Abstract
Rationale: Obesity is associated with pulmonary arterial hypertension (PAH), but its impact on outcomes such as health-related quality of life (HRQoL), hospitalizations, and survival is not well understood.
Objectives: To assess the effect of obesity on HRQoL, hospitalizations, and survival in patients with PAH.
Methods: We performed a cohort study of adults with PAH from the Pulmonary Hypertension Association Registry, a prospective multicenter registry. Multivariate linear mixed-effects regression was used to examine the relationship between weight categories and HRQoL using the Short Form-12 and emPHasis-10. We used multivariable negative binomial regression to estimate hospitalization incidence rate ratios (IRRs) and Cox regression to estimate hazard ratios (HRs) for transplant-free survival by weight status.
Results: A total of 767 subjects were included (mean age of 57 years, 74% female, 33% overweight, and 40% with obesity), with median follow-up duration of 527 days. Overweight patients and patients with obesity had higher baseline emPHasis-10 scores (worse HRQoL), which persisted over time (P < 0.001). Patients who are overweight and obese have a trend toward increased incidence of hospitalizations compared with normal-weight patients (IRR, 1.34; 95% confidence interval [95% CI], 0.94–1.92 and IRR, 1.33; 95% CI 0.93–1.89, respectively). Overweight patients and patients with obesity had lower risk of transplant or death compared with normal-weight patients (HR, 0.45; 95% CI, 0.25–0.80 and HR, 0.39; 95% CI, 0.22–0.70, respectively).
Conclusions: In a large multicenter, prospective cohort of PAH, patients who were overweight or obese had worse disease-specific HRQoL despite better transplant-free survival compared with normal-weight patients. Future interventions should address the specific needs of these patients.
Keywords: pulmonary arterial hypertension, obesity, quality of life, hospitalization, survival analysis
Pulmonary arterial hypertension (PAH) is characterized by elevated pulmonary artery pressure, right ventricular dysfunction, exercise limitation, and limited life expectancy. Historically described as a disease of young, otherwise healthy women, the epidemiology of PAH has evolved over time (1). Currently, patients with PAH are older and approximately one-third are obese (2–4). Although human and experimental models suggest that obesity may predispose individuals to the development of PAH (5–7), in some studies, obesity has been associated with better survival, a phenomenon coined the “obesity paradox” (4).
PAH exerts a significant negative impact on health-related quality of life (HRQoL) (8). As the focus of PAH care expands beyond improving survival, patient-centered outcomes such as HRQoL and hospitalizations have become a therapeutic target and included as outcome measures in recent clinical trials (9). Although obesity is associated with worse HRQoL in the general population, less is known about the effect of obesity on HRQoL in PAH (10, 11). Studies of the impact of obesity on survival in PAH have conflicting results. Obesity was associated with better survival in the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) and decreased in-hospital mortality in an inpatient claims dataset (4, 12); however, it was not associated with decreased mortality in the French PAH Registry (13).
We aimed to assess the association of obesity with HRQoL, hospitalizations, and survival in patients with PAH using data from the prospective Pulmonary Hypertension Association Registry (PHAR), a multicenter registry of patients with PAH in the United States. We hypothesized that overweight patients and patients with obesity with PAH would be associated with worse HRQoL but better overall survival compared with normal-weight patients.
Methods
Study Sample
We studied patients with newly diagnosed or established PAH who enrolled in the PHAR between 2015 and September 2019 at one of the 50 participating pulmonary hypertension care centers. We included patients age 18 or more with a diagnosis of PAH. We excluded those with pulmonary veno-occlusive disease, persistent pulmonary hypertension of the newborn, and chronic thromboembolic pulmonary embolism. Patients with missing body mass index (BMI) data at enrollment and those classified as underweight (BMI < 18.5 kg/m2) were also excluded, as the number of underweight patients was insufficient to draw meaningful conclusions.
Clinical Variables
Patients enrolled into the registry have demographics, clinical parameters, and hemodynamics from their diagnostic right heart catheterization recorded at their initial visit and are seen in follow-up as clinically indicated, with data collected in approximately 6-month intervals (14). Anthropometric data are collected at the initial and subsequent visits. Weight status was defined by BMI as normal weight (BMI = 18.5–24.9 kg/m2), overweight (BMI = 25.0–29.9 kg/m2), and obese (BMI ≥ 30.0 kg/m2). Percentage predicted 6-minute walk distance (6MWD) was calculated from the ratio of the actual 6MWD to the predicted 6MWD derived from sex-specific reference equations (15).
Study Outcomes
The PHAR administers the following two HRQoL questionnaires at each visit: the Medical Outcome Study Short Form-12 (SF-12) and the emPHasis-10 (e10) (16, 17). The SF-12 is an abbreviated form of the Medical Outcome Study Short Form-36 (SF-36) and is divided into physical and mental domains, which are each scored from 0 to 100, with higher scores indicating better HRQoL. The e10 is a pulmonary hypertension–specific HRQoL instrument and is scored from 0 to 50, with higher scores indicating worse HRQoL (16). At each visit, patients report any interval hospitalizations from their last visit. Death or lung transplantation were tracked by the research coordinator at each participating site.
