Abstract
RNA-binding motif protein 3 is a molecular marker of hypothermia that has proved neuroprotective in neurodegenerative disease models. However, its relationship to the well-recognized therapeutic effect of hypothermia in ischaemic stroke had not been studied. In this work, the expression of RNA-binding motif protein 3 was investigated in ischaemic animal models subjected to systemic and focal brain hypothermia, specifically the effects of RNA-binding motif protein 3 silencing and overexpression on ischaemic lesions. Moreover, the association of RNA-binding motif protein 3 levels with body temperature and clinical outcome was evaluated in two independent cohorts of acute ischaemic stroke patients (n = 215); these levels were also determined in a third cohort of 31 patients derived from the phase III EuroHYP-1 trial of therapeutic cooling in ischaemic stroke. The preclinical data confirmed the increase of brain RNA-binding motif protein 3 levels in ischaemic animals subjected to systemic and focal hypothermia; this increase was selectively higher in the cooled hemisphere of animals undergoing focal brain hypothermia, thus confirming the direct effect of hypothermia on RNA-binding motif protein 3 expression, while RNA-binding motif protein 3 up-regulation in ischaemic brain regions led to functional recovery. Clinically, patients with body temperature <37.5°C in the first two cohorts had higher RNA-binding motif protein 3 values at 24 h and good outcome at 3 months post-ischaemic stroke, while RNA-binding motif protein 3 levels in the cooled third cohort tended to exceed those in placebo-treated patients. These results make RNA-binding motif protein 3 a molecular marker associated with the effect of hypothermia in ischaemic stroke and suggest its potential application as a promising protective target.
Keywords: hypothermia, neuroprotection, RBM3, stroke, translational study
Hypothermia is an effective neuroprotective treatment in stroke. We have demonstrated that the expression of RBM3 is increased under hypothermia treatment in animal models of ischaemia and stroke patients. These results evidence the potential application of RBM3 as a promising protective target against ischaemia.
Graphical Abstract
Graphical Abstract.
Introduction
Hypothermia is one of the most effective neuroprotective treatments in preclinical models of ischaemic stroke (IS) (Castillo et al., 1998; van der Worp et al., 2007; Campos et al., 2012; Vieites-Prado et al., 2016). However, its clinical translation remains hampered by side effects such as shivering, hypotension, arrhythmia and increased risk of pneumonia that usually require sedation or anaesthesia (Darwazeh and Yan, 2013; Geurts et al., 2017). We recently showed that non-invasive focal hypothermia applied to the cerebral ischaemic region decreases the side effects of cooling stress in awake animals while retaining benefits similar to those of systemic hypothermia; unfortunately, clinical translation remains a daunting challenge since the thickness of human skull effectively insulates the brain, thus requiring the use of prolonged skin-damaging cold to achieve targetintracerebral temperatures (Vieites-Prado et al., 2016).
Despite theseclinical limitations, the physiology underpinning the therapeutic effects of hypothermia provides key protective targets for reducing ischaemic lesions (Han et al., 2012). At the same time as hypothermia down-regulates global protein synthesis and cell metabolism, it up-regulates cold shock proteins (CSPs). The two main CSPs described in mammals are cold-inducible RNA-binding protein (CIRP) and RNA-binding motif protein 3 (RBM3). While CIRP is detrimental in enhancing the inflammatory response (Zhou et al., 2014), interest in RBM3 has significantly increased due to its critical role in the protective effect of hypothermia (Zhou et al., 2014; Knott, 2015; Zhu et al., 2016a).
RMB3 is a glycine-rich protein (17 kDa) that promotes global protein synthesis at 32°C by accelerating ribosome assembly, stabilizing mRNA and decreasing microRNA expression (Dresios et al., 2005). In perinatal asphyxia models, RBM3 mediates rescue from apoptotic neuronal death during therapeutic cooling (Wellmann et al., 2010). Up-regulated RBM3 expression is associated with hibernation: it helps restore brain activity in awakening animals and protects cells against cold damage (Peretti et al., 2015; Wood, 2015). In mouse models of Alzheimer’s disease, RBM3 mediates protective cooling effects by reducing synaptic loss (Knott, 2015; Zhu et al., 2016a). More recently, RBM3 has been found to stimulate neuronal differentiation and inhibit hypoxic-ischaemia-induced apoptosis in the two main areas of persistent adult neurogenesis, the subventricular and subgranular zones (Zhu et al., 2019). However, although cooling is a well-recognized therapy in cerebral ischaemia, a role for RBM3 has yet to be sought in this regard. We therefore evaluated the effect of systemic and focal hypothermia on cerebral RBM3 expression in ischaemic animals and analysed the relationship between changes in blood RBM3 levels and body temperature in IS patients.
