Abstract
Rationale: Sex hormones play a role in pulmonary arterial hypertension (PAH), but the menstrual cycle has never been studied.
Objectives: We conducted a prospective observational study of eight women with stable PAH and 20 healthy controls over one cycle.
Methods: Participants completed four study visits 1 week apart starting on the first day of menstruation. Relationships between sex hormones, hormone metabolites, and extracellular vesicle microRNA (miRNA) expression and clinical markers were compared with generalized linear mixed modeling.
Results: Women with PAH had higher but less variable estradiol (E2) levels (P < 0.001) that tracked with 6-minute walk distance (P < 0.001), N-terminal prohormone of brain natriuretic peptide (P = 0.03) levels, and tricuspid annular plane systolic excursion (P < 0.01); the direction of these associations depended on menstrual phase. Dehydroepiandrosterone sulfate (DHEA-S) levels were lower in women with PAH (all visits, P < 0.001). In PAH, each 100-μg/dl increase in DHEA-S was associated with a 127-m increase in 6-minute walk distance (P < 0.001) and was moderated by the cardioprotective E2 metabolite 2-methoxyestrone (P < 0.001). As DHEA-S increased, N-terminal prohormone of brain natriuretic peptide levels decreased (P = 0.001). Expression of extracellular vesicle miRNAs-21, -29c, and -376a was higher in PAH, moderated by E2 and DHEA-S levels, and tracked with hormone-associated changes in clinical measures.
Conclusions: Women with PAH have fluctuations in cardiopulmonary function during menstruation driven by E2 and DHEA-S. These hormones in turn influence transcription of extracellular vesicle miRNAs implicated in the pathobiology of pulmonary vascular disease and cancer.
Keywords: estradiol, dehydroepiandrosterone sulfate, menstrual cycle, pulmonary hypertension, microRNA
Pulmonary arterial hypertension (PAH) was first described in premenopausal women (1). Anecdotally, PAH can be unmasked or exacerbated by oral contraceptive pills (OCP) and pregnancy (2). In men and postmenopausal women, higher estradiol (E2) and lower dehydroepiandrosterone sulfate (DHEA-S) levels are associated with the risk and severity of PAH (3–5). No studies have accounted for cyclical hormonal changes with menstruation in premenopausal women with PAH. The menstrual cycle has not been leveraged to provide mechanistic insight into how sex hormones contribute to PAH pathogenesis.
In healthy women and women with asthma (also characterized by a female sex bias), diffusing capacity of the lung for carbon monoxide (DlCO) varies over the menstrual cycle, but the mechanisms that govern these fluctuations may differ in health versus disease (6–8). Hormonal variation in pre- and postmenopausal women is associated with increased VEGF (vascular endothelial growth factor) levels and extracellular vesicle (EV) formation (6, 9–11). We have shown that EVs can prevent and reverse experimental pulmonary hypertension and that there is a microRNA (miRNA) signature of exosomes in human PAH that promotes vascular proliferation (12, 13). E2 increases circulation of hematopoietic progenitors leading to microvessel growth in the heart and lungs (14–17). The proinflammatory E2 metabolite 16α-hydroxyestrone (16-OHE1) as compared with the 2-compounds (2-methoxyestrone [2-ME] and 2-methoxyestradiol) has been linked to cancer angioinvasion and PAH (18–20). A pilot study of the E2 receptor modulator fulvestrant reduced circulating hematopoietic progenitors and 16-hydoxyestradiol in postmenopausal women (21). Finally, DHEA reduces oxidative stress, is required for nitric oxide synthesis, and has a number of purported cardiopulmonary benefits (3, 22, 23). Whether low DHEA-S levels occur in premenopausal women and vary with the menstrual cycle in PAH is unknown. As there are ongoing clinical trials manipulating E2 and DHEA in premenopausal women with PAH (NCT03528902; NCT03648385), it is critical to address these gaps in knowledge.
We sought to understand the relationship between cyclic sex hormone and metabolite changes and cardiopulmonary function in women with PAH as compared with healthy controls over one menstrual cycle. We hypothesized that 1) hormone levels (specifically higher E2 and lower DHEA-S levels) and menstrual cycle phase would be associated with decrements in measures of cardiopulmonary function, 2) the impact of E2 and DHEA-S and menstrual phase on these measures would be greater in PAH, and 3) relationships between hormone levels and clinical endpoints would be moderated by E2 metabolites. We also explored the relationship between hormone fluctuation and miRNA expression from circulating EVs in PAH as compared with controls; we focused on miRNAs implicated in angioinvasion in cancer or in PAH in an effort to gain mechanistic insight into hormone-driven effects on heart–lung function.
Methods
Study Design
We performed a prospective study of premenopausal women with PAH as compared with healthy controls during one menstrual cycle. Participants were assessed on a weekly basis for four visits with the first day of week 1 defined as day 1 of menstruation. Participants called study staff on the first day of menstruation and study visits were arranged within 48 hours and then at fixed weekly intervals thereafter. Patients with PAH were enrolled from the Rhode Island Hospital Pulmonary Hypertension Center. Controls were recruited from the Women’s Medicine Collaborative of the Lifespan Hospital System, a center focused on the medical needs of healthy women, as well as hospital staff and local advertisements. This study was approved by the Lifespan Institutional Review Board (IRB #024112) and informed consent was obtained from all participants.
Study Sample
Case definition
We included menstruating women with World Symposium Group 1 PAH. Traditional hemodynamic criteria (24) were required, as was prior pulmonary testing (pulmonary function testing, chest computed tomography, and ventilation–perfusion scans) excluding concurrent chronic lung disease and chronic thromboembolic disease. Patients on targeted PAH therapy were enrolled provided they had at least 4 weeks of stability (no hospitalizations, new PAH therapy, or PAH medication adjustments). Patients who had acute issues arise during the study period discontinued subsequent visits and were invited to repeat the entire study protocol once they were clinically stable.
Control definition
Women with a history of ≥12 months of regular menstrual cycles and no prior history of chronic heart or lung disease were eligible for screening. Participants were enrolled if they denied subjective cardiopulmonary symptoms and had normal screening spirometry.
Enrollment was coterminous for PAH cases and controls and therefore not matched. Women <18 years old or who were pregnant or breastfeeding were excluded. OCP use was not an absolute exclusion criterion.
Clinical Variables
Participant demographics, anthropometrics, smoking, medication, and obstetric and gynecologic history was collected at enrollment.
Peripheral Blood Markers and Study Assessments
Sex hormone levels, E2 metabolites, and angiogenesis markers (VEGF-R2 [VEGF receptor 2], angiopoietin-2, and EVs) were measured from peripheral blood as detailed in the online supplement. Investigators and study personnel involved in their measurement were blinded to case–control status, the temporal order of samples, and time point in menstrual cycle. Values of all bioassays above or below the limits of detection were assigned a value of the upper or lower detection limit, respectively. Markers of cardiopulmonary function including 6-minute walk distance (6MWD), DlCO, N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels, and tricuspid annular plane systolic excursion (TAPSE) from echocardiograms (in PAH cases only) were measured weekly using standardized methods with study staff and investigators blinded to case–control status (except for TAPSE) and visit timing (see online supplement).
Statistical Analysis
All analyses were conducted using SAS Software 9.4 (SAS Inc.). Summary data are presented as median and first and third quartiles and categorical data as frequency with percentage. Sex hormones (E2, DHEA-S), their metabolites (2-ME), and NT-proBNP were natural log-transformed. Outcomes of interest (6MWD, DlCO, NT-proBNP, TAPSE) were modeled as a function of sex hormone (E2, DHEA-S) and/or metabolite (2-ME) over time (menstrual cycle, four time points) between women with PAH and controls (three-way and four-way interactions) using generalized linear mixed modeled with sandwich estimation assuming normal or lognormal distributions (where appropriate) with the GLIMMIX procedure. All interval estimates were calculated for 95% confidence. Alpha was established at the 0.05 level.
