 18 months over the years 2017 and 2018. An institutional review board (IRB) at the CDC and each of the respective IRBs for the three state cancer registries approved this cohort study.
Variables
Our primary exposure of interest was the rurality of the census tract where the ovarian cancer patient lived at the time of diagnosis. This variable was created based on the 6-category National Center for Health Statistics (NCHS) rural-urban classification scheme framework.29 A rural census tract was defined as a non-metropolitan population with an urban cluster population of 10,000–49,999 persons or a non-metropolitan/non-core population. An urban census tract was defined as populations greater than 50,000 persons. This binary cut-point was chosen in accordance with NCHS recommendations and compared against metropolitan and micropolitan statistical areas, proximity to metropolitan centers, urbanization county maps, and growth rate maps in the three states for appropriateness.29,30
Our primary outcome was the specialty of the physician who performed the primary ovarian cancer-directed surgery. This variable was operationalized as gynecologic oncologist versus another specialty (i.e., general surgeon, obstetrician-gynecologist, and other/unknown). Cancer-directed surgery refers to surgery with the main purpose of removing and/or debulking the ovarian cancer. Diagnostic procedures that have no impact on cancer removal (i.e., paracentesis or tapping a pleural effusion), are not considered cancer-directed surgery. Laparotomy and operative laparoscopy without intention of resection of any ovarian mass may be staging, but not cancer-directed surgery. Incidental surgeries that discover cancer, but do not treat cancer, are not considered cancer-directed surgery. Our secondary outcome was referral to a gynecologic oncologists (binary). ‘Referral’ means that there was documentation of the referring provider suggesting, recommending, or scheduling their patient to see a gynecologic oncologist for surgical care. Referrals were documented irrespective of whether they resulted in a visit.
Covariates were selected using a theoretical framework and directed acyclic graphs. They are age at diagnosis, Charlson comorbidity score at diagnosis, census tract percentage of residents with less than a high school level of education, census tract median income, insurance status of the patient at time of surgery, the patient’s race/ethnicity, and stage of cancer at diagnosis. The Charlson comorbidity score was calculated based on the original conditional weighting of patient comorbidities at time of diagnosis, meaning the ovarian cancer tumor is not included in the score.31–33 Patient race/ethnicity was determined from the medical record, and due to the limited number of non-white patients, was categorized as non-Hispanic white versus non-white. Stage was obtained from the medical record and reported according to the International Federation of Gynecology and Obstetrics (FIGO 2013, www.figo.org).
In the description of our study population, we detailed the histologies of patients’ tumors as epithelial or non-epithelial disease in accordance with ICD-O-3 morphology codes.34 Grade was classified according to Surveillance, Epidemiology, and End Results (SEER) standards and site of origin was classified as ovarian (ICD-O-3 code C56.9), fallopian tube (C57.0), or peritoneal (C48.1, C48.2, and C48.8) cancers.35 Distance to surgical care was calculated as straight distance miles using latitudes and longitudes for Great Circle Distance in ArcGIS between the patient’s residence at diagnosis and the location of the primary cancer-directed surgical care. Hospital type was obtained through the American Hospital Association (AHA) Annual Survey of Hospitals and was categorized as government (federal, state and local government hospitals) and private (non-profit private and private investor-owned).
Theoretical Framework
The selection of covariates for analyses was based on the Behavioral Model of Health Services Use, which was first published by Andersen in 1973 as an adaptation of his earlier and broader behavioral model published in 1968. This model provides a casual framework for multilevel modeling of patient- and system-level factors impacting utilization of a specialist.36–38 The theory has three major constructs including predisposing factors, enabling factors, and need factors.36–39 Predisposing factors are subcategorized into demographic factors, social structure factors, and beliefs.36–39 Enabling factors are subcategorized into family and community level factors.36–39 Finally, need factors, sometimes referred to as illness factors, are subcategorized into perceived factors and evaluated/proven need factors.36–39 Our exposure of interest was a community level enabling factor, while the other categorization of our covariates can be found in Supplemental Image 1.
Statistical Analysis
We compared the patient, tumor, and treatment characteristics of women at the time of cancer diagnosis who had rural versus urban residences. All comparisons used an alpha of 0.05. Categorical variables were compared against rurality using a Pearson Chi-squared test and rurality was compared by continuous variables using a 2-sample independent group t-test.
We created three multivariable logistic regression models to investigate our primary and secondary aims. The multivariable model for our primary aim calculated the adjusted odds of receiving surgery from a gynecologic oncologist (versus a non-specialist) among all ovarian cancer patients who had cancer-directed surgery after adjusting for rurality and all previously described covariates. The second multivariable model estimated the adjusted odds of receiving a surgical referral to a gynecologic oncologist (versus not receiving a referral to a specialist) among all ovarian cancer patients who had cancer-directed surgery after adjusting for rurality and all covariates stated previously. The third model estimated the adjusted odds of receiving surgery from a gynecologic oncologist (versus a non-specialist) among the patients that received surgical care and received a referral to a gynecologic oncologist prior to their surgical care after adjusting for rurality, age at diagnosis, and stage at diagnosis. We limited the number of covariables in the third multivariable logistic regression model to prevent over parameterization.40 Covariates were selected a priori.
For our third aim, we subdivided our cohort into two strata: women who received surgery from a gynecologic oncologist and women who received surgery from another specialty. Within each stratum, we compared rural and urban women by their receipt of cytoreductive surgery, amount of tumor remaining, hospital type, great circle distance to surgical care, time from diagnosis-to-surgery among women who received adjuvant chemotherapy, and time from diagnosis-tosurgery among women who did not receive adjuvant chemotherapy.41 We also compared rural women and urban women in each stratum with their counterpart in the other stratum. Finally, we conducted an exploratory analysis among the subset of women who did not have surgery with a gynecologic oncologist, by comparing surgeon specialties and reasons for not being referred to a gynecologic oncologist by rurality.