Materials and methods
Animal handling
All experimental protocols were approved by the local Animal Care Committee according to European Union rules (86/609/CEE, 2003/65/CE and 2010/63/EU). The manuscript conforms to Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Male 280–330 g Sprague-Dawley rats were housed individually, in stable environmental conditions (23°C, 40% relative humidity, 12-h light-dark cycle), with free access to food and water. Younger 130–150 g rats were used for premature viral injections.
Cerebral ischaemia
Transient focal ischaemia (45 min) was induced by intraluminal occlusion of the middle cerebral artery as previously described (Vieites-Prado et al., 2016; Fernandez-Susavila et al., 2017). Cerebral blood flow was monitored during surgery and evaluated by MRI during occlusion, before hypothermic/normothermic treatment, to confirm uniformity of the initial ischaemic lesion in all animals (detailed protocol in Supplementary material).
MRI
MRI was performed using a 9.4 T system (Bruker BioSpin, Billerica, MA, USA) with 440 mT/m gradients combining a linear birdcage resonator (7 cm diameter) for signal transmission with a 2 × 2 surface coil array for signal detection (details in Supplementary material).
Inducing systemic and focal hypothermia
Systemic hypothermia was induced to a rectal and brain temperature of 32°C for 4 h under 2% sevoflurane anaesthesia animal models using a rectal thermostat-controlled pad (Neos Biotec), as previously described (Vieites-Prado et al., 2016). Animals were then returned to their home cages for spontaneous rewarming. Normothermic systemic controls were also maintained for 4 h at 37°C (rectal and brain temperature). We found differences lower than 2°C between rectal and brain temperature, measured either via non-invasive magnetic resonance thermometry or by means of invasive temperature probes (Vieites-Prado et al., 2016). Based on these results, we selected rectal temperature as a reliable monitoring approach that reflected brain temperature throughout the experimental procedures.
Brain focal hypothermia was induced for 24 h via a metallic spiral tube implanted over the temporal skull and connected to a thermal bath, allowing free movement. The tube was also implanted for 24 h in normothermic focal controls, after which the tube was removed and the animals returned to their home cages. This protocol selectively cools the targeted hemisphere to 32°C by the spiral device without affecting body temperature (Vieites-Prado et al., 2016). Brain RBM3 expression was measured by quantitative polymerase chain reaction (qPCR) and western blot (WB) 3 h after cooling (details in Supplementary material; Supplementary Tables 1 and 2). Animals were randomly assigned to the experimental groups (n = 3) using the GraphPad software randomization tool (www.graphpad.com).
Brain RBM3 silencing and enhancing in ischaemic animals
We evaluated the impact on cerebral ischaemia of silenced or enhanced RBM3 expression using two different constructs of adeno-associated virus (AAV) (Vector Biosystems, PA, USA) and their respective empty vectors: AAV2-CamKII-rat-Rbm3-CamKII-eGFP and AAV2-GFP-U6-rat-Rbm3-shRNA (GenBank access number for RBM3: NM_053696). All animals weighing 130–150 g were injected four times with 2 µl virus in the somatosensory cortex. Stereotaxic injection of 0.2 µl/min was performed using 30 Ga cemented Hamilton syringes (Hamilton, NV, USA) under microinjector control (Stoelting Co., Wood Dale, IL, USA). The needle was left in place for 10 additional minutes to avoid reflux. Animals were divided into four experimental groups: (i) RBM3 overexpression; (ii) RBM3 overexpressing vector without the insert; (iii) RBM3 silencing; and (iv) RBM3 silencing vector without the shRNA sequence. T2*-weighted MRI was performed after stereotaxic surgery to confirm correct injection positioning or to discard needle-induced haemorrhage. Infected animals were allowed to recover from surgery and express the vectors for 4 weeks before the ischaemic injury was induced. Animals were randomly assigned to the experimental groups (n = 8) using the GraphPad software randomization tool (www.graphpad.com).