Statistical Analysis
Baseline data were summarized by weight strata. We fit mixed-effects generalized linear regression models with random intercepts to account for the repeated measures of weight. We adjusted these models for variables determined a priori as potential confounders of the association of weight strata and HRQoL and significantly associated with HRQoL on univariate analysis (age, sex, race/ethnicity, etiology, marital status, employment status, use of supplemental oxygen, and referral to lung transplantation). We assessed effect modification by age, sex, and race by adding an interaction term between these variables and weight strata. Using negative binomial regression, we estimated hospitalization incidence rate ratios (IRRs) by weight strata and included an offset term denoting follow-up time to account for differential length of follow-up between individuals. We adjusted these models for the following a priori determined potential confounders: age, sex, race/ethnicity, etiology, cardiac index, right atrial pressure (RAP), receipt of combination PAH therapy, use of a parenteral prostacyclin analog, use of supplemental oxygen, and referral to lung transplantation. We conducted sensitivity analysis for the hospitalization models, restricting the dataset to patients with idiopathic PAH (IPAH). Kaplan-Meier curves and the log-rank test were used to compare transplant-free survival among different weight strata. Cox proportional hazards models were used to estimate hazard ratios (HRs) for transplant-free survival by weight stratum, adjusting for the same potential confounders as the hospitalization models. Subjects were censored at the time of last follow-up before March 1, 2020. We did not impute missing variables or outcomes. For the survival analysis, we conducted the following two additional sensitivity analyses: 1) exclusion of lung transplantation as an outcome to account for potential differences in transplant candidacy between individuals with obesity and those without obesity; 2) additional analyses using only incident patients who had PAH diagnosed within 6 months of enrollment into PHAR. Statistical analyses were performed using R version 3.6.1 (18) and R packages lme4 (19), MASS (20), and survival (21).
Results
Clinical Characteristics
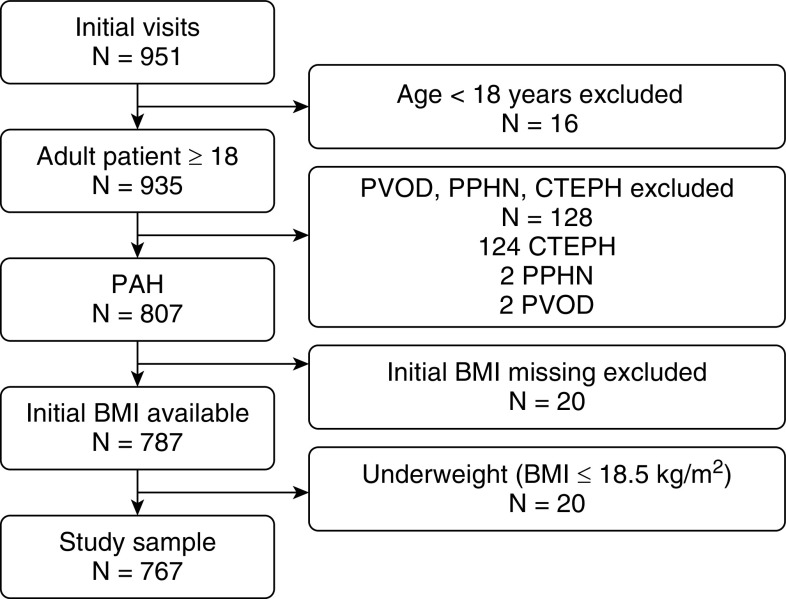
There were 951 subjects enrolled in PHAR by April 2019, of whom a total of 767 patients comprised our study sample (Figure 1). The baseline characteristics stratified by weight are presented in Table 1. One-third of patients were overweight, and 40% had obesity. The mean age of our study cohort was 57 years, the majority were female (75%) and non-Hispanic white (68%), with no differences among the weight strata. The two most common etiologies observed were connective tissue disease–related PAH and IPAH. Patients with obesity were more likely to have IPAH than associated PAH compared with overweight or normal-weight patients. No significant differences were observed in levels of education, employment status, and marital status. Median follow-up time was similar (502, 512, and 557 days for normal-weight, overweight, and obese subjects, respectively; P = 0.79).
Figure 1.
Flowchart of study inclusion. BMI = body mass index; CTEPH = chronic thromboembolic pulmonary hypertension; PAH = pulmonary arterial hypertension; PPHN = persistent pulmonary hypertension of the newborn; PVOD = pulmonary veno-occlusive disease.
Table 1.
Baseline characteristics of adult patients with PAH enrolled in PHAR (n = 767)
| Normal-Weight Patients with PAH (n = 204) | Overweight Patients with PAH (n = 259) | Patients with PAH and Obesity (n = 304) | |
|---|---|---|---|
| Age, yr | 55 ± 17 | 57 ± 16 | 55 ± 15 |
| Sex, F, % | 74 | 72 | 80 |
| Race/ethnicity | |||
| Non-Hispanic white | 63 | 69 | 69 |
| African American | 17 | 12 | 10 |
| Hispanic | 7 | 9 | 14 |
| Asian | 8 | 3 | 2 |
| Other | 3 | 7 | 5 |
| BMI, kg/m2 | 22.7 (20.9–23.8) | 27.5 (26.5–28.5) | 35.2 (32.2–39.0) |
| Diagnosis, % | |||
| Idiopathic | 29 | 36 | 48 |
| Heritable | 2 | 3 | 4 |
| Drug/toxin-induced | 7 | 14 | 13 |
| Connective tissue disease | 44 | 33 | 24 |