Results
The average age of the women in this study was 61.7 years among rural women (N=252) and 60.8 years among urban women (N=552). The majority of women in the study had Charlson scores of zero (rural and urban, 79% and 81%), lived in census tracts with 0–10% of their residents having less than a high school level of education (50% and 69%), had poorly to undifferentiated grade tumors (63% and 66%), had epithelial histologies (97% and 97%), were insured (96% and 96%), had a primary site of the ovary (83% and 81%), were of non-Hispanic white race/ethnicity (99% and 91%), had stage III and IV cancer (66% and 64%), and had surgery performed by a gynecologic oncologist (73% and 88%) (Table 1). Per two-sample Chi-square tests, rural women differed from urban women in percentage of the census tract with less than a high school education, average income of their census tract, distance to surgeon, race/ethnicity, and surgeon specialty (Table 1).
Table 1.
Patient and Tumor Characteristics Among Women Who Received Surgical Care for Ovarian Cancer Treatment
| Population: Women Who Received Surgical Care for Ovarian Cancer Treatment N=804 | ||||
|---|---|---|---|---|
| Rural N=252 | Urban N=552 | P value | ||
| % | % | |||
| Age (Years) | 18–45 | 12 | 11 | 0.059 |
| 46–60 | 32 | 36 | ||
| 61–75 | 37 | 41 | ||
| 76–89 | 19 | 12 | ||
| Charlson Score | 0 | 79 | 81 | 0.756 |
| 1 | 15 | 14 | ||
| 2+ | 6 | 5 | ||
| Census Tract Percentage of Residents with Less Than a High School Education |
0–10% | 50 | 69 | <0.001 |
| 11–20% | 39 | 25 | ||
| 21%+ | 11 | 6 | ||
| Census Tract Median Income | $1–39,999 | 29 | 19 | <0.001 |
| $40,000–50,999 | 44 | 19 | ||
| $51,000–65,999 | 22 | 28 | ||
| $66,000+ | 5 | 35 | ||
| Distance to Surgeon (Miles) | 0–20 | 15 | 68 | <0.001 |
| 21–60 | 38 | 21 | ||
| 61+ | 47 | 11 | ||
| Grade | Well-Moderately Differentiated | 23 | 22 | 0.764 |
| Poorly-Undifferentiated | 63 | 66 | ||
| Unknown | 13 | 12 | ||
| Histology | Epithelial | 97 | 97 | 0.842 |
| Non-Epithelial | 3 | 3 | ||
| Insurance Status | Insured | 96 | 96 | 0.991 |
| Uninsured | 4 | 4 | ||
| Primary Site | Ovary | 83 | 81 | 0.517 |
| Fallopian Tube and Peritoneum | 17 | 19 | ||
| Race/Ethnicity | Non-Hispanic White | 99 | 91 | <0.001 |
| Non-White | 1 | 9 | ||
| Receipt of Neoadjuvant Chemotherapy Care | Yes | 12 | 10 | 0.405 |
| No | 88 | 90 | ||
| Stage | I | 24 | 28 | 0.540 |
| II | 9 | 7 | ||
| III | 49 | 45 | ||
| IV | 17 | 19 | ||
| Unknown | 1 | 1 | ||
| Surgeon Specialty | Gynecologic Oncologist |
73 | 88 | <0.001 |
| General Surgeon | 10 | 2 | ||
| Obstetrician-Gynecologist | 13 | 8 | ||
| Other/Unknown | 4 | 2 | ||
Bolding indicates a significance at 0.05.
60 miles was selected a priori due to theoretically meaningful travel/drive times in a rural state and due to concerns a larger cut point was needed for the rural states than the 50 mile cut point selected in Stewart et.al. 2014 for the continental United States.
Among all women treated surgically, rural women had lower odds of receiving both a surgical referral to a gynecologic oncologist (odds ratio (OR) 0.37, 95% confidence interval (CI) 0.23–0.59) and surgery from a gynecologic oncologist (OR 0.37, 95% CI 0.24–0.58) (Table 2). Likewise, similar patterns were observed in older women (76–89 years) versus 18–45-year-old women. In contrast, women who had stage III/ IV and unknown cancer (versus I/II) had greater odds of receiving a referral to a gynecologic oncologist (OR 2.02, 95% CI 1.29–3.19) and receiving surgery from a gynecologic oncologist (2.30, 95% CI 1.52–3.48). Lastly, among all women treated surgically, the odds of receiving a referral to a gynecologic oncologist were also nearly significantly lower in women with Charlson scores of 2+ (versus zero) (OR 0.44, 95% CI 0.20–1.00).
Table 2.