RBM3 levels in IS patients
RBM3 blood levels were measured in two independent IS cohorts: one from the Clinical Hospital of Santiago de Compostela, and the other from Dr. Josep Trueta University Hospital in Girona, following approval by the respective ethics committees. The study was carried out in accordance with the Declaration of Helsinki of the World Medical Association, fulfilling the criteria of the STROBE Statement (Guidelines for reporting observational studies). Application of the inclusion and exclusion criteria (Supplementary Tables 3 and 4) yielded a total of 215 eligible patients (113 from Santiago and 102 from Girona). RBM3 levels were determined at admission and at 24 h after stroke onset. Body temperature was measured at admission and for the first 24 h. Hyperthermia was defined as an axillary temperature ≥37.5°C and normothermia as <37.5°C (details in Supplementary material).
RBM3 levels in cooled IS patients
Blood samples for RBM3 analysis were obtained from the phase III EuroHYP-1 trial of therapeutic cooling in IS patients (van der Worp et al., 2014). The trial randomized patients to hypothermic treatment plus standard care or standard care alone (controls). In the hypothermic arm, cooling was started within 6 h of symptom onset and within 90 min of starting thrombolysis (or within 90 min of hospital admission in those not treated with thrombolysis) using 20 ml/kg refrigerated normal saline (4°C) IV over 30–60 min or a pre-specified surface cooling method. Cooling was maintained at 34–35°C for 24 h using a surface or endovascular technique. Thereafter, patients were passively rewarmed at a rate of 0.2 ± 0.1°C/h until rectal or bladder temperature reached 36.0°C, after which the cooling device was disconnected. All patients received treatment for acute IS and secondary prevention according to published guidelines. Pre-defined per-protocol analyses were performed in all patients whose body temperature was maintained at ≤35.0°C for at least 6 h during active cooling. We selected 31 patients (14 treated and 17 controls) for the study based on blood sample availability. Sample collection for biomarker measurement was pre-specified at three time-points: basal (pre-cooling baseline); 26 h (2 h after rewarming) and 72 h after stroke onset.
RBM3 analysis in human blood samples
Serum RBM3 levels were measured using commercial ELISA kits following manufacturer instructions (Biotez RBM3 ELISA, Berlin, Germany).
Statistical analysis
All preclinical data were analysed in GraphPad Prism 5.01 as the mean and standard error of the mean. One- or two-way ANOVA followed by post hoc Bonferroni evaluation was used to determine significant difference between multiple groups, with P set at <0.05. Investigators responsible for the assessment of outcomes (infarct size, behaviour and molecular biology analyses) were blinded to treatment groups (coded animals, samples and MRI images). Investigators performing surgeries could not be blinded to the hypothermic treatment (systemic and focal) due to the differences in the experimental procedure. Sample size was established based on our previous study where we have already evaluated the effect of systemic and focal hypothermia in the same experimental ischaemic animal model (Vieites-Prado et al., 2016).
Clinical data were analysed blind in SPSS 21.0 (IBM, USA) as percentages for categorical variables and as the mean ± standard deviation (SD) or median and range (25th to 75th percentiles) for continuous variables depending on their adjustment to normality, assessed by Kolmogorov–Smirnov and Lilliefors tests; correlations were performed using Spearman coefficients. Differences between patients according to outcome group were determined by ANOVA and chi-square tests. Logistic regression analysis was adjusted by the clinically significant variables identified in the bivariate analysis and the results shown as odds ratios (ORs) with 95% confidence intervals (95% CI).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.