Results
A total of eight women with PAH were enrolled. Three participants had clinical events unrelated to their PAH or personal logistical issues arise over the course of the study protocol and initially did not complete all study visits. They were reenrolled after these issues resolved, clinical stability and enrollment criteria were reconfirmed, and they completed all study visits over one menstrual cycle. In these participants, all data for available visits were analyzed and nested within subject. Twenty controls were enrolled and all completed the study protocol during one menstrual cycle. Women with PAH tended to be slightly older and more diverse with higher body mass index, were more likely to be smokers, and were more likely to have irregular menstrual periods by self-report as compared with controls. Only one control participant was taking OCPs. Among women with PAH, idiopathic PAH was the most common subtype (38%), and, as was required for study enrollment, participants were well controlled on PAH therapy (63% were functional class II; 75% were on combination therapy) (Table 1).
Table 1.
Subject characteristics
| Variables | PAH Cases | Controls |
|---|---|---|
| Number | 8 | 20 |
| Age, yr | 37 (31–42) | 28 (24–40) |
| Race, n (%) | ||
| White | 5 (63) | 15 (75) |
| Black | 1 (12) | 3 (15) |
| Asian | 0 (0) | 1 (5) |
| American Indian or Alaska Native | 0 (0) | 0 (0) |
| Native Hawaiian or other Pacific Islander | 0 (0) | 0 (0) |
| Other | 2 (25) | 1 (5) |
| Ethnicity, n (%) | ||
| Hispanic | 2 (25) | 1 (5) |
| Not Hispanic | 6 (75) | 19 (95) |
| BMI, kg/m2 | 30 (28–31) | 25 (20–32) |
| Current smoker, n (%) | 3 (38) | 0 (0) |
| Ever-smoker, n (%) | 5 (63) | 2 (10) |
| Pack years among ever-smokers | 4 (1–15) | 5 (3–8) |
| Hormonal factors | ||
| Current oral contraceptive pill use, n (%) | 0 (0) | 1 (5) |
| Age at menarche, yr | 13 (12–13) | 13 (13–13) |
| Regular menstrual periods, n (%) | 5 (63) | 19 (95) |
| Irregular menstrual periods, n (%) | 3 (38) | 1 (5) |
| Number of pregnancies | 3 (2–3) | 0 (0–2) |
| Number of live births | 2 (1–2) | 0 (0–2) |
| Surgical removal of ovaries or uterus | 0 (0) | 0 (0) |
| Diagnosis, n (%) | ||
| Idiopathic | 3 (38) | — |
| Heritable | 2 (25) | — |
| Connective tissue disease | 2 (25) | — |
| Drug-toxin induced | 1 (12) | — |
| WHO functional class, n (%) | ||
| I | 1 (12) | — |
| II | 5 (63) | — |
| III | 2 (25) | — |
| IV | 0 (0) | — |
| Hemodynamics | ||
| Right atrial pressure, mm Hg | 8 (6–10) | — |
| Mean pulmonary artery pressure, mm Hg | 42 (39–47) | — |
| Cardiac output, L/min | 6 (5–6) | — |
| Pulmonary capillary wedge pressure, mm Hg | 11 (9–11) | — |
| Pulmonary vascular resistance, Wood units | 5 (4–6) | — |
| PAH therapies, n (%) | ||
| Calcium channel blockers | 2 (25) | — |
| Phosphodiesterase type 5 inhibitors | 6 (75) | — |
| Endothelin receptor antagonists | 4 (50) | — |
| Prostacyclin analogs | 4 (50) | — |
| Combination therapy | 6 (75) | — |
Definition of abbreviations: BMI = body mass index; PAH = pulmonary arterial hypertension; WHO = World Health Organization.
Data represented as n (%) [where (%) equals column frequency] or median (interquartile range).
Hormone Fluctuations during the Menstrual Cycle
Changes in hormone levels over the course of one cycle (four study visits) are depicted in Figure 1 and Table 2. Women with PAH tended to have much lower variability in E2 levels as compared with controls over the cycle (i.e., controls had wider confidence intervals despite having twice the sample size). Levels of E2 were higher for PAH cases than controls in the follicular phase (visit 1 and 2) and at the end of the luteal phase (visit 4) (P < 0.001) (Figure 1A). Levels of DHEA-S were lower in PAH cases (red) as compared with controls (blue) throughout the menstrual cycle (all visits, P < 0.001) (Figure 1B).
Figure 1.
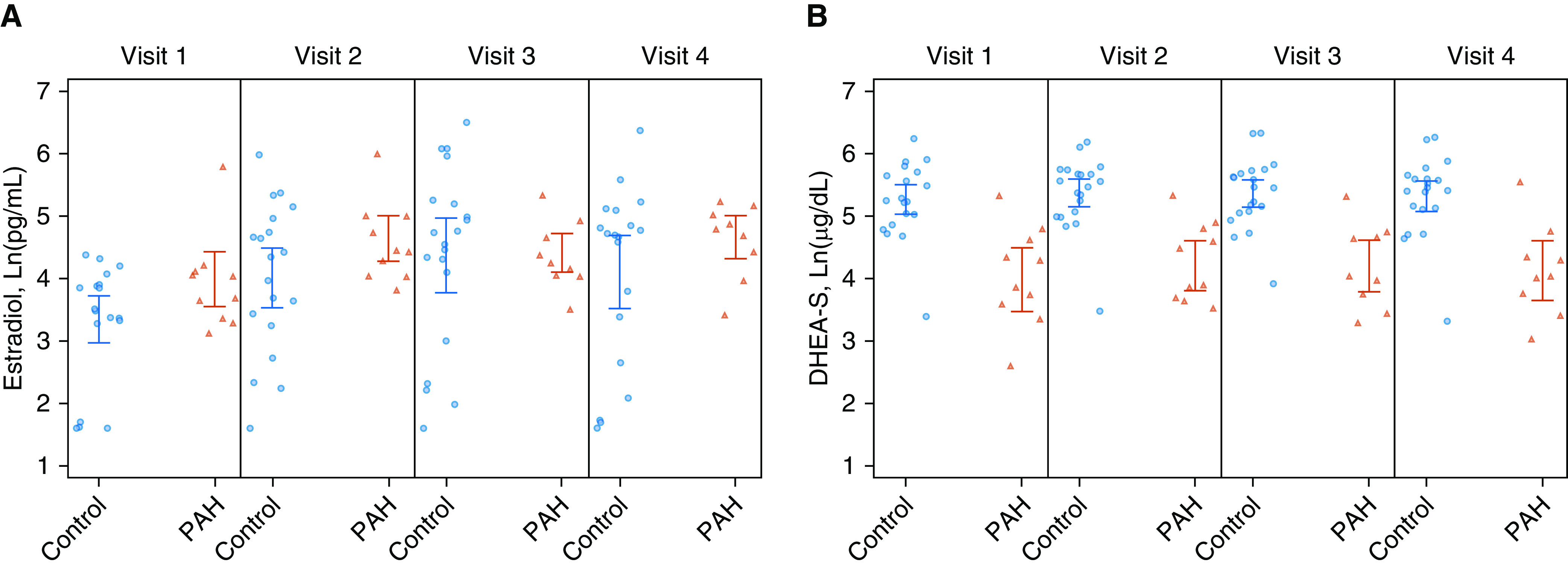
Hormone fluctuations over the course of the menstrual cycle. (A) Mean Ln(estradiol) levels over the menstrual cycle (visit 1, 2, 3, and 4) between pulmonary arterial hypertension (PAH) cases (red) and controls (blue) with 95% confidence intervals. (B) Mean Ln(DHEA-S) levels over the menstrual cycle (visit 1, 2, 3, and 4) between PAH cases (red) and controls (blue) with 95% confidence intervals. DHEA-S = dehydroepiandrosterone sulfate; Ln = natural log; PAH = pulmonary arterial hypertension.