Adjusted Odds of Receiving a Surgical Referral and Surgery from a Gynecologic Oncologist Among Women Who had Ovarian Cancer-directed Surgery
| Population: Women Who Received Surgical Care for Ovarian Cancer Treatment N=804 | Odds of Receiving a Surgical Referral to a Gynecologic Oncologist (Versus No Referral) N=669 versus N=135 | Odds of Receiving Surgery from a Gynecologic Oncologist (Versus Non-specialist) N=672 versus N=132 | |||
|---|---|---|---|---|---|
| OR* | 95% CI | OR* | 95% CI | ||
| Rurality | Urban | Reference | |||
| Rural | 0.37 | 0.23–0.59 | 0.37 | 0.24–0.58 | |
| Age (Years) | 18–45 | Reference | |||
| 46–60 | 0.99 | 0.45–2.17 | 1.10 | 0.57–2.13 | |
| 61–75 | 0.81 | 0.37–1.77 | 1.03 | 0.52–2.03 | |
| 76–89 | 0.33 | 0.14–0.75 | 0.43 | 0.21–0.91 | |
| Charlson Score | 0 | Reference | |||
| 1 | 0.84 | 0.46–1.53 | 1.08 | 0.60–1.93 | |
| 2+ | 0.44 | 0.20–1.00 | 0.61 | 0.27–1.36 | |
| Census Tract Percentage of Residents with Less Than a High School Education | 0–10% | Reference | |||
| 11–20% | 0.85 | 0.50–1.44 | 0.47 | 0.47–1.25 | |
| 21%+ | 0.48 | 0.21–1.10 | 0.24 | 0.24–1.07 | |
| Census Tract Median Income |
$1–39,999 | Reference | |||
| $40,000–50,999 | 0.56 | 0.29–1.07 | 0.59 | 0.33–1.06 | |
| $51,000–65,999 | 0.61 | 0.29–1.26 | 0.74 | 0.38–1.42 | |
| $66,000+ | 0.52 | 0.23–1.21 | 0.52 | 0.25–1.10 | |
| Insurance Status | Insured | Reference | |||
| Uninsured | 0.56 | 0.20–1.63 | 0.73 | 0.27–1.95 | |
| Race/Ethnicity | Non-Hispanic White | Reference | |||
| Non-White | 1.10 | 0.39–3.10 | 1.02 | 0.42–2.48 | |
| Stage | I/II | Reference | |||
| III/IV + Unknown | 2.02 | 1.29–3.19 | 2.30 | 1.52–3.48 | |
Bolding indicates a significance at 0.05. All odds ratios are adjusted for all variables in table.
Among the 669 women who received a surgical referral to a gynecologic oncologist, 30 women (5%) did not receive surgery from a gynecologic oncologist (Table 3). Among the women that received a surgical referral to a gynecologic oncologist, rural residence at diagnosis (versus urban) did not statistically impact the odds of receiving surgery from a gynecologic oncologist (OR 0.56, 95% CI 0.26–1.20). Likewise, age categories (46–60, 61–75, and 76–89-year-old women versus 18–45-year-old women) did not impact the odds of receiving surgery from a gynecologic oncologist (OR 1.32, 1.88, 1.69; 95% CI 0.47–3.70, 0.63–5.62, 0.39–7.21, respectively). However, after receiving a referral to a gynecologic oncologist, women with stage III/ IV and unknown cancer continued to have greater odds of receiving surgery from a gynecologic oncologist (OR 2.27, 95% CI 1.06–4.86).
Table 3.
Adjusted Odds of Receiving Surgery from a Gynecologic Oncologist Among Women Who Were Referred to a Gynecologic Oncologist for Surgical Care
| Sub-Analysis Population: Women Who Had Surgery and Received a Surgical Referral to a Gynecologic Oncologist Prior to Surgery N=669 | Odds of Receiving Surgery from a Gynecologic Oncologist (Versus Non-specialist) N=639 versus N=30 |
||
|---|---|---|---|
| OR* | 95% CI | ||
| Rurality | Urban | Reference | |
| Rural | 0.56 | 0.26–1.20 | |
| Age (Years) | 18–45 | Reference | |
| 46–60 | 1.32 | 0.47–3.70 | |
| 61–75 | 1.88 | 0.63–5.62 | |
| 76–89 | 1.69 | 0.39–7.21 | |
| Stage | I/II | Reference | |
| III/IV + Unknown | 2.27 | 1.06–4.86 | |
Bolding indicates a significance at 0.05. All odds ratios are adjusted for all variables in table.
There was no difference between rural and urban women in surgery received, amount of tumor remaining, and time from diagnosis-to-surgery for women who did not have adjuvant chemotherapy among women who received surgery from a gynecologic oncologist and among women who did not receive surgery from a gynecologic oncologist (Table 4). Among women who received surgery from a gynecologic oncologist, rural women were more likely than urban women to have surgery at a government hospital (45% versus 33%, p value 0.013) and to have a shorter time from diagnosis-to-surgery for women who had adjuvant chemotherapy (mean: 76 versus 105 days, p value 0.039). Additionally, among women who received surgery from a gynecologic oncologist, rural women were more likely than urban women to travel a greater distance to surgery (mean: 80.7 miles versus 24.9 miles, p value <0.001).
Table 4.