Results
Temporal profile of brain RBM3 expression in hypothermia
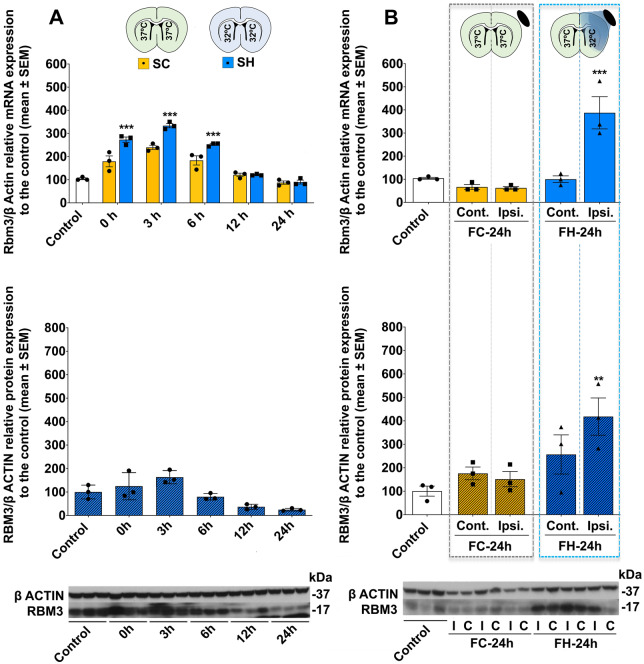
We began by measuring brain RBM3 expression at different time-points in healthy animals subjected to systemic hypothermia or normothermia + anaesthesia. After cooling for 4 h, all animals were sacrificed at 0, 3, 6, 12 or 24 h. Rbm3 mRNA expression in normothermic conditions after 4 h showed an increase over baseline at 3 h. The increase was greater in cooled animals. The same increasing profile was observed in RBM3 protein levels (Fig. 1A, Supplementary Tables 5 and 6). Based on these findings, RBM3 expression was determined 3 h post-cooling in subsequent study groups. Post-focal hypothermic quantification showed selective increases in Rbm3 mRNA and RBM3 protein levels in the cooled brain region of healthy animals versus controls. Focal normothermic conditions (24 h with the cooling device without hypothermia in awake conditions) did not significantly affect Rbm3 mRNA or RBM3 protein expression (Fig. 1B, Supplementary Tables 5 and 6). All animals which passed the surgical inclusion criteria completed the hypothermic experimental procedures.
Figure 1.
Temporal profile of RBM3 expression after hypothermia. (A) Above: Temporal brain Rbm3 mRNA levels after 4 h of SH (target temperature 32°C) or normothermia (target temperature 37°C). Bottom: Temporal brain RBM3 protein expression after 4 h of SH. (B) Above: Brain Rbm3 mRNA expression in healthy animals under FC conditions for 24 h (FC-24h, target temperature 37°C) or FH for 24 h (FH-24h, target temperature 32°C) followed by 3 h of recovery. Bottom: Brain RBM3 protein expression in healthy animals subjected to FC-24h or FH-24h. Rbm3 mRNA levels were relativized to β-actin then normalized to untreated controls. RBM3 protein expression was relativized to β-actin then normalized to control animal expression. WB bands corresponding to β-actin and RBM3 (37 and 17 kDa, respectively) are shown below. Data are shown as mean ± SEM. **P < 0.01 or ***P < 0.001; using one-way or two-way ANOVA followed by a post hoc Bonferroni test (n = 3/group). FC = focal hypothermia control group; FH = focal hypothermia group; SC = Systemic normothermia control groups; SH = systemic hypothermia group. Full-size WB gels are available in Supplementary material.
RBM3 expression in cooled ischaemic animals
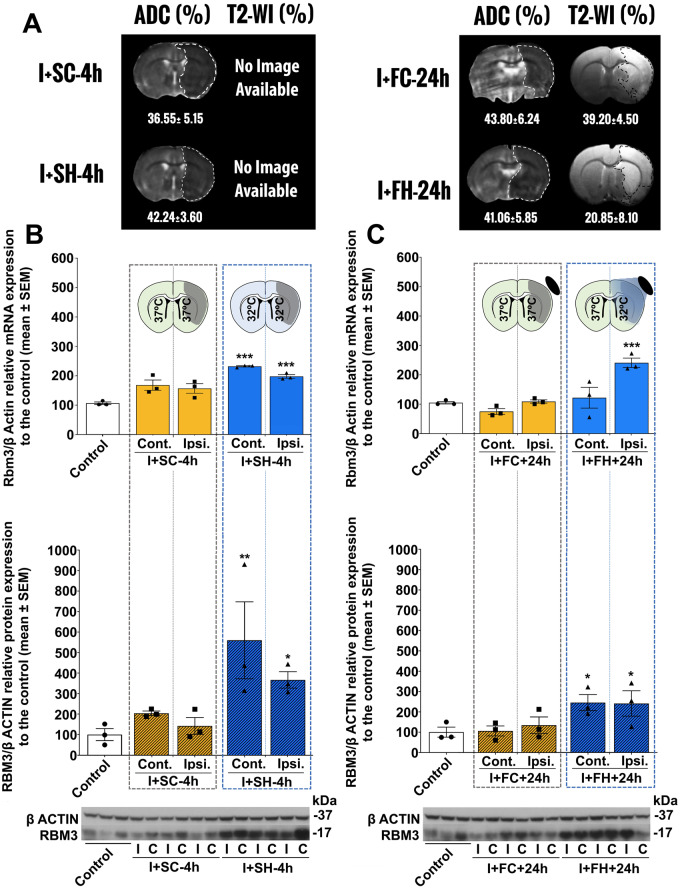
Having established the effect of temperature in healthy animals, Rbm3 mRNA and protein levels were then quantified in ischaemic animals undergoing systemic or focal hypothermia and their respective normothermic controls. MRI data determined using apparent diffusion coefficient maps confirmed baseline lesion volumes between 35% and 45% of the ipsilateral hemisphere in each animal (Fig. 2A), before cooling treatment.