Table 2.
Levels of sex hormone, metabolite, and cardiopulmonary markers in healthy controls and pulmonary arterial hypertension cases over one menstrual cycle (four study visits)
| Visit | Variable | Healthy Controls |
PAH Cases |
||||
|---|---|---|---|---|---|---|---|
| Median | Q1–Q3 | Min–Max | Median | Q1–Q3 | Min–Max | ||
| 1 | Estradiol, pg/ml | 33.8 | 26.6–49.9 | 5.0–79.8 | 47.9 | 29.1–60.9 | 22.9–330.2 |
| DHEA-S, μg/dl | 190.6 | 130.3–301.9 | 30.0–512.9 | 59.1 | 37.0–101.4 | 13.6–206.4 | |
| 16-OHE1, pg/ml | 6.0 | 6.0–6.0 | 6.0–181.3 | 6.0 | 6.0–6.0 | 6.0–6.0 | |
| 2-ME, pg/ml | 7.2 | 6.0–12.2 | 5.1–53.0 | 8.0 | 6.0–13.7 | 1.7–31.8 | |
| 6MWD, m | 496 | 384–530 | 320–613 | 429 | 385–499 | 238–586 | |
| NT-proBNP, pg/ml | 38.1 | 14.2–60.9 | 5.0–287.1 | 91.8 | 25.5–133.0 | 11.4–317.3 | |
| TAPSE, cm | — | — | — | 2.3 | 2.1–2.4 | 1.7–3.0 | |
| |
|
||||||
| Healthy Controls |
PAH Cases |
||||||
| 2 | Variable | Median | Q1–Q3 | Min–Max | Median | Q1–Q3 | Min–Max |
| Estradiol, pg/ml | 64.1 | 28.5–129.0 | 5.0–399.4 | 85.6 | 57.4–148.4 | 45.6–403.4 | |
| DHEA-S, μg/dl | 249.6 | 152.9–301.9 | 32.5–483.0 | 66.0 | 40.0–122.7 | 34.1–206.4 | |
| 16-OHE1, pg/ml | 6.0 | 6.0–6.0 | 2.1–295.9 | 6.0 | 6.0–6.0 | 6.0–6.0 | |
| 2-ME, pg/ml | 9.6 | 6.0–15.8 | 4.1–41.3 | 9.8 | 6.0–16.0 | 6.0–22.9 | |
| 6MWD, m | 497 | 413–534 | 331–628 | 434 | 379–490 | 263–579 | |
| NT-proBNP, pg/ml | 25 | 5.4–46.1 | 5.0–257.2 | 50.4 | 9.9–86.4 | 5.0–242.2 | |
| TAPSE, cm | — | — | — | 2.3 | 2.1–2.5 | 1.9–3.3 | |
| |
|
||||||
| Healthy Controls |
PAH Cases |
||||||
| 3 | Variable | Median | Q1–Q3 | Min–Max | Median | Q1–Q3 | Min–Max |
| Estradiol, pg/ml | 103.5 | 35.2–186.8 | 5.0–665.1 | 70.8 | 58.0–105.6 | 33.4–206.4 | |
| DHEA-S, μg/dl | 237.5 | 159.2–301.9 | 50.4–561.2 | 56.8 | 42.9–106.7 | 27.1–204.4 | |
| 16-OHE1, pg/ml | 6.0 | 6.0–6.0 | 2.1–267.7 | 6.0 | 6.0–6.0 | 6.0–7.2 | |
| 2-ME, pg/ml | 17.1 | 7.8–34.8 | 3.3–73.0 | 12.3 | 6.0–29.4 | 5.6–36.2 | |
| 6MWD, m | 491 | 386–516 | 320–624 | 398 | 335–498 | 183–555 | |
| NT-proBNP, pg/ml | 26.0 | 5.9–57.4 | 5.0–190.6 | 75.2 | 23.3–112.2 | 5.0–174.2 | |
| TAPSE, cm | — | — | — | 2.2 | 2.0–2.5 | 1.8–2.9 | |
| |
|
||||||
| Healthy Controls |
PAH Cases |
||||||
| 4 | Variable | Median | Q1–Q3 | Min–Max | Median | Q1–Q3 | Min–Max |
| Estradiol, pg/ml | 108.9 | 20.5–145.5 | 5.0–584.1 | 121.5 | 84.8–151.4 | 30.6–188.7 | |
| DHEA-S, μg/dl | 228.1 | 167.3–278.7 | 27.7–528.5 | 57.4 | 43.4–77.5 | 20.9–257.2 | |
| 16-OHE1, pg/ml | 6.0 | 6.0–6.0 | 6.0–75.2 | 6.0 | 6.0–6.0 | 6.0–6.0 | |
| 2-ME, pg/ml | 13.3 | 6.0–35.9 | 5.0–91.8 | 12.4 | 8.9–27.7 | 3.1–69.4 | |
| 6MWD, m | 487 | 410–532 | 319–606 | 428 | 384–478 | 210–566 | |
| NT-proBNP, pg/ml | 34.1 | 8.1–58.6 | 5.0–172.4 | 43.8 | 30.9–99.5 | 5.0–290.0 | |
| TAPSE, cm | — | — | — | 1.9 | 1.8–2.3 | 1.7–2.5 | |
Definition of abbreviations: 2-ME = 2-methoxyestrone; 6MWD = 6-minute walk distance; 16-OHE1 = 16α-hydroxyestrone; DHEA-S = dehydroepiandrosterone-sulfate; min = minimum; max = maximum; NT-proBNP = N-terminal prohormone of brain natriuretic peptide; PAH = pulmonary arterial hypertension; Q = quartile; TAPSE = tricuspid annular plane systolic excursion.