Investigating Surgical Care Disparities Among Women Who Received Surgery for Ovarian Cancer Treatment
| Women Who Received Surgery from a Gynecologic Oncologist N=672 | Women Who Did Not Receive Surgery from a Gynecologic Oncologist N=132 | Comparison of Women Who Received Care by a Gynecologic Oncologist (Versus Non-Specialist) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rural Women | Urban Women | ||||||||
| Rural N=185 | Urban N=487 | P value | Rural N=67 | Urban N=65 | P value | P value | P value | ||
| % | % | % | % | ||||||
| Received Cytoreductive SurgeryA |
Yes | 89 | 91 | 0.320 | 69 | 65 | 0.884 | <0.001 | <0.001 |
| No | 11 | 9 | 31 | 35 | |||||
| Amount of Tumor RemainingA |
≤1cm | 63 | 64 | 0.775 | 39 | 26 | 0.173 | <0.001 | <0.001 |
| >1cm | 24 | 22 | 12 | 22 | |||||
| UnknownB | 12 | 14 | 49 | 52 | |||||
| Hospital Type Where Surgery was ReceivedC | Government | 45 | 33 | 0.013 | 38 | 28 | 0.271 | 0.355 | 0.288 |
| Private | 55 | 66 | 62 | 72 | |||||
| Distance to Surgery (Miles) |
Mean (95% CI) | 80.7 (74.3–87.1) | 24.9 (22.0–27.9) | <0.001 | 56.7 (10.1–103.4) | 24.2 (4.6–43.8) | 0.206 | 0.126 | 0.932 |
| Standard Deviation (Range) |
44.3 (0–225) | 32.6 (0–285) | 191.2 (1–1526) | 79.1 (1–617) | |||||
| Time from Diagnosis to Surgery (Days) among patients who received adjuvant chemotherapyD |
Mean (95% CI) | 75.7 (54.8–96.7) | 104.9 (87.7–122.1) | 0.039 | -- | -- | -- | -- | -- |
| Range | 0–230 | 0–288 | -- | -- | |||||
| Time from Diagnosis to Surgery (Days) among patients who did not receive adjuvant chemotherapyD |
Mean (95% CI) | 8.7 (6.4–10.9) | 10.0 (7.4–12.6) | 0.564 | 6.7 (3.2–10.2) | 5.0 (1.9–8.2) | 0.486 | 0.376 | 0.175 |
| Range | 0–97 | 0–376 | 0–69 | 0–69 | |||||
Indicates results were not reported due to N<5.
Bolding indicates a significance at 0.05.
Cytoreduction is standard of care surgery, also known as optimal debulking, and involves removal of as much cancer as possible in the pelvis and/or abdomen.
Persons in this category either had cytoreductive surgery and the tumor remaining was unknown, or they did not have cytoreductive surgery and tumor remaining was not reported.
Unknown hospital type was not included in this analysis. Government hospitals are based on Healthcare Cost and Utilization Project (HPUC) and defined as a government owned, nonfederal, public hospital.
Date of diagnosis coincides with the first mention and concern of an ovarian cancer in the medical record. Date of diagnosis does not require histologic confirmation of this cancer.
Rural women who were treated surgically by a gynecologic oncologist were more likely to receive cytoreductive surgery than rural women who were not treated surgically by a gynecologic oncologist (89% versus 69%, p value <0.001). As well, rural women treated surgically by a gynecologic oncologist were more likely to have more complete tumor removal to one centimeter or less (63% versus 39%, p value <0.001). This difference in complete tumor removal to one centimeter or less persisted when we limited our analysis to stages III and IV patients (56% versus 41%, p value 0.014). Rural women treated surgically by a gynecologic oncologist (versus non-gynecologic oncologist) were not more likely to have greater time from diagnosis-to-surgery for women who did not have adjuvant chemotherapy. Similar trends were seen in urban women when compared by surgeon specialty. Neither rural women nor urban women experienced differences in travel distance and hospital type by surgeon specialty (Table 4). Urban patients (all stages: 64% versus 26%, p value <0.001; stages III and IV disease:53% versus 20%, p value 0.004) with a gynecologic oncologist surgeon versus a non-gynecologic oncologist surgeon were also less likely to receive complete cytoreductive surgery.
The subgroup of women who did not have surgery performed by a gynecologic oncologist had surgery performed by general surgeons or obstetrician-gynecologists (Table 5). No differences in surgeon specialty were observed by rurality. More urban (versus rural) women in this subgroup saw a gynecologic oncologist after their initial surgery with a non-gynecologic oncologist (37% versus 12%).
Table 5.
Information on Women Who Did Not Have Surgery with a Gynecologic Oncologist (N=132)
| Rural N=67 | Urban N=65 | P value | ||
|---|---|---|---|---|
| % | % | |||
| Specialty of Non-Gynecologic Oncologist Physician Who Performed Surgery | General Surgeon | 36 | 20 | 0.142 |
| Obstetrician-Gynecologist | 48 | 63 | ||
| Other or Unknown | 17 | 17 | ||
| Reason Patient was Not Referred to a Gynecologic Oncologist^ | There was no gynecologic oncologist practicing at the hospital where the surgery was performed | 18 | 14 | 0.022 |
| Insurance issues | 0 | 0 | ||
| Patient is too ill or died | 4 | 5 | ||
| Patient was referred to a gynecologic oncologist after surgical care | 12 | 37 | ||
| Other or Unknown | 66 | 45 |
Bolding indicates a significance at 0.05.
Registrars were instructed to review the medical record and record the above preset list of reasons for the patient not being referred to a gynecologic oncologist for surgical care. The registrars were also able to write in responses if they were unsure of the best category for the reason. This was a select all that apply response-option question. The selection of a response indicates it was stated in the medical record by the physician as a reason for not providing a referral or performing the surgery themselves. For example, for insurance issues to be selected, the physician would need to state insurance issues were a reason that the patient was not referred. It does not indicate that the abstractor verified that all patients were insured.