Figure 2.
Hypothermia reduces ischaemic lesion volume and enhances RBM3 expression. (A) MRI assessments of ischaemic injury. Apparent diffusion coefficient maps were recorded before treatment (during surgical ischaemia induction) and T2 images were acquired after 24 h. Since animals subjected to systemic normothermia or SH were sacrificed 3 h after treatment (7 h after hypothermia induction), no T2 images at 24 h are available for these groups. (B) Above: Brain Rbm3 mRNA levels in ischaemic animals subjected to systemic normothermia (I+SC-4h, target temperature 37°C) or SH (I+SH-4h, target temperature 32°C) followed by 3 h of recovery. Below: RBM3 protein expression in animals subjected to I+SC-4h or I+SH-4h, followed by 3 h of recovery. (C) Above: Brain RBM3 mRNA levels in ischaemic animals subjected to focal normothermia (I+FC-24h, target temperature 37°C) or FH (I+FH-24h, target temperature 32°C) followed by 3 h of recovery; Below: Brain RBM3 protein expression in ischaemic animals subjected to I+FC-24h or I+FH-24h, followed by 3 h of recovery. Rbm3 mRNA levels were relativized to β-actin then normalized to the expression in untreated controls; RBM3 protein expression was relativized to β-actin then normalized to the expression in controls. WB bands corresponding to β-actin and RBM3 (37 and 17 kDa, respectively) are shown below. Data are shown as mean ± SEM. **P < 0.01 or ***P < 0.001; using one-way or two-way ANOVA followed by a post hoc Bonferroni test (n = 3/group). FC = focal hypothermia control group; FH = focal hypothermia group; SC = systemic normothermia control groups; SH = systemic hypothermia group. Full-size WB gels are available in Supplementary material.
In ischaemic animals undergoing systemic normothermia, Rbm3 mRNA and RBM3 protein expression tended to increase in both hemispheres compared with healthy controls. This increase was significantly enhanced by systemic hypothermia in both hemispheres (Fig. 2B, Supplementary Tables 3 and 4). In ischaemic animals undergoing focal hypothermia, Rbm3 mRNA expression increased in the ipsilateral hemisphere ischaemic region where the cooling spiral was located. RBM3 protein analysis confirmed the focal hypothermia-induced increase in RBM3, although it was also observed in the contralateral region (Fig. 2C, Supplementary Tables 5 and 6).
Analysis of brain RBM3 silencing and enhancing in ischaemic damage
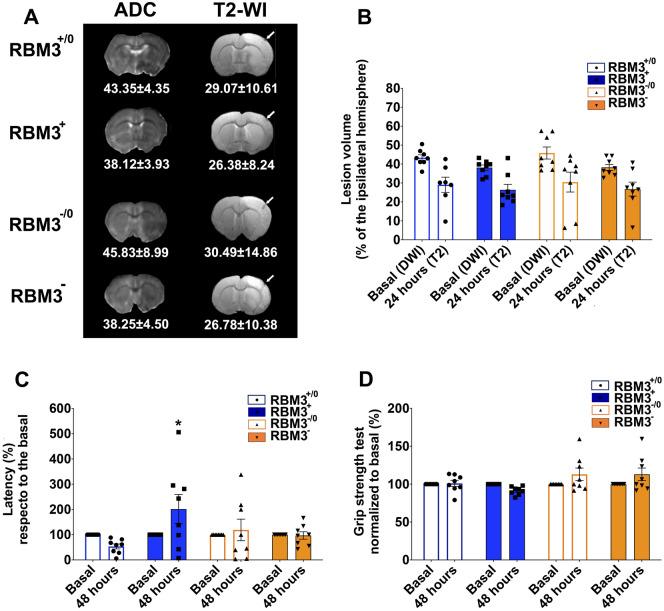
To evaluate the protective effect of RBM3 on infarct volume reduction, we induced artificial expression (up-regulation and down-regulation) in the ipsilateral ischaemic cortical region (before inducing ischaemia) using AAV infection followed by confirmatory MRI. AAV injection selectively infected cortical neurons determined by co-localization with NeuN (neuronal marker) and green fluorescent protein (GFP) as reporter gene (Fig. 3A and B). No GFP co-localization was observed with markers of astrocytes [glial fibrillary acidic protein (GFAP)] or microglia [ionized calcium-binding adaptor molecule 1 (Iba1)]. One month after virus injection, animals underwent cerebral ischaemia to evaluate the effect of RBM3 overexpression or silencing on ischaemic damage. Following the same protocol used for inducing cerebral ischaemia, all animals were scanned by MRI to confirm uniformity of basal infarct volume (Fig. 4A). Analysis of infarct volume 24 h post-ischaemia showed no significant impact on ischaemic damage by local changes in RBM3 expression (Fig. 4B), although rotarod testing revealed functional recovery in animals with up-regulated protein expression (Fig. 4C and D).