| HIV-related | 4 | 1 | — |
| Portopulmonary hypertension | 6 | 9 | 6 |
| Congenital heart disease | 7 | 3 | 5 |
| Highest level of education (n = 765) | |||
| Some schooling | 55 | 54 | 52 |
| Completed high school, GED, vocational, or business | 36 | 40 | 39 |
| Trade school | 9 | 6 | 9 |
| College or graduate studies | |||
| Occupation, % | |||
| Homemaker/student/retired | 68 | 73 | 70 |
| Employed part-time or full-time | 27 | 25 | 28 |
| Unemployed | 5 | 3 | 2 |
| Marital status, % | |||
| Married/living with partner | 57 | 56 | 56 |
| Widowed/divorced/separated | 21 | 26 | 27 |
| Never married | 20 | 16 | 16 |
| Decline to answer | 1 | 2 | 1 |
| WHO functional class, % (n = 726) | |||
| I | 10 | 7 | 5 |
| II | 36 | 36 | 30 |
| III | 46 | 50 | 57 |
| IV | 7 | 7 | 7 |
| 6-minute walk distance, m (n = 569) | 370 ± 135 | 341 ± 124 | 307 ± 117 |
| Percent predicted 6-minute walk distance, % | 73 ± 36 | 66 ± 30 | 60 ± 29 |
| Supplemental oxygen use, % | 40 | 40 | 44 |
| Baseline hemodynamics | |||
| Right atrial pressure, mm Hg (n = 728) | 7 (4–12) | 9 (5–13) | 10 (7–15) |
| Mean pulmonary artery pressure, mm Hg (n = 744) | 48 ± 15 | 49 ± 14 | 51 ± 13 |
| Pulmonary artery wedge pressure, mm Hg (n = 712) | 9 (7–12) | 10 (7–14) | 12 (8–15) |
| Cardiac output, L/min (n = 700) | 3.65 (2.94–4.74) | 4.00 (3.21–5.15) | 4.18 (3.60–5.20) |
| Cardiac index, L/min/m2 (n = 680) | 2.15 (1.71–2.71) | 2.15 (1.80–2.70) | 2.10 (1.74–2.59) |
| Pulmonary vascular resistance, WU (n · 664) | 10.0 (7.0–14.3) | 9.0 (5.8–12.4) | 8.7 (6.0–12.2) |
| Pulmonary vascular resistance index, WU.m2 (n = 664) | 16.8 (12.0–23.0) | 16.9 (10.5–23.5) | 18.0 (12.0–24.5) |
| Pulmonary arterial compliance, ml/mm Hg (n = 505) | 1.04 (0.79–1.37) | 1.14 (0.76–1.69) | 1.17 (0.84–1.71) |
| Quality of life measures at time of enrollment | |||
| EmPHasis-10 (n = 757) | 23 (11–33) | 27 (16–34) | 28 (19–36) |
| SF-12 physical score (n = 757) | 34 ± 7 | 34 ± 7 | 34 ± 7 |
| SF-12 mental score (n = 757) | 49 ± 8 | 48 ± 8 | 48 ± 9 |
| Lost to follow-up, n (%) | 19 (9) | 16 (6) | 19 (6) |
| Referred for lung transplant evaluation, n (%) | 11 (5) | 16 (6) | 11 (4) |
| Median follow-up time, d | 502 (275–795) | 512 (321–812) | 557 (321–846) |
Definition of abbreviations: BMI = body mass index; GED = General Educational Development; PAH = pulmonary arterial hypertension; PHAR = Pulmonary Hypertension Association Registry; SF-12 = Short Form-12; WHO = World Health Organization; WU = Wood units.
Values are presented as mean ± SD or median (interquartile range) unless otherwise indicated.
A greater proportion of patients with obesity were classified as World Health Organization (WHO) functional class III or IV (P = 0.02). The 6MWD and percentage predicted 6MWD decreased with increasing weight stratum (370, 341, and 307 m and 73, 66, and 60% for normal-weight, overweight, and obese subjects, respectively; P < 0.001). Patients with obesity had higher RAP, mean PA pressure, and pulmonary artery wedge pressure compared with normal-weight and overweight patients. Patients with obesity had a higher cardiac output but a similar cardiac index. Pulmonary vascular resistance was lower and pulmonary arterial compliance was higher in patients with obesity, although there was no difference in pulmonary vascular resistance index between weight strata.
There were no differences in PAH-specific therapy classes by strata (Table 2); however, overweight patients were less likely to be on a combination regimen (23% compared with 37% and 32% for normal-weight patients and patients with obesity, respectively; P = 0.006).
Table 2.
Initial PAH-specific therapies
| Normal-Weight Patients with PAH (n = 204) | Overweight Patients with PAH (n = 259) | Patients with PAH and Obesity (n = 304) | ||
|---|---|---|---|---|
| Phosphodiesterase-5-inhibitors, % | 46 | 38 | 40 | |
| Endothelin receptor antagonists, % | 33 | 26 | 30 | |
| Inhaled prostacyclin analog, % | 2 | 2 | 2 | |
| Parenteral prostacyclin analog, % | 14 | 11 | 12 | |
| Riociguat, % | 1 | 1 | 3 | |
| Selexipag, % | 4 | — | 3 | |
| Any medication combination, % | 37 | 23 | 32 |
Definition of abbreviation: PAH = pulmonary arterial hypertension.