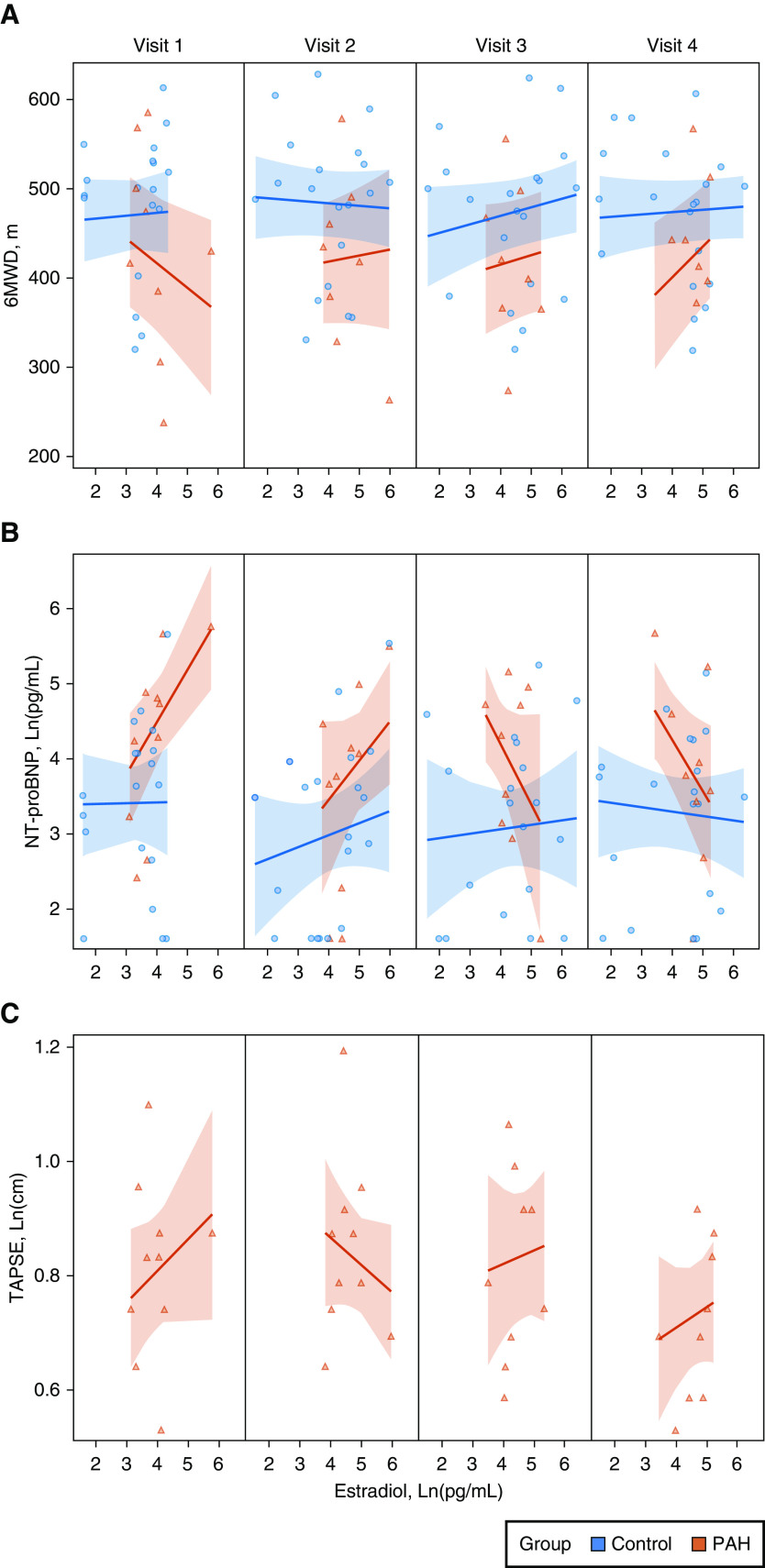
Estradiol and Markers of Cardiopulmonary Function
The relationship between E2 and 6MWD was moderated by case–control status and by menstrual phase (P < 0.001) (see Figure 2A). Women with PAH had shorter 6MWD and higher NT-proBNP levels than controls (as expected) and significant but inverse associations between E2 and 6MWD, as well as E2 and NT-proBNP, respectively, over the cycle. For example, each 1-pg/ml increase in Ln(E2) was associated with a 28-m decrease in 6MWD in the follicular phase (visit 1) and a 34-m increase in 6MWD in the luteal phase (visit 4) in PAH cases. Similarly, as E2 levels increased in the follicular phase (visits 1 and 2), NT-proBNP levels increased, but this relationship reversed in the luteal phase (visits 3 and 4) (P = 0.03) (Figure 2B). There was no evidence of a relationship between E2 and 6MWD or E2 and NT-proBNP over the cycle in controls. There was also a variable relationship between E2 levels and TAPSE in PAH cases; as E2 increased, TAPSE fluctuated depending on menstrual phase and study visit (Figure 2C). Results were unchanged when age was included in the models for 6MWD, NT-proBNP, and TAPSE. We conducted sensitivity analyses in which we excluded nulliparous controls (all women with PAH had at least one prior pregnancy); we observed more variability in the effect estimates between levels of E2 and endpoints (6MWT, NT-proBNP) in controls, but the interactions with case–control status and study visit, respectively, remained unchanged and significant. DlCO was higher for controls than cases (P < 0.001), regardless of E2 level and phase (P = 0.23) (data not shown).
Figure 2.
Estradiol levels during the menstrual cycle (visit 1, 2, 3, and 4) and the relationship to clinical endpoints in pulmonary arterial hypertension cases (PAH) (red) and controls (blue) with 95% confidence bands. (A) 6MWD, meters (m). (B) NT-proBNP levels, Ln (pg/ml). (C) TAPSE, Ln (centimeters) (cm) in PAH cases only. 6MWD = 6-minute walk distance; NT-proBNP = N-terminal prohormone of brain natriuretic peptide; TAPSE = tricuspid annular plane systolic excursion.
Dehydroepiandrosterone Sulfate and Markers of Cardiopulmonary Function
The relationship between DHEA-S and 6MWD was moderated by case–control status throughout the cycle (P < 0.001) (Figure 3A). Only women with PAH had evidence of longer 6MWD with increases in DHEA-S levels. Each unit increase in Ln(DHEA-S) was associated with a 94-m increase in 6MWD in women with PAH (in a normal distribution without natural log transformation, each 100-μg/dl increase in DHEA-S was associated with an increase of 127 m). This association was consistent across the follicular and luteal phase (i.e., there was no evidence of interaction by study visit). Given the time-invariant nature of this relationship, the main effect (6MWD regressed on DHEA-S) is represented in Figure 3B. There was an inverse relationship between DHEA-S levels and NT-proBNP levels such that as DHEA-S levels increased, NT-proBNP levels decreased, an association that varied by case–control status and visit (P = 0.001) (Figure 3C). In PAH, as DHEA-S levels increased, TAPSE increased during menstruation and the follicular and early luteal phases (visits 1, 2, and 3) (P = 0.003) (Figure 3D). Results were unchanged when age was included in the models for 6MWD, NT-proBNP, and TAPSE. Exclusion of nulliparous controls and the control subject taking OCPs, respectively, did not change the results. There was no evidence of a relationship between DHEA-S levels and DlCO over the course of the study in cases or controls (data not shown).
Figure 3.
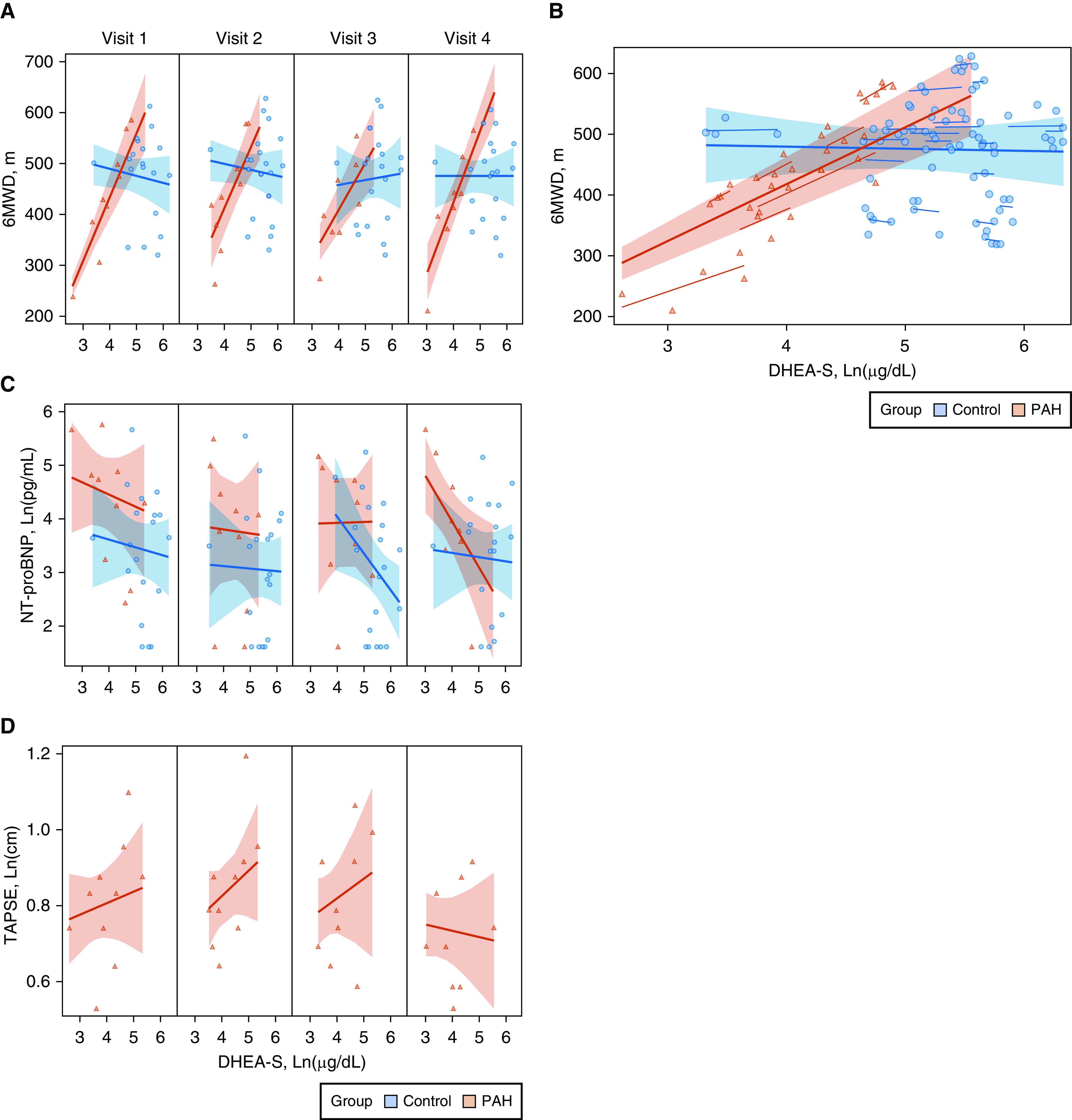
Dehydroepiandrosterone sulfate (DHEA-S) levels and the relationship to clinical endpoints in pulmonary arterial hypertension cases (PAH) (red) and controls (blue) with 95% confidence bands. (A) DHEA-S levels during the menstrual cycle (visit 1, 2, 3, and 4) and the relationship to 6-minute walk distance (6MWD), meters (m). (B) Overall relationship between DHEA-S and 6MWD, all visits. (C) NT-proBNP levels, Ln (pg/ml). (D) TAPSE, Ln (centimeters) (cm) in PAH cases only. NT-proBNP = N-terminal prohormone of brain natriuretic peptide; TAPSE = tricuspid annular plane systolic excursion.