Discussion
There is a large rural-urban difference in receipt of ovarian cancer surgery by a specialist among ovarian cancer patients that had surgical care. Rural ovarian cancer patients have 63% lower odds of receiving surgery by a gynecologic oncologist and receiving a surgical referral to a gynecologic oncologist. The adjusted odds ratio results obtained in our study agree with prior studies, but exceed the magnitudes previously reported.16–18 It is unclear why rural women who receive ovarian cancer-directed surgery are less likely to be given a referral to a gynecologic oncologist. It is possible rural general surgeons and obstetrician-gynecologists are more comfortable performing ovarian cancer surgery, are unaware of or place less importance on the guideline recommendation, are more likely to place importance on local care and geographic convenience, or are more likely to care for patients who prefer local care.8,42,43 It is also possible barriers in rural referral networks, such as long wait times and poor provider-to-provider communication, reduce rural providers’ perceived ability to make a referral to a gynecologic oncologist.8,12,24,44 Additionally, it is possible rural providers perceive their patients have greater patient-level barriers, such as transportation limitations, financial concerns, and apprehension about receiving care at a higher level care center, and thus selectively do not make the recommended referral.24,45
Among the ovarian cancer patients who received a surgical referral to a gynecologic oncologist, rural women were as likely to receive surgery from a specialist. Consequently, it appears the disparity in receipt of surgery from a gynecologic oncologist may be largely due to referral differences versus patient-level differences. Furthermore, given that rural women have lower odds of receiving surgery from a gynecologic oncologist independent of age, disease severity, and the socioeconomic status of the census tract, it seems unlikely that the difference in specialist surgical care is due to patient wellness and the local community resources available. It is possible this study failed to detect all rural barriers patients face after receiving a referral, such as differences that extend from disparities in provider encouragement and patient-provider relationships.46,47 Rural cancer patients have been shown to play a less active role in care decisions and in researching alternative options.46,47 They have also been shown to choose local care more often, especially if they have a strong relationship with their provider.24,48 It is also possible the importance of traveling to a gynecologic oncologist for surgical care is not communicated to rural patients effectively.24 Studies have shown rural patients utilize healthcare and specialists less.49 Thus, extended explanations about why it is important to seek surgical care from a gynecologic oncologist may be important for rural patients. Further studies may help to explain the disparities rural patients face after receiving referrals to surgical specialists.
A surgical referral to a gynecologic oncologist was 67% less likely in older women ages 76–89 versus 18–45 years old, 56% less likely (nearly significant) in women with Charlson scores of 2+ versus 0, and 102% more likely in women who had late stage cancer. These findings are not surprising given the Behavioral Model of Health Services Use, which suggests age is a predisposing factor impacting the need for a specialist, and overall health (proxied by the Charlson score) and stage are the perceived and evaluated factor providers and patients utilize in care decision-making.36–38 Young women may be more motivated to obtain care through a specialist for fertility preservation, and older women may face greater barriers obtaining transportation to Centers of Excellence.50 Women with greater comorbidities may need more ancillary services during and after their surgical care.19 Advanced stage patients may be more often referred to a gynecologic oncologist due to a higher chance of having a preoperative diagnosis or being perceived to require a more technically difficult operation with a larger amount of lymph node and organ sampling/removal.51 However, women without suspicion of cancer prior to surgery, should have still received cancer-directed surgery with a gynecologic oncologist after their cancer was discovered regardless of stage.
Rural women traveled 56 miles further than their urban counterparts when receiving surgical care by a specialist. This agrees with the Centers of Excellence Model of practicing gynecologic oncologists, within which, specialists are located in urban centers.19 The Centers of Excellence Model is preferred by some gynecologic oncologists. Some gynecologic oncologists have reported select quality-of-care concerns about performing operations at non-specialized facilities or rural hospitals.19,52 Furthermore, rural women with a non-gynecologic oncologist traveled 32 miles further than urban women with a gynecologic oncologist. While the difference among rural and urban patients was not statistically different by surgeon specialty, the difference in average distance to surgery for rural women receiving surgery by a gynecologic oncologist was 24 miles further than rural women receiving surgery from a non-gynecologic oncologist, while it was less than one mile difference by surgeon specialty for urban women. Promising strategies to lessen the resulting rural-urban distance disparities would be useful. When receiving care from a non-specialist, there was no statistical difference in travel distance between rural and urban patients. Furthermore, women traveled shorter distances to non-specialists, which likely suggests often non-specialists are local providers. The specific drivers of local care can be further defined in future studies.
Receiving surgical care by a gynecologic oncologist increased the odds of receiving cytoreduction and having optimal cytoreduction with removal of residual tumor to less than one centimeter. This is consistent with prior literature and remains a resounding reason that women with ovarian cancer are recommended to be referred to a surgical specialist.53,54 Having surgery performed by a gynecologic oncologist (versus another provider type) reduced rural women’s’ time from diagnosis-to-surgery for women receiving adjuvant chemotherapy by about one month. This finding could be due to greater care coordination, or reduced courses of chemotherapy treatment. Further research is needed into disparities in health service among ovarian cancer patients receiving adjuvant chemotherapy.
Finally, while we were unable to assess exact reasons patients were not referred due to missing information, three times more urban women saw a gynecologic oncologist for at least a consult after surgery relative to rural women. This may be concerning because it suggests a potential continuation of rural-urban differences in specialty care even after emergency surgery or non-specialist surgical care. This finding warrants further investigation.
Strengths and Limitations
The quality and representativeness of the data are strengths of this study. The data were created from statewide population-based cancer surveillance and were abstracted by highly trained cancer registrars. The specific study variables were collected using a standardized tool and included thorough quality control checks to ensure appropriateness and accurateness of reported values. Complete cancer surveillance data for ovarian cancer is generally three years behind and often times more for special studies. Since 2010, there have not been substantial changes in guidelines pertaining to the importance of surgical care. As well, the distribution of Centers of Excellence likely has changed very little in these three states in the last 10 years. In addition, all sampled cases were histologically confirmed, limiting diagnostic misclassification.