Figure 3.
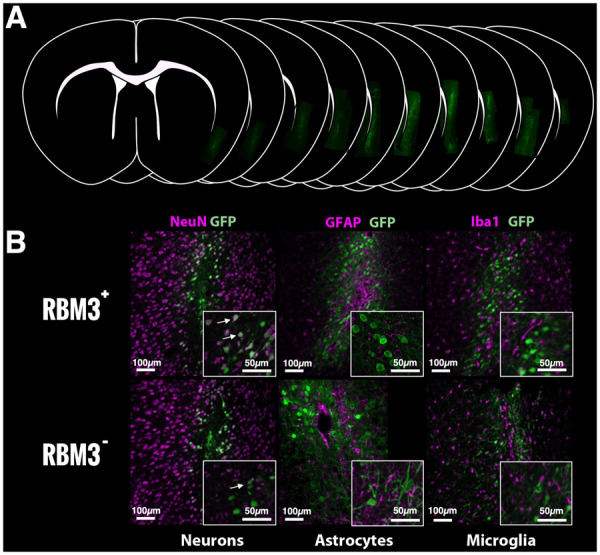
Histological analysis of RBM3 in brain tissue. (A) Cortical AAV infection 1 month before ischaemia induction. (B) GFP immunofluorescence with neuronal (NeuN), astrocyte (GFAP) and microglial (Iba1) markers. White arrows represent the co-localization observed only for GFP and NeuN.
Figure 4.
Effect of RBM3 expression on neurological ischaemic damage. (A) MRI assessments of ischaemic injury and ischaemic lesion volume in AAV-infected animals. Apparent diffusion coefficient maps were recorded before treatment (during surgical ischaemia induction) and T2 images were acquired after 24 h. White arrows show the needle track after virus injection. (B) Representation of the ischaemic injury determined in the four groups studied. Functional deficit in AAV-infected animals determined by grip (C) and rotarod tests (D) respect to the basal conditions (before ischaemia) and 48 h after ischaemia. RBM3+: animals infected with AAV2-CamKII-rat-Rbm3-CamKII-eGFP used to enhance RBM3 expression; RBM3+/0: control group infected with the same empty vector; RBM3−: animals infected with AAV2-GFP-U6-rat-Rbm3-shRNA used to silence RBM3 expression; RBM3−/0: control group infected with the same empty vector. Data are shown as mean ± SEM (n = 8/group).