Estrogen Metabolites and Markers of Cardiopulmonary Function
There was no evidence of a difference between 2-ME between PAH cases and controls over time (all P > 0.60) (Figure E1 in the online supplement). Over the course of the cycle, 2-ME increased for both groups (P < 0.001). There was no evidence of a difference between PAH cases and controls between 2-ME levels and 6MWD (P = 0.62), NT-proBNP (P = 0.26), or DlCO (P = 0.54) over time. In women with PAH, as 2-ME increased, the association with TAPSE varied over the cycle (P = 0.001) (Figure E2). We measured additional metabolites including 2-methoxyestradiol, 16-OHE1, and 16-hydroxyestradiol, but too many values were below the lower limit of detection in both cases and controls, rendering informative analyses impossible (Table 2).
PAH Moderates the Relationship between DHEA-S, 2-ME, and 6-Minute Walk Distance
There was evidence of significant moderation between case–control status over time by 2-ME and DHEA-S levels on 6MWD. As is depicted in the three-dimensional plots in Figure 4, in women with PAH, the relationship between lower levels of Ln(DHEA-S) and shorter 6MWD was moderated by lower Ln(2-ME) levels (P < 0.001). This relationship did not exist in controls. There was no evidence of moderation in the models for E2 (data not shown).
Figure 4.
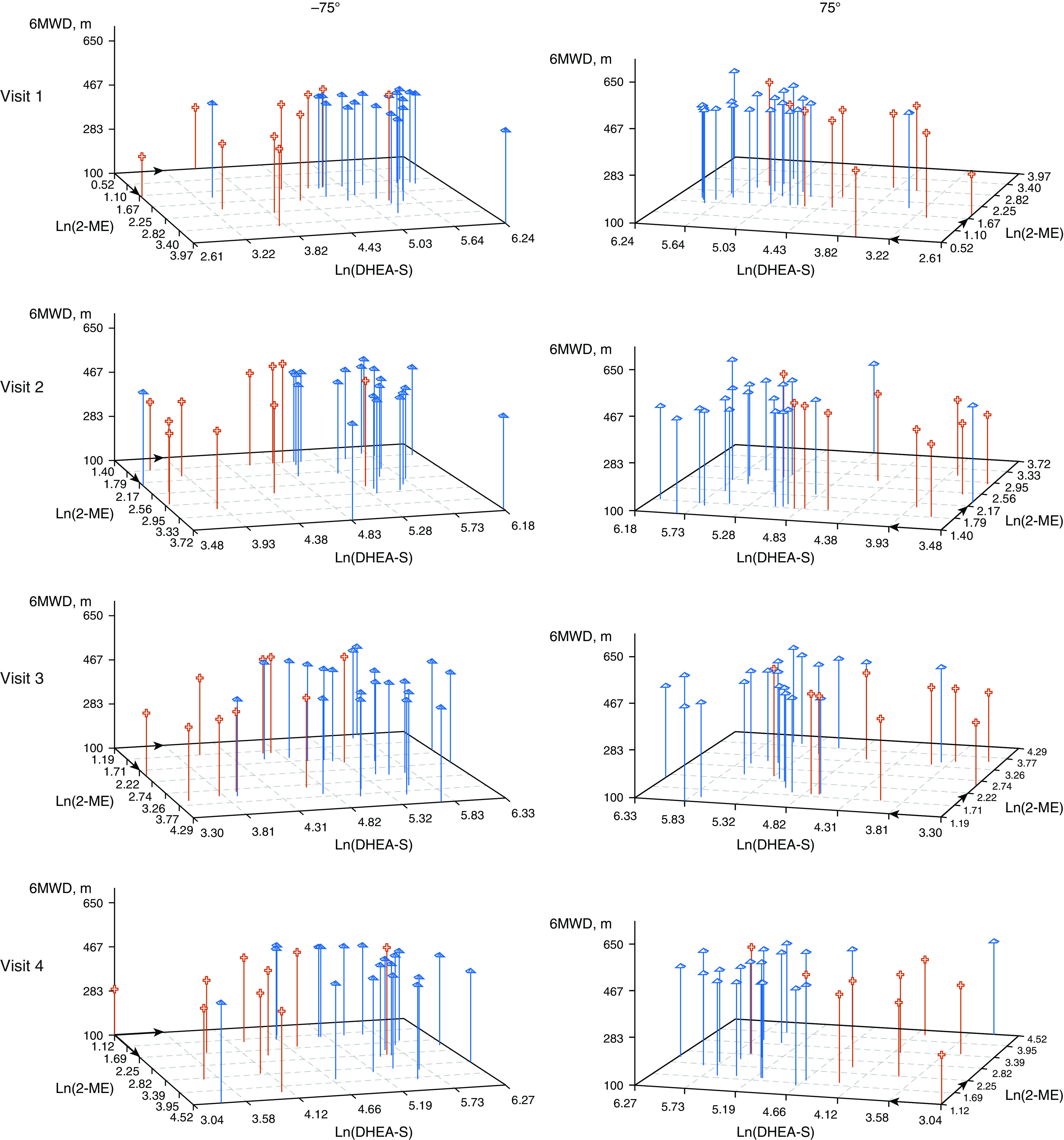
Relationship between 6-minute walk distance (6MWD) by Ln(DHEA-S) levels and Ln(2-ME) levels in pulmonary arterial hypertension cases (red cross) and controls (blue pyramids) over time (visits 1, 2, 3, 4); 180° rotation is provided for interpretation. 2-ME = 2-methoxyestrone; DHEA-S = dehydroepiandrosterone sulfate.