Quality of Life
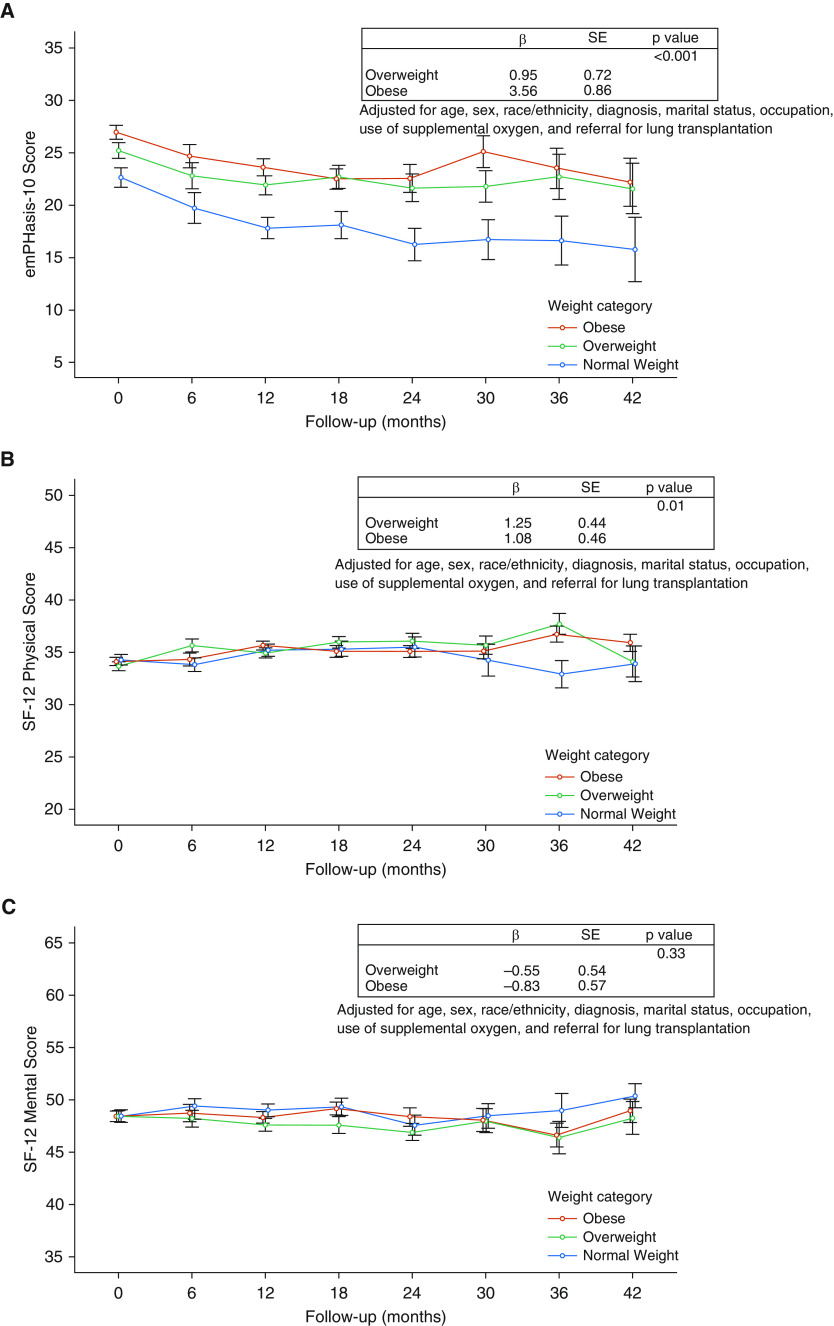
Overweight patients and patients with obesity had higher baseline e10 scores (worse HRQoL) compared with normal-weight patients (P < 0.001 and 0.01, respectively), whereas SF-12 physical and mental scores did not differ among weight strata. The difference in baseline e10 scores persisted over time, with the overweight group scoring on average 1.0 points higher and patients with obesity scoring on average 3.6 points higher than normal-weight patients (overall P < 0.001) after multivariate adjustment (Figure 2A). We did observe statistically significant differences in the SF-12 physical score over time; however, the magnitude of score difference by weight stratum was not clinically significant (Figure 2B). We did not observe any differences in the SF-12 mental scores by weight strata over time (Figure 2C).
Figure 2.
Health-related quality of life scores (HRQoL) by weight categories. Mean HRQoL measures and 95% confidence intervals for different weight categories. β coefficients are reported from multivariable mixed-effects generalized linear regression models. (A) emPHasis-10 scores (high score = worse HRQoL). (B) Short Form-12 physical scores (higher score = better HRQoL). (C) Short Form-12 mental scores (higher score = better HRQoL). SE = standard error; SF-12 = Short Form-12.
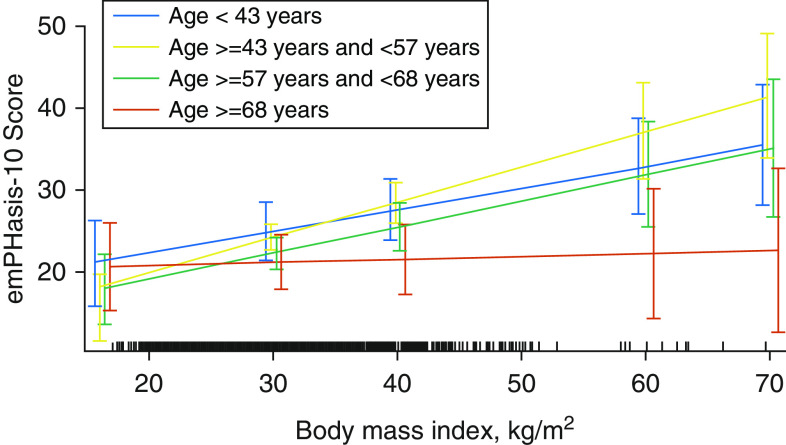
We found significant effect modification of the relationship between e10 and weight strata by quartiles of age (P for interaction < 0.007). BMI was strongly associated with worse e10 scores in all but the highest quartile of age (≥68 yr) (Figure 3). We did not observe any significant effect modification of the relationship between HRQoL and weight strata by sex or race.
Figure 3.
Interaction plot showing age as an effect modifier between body mass index and emPHasis-10 score, with sample density shown along the x-axis.