Our primary outcome indicated the primary surgeon. The involvement of a specialist in a consulting or standby role was not abstracted. However, future investigations could investigate patterns of care across different consulting methods. Not all potential covariates were available in the dataset, such as patient-level wealth, strength of social support network, and attitudes about specialists.55 Actual driving distance was not available, but straight mile distance served as a proxy. This likely made our estimates of travel distance conservative. The findings in these three Midwestern states do not necessarily represent all women diagnosed with ovarian cancer in the United States. As well, they are a focused investigation of ovarian cancer patients that pursued cancer-directed surgery, and thus findings are not generalizable of all ovarian cancer patients, such as those pursuing palliative treatment. This study was designed by the CDC to be able to detect differences among women living in rural areas compared to others, as opposed to assessing other documented disparities in treatment, such as racial and ethnic differences. While regionalization patterns were well established in these states prior to 2010 to 2012, patterns of care may not be generalizable across time, and may not be generalizable to other states. There are nuances to treating ovarian cancer. While we analyzed the outcome of cytoreductive surgery for the cohort overall similar to previously published studies, we acknowledge some specific cases may not have required cytoreduction and appropriately received an alternative therapy. 56–59
Conclusion
Independent of census tract-level socioeconomic status, rural women were significantly less likely to receive a referral to, and surgery from, a gynecologic oncologist. Among women that received a surgical referral to a gynecologic oncologist, rural women were as likely as their urban counterparts to receive surgical care by a specialist. Rural women traveled further than urban women when they received surgical care from a specialist. Additionally, rural women who received care from a gynecologic oncologist (versus a non-specialist) were more likely to have guideline-recommended cytoreduction surgery and tumor removal to ≤1cm. As a result, rural women who are treated by a non-specialist are at greater risk of receiving substandard care and unnecessarily high recurrence and death rates. Further research in this area will help to determine the causes of the rural-urban differences in referral rates to specialists and into disparities faced by rural women after a surgical referral is received to a specialist. Likewise, system-level efforts that attempt to reduce the barriers rural ovarian cancer patients face when seeking surgical care from a gynecologic oncologist, such as travel burdens and greater time from diagnosis to surgery may be helpful.
Supplementary Material
Covariate Selection through the Behavioral Model of Health Service Use
Highlights.
Rural ovarian cancer patients are 63% less likely to receive a referral to a gynecologic oncologist for surgery
Rural ovarian cancer patients are significantly less likely to receive surgery from a gynecologic oncologist
After a surgical referral, rural ovarian cancer patients are just as likely to receive surgery from a specialist
Specialist-provided surgery increases receipt of cytoreduction and complete tumor removal for rural ovarian cancer patients
Rural women (versus urban) who receive surgery from a gynecologic oncologist travel farther to surgical care
Acknowledgements
We would like to thank Jeff Steffens, BS CTR, the MCR-ARC Quality Assurance staff, and the staff of facilities throughout Missouri, Iowa, and Kansas’ central cancer registries for their dedication and desire for continuous quality improvement and submitting their reportable cases to MCR-ARC. We particularly want to thank staff of the 50 facilities in Missouri and the facilities in Iowa and Kansas who participated in this project for their willingness to take on extra responsibilities to make this project a success. We acknowledge, appreciate, and sincerely thank the women with ovarian cancer in this study for this opportunity. We acknowledge the National Institute of Health for their funding of the University of Iowa Medical Scientist Training Program [NIH T32GM007337].
Funding Sources
This project was conducted by the CDC, Westat and the state cancer registries of Iowa, Kansas, and Missouri, and funded under CDC contract 200-2014-61258. The Iowa Cancer Registry is also funded in part with Federal funds from NIH/NCI contract HHSN261201800012I and cancer center support grant NIH/NCI P30CA086862. The Kansas Cancer Registry is also funded by the Kansas Department of Health and Environment. The Missouri Cancer Registry core activities are supported in part by a cooperative agreement between CDC and the Missouri Department of Health and Senior Services (DHSS) (U58DP006299-01/02) and a Surveillance Contract between DHSS and the University of Missouri.
Footnotes
Conflict of Interest Statement:
The authors have no conflict of interests to disclose.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Surveillance E, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Populations - Total U.S. (1969–2017) <Katrina/Rita Adjustment> - Linked To County Attributes - Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released December 2018. [Google Scholar]
- 2.U.S. Preventive Services Task Force. Risk assessment gc, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–81. [DOI] [PubMed] [Google Scholar]
- 3.Henderson JT WE, Sawaya GF. Screening for Ovarian Cancer: An Evidence Review for the US Preventive Services Task Force Evidence Synthesis No. 157. AHRQ Publication No. 17–05231-EF1. Rockville, MD: Agency for Healthcare Research and Quality; 2018. [PubMed] [Google Scholar]