Extracellular Vesicle miRNA Expression Varies in PAH Cases versus Controls according to Time and Hormone Levels
Ninety-four miRNAs were significantly expressed in EVs from PAH cases as compared with controls; many miRNAs were significantly associated with E2 and DHEA-S in women with PAH (data not shown). We focused on three miRNAs previously linked with sex hormone biology and the pathobiology of pulmonary vascular disease. Expression of miRNA-21, -29c, and -376a were increased in PAH cases as compared with controls and had more fluctuation over the course of the cycle. Levels of E2 significantly moderated the relationship between miRNA-29c and -376a expression, PAH status, and menstrual phase, respectively (P < 0.001 for both). In women with PAH, increasing levels of E2 were associated with decreased miRNA-29c expression in the follicular phase (visits 1 and 2) and increased expression in the luteal phase (visits 3 and 4) (Figure E3A)—a pattern not observed in controls and similar to that seen between E2 levels and clinical measures. Increasing E2 levels were associated with decreased miRNA-376a expression in PAH cases, but no relationship was observed for controls (Figure E3B). Increasing levels of E2 were associated with decreased miRNA-21 expression in PAH cases but increased expression in controls (P < 0.001), but these relationships did not significantly vary over the menstrual cycle (P = 0.22) (Figure E3C). The relationship between miRNA expression, PAH status, and menstrual phase was significantly moderated by DHEA-S levels for miRNA-29c (P < 0.001), miRNA-376a (P = 0.001) and possibly miRNA-21 (P = 0.06). For example, increasing levels of DHEA-S were associated with increasing expression of miRNA-29c and -376a over the course of the cycle in PAH cases, whereas the relationships in controls tended to be static or reversed (Figures E4A and E4B). The DHEA-S-associated fold increases in miRNA-29c and -376a expression tracked with longer 6MWD and higher TAPSE in women with PAH.
Additional Hormones and Angiogenesis Markers of Interest
Other hormones known to fluctuate during the menstrual cycle (luteinizing hormone, progesterone) failed to have significant associations with endpoints or moderators of interest in participants with PAH that were distinguishable from controls (data not shown). Although there were some significant associations detected between E2 and DHEA-S levels, VEGF-R2 and angiopoietin-2 levels, and cardiopulmonary measures in PAH cases as compared with controls, no consistent signals emerged (data not shown).
Discussion
Premenopausal women with PAH have significant fluctuations in markers of cardiopulmonary function (6MWD, NT-proBNP, TAPSE) over the course of the menstrual cycle that may be driven by E2 and DHEA-S and are distinct from controls. In women with PAH, E2 levels tended be higher and vary less as compared with controls and have variable, inverse relationships with clinical endpoints that depended on menstrual cycle timing. As in men and postmenopausal women, levels of DHEA-S were lower in premenopausal women as compared with controls and—unlike E2—had consistent, robust associations with clinical measures such that lower levels were associated with worse clinical measures of PAH across the cycle. The cardioprotective E2 metabolite 2-ME moderated the relationship between DHEA-S and 6MWD but not E2 and cardiopulmonary outcomes in women with PAH. Women with PAH tended to have higher expression of EV miRNAs-21, -29c, and -376a, which were moderated by E2 and DHEA-S levels and tracked with sex hormone–associated changes in clinical measures. This is the first study to characterize the menstrual cycle in women with PAH, to document cyclical fluctuations in PAH clinical metrics that are associated with major hormones of biological import, and to identify potential mechanistic mediators in the relationship between sex hormones and PAH disease activity.
Although we and others have previously linked higher circulating E2 levels to the risk and severity of PAH in both men and postmenopausal women (3–5), these relationships have not been robustly studied in young women who are still menstruating. In a brief journal correspondence, Yuan and colleagues described lower levels of circulating E2 as compared with age-matched controls in premenopausal women with incident idiopathic PAH in the follicular phase of menstruation (25). In our study, the tendency for E2 to be higher but less variable (also observed in the Yuan study) in women with PAH mirrors our prior observations that postmenopausal women with PAH treated with the aromatase inhibitor anastrozole had lesser reductions in circulating E2 as compared with anastrozole-treated women with breast cancer (26). Elevated E2 levels may therefore be a hallmark of the PAH disease process itself.
The question of whether E2 is beneficial or harmful has been the subject of intense debate in human and experimental studies and has been dubbed the “estrogen paradox” and the “estrogen puzzle” of PAH (27, 28). Our data confirm this conundrum and suggest pleiotropism as higher E2 levels were associated with “better” and “worse” endpoints depending on menstrual cycle phase. This observation suggests that clinical trials targeted at estrogen signaling should include comprehensive and right ventricle (RV)-specific endpoints over a sufficient duration. The randomized clinical trial of anastrozole in PAH (NCT03229499) does not include premenopausal women. In our ongoing trial of DHEA in PAH (NCT03648385), which includes premenopausal women, menstrual cycle–related variability should be minimized by the crossover design, a consistent number of cycles in each 18-week treatment period, and the measurement of all sex hormones and their metabolites at each study visit.
Estrogen metabolites have dimorphic and tissue-specific effects and have been linked to cancer, cardiovascular health, and PAH. In a cohort of patients with heritable PAH with two premenopausal women, lower ratios of 2/16 E2 metabolites were reported (19). This is somewhat consistent with our findings that lower 2-ME levels moderated the relationship between lower DHEA-S levels (but surprisingly not E2 levels) and shorter 6MWD. The active metabolite 2-methoxyestradiol has been implicated in experimental models of PAH (29–31), and of the 2-compounds, 2-ME is relatively inactive. Although not directly measured, lower levels of DHEA-S in PAH may modulate catechol-O-methyltransferase and lead to lower levels of 2-ME; catechol-O-methyltransferase metabolizes the 2-compounds and has been speculated to modify breast cancer risk (32). We were unable to detect signals with 2-methoxyestradiol (the active metabolite), 16-OHE1, and 16-hydroxyestradiol levels because of a floor effect in both cases and controls. Recently, 16-OHE1 and 16-hydroxyestradiol levels were reported to be altered in a cohort of men and postmenopausal women (mean age for both sexes 57 yr) with idiopathic PAH as compared with controls (33). The fact that E2 and its metabolites have now been shown in a number of studies to differ between patients with PAH and controls (despite critical differences in study populations such as age, menopausal status, and PAH subtype) also points to a critical role for E2 in PAH pathogenesis.
The robust associations between DHEA-S levels in patients with PAH (but not controls) and clinical endpoints were largely independent of time and were directionally consistent. Although it is possible that the relationship between DHEA-S levels and 6MWD in women with PAH is in-part explained by effects on the systemic musculature, this is unlikely to explain the associations with TAPSE and NT-proBNP levels. Our prior work demonstrated associations between lower DHEA-S levels and worse hemodynamics (right atrial pressure and pulmonary vascular resistance) in both sexes and mortality in postmenopausal women (3, 5). Low levels of DHEA-S and its metabolites were associated with mortality in an unbiased metabolomics analysis in patients with PAH (23). DHEA binds to an endothelial cell–surface receptor coupled to nitric oxide synthase and regulates endothelin-1 production (34, 35), two major treatment targets in the PAH causal pathway. Human pulmonary artery endothelial cells actively metabolize DHEA, and treatment of these cells from patients with PAH decreases STAT3 activation (36). In multiple experimental models of PAH, DHEA prevents and rescues pulmonary vascular and RV dysfunction (22). Together with the current findings, this provides a strong rationale for our ongoing clinical trial of DHEA in patients with PAH.
Levels of E2 and DHEA-S in PAH cases but not controls modulated the expression of miRNA-21, -29c, and -376a in circulating EVs and mirrored sex hormone–based associations with clinical markers. These miRNAs have been implicated in the pathobiology of PAH, vascular ischemic injury, and cancer (37–42). This observation, along with the greater variability in miRNA expression over the cycle in PAH cases as compared with controls, suggests that E2 and DHEA-S stimulate hematopoietic signals and transcription of EV miRNA cargo to influence vascular and cardiopulmonary homeostasis in PAH. Higher E2 levels were associated with lower miRNA-21 expression in women with PAH, whereas controls demonstrated the inverse relationship throughout the cycle. miRNA-21 has been identified as a central regulator of bone morphogenetic protein and Rho-associated kinase signaling, moderating angiogenesis and vascular tone in PAH (37). Increasing levels of DHEA-S and E2 (in the luteal phase only) were associated with greater expression of EV miRNA-29c. Expression of miRNA-376a correlated directly with DHEA but inversely with E2 levels. In Bmpr2 mutant mice, 16α-OHE1 leads to upregulation of miR-29, which plays a key role in cellular energetics and metabolism (38, 43). The family of miRs that includes miR-376a have antiproliferative and antimigratory effects on tumor cells and downregulate cyclin-dependent kinases, an emerging therapeutic target in PAH (44–46). Whether these observations have a bearing on E2 and DHEA modulation as a treatment strategy in PAH is impossible to conclude given the potential for dynamic feedback over the cycle in menstruating women.