All-Cause Hospitalization and Transplant-Free Survival
Of the total 767 patients, 685 (89%) had at least one subsequent follow-up visit, 28 subjects (4%) were not yet due for a follow-up visit, and 54 (7%) were lost to follow-up. Overweight patients and patients with obesity appeared to have a higher incidence rate of hospitalization compared with normal-weight individuals, but the results were not statistically significant (IRR, 1.34; 95% confidence interval [95% CI], 0.94–1.92; P = 0.09 and IRR, 1.33; 95% CI, 0.93–1.89; P = 0.09, respectively) after adjusting for age, sex, race/ethnicity, PAH etiology, cardiac index, RAP, combination therapy, parenteral prostacyclin analog use, use of supplemental oxygen, and referral for lung transplantation. Limiting the cohort to patients with IPAH only showed similar nonsignificant trends for increased hospitalization rates (IRR, 1.23; 95% CI, 0.60–2.56; P = 0.55 for overweight patients and IRR, 1.48; 95% CI, 0.76–2.93; P = 0.24 for patients with obesity).
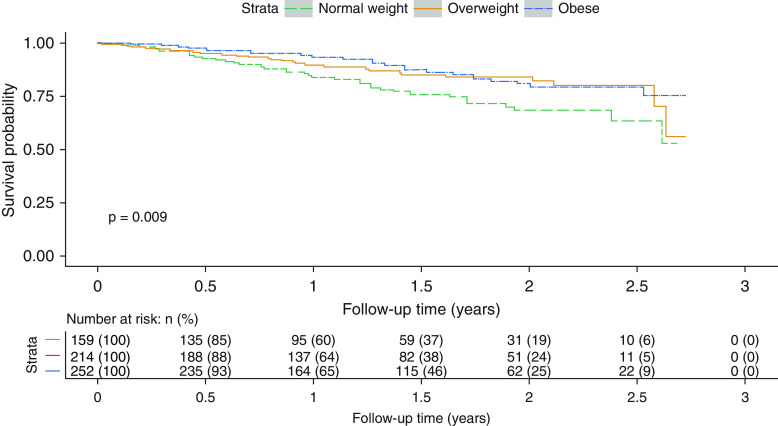
At the end of the follow-up period, 94 patients (12%) had died, and 12 (2%) underwent lung transplantation. Overweight individuals and those with obesity had longer transplant-free survival compared with normal-weight patients (Figure 4; P = 0.009). Higher weight stratum was associated with lower risk of death or lung transplant (HR, 0.45; 95% CI, 0.25–0.80; P = 0.007 for overweight patients and HR, 0.39; 95% CI, 0.22–0.70; P = 0.002 for patients with obesity) when adjusted for age, sex, race/ethnicity, PAH etiology, cardiac index, RAP, combination therapy, parenteral prostacyclin analog use, use of supplemental oxygen, and referral for lung transplantation. Overall survival excluding lung transplant was similar (HR, 0.48; 95% CI, 0.25–0.89; P = 0.02 for overweight patients and HR, 0.43; 95% CI, 0.23–0.78; P = 0.006 for patients with obesity). The findings were similar in the 386 patients with incident PAH (HR, 0.47; 95% CI, 0.22–0.99; P = 0.05 for overweight patients and HR, 0.39; 95% CI, 0.19–0.83; P = 0.01 for patients with obesity) and in the 245 patients with IPAH (HR, 0.31; 95% CI, 0.11–0.89; P = 0.03 for overweight patients and HR, 0.15; 95% CI, 0.05–0.44; P < 0.001 for patients with obesity).
Figure 4.
Transplant-free survival by weight status. Kaplan-Meier plot of transplant-free survival for weight categories from time of Pulmonary Hypertension Association Registry enrollment over a 2-year follow-up period.
Discussion
In a large multicenter prospective cohort of patients with PAH, we found that overweight patients and patients with obesity with PAH had worse pulmonary hypertension–specific HRQoL. The impairment in pulmonary hypertension–specific HRQoL persisted in follow-up despite treatment with PAH therapies. Overweight patients and those with obesity had a trend toward increased incidence rates for hospitalizations when compared with normal-weight individuals. Despite this, overweight individuals and individuals with obesity had better overall transplant-free survival compared with the normal-weight patients, consistent with an obesity paradox in PAH.
Forty percent of patients had obesity, compared with 39% in REVEAL (22) and 30% in the French PAH Registry (13). In our study, overweight patients and patients with obesity had worse pulmonary hypertension–specific HRQoL both at baseline and follow-up. These findings are consistent with the effect of obesity in the general population and other diseases (23, 24). In a prospective cohort of patients with chronic obstructive pulmonary disease (COPD), weight stratum was associated with both worse disease-specific HRQoL and general quality of life measured by SF-36 (25). A greater number of comorbidities were identified among patients with obesity as a potential explanation. In contrast, we found a significant interaction between age and weight stratum, with a greater association between BMI and pulmonary hypertension–specific HRQoL among younger patients who are less likely to have other medical conditions. Physical limitations, on the other hand, likely play a role (24); individuals with obesity had shorter absolute and relative 6MWD and higher WHO functional class. E10 assesses breathlessness, fatigue, lack of energy, social restrictions, and concerns regarding effects on patient’s significant others, and it is possible that patients with obesity experience these differently compared with counterparts without obesity(16, 24).
We found statistically significant differences in the physical component score of the SF-12 over time between overweight, obese, and normal-weight patients; however, the magnitude of the difference was small and likely not clinically meaningful (26). We observed no differences in the mental component score of the SF-12 over time between weight strata. Questions remain as to whether disease-specific measures are more sensitive than general questionnaires such as the SF-12 or SF-36. A study comparing various measures of HRQoL in COPD showed poor correlation between a disease-specific tool and the SF-36 (27, 28). Among patients with PAH, the performance of the SF-36 is mixed. In one analysis of the Sildenafil Use in PAH trial, sildenafil initiation resulted in significant improvements in SF-36 scores (29); however, the minimally important difference in SF-36 scores was much higher than expected in responders versus nonresponders in a post hoc analysis of the same trial (30). Likewise, in another PAH cohort, SF-36 scores poorly correlated with WHO functional class compared with a disease-specific tool (31). To our knowledge, there are no published studies directly comparing the e10 with the SF-12 or SF-36. The e10 has face validity and correlates with both WHO functional class as well as overall mortality among patients with PAH (16, 32). Therefore, in our study, the SF-12 may have lacked sensitivity in distinguishing differences in HRQoL when compared with a pulmonary hypertension–specific tool.