- 4.Carlson KJ SS, Singer DE. Screening for Ovarian Cancer. Ann Intern Med. 1994;121:124–132. doi: 10.7326/0003-4819-121-2-199407150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Averette HE, Wrennick A, Angioli R. HISTORY OF GYNECOLOGIC ONCOLOGY SUBSPECIALTY. Surgical Clinics of North America. 2001;81(4):747–751. [DOI] [PubMed] [Google Scholar]
- 6.Guidelines for Referral to a Gynecologic Oncologist: Rationale and Benefits. Gynecol Oncol. 2000;78(3):S1–S13. [DOI] [PubMed] [Google Scholar]
- 7.Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006;98(3):172–180. [DOI] [PubMed] [Google Scholar]
- 8.Mercado C, Zingmond D, Karlan BY, et al. Quality of care in advanced ovarian cancer: the importance of provider specialty. Gynecol Oncol. 2010;117(1):18–22. [DOI] [PubMed] [Google Scholar]
- 9.Vernooij F, Heintz P, Witteveen E, van der Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: a systematic review. Gynecol Oncol. 2007;105(3):801–812. [DOI] [PubMed] [Google Scholar]
- 10.Chan JK, Kapp DS, Shin JY, et al. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstet Gynecol. 2007;109(6):1342–1350. [DOI] [PubMed] [Google Scholar]
- 11.Rim SH, Polonec L, Stewart SL, Gelb CA. A national initiative for women and healthcare providers: CDC’s Inside Knowledge: Get the Facts About Gynecologic Cancer campaign. Journal of Women’s Health. 2011;20(11):1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart SL, Rim SH, Richards TB. Gynecologic Oncologists and Ovarian Cancer Treatment: Avenues for Improved Survival. Journal of Women’s Health. 2011;20(9):1257–1260. [DOI] [PubMed] [Google Scholar]
- 13.Verleye L, Vergote I, van der Zee AGJ. Patterns of care in surgery for ovarian cancer in Europe. European Journal of Surgical Oncology. 2010;36:S108–S114. [DOI] [PubMed] [Google Scholar]
- 14.Escayola C, Torrent JJ, Ferron G, Quenet F, Querleu D. When and Who Should Perform Epithelial Ovarian Cancer Surgery? International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2018;28(3):594–599. [DOI] [PubMed] [Google Scholar]
- 15.Shakeel S, Elit L, Akhtar-Danesh N, Schneider L, Finley C. Care Delivery Patterns, Processes, and Outcomes for Primary Ovarian Cancer Surgery: A Population-Based Review Using a National Administrative Database. J Obstet Gynaecol Can. 2017;39(1):25–33. [DOI] [PubMed] [Google Scholar]
- 16.Carney ME, Lancaster JM, Ford C, Tsodikov A, Wiggins CL. A population-based study of patterns of care for ovarian cancer: who is seen by a gynecologic oncologist and who is not? Gynecol Oncol. 2002;84(1):36–42. [DOI] [PubMed] [Google Scholar]
- 17.Mercado C, Zingmond D, Karlan BY, et al. Quality of care in advanced ovarian cancer: The importance of provider specialty. Gynecol Oncol. 2010;117(1):18–22. [DOI] [PubMed] [Google Scholar]
- 18.Tracey E, Hacker NF, Young J, Armstrong BK. Effects of Access to and Treatment in Specialist Facilities on Survival From Epithelial Ovarian Cancer in Australian Women: A Data Linkage Study. International Journal of Gynecologic Cancer. 2014;24(7):1232. [DOI] [PubMed] [Google Scholar]
- 19.Ricci S, Tergas AI, Long Roche K, et al. Geographic disparities in the distribution of the U.S. gynecologic oncology workforce: A Society of Gynecologic Oncology study. Gynecologic oncology reports. 2017;22:100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart SL, Cooney D, Hirsch S, et al. The Effect of Gynecologic Oncologist Availability on Ovarian Cancer Mortality. World J Obstet Gynecol. 2014;3(2):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergin RJ, Emery J, Bollard RC, et al. Rural-Urban Disparities in Time to Diagnosis and Treatment for Colorectal and Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2018;27(9):1036–1046. [DOI] [PubMed] [Google Scholar]
- 22.Gentil J, Dabakuyo TS, Ouedraogo S, Poillot ML, Dejardin O, Arveux P. For patients with breast cancer, geographic and social disparities are independent determinants of access to specialized surgeons. A eleven-year population-based multilevel analysis. BMC Cancer. 2012;12:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlton M, Schlichting J, Chioreso C, Ward M, Vikas P. Challenges of Rural Cancer Care in the United States. Oncology (Williston Park). 2015;29(9):633–640. [PubMed] [Google Scholar]
- 24.Weeks KS, West M, Lynch CF, et al. Patient and Provider Perspectives on Barriers to Accessing Gynecologic Oncologists for Ovarian Cancer Surgical Care. Under reveiw at Women’s Health Reports. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham S, Hallisey E, Wilt G, Flanagan B, Rodriguez JL, Peipins L. Sociodemographic disparities in access to ovarian cancer treatment. Annals of Cancer Epidemiology. 2019;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilhelmina Ross JJ-T, Diane Ng, Maricarmen Traverso-Ortiz. Methods of Conducting the Patterns of Ovarian Cancer Care and Survival in the Midwestern Region of the United States. NAACCR- IACR Conference Abstract Program.June 9-13:27. [Google Scholar]
- 27.Stewart SL, Rim SH, Trivers KF. Summary and impact of ovarian cancer research and programmatic activities at the Centers for Disease Control and Prevention. Journal of women’s health (2002). 2010;19(8):1427–1432. [DOI] [PubMed] [Google Scholar]
- 28.Shalowitz DI, Vinograd AM, Giuntoli RL, 2nd. Geographic access to gynecologic cancer care in the United States. Gynecol Oncol. 2015;138(1):115–120. [DOI] [PubMed] [Google Scholar]
- 29.Ingram DD, Franco SJ. 2013. NCHS Urban-Rural Classification Scheme for Counties. Vital and health statistics Series 2, Data evaluation and methods research. 2014(166):1–73. [PubMed] [Google Scholar]