Our study has limitations. Our “time zero” for the protocol was the first day of menstruation. We did not confirm ovulation by luteinizing hormone surge or temperature change in real time. We required clinical stability without changes in PAH medications before study enrollment to minimize the risk of bias and confounding. The scope of inference for our conclusions therefore applies only to patients with PAH with stable and treated disease. The study is underpowered to detect differences among women with PAH on different treatment regimens, and most (75%) were on combination PAH therapy. We did not measure activity levels or environmental or dietary exposures, which may have differed between women with PAH and healthy controls. Weekly echo-based measurements of RV function were not performed in controls as these were unlikely to be informative and likely too insensitive (or imprecise) to detect subtle week-to-week changes in RV function in health and perhaps also treated/stable PAH. Our analyses do not account for a potential “lag” effect between hormone, metabolites, miRNAs, and endpoints—the potential for time-based interactions are limitless and we are restricted by sample size.
Conclusions
This is the first study to leverage the menstrual cycle in PAH. Women with PAH had unique and dynamic associations between circulating E2 and DHEA-S, menstrual phase, and cardiopulmonary function as compared with healthy controls. E2 and DHEA-S–driven associations with heart–lung function were moderated by E2 metabolites and tracked with altered EV miRNA transcription in women with PAH, suggesting a critical role for these hormones in PAH pathogenesis.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank all study subjects for participating in this study.
Footnotes
Supported by the CHEST Foundation Research Grant in Pulmonary Arterial Hypertension (C.E.V.), an Institutional Development Award from the National Institute of General Medical Sciences (P20 GM103652) (E.O.H. and C.E.V.), U.S. National Institutes of Health R01-HL141268 (C.E.V.), the British Heart Foundation, through grants RE/13/5/30177, RG/16/2/32153, BBSRC iCASE PhD award (BB/N503691/1) (N.D. and M.R.M.), and the Wellcome Trust (108,468/z/15/z) (R.A.).
Author Contributions: G.L.B.: data analysis and interpretation, and drafting and revision of the manuscript. T.W.: study design and coordination, data collection, and drafting and revision of the manuscript. J.A., A.S.B., M.D., E.O.H., and M.P.: extracellular vesicle and microRNA measurement, analysis and interpretation, and revision of the manuscript. M.A. and G.B.: subject recruitment and study visit execution. R.A., N.D., and M.R.M.: hormone metabolite measurement and interpretation, and revision of the manuscript. J.R.K., C.J.M., and M.W.: subject recruitment, and revision of the manuscript. A.P.: echocardiogram interpretation, and revision of the manuscript. C.E.V.: study concept and design, data analysis and interpretation, and drafting and revision of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dresdale DT, Schultz M, Michtom RJ. Primary pulmonary hypertension. I. Clinical and hemodynamic study. Am J Med. 1951;11:686–705. doi: 10.1016/0002-9343(51)90020-4. [DOI] [PubMed] [Google Scholar]
- 2.Morse JH, Horn EM, Barst RJ. Hormone replacement therapy: a possible risk factor in carriers of familial primary pulmonary hypertension. Chest. 1999;116:847. doi: 10.1378/chest.116.3.847. [DOI] [PubMed] [Google Scholar]
- 3.Baird GL, Archer-Chicko C, Barr RG, Bluemke DA, Foderaro AE, Fritz JS, et al. Lower DHEA-S levels predict disease and worse outcomes in post-menopausal women with idiopathic, connective tissue disease- and congenital heart disease-associated pulmonary arterial hypertension. Eur Respir J. 2018;51:1800467. doi: 10.1183/13993003.00467-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu WH, Yuan P, Zhang SJ, Jiang X, Wu C, Li Y, et al. Impact of pituitary-gonadal axis hormones on pulmonary arterial hypertension in men. Hypertension. 2018;72:151–158. doi: 10.1161/HYPERTENSIONAHA.118.10963. [DOI] [PubMed] [Google Scholar]
- 5.Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, et al. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med. 2016;193:1168–1175. doi: 10.1164/rccm.201509-1785OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farha S, Asosingh K, Laskowski D, Licina L, Sekiguchi H, Losordo DW, et al. Pulmonary gas transfer related to markers of angiogenesis during the menstrual cycle. J Appl Physiol (1985) 2007;103:1789–1795. doi: 10.1152/japplphysiol.00614.2007. [Published erratum appears in J Appl Physiol 104:903.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farha S, Asosingh K, Laskowski D, Hammel J, Dweik RA, Wiedemann HP, et al. Effects of the menstrual cycle on lung function variables in women with asthma. Am J Respir Crit Care Med. 2009;180:304–310. doi: 10.1164/rccm.200904-0497OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sansores RH, Abboud RT, Kennell C, Haynes N. The effect of menstruation on the pulmonary carbon monoxide diffusing capacity. Am J Respir Crit Care Med. 1995;152:381–384. doi: 10.1164/ajrccm.152.1.7599851. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal R, Prelevic G, Conway GS, Payne NN, Ginsburg J, Jacobs HS. Serum vascular endothelial growth factor concentrations in postmenopausal women: the effect of hormone replacement therapy. Fertil Steril. 2000;73:56–60. doi: 10.1016/s0015-0282(99)00476-8. [DOI] [PubMed] [Google Scholar]
- 10.Toth B, Nikolajek K, Rank A, Nieuwland R, Lohse P, Pihusch V, et al. Gender-specific and menstrual cycle dependent differences in circulating microparticles. Platelets. 2007;18:515–521. doi: 10.1080/09537100701525843. [DOI] [PubMed] [Google Scholar]
- 11.Möller B, Rasmussen C, Lindblom B, Olovsson M. Expression of the angiogenic growth factors VEGF, FGF-2, EGF and their receptors in normal human endometrium during the menstrual cycle. Mol Hum Reprod. 2001;7:65–72. doi: 10.1093/molehr/7.1.65. [DOI] [PubMed] [Google Scholar]
- 12.Klinger JR, Pereira M, Del Tatto M, Brodsky AS, Wu KQ, Dooner MS, et al. Mesenchymal stem cell extracellular vesicles reverse Sugen/hypoxia pulmonary hypertension in rats. Am J Respir Cell Mol Biol. 2020;62:577–587. doi: 10.1165/rcmb.2019-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aliotta JM, Pereira M, Wen S, Dooner MS, Del Tatto M, Papa E, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. 2016;110:319–330. doi: 10.1093/cvr/cvw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, et al. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation. 2006;113:1605–1614. doi: 10.1161/CIRCULATIONAHA.105.553925. [DOI] [PubMed] [Google Scholar]
- 15.Nematbakhsh M, Ghadesi M, Hosseinbalam M, Khazaei M, Gharagozloo M, Dashti G, et al. Oestrogen promotes coronary angiogenesis even under normoxic conditions. Basic Clin Pharmacol Toxicol. 2008;103:273–277. doi: 10.1111/j.1742-7843.2008.00286.x. [Published erratum appears in Basic Clin Pharmacol Toxicol 104:417.] [DOI] [PubMed] [Google Scholar]
- 16.Jesmin S, Sakuma I, Hattori Y, Kitabatake A. In vivo estrogen manipulations on coronary capillary network and angiogenic molecule expression in middle-aged female rats. Arterioscler Thromb Vasc Biol. 2002;22:1591–1597. doi: 10.1161/01.atv.0000034929.42459.0d. [DOI] [PubMed] [Google Scholar]
- 17.Masuda H, Kalka C, Takahashi T, Yoshida M, Wada M, Kobori M, et al. Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ Res. 2007;101:598–606. doi: 10.1161/CIRCRESAHA.106.144006. [DOI] [PubMed] [Google Scholar]