In our cohort, overweight patients and patients with obesity tended to be more likely to be hospitalized when compared with normal-weight counterparts. This association was independent of baseline hemodynamics, supplemental oxygen use, referral for lung transplantation, and PAH medications (factors that reflect underlying disease severity). As normal-weight patients had increased prevalence of associated PAH that may lead to hospitalizations, we restricted the cohort to IPAH only and found similar effect estimates. Although the reason for hospitalization was not available for analysis, there are several potential explanations for this phenomenon. First, we note that overweight patients and patients with obesity had shorter 6MWD and were more likely to be WHO functional class III or IV, representing a greater degree of functional limitation, which could lower a patient’s threshold for hospitalization for both PAH-related and unrelated events. In addition, with a higher pulmonary artery wedge pressure observed in the overweight patients and those with obesity, without provocative testing, it is possible that these patients may have occult left heart disease, particularly heart failure with preserved ejection fraction (HFpEF) (33, 34). Pulmonary hypertension in the setting of HFpEF is associated with a greater risk for hospitalizations than precapillary PH disease (35). Finally, an obese body habitus limits the utility of physical exam findings to assess volume status and may mask the degree of lower extremity edema or ascites. Therefore, overweight patients with PAH and patients with obesity and PAH may be more difficult to manage as outpatients from a volume balance perspective, resulting in more hospitalizations for volume overload or iatrogenic overdiuresis.
The term “obesity paradox” was first used to describe a phenomenon by which patients with obesity and systolic heart failure survive longer than their counterparts without obesity (36, 37). A similar pattern has been observed in patients with PAH, despite observational data showing that obesity is a risk factor for developing PAH (4, 12, 38, 39). Our study further supports this paradox using real-world, prospective data. Significant differences in transplant-free survival persisted after adjusting for differences among the weight strata in PAH etiology, hemodynamic profile, use of supplemental oxygen, referral for lung transplantation, and PAH medications. Pulmonary vascular resistance was lower for the overweight and obese groups, although resistances were similar when indexed to body surface area and are unlikely to explain the differences in survival observed. Furthermore, patients with obesity had higher pulmonary artery wedge pressures, likely reflecting a greater prevalence of concomitant HFpEF, which results in a lower transpulmonary gradient and PVR. A similar association between body habitus and survival was found in the United States–based REVEAL registry but not in the French PAH Registry (4, 13). Because obesity is a potential barrier for lung transplant, we performed analysis for survival alone to account for different rates of transplant referral and listing between obese and nonobese groups, with similar results.
We also found differences in PAH-directed medications, namely, normal-weight patients were most likely to be on combination therapy (37%), followed by patients with obesity (32%) and then overweight patients (23%) despite similar cardiac index and pulmonary vascular resistance index. Normal-weight patients had higher pulmonary vascular resistance and lower pulmonary arterial capacitance and a greater proportion of connective tissue disease–related PAH, which may explain the high rates of combination therapy use compared with overweight patients and patients with obesity. Furthermore, clinicians may be more likely to treat patients with obesity with multiple agents because of perceived higher risk from associated comorbidities (e.g., HFpEF and sleep disordered breathing) or in response to higher symptom burden, more restricted 6MWD, or more frequent hospitalizations. This observation also raises the possibility of differences in pharmacokinetics or treatment effects in overweight patients and patients with obesity. Patients with higher weight may receive higher relative doses of medications because of weight-based dosing (for parenteral therapy) or may experience increased tolerance to medications because of lower effective drug concentrations in setting of larger volume distribution. More information on dosing and treatment response as well as assessment of cardiorespiratory fitness are warranted. At the current time, no causal relationship has been described between obesity and survival, and thus, these results should not be applied in the management of individual patients.
There are several strengths to our study. This is the largest prospective study of the association between obesity and comprehensive patient outcomes in PAH. The PHAR cohort is likely highly generalizable to the PAH population within the United States, with recruitment from centers throughout the country and a greater proportion of nonwhite and male patients, who are often underrepresented in PAH studies. Finally, our study is also one of the largest ever using the e10 questionnaire in PAH.
There are some limitations to our study. Seven percent of the patients were lost to follow-up. Because of ongoing recruitment, ∼4% of patients had only an initial visit recorded at the time of analysis and were not yet due for their follow-up visit. Any differences between this group and our overall cohort should not lead to bias considering the noninformative nature of the missingness. In addition, we adjusted for follow-up time in all analyses to incorporate the variable follow-up time. We excluded underweight patients from our analysis, limiting the generalizability to this population. Comorbid conditions associated with obesity, such as diabetes mellitus and sleep disordered breathing, were not available and may explain some of the differences observed in HRQoL and hospitalization between weight strata but would not account for differences in mortality. Lung transplant referral patterns may differ among centers, and BMI may impact transplant eligibility. Although we ran sensitivity analysis for survival excluding lung transplantation, we may not have necessarily captured all the factors that influence lung transplant referral. Finally, because many of the proposed mechanisms underlying the obesity paradox point to metabolic dysfunction with BMI as a surrogate measurement, it would have been helpful to further characterize obesity phenotypes using additional anthropometric measurements or using serum metabolomic profiles, which were not available.