- 30.Eathington L 2000–2009 Population Growth in the Midwest: Urban and Rural Dimensions. 2010. [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 32.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. [DOI] [PubMed] [Google Scholar]
- 33.Radovanovic D, Seifert B, Urban P, et al. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart (British Cardiac Society). 2014;100(4):288–294. [DOI] [PubMed] [Google Scholar]
- 34.Matz M, Coleman MP, Sant M, et al. The histology of ovarian cancer: worldwide distribution and implications for international survival comparisons (CONCORD-2). Gynecol Oncol. 2017;144(2):405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruhl J WE, Hofferkamp J, et al. Grade Manual. Springfield, IL: February 2019. 62704–4194. [Google Scholar]
- 36.Andersen R, Newman JF. Societal and individual determinants of medical care utilization in the United States. Milbank Mem Fund Q Health Soc. 1973;51(1):95–124. [PubMed] [Google Scholar]
- 37.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36(1):1–10. [PubMed] [Google Scholar]
- 38.Derose KP, Gresenz CR, Ringel JS. Understanding disparities in health care access--and reducing them--through a focus on public health. Health Aff (Millwood). 2011;30(10):1844–1851. [DOI] [PubMed] [Google Scholar]
- 39.Andersen RM. Revisiting the Behavioral Model and Access to Medical Care: Does it Matter? Journal of Health and Social Behavior. 1995;36(1):1–10. [PubMed] [Google Scholar]
- 40.Agresti A An introduction to categorical data analysis. John Wiley & Sons; 2018. [Google Scholar]
- 41.Chi DS, Hoskins WJ. Primary surgical management of ovarian cancer In: Ovarian Cancer. Springer; 2000:75–87. [DOI] [PubMed] [Google Scholar]
- 42.Ward MM, Jaana M, Wakefield DS, et al. What would be the effect of referral to high‐volume hospitals in a largely rural state? The Journal of Rural Health. 2004;20(4):344–354. [DOI] [PubMed] [Google Scholar]
- 43.Stewart GD, Long G, Tulloh BR. Surgical service centralisation in Australia versus choice and quality of life for rural patients. Medical Journal of Australia. 2006;185(3):162–163. [DOI] [PubMed] [Google Scholar]
- 44.Stewart SL, Cooney D, Hirsch S, et al. The Effect of Gynecologic Oncologist Availability on Ovarian Cancer Mortality. World J Obstet Gynecol. 2014;3(2):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart SL, Townsend JS, Puckett MC, Rim SH. Adherence of Primary Care Physicians to Evidence-Based Recommendations to Reduce Ovarian Cancer Mortality. Journal of women’s health (2002). 2016;25(3):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butow PN, Phillips F, Schweder J, et al. Psychosocial well-being and supportive care needs of cancer patients living in urban and rural/regional areas: a systematic review. Supportive Care in Cancer. 2012;20(1):1–22. [DOI] [PubMed] [Google Scholar]
- 47.Ziebland S, Evans J, McPherson A. The choice is yours? How women with ovarian cancer make sense of treatment choices. Patient education and counseling. 2006;62(3):361–367. [DOI] [PubMed] [Google Scholar]
- 48.McCall K, Rice AM. What influences decisions around the place of care for terminally ill cancer patients? International Journal of Palliative Nursing. 2005;11(10):541–547. [DOI] [PubMed] [Google Scholar]
- 49.Chan L, Hart LG, Goodman DC. Geographic Access to Health Care for Rural Medicare Beneficiaries. The Journal of Rural Health. 2006;22(2):140–146. [DOI] [PubMed] [Google Scholar]
- 50.Abdel-Hady E-S, Abdel-Hady Hemida R, Gamal A, El-Shamey M. Fertility sparing surgery for ovarian tumors in children and young adults. Archives of Gynecology and Obstetrics. 2012;285(2):469–471. [DOI] [PubMed] [Google Scholar]
- 51.Angioli R, Plotti F, Palaia I, et al. Update on lymphadenectomy in early and advanced ovarian cancer. Current Opinion in Obstetrics and Gynecology. 2008;20(1). [DOI] [PubMed] [Google Scholar]
- 52.Killeen S, O’sullivan M, Coffey J, Kirwan W, Redmond H. Provider volume and outcomes for oncological procedures. British Journal of Surgery: Incorporating European Journal of Surgery and Swiss Surgery. 2005;92(4):389–402. [DOI] [PubMed] [Google Scholar]
- 53.Vernooij F, Heintz P, Witteveen E, van der Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: A systematic review. Gynecol Oncol. 2007;105(3):801–812. [DOI] [PubMed] [Google Scholar]
- 54.Chan JK, Kapp DS, Shin JY, et al. Influence of the Gynecologic Oncologist on the Survival of Ovarian Cancer Patients. Obstetrics & Gynecology. 2007;109(6). [DOI] [PubMed] [Google Scholar]
- 55.Mao L, Yang J, Deng G. Mapping rural-urban disparities in late-stage cancer with high-resolution rurality index and GWR. Spatial and spatio-temporal epidemiology. 2018;26:15–23. [DOI] [PubMed] [Google Scholar]
- 56.Lin JJ, Egorova N, Franco R, Prasad-Hayes M, Bickell NA. Ovarian Cancer Treatment and Survival Trends Among Women Older Than 65 Years of Age in the United States, 1995–2008. Obstet Gynecol. 2016;127(1):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thrall MM, Goff BA, Symons RG, Flum DR, Gray HJ. Thirty-Day Mortality After Primary Cytoreductive Surgery for Advanced Ovarian Cancer in the Elderly. Obstetrics & Gynecology. 2011;118(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dahm-Kähler P, Palmqvist C, Staf C, Holmberg E, Johannesson L. Centralized primary care of advanced ovarian cancer improves complete cytoreduction and survival - A population-based cohort study. Gynecol Oncol. 2016;142(2):211–216. [DOI] [PubMed] [Google Scholar]
- 59.Thrall MM, Goff BA, Symons RG, Flum DR, Gray HJ. Thirty-day mortality after primary cytoreductive surgery for advanced ovarian cancer in the elderly. Obstet Gynecol. 2011;118(3):537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Covariate Selection through the Behavioral Model of Health Service Use