- 18.White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, et al. Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation. 2012;126:1087–1098. doi: 10.1161/CIRCULATIONAHA.111.062927. [DOI] [PubMed] [Google Scholar]
- 19.Austin E, Cogan J, West J, Hedges L, Hamid R, Dawson EP, et al. Alterations in oestrogen metabolism: implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J. 2009;34:1093–1099. doi: 10.1183/09031936.00010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hood KY, Montezano AC, Harvey AP, Nilsen M, MacLean MR, Touyz RM. Nicotinamide adenine dinucleotide phosphate oxidase-mediated redox signaling and vascular remodeling by 16α-hydroxyestrone in human pulmonary artery cells: implications in pulmonary arterial hypertension. Hypertension. 2016;68:796–808. doi: 10.1161/HYPERTENSIONAHA.116.07668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawut SM, Pinder D, Al-Naamani N, McCormick A, Palevsky HI, Fritz J, et al. Fulvestrant for the treatment of pulmonary arterial hypertension. Ann Am Thorac Soc. 2019;16:1456–1459. doi: 10.1513/AnnalsATS.201904-328RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hester J, Ventetuolo C, Lahm T. Sex, gender, and sex hormones in pulmonary hypertension and right ventricular failure. Compr Physiol. 2019;10:125–170. doi: 10.1002/cphy.c190011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes CJ, Ghataorhe P, Wharton J, Rue-Albrecht KC, Hadinnapola C, Watson G, et al. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation. 2017;135:460–475. doi: 10.1161/CIRCULATIONAHA.116.024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(Suppl):D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Yuan P, Gao L, Jing ZC. Complexities of oestradiol pharmacology in pulmonary arterial hypertension. Eur Respir J. 2013;41:1466–1467. doi: 10.1183/09031936.00176212. [DOI] [PubMed] [Google Scholar]
- 26.Kawut SM, Archer-Chicko CL, DeMichele A, Fritz JS, Klinger JR, Ky B, et al. Anastrozole in pulmonary arterial hypertension. a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2017;195:360–368. doi: 10.1164/rccm.201605-1024OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Naamani N, Ventetuolo CE. Another piece in the estrogen puzzle of pulmonary hypertension. Am J Respir Crit Care Med. 2020;201:274–275. doi: 10.1164/rccm.201910-1982ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014;307:L7–L26. doi: 10.1152/ajplung.00337.2013. [DOI] [PubMed] [Google Scholar]
- 29.Docherty CK, Nilsen M, MacLean MR. Influence of 2-methoxyestradiol and sex on hypoxia-induced pulmonary hypertension and hypoxia-inducible factor-1-α. J Am Heart Assoc. 2019;8:e011628. doi: 10.1161/JAHA.118.011628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao S, Jiang L, Fu C, Wu X, Liu Z, Song J, et al. 2-Methoxyestradiol attenuates chronic-intermittent-hypoxia-induced pulmonary hypertension through regulating microRNA-223. J Cell Physiol. 2019;234:6324–6335. doi: 10.1002/jcp.27363. [DOI] [PubMed] [Google Scholar]
- 31.Tofovic SP, Zhang X, Jackson EK, Dacic S, Petrusevska G. 2-Methoxyestradiol mediates the protective effects of estradiol in monocrotaline-induced pulmonary hypertension. Vascul Pharmacol. 2006;45:358–367. doi: 10.1016/j.vph.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Goodman JE, Lavigne JA, Wu K, Helzlsouer KJ, Strickland PT, Selhub J, et al. COMT genotype, micronutrients in the folate metabolic pathway and breast cancer risk. Carcinogenesis. 2001;22:1661–1665. doi: 10.1093/carcin/22.10.1661. [DOI] [PubMed] [Google Scholar]
- 33.Denver N, Homer NZM, Andrew R, Harvey KY, Morrell N, Austin ED, et al. Estrogen metabolites in a small cohort of patients with idiopathic pulmonary arterial hypertension. Pulm Circ. 2020;10:2045894020908783. doi: 10.1177/2045894020908783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Lin AS, Li Y, Reiter CEN, Ver MR, Quon MJ. Dehydroepiandrosterone stimulates phosphorylation of FoxO1 in vascular endothelial cells via phosphatidylinositol 3-kinase- and protein kinase A-dependent signaling pathways to regulate ET-1 synthesis and secretion. J Biol Chem. 2008;283:29228–29238. doi: 10.1074/jbc.M802906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huerta-García E, Ventura-Gallegos JL, Victoriano ME, Montiél-Dávalos A, Tinoco-Jaramillo G, López-Marure R. Dehydroepiandrosterone inhibits the activation and dysfunction of endothelial cells induced by high glucose concentration. Steroids. 2012;77:233–240. doi: 10.1016/j.steroids.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Paulin R, Meloche J, Jacob MH, Bisserier M, Courboulin A, Bonnet S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2011;301:H1798–H1809. doi: 10.1152/ajpheart.00654.2011. [DOI] [PubMed] [Google Scholar]
- 37.Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125:1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, Talati M, Fessel JP, Hemnes AR, Gladson S, French J, et al. Estrogen metabolite 16α-hydroxyestrone exacerbates bone morphogenetic protein receptor type II-associated pulmonary arterial hypertension through microRNA-29-mediated modulation of cellular metabolism. Circulation. 2016;133:82–97. doi: 10.1161/CIRCULATIONAHA.115.016133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He J, Gao Y, Wu G, Lei X, Zhang Y, Pan W, et al. Molecular mechanism of estrogen-mediated neuroprotection in the relief of brain ischemic injury. BMC Genet. 2018;19:46. doi: 10.1186/s12863-018-0630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao S, Liu Y, Yao Z, Zhao Y, Wang H, Xu Y, et al. MicroRNA-376a regulates cell proliferation and apoptosis by targeting forkhead box protein P2 in lymphoma. Oncol Lett. 2018;16:3169–3176. doi: 10.3892/ol.2018.9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Chen Y, Wang H, Zheng X, Li C, Han Z. miR-376a inhibits breast cancer cell progression by targeting neuropilin-1 NR. OncoTargets Ther. 2018;11:5293–5302. doi: 10.2147/OTT.S173416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertero T, Handen AL, Chan SY. Factors associated with heritable pulmonary arterial hypertension exert convergent actions on the mIR-130/301-vascular matrix feedback loop. Int J Mol Sci. 2018;19:2289. doi: 10.3390/ijms19082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Austin EDHR, Hamid R, Hemnes AR, Loyd JE, Blackwell T, Yu C, et al. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol Sex Differ. 2012;3:6. doi: 10.1186/2042-6410-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Formosa A, Markert EK, Lena AM, Italiano D, Finazzi-Agro’ E, Levine AJ, et al. MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c, miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p, mapped to the 14q32.31 locus, regulate proliferation, apoptosis, migration and invasion in metastatic prostate cancer cells. Oncogene. 2014;33:5173–5182. doi: 10.1038/onc.2013.451. [DOI] [PubMed] [Google Scholar]
- 45.Wang F, Yu J, Yang GH, Wang XS, Zhang JW. Regulation of erythroid differentiation by miR-376a and its targets. Cell Res. 2011;21:1196–1209. doi: 10.1038/cr.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss A, Neubauer MC, Yerabolu D, Kojonazarov B, Schlueter BC, Neubert L, et al. Targeting cyclin-dependent kinases for the treatment of pulmonary arterial hypertension. Nat Commun. 2019;10:2204. doi: 10.1038/s41467-019-10135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.