An overweight or obese body habitus is associated with increased disease burden in a large, multicenter, prospective PAH cohort. Despite significantly worse PH-specific HRQoL and a trend toward higher rates of hospitalization in overweight patients and patients with obese, these patients had better survival compared with normal-weight patients. Further deep phenotyping of patients’ fat distribution and cardiometabolic risk profiles is warranted to understand the underlying mechanism for these findings. Future interventions in PAH should address the specific needs of overweight patients and patients with obesity and may include support groups and targeted strategies for weight management.
Supplementary Material
Acknowledgments
Acknowledgment
The Pulmonary Hypertension Association Registry (PHAR) is supported by Pulmonary Hypertension Care Centers, Inc., a supporting organization of the Pulmonary Hypertension Association. The authors thank the other investigators, the staff, and particularly the participants of the PHAR for their valuable contributions. A full list of participating PHAR sites and institutions can be found at www.PHAssociation.org/PHAR.
Additional Pulmonary Hypertension Association Registry Investigators: Roblee Allen, M.D., Sonja Bartolome, M.D., Raymond Benza, M.D., Todd Bull, M.D., Linda Cadaret, M.D., Michael Eggert, M.D., Jean Elwing, M.D., Jeffrey Fineman, M.D., Raymond Foley, D.O., H. James Ford, M.D., Robert Frantz, M.D., Russel Hirsch, M.D., James Grinnan, M.D., D. Dunbar Ivy, M.D., Steven Kawut, M.D., M.S., Jamie Kennedy, M.D., James Klinger, M.D., Peter Leary, M.D., Ph.D., Sula Mazimba, M.D., M.P.H., Gautam Ramani, M.D., Amresh Raina, M.D., James Runo, M.D., John Swisher, M.D., Ph.D., John Ryan, M.D., Nidhy Varghese, M.D., R. James White, M.D., Ph.D., Timothy Williamson, M.D., Delphine Yung, M.D., Roham Zamanian, M.D., and Dianne Zwicke, M.D.
Footnotes
Supported by the U.S. National Institutes of Health K23 HL141584, Aldrighetti Research Award for Young Investigators.
Author Contributions: N.A.-N. was responsible for the conception and design of the work. J.M., R.F., and N.A.-N. were responsible for the analysis of the data. J.M., R.F., D.B., E.B.-R., C.B., M.C., T.D.M., J.F., A.H., E.M.H., M.L., S.M., J.W.M., K.P., J.R., J.S., O.S., M.S., T.T., C.V., and N.A.-N. made contributions to the acquisition of and interpretation of the data and drafting and revising the manuscript for important intellectual content. All authors were responsible for ensuring the accuracy and integrity of the work and have given final approval for its publication.
A complete list of additional PHAR Investigators may be found before the beginning of the References.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the PHAR Investigators, Roblee Allen, Sonja Bartolome, Raymond Benza, Todd Bull, Linda Cadaret, Michael Eggert, Jean Elwing, Jeffrey Fineman, Raymond Foley, H. James Ford, Robert Frantz, Russel Hirsch, James Grinnan, D. Dunbar Ivy, Steven Kawut, Jamie Kennedy, James Klinger, Peter Leary, Sula Mazimba, Gautam Ramani, Amresh Raina, James Runo, John Swisher, John Ryan, Nidhy Varghese, R. James White, Timothy Williamson, Delphine Yung, Roham Zamanian, and Dianne Zwicke
References
- 1.Thenappan T, Ryan JJ, Archer SL. Evolving epidemiology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:707–709. doi: 10.1164/rccm.201207-1266ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 3.Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186:790–796. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 4.Poms AD, Turner M, Farber HW, Meltzer LA, McGoon MD. Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest. 2013;144:169–176. doi: 10.1378/chest.11-3241. [DOI] [PubMed] [Google Scholar]
- 5.Taraseviciute A, Voelkel NF. Severe pulmonary hypertension in postmenopausal obese women. Eur J Med Res. 2006;11:198–202. [PubMed] [Google Scholar]
- 6.Irwin DC, Garat CV, Crossno JT, Jr, MacLean PS, Sullivan TM, Erickson PF, et al. Obesity-related pulmonary arterial hypertension in rats correlates with increased circulating inflammatory cytokines and lipids and with oxidant damage in the arterial wall but not with hypoxia. Pulm Circ. 2014;4:638–653. doi: 10.1086/678510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haque AK, Gadre S, Taylor J, Haque SA, Freeman D, Duarte A. Pulmonary and cardiovascular complications of obesity: an autopsy study of 76 obese subjects. Arch Pathol Lab Med. 2008;132:1397–1404. doi: 10.5858/2008-132-1397-PACCOO. [DOI] [PubMed] [Google Scholar]
- 8.Delcroix M, Howard L. Pulmonary arterial hypertension: the burden of disease and impact on quality of life. Eur Respir Rev. 2015;24:621–629. doi: 10.1183/16000617.0063-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galiè N, Barberà JA, Frost AE, Ghofrani H-A, Hoeper MM, McLaughlin VV, et al. AMBITION Investigators. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373:834–844. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 10.Ul-Haq Z, Mackay DF, Fenwick E, Pell JP. Meta-analysis of the association between body mass index and health-related quality of life among adults, assessed by the SF-36. Obesity (Silver Spring) 2013;21:E322–E327. doi: 10.1002/oby.20107. [DOI] [PubMed] [Google Scholar]
- 11.van Nunen AM, Wouters EJ, Vingerhoets AJ, Hox JJ, Geenen R. The health-related quality of life of obese persons seeking or not seeking surgical or non-surgical treatment: a meta-analysis. Obes Surg. 2007;17:1357–1366. doi: 10.1007/s11695-007-9241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal M, Agrawal S, Garg L, Lavie CJ. Relation between obesity and survival in patients hospitalized for pulmonary arterial hypertension (from a nationwide inpatient sample database 2003 to 2011) Am J Cardiol. 2017;120:489–493. doi: 10.1016/j.amjcard.2017.04.051. [DOI] [PubMed] [Google Scholar]
- 13.Weatherald J, Huertas A, Boucly A, Guignabert C, Taniguchi Y, Adir Y, et al. Association between BMI and obesity with survival in pulmonary arterial hypertension. Chest. 2018;154:872–881. doi: 10.1016/j.chest.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Gray MP, Kawut SM. The pulmonary hypertension association registry: rationale, design, and role in quality improvement. Adv Pulm Hypertens. 2018;16:185–188. [Google Scholar]
- 15.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 16.Yorke J, Corris P, Gaine S, Gibbs JSR, Kiely DG, Harries C, et al. emPHasis-10: development of a health-related quality of life measure in pulmonary hypertension. Eur Respir J. 2014;43:1106–1113. doi: 10.1183/09031936.00127113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware J, Jr, Kosinski M, Keller SDA. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 18.R Foundation for Statistical Computing Team. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 19.Bates DMM, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 20.Venables WN, Ripley BD. Modern applied statistics with S. New York: Springer; 2002. [Google Scholar]
- 21.Therneau TM, Grambsh PM. Modeling survival data: extending the cox model. New York: Springer; 2000. [Google Scholar]
- 22.Farber HW, Miller DP, Poms AD, Badesch DB, Frost AE, Muros-Le Rouzic E, et al. Five-Year outcomes of patients enrolled in the REVEAL Registry. Chest. 2015;148:1043–1054. doi: 10.1378/chest.15-0300. [DOI] [PubMed] [Google Scholar]
- 23.Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health-related quality of life. Clin Obes. 2017;7:273–289. doi: 10.1111/cob.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forhan M, Gill SV. Obesity, functional mobility and quality of life. Best Pract Res Clin Endocrinol Metab. 2013;27:129–137. doi: 10.1016/j.beem.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Lambert AA, Putcha N, Drummond MB, Boriek AM, Hanania NA, Kim V, et al. COPDGene Investigators. Obesity is associated with increased morbidity in moderate to severe COPD. Chest. 2017;151:68–77. doi: 10.1016/j.chest.2016.08.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyrwich KW, Spertus JA, Kroenke K, Tierney WM, Babu AN, Wolinsky FD Heart Disease Expert Panel. Clinically important differences in health status for patients with heart disease: an expert consensus panel report. Am Heart J. 2004;147:615–622. doi: 10.1016/j.ahj.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 27.Wilke S, Janssen DJ, Wouters EF, Schols JM, Franssen FM, Spruit MA. Correlations between disease-specific and generic health status questionnaires in patients with advanced COPD: a one-year observational study. Health Qual Life Outcomes. 2012;10:98. doi: 10.1186/1477-7525-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olschewski H, Hoeper MM, Behr J, Ewert R, Meyer A, Borst MM, et al. Long-term therapy with inhaled iloprost in patients with pulmonary hypertension. Respir Med. 2010;104:731–740. doi: 10.1016/j.rmed.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Pepke-Zaba J, Gilbert C, Collings L, Brown MC. Sildenafil improves health-related quality of life in patients with pulmonary arterial hypertension. Chest. 2008;133:183–189. doi: 10.1378/chest.07-0592. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert C, Brown MCJ, Cappelleri JC, Carlsson M, McKenna SP. Estimating a minimally important difference in pulmonary arterial hypertension following treatment with sildenafil. Chest. 2009;135:137–142. doi: 10.1378/chest.07-0275. [DOI] [PubMed] [Google Scholar]
- 31.Twiss J, McKenna S, Ganderton L, Jenkins S, Ben-L’Amri M, Gain K, et al. Psychometric performance of the CAMPHOR and SF-36 in pulmonary hypertension. BMC Pulm Med. 2013;13:45. doi: 10.1186/1471-2466-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Favoccia C, Kempny A, Yorke J, Armstrong I, Price LC, McCabe C, et al. EmPHasis-10 score for the assessment of quality of life in various types of pulmonary hypertension and its relation to outcome. Eur J Prev Cardiol. 2019;26:1338–1340. doi: 10.1177/2047487318819161. [DOI] [PubMed] [Google Scholar]
- 33.Vachiéry JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53:1801897. doi: 10.1183/13993003.01897-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Opitz CF, Hoeper MM, Gibbs JSR, Kaemmerer H, Pepke-Zaba J, Coghlan JG, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol. 2016;68:368–378. doi: 10.1016/j.jacc.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 35.Vanderpool RR, Saul M, Nouraie M, Gladwin MT, Simon MA. Association between hemodynamic markers of pulmonary hypertension and outcomes in heart failure with preserved ejection fraction. JAMA Cardiol. 2018;3:298–306. doi: 10.1001/jamacardio.2018.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 37.Clark AL, Chyu J, Horwich TB. The obesity paradox in men versus women with systolic heart failure. Am J Cardiol. 2012;110:77–82. doi: 10.1016/j.amjcard.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazimba S, Holland E, Nagarajan V, Mihalek AD, Kennedy JLW, Bilchick KC. Obesity paradox in group 1 pulmonary hypertension: analysis of the NIH-Pulmonary Hypertension registry. Int J Obes. 2017;41:1164–1168. doi: 10.1038/ijo.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zafrir B, Adir Y, Shehadeh W, Shteinberg M, Salman N, Amir O. The association between obesity, mortality and filling pressures in pulmonary hypertension patients; the “obesity paradox”. Respir Med. 2013;107:139–146. doi: 10.1016/j.rmed.2012.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.