Association between blood RBM3 levels, temperature and outcome in IS patients
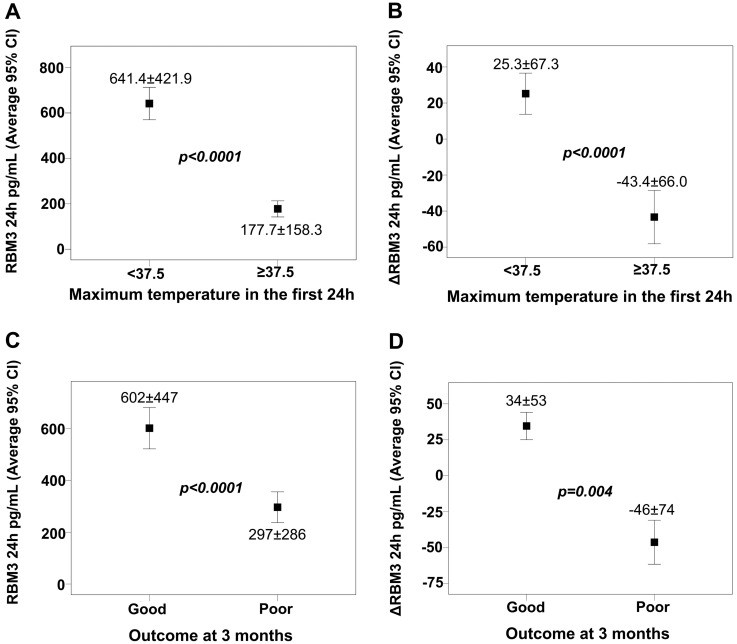
IS patient characteristics in both cohorts (Supplementary Table 7) showed a negative correlation between basal RBM3 levels and body temperature at admission (Spearman coefficient −0.150, P = 0.028) and at 24 h (Spearman coefficient −0.662, P < 0.0001). Individual correlation data (Supplementary Table 8) showed that normothermic (<37.5°C) patients had higher RBM3 values at 24 h than hyperthermic (≥37.5°C) patients (Fig. 5A). Interestingly, mean variation between the RBM3 levels determined at admission and 24 h after stroke onset (ΔRBM3) was positive in normothermic patients versus negative in hyperthermic patients (Fig. 5B).
Figure 5.
RBM3 is associated with a good outcome and is temperature-dependent. (A) RBM3 levels at 24 h and (B) ΔRBM3 in normothermic (<37.5°C) and hyperthermic (≥37.5°C) IS patients. (C) RBM3 levels at 24 h and (D) ΔRBM3 in IS patients with poor and good clinical outcome at 3 months. ΔRBM3 was defined as % variation of RBM3 at 24 h respect to the basal levels.
Having observed several variables related to temperature (Supplementary Table 9), we evaluated ORs for the association between RBM3 and maximum temperature at 24 h <37.5°C by performing a logistic regression analysis using the variables identified as clinically and statistically significant in the model. The analysis revealed an independent association between maximum temperature at 24 h <37.5°C and RBM3 levels at 24 h ≥300 pg/ml (obtained from the ROC curve), with an adjusted OR of 15.83 (95% CI: 6.71–37.39; P < 0.0001) (Table 1).
Table 1.
Multivariate analysis
| OR not adjusted | 95% CI | P | OR adjusted | 95% CI | P | |
|---|---|---|---|---|---|---|
| Center (cat) | 1.32 | 0.76–2.31 | 0.325 | 1.22 | 0.43–3.50 | 0.708 |
| Glycaemia | 0.99 | 0.99–0.99 | 0.002 | 0.99 | 0.99–1.00 | 0.177 |
| Leukocytes | 0.82 | 0.74–0.90 | <0.0001 | 0.79 | 0.69–0.93 | 0.004 |
| Fibrinogen | 0.99 | 0.99–0.99 | <0.0001 | 0.99 | 0.99–1.00 | 0.070 |
| Systemic fibrinolysis (cat) | 2.86 | 1.53–5.36 | 0.001 | 2.81 | 0.91–8.74 | 0.074 |
| Endovascular treatment (cat) | 0.40 | 0.17–0.97 | 0.042 | 0.49 | 0.12–1.97 | 0.317 |
| Previous Rankin | 0.76 | 0.58–0.99 | 0.040 | 0.94 | 0.65–1.37 | 0.764 |
| NIHSS on admission | 0.91 | 0.87–0.96 | <0.0001 | 0.92 | 0.86–0.98 | 0.014 |
| RBM3 at 24 h ≥300 pg/ml | 16.11 | 7.81–33.26 | <0.0001 | 15.83 | 6.71–37.39 | <0.0001 |
Dependent variable: maximum temperature in the first 24 h <37.5˚C, not adjusted and adjusted by clinically relevant variables found in our study.
Cat = categorized; OR = odds ratio.
We observed a clear relationship between RBM3 levels at 24 h and functional outcome at 3 months measured on the modified Rankin Scale (mRS) (Fig. 5C). In agreement with the trend observed with temperature, mean ΔRBM3 was positive in patients with good outcome versus negative in those with poor outcome (Fig. 5D). In addition, logistic regression analysis revealed that an ΔRBM3 value ≥10 (obtained from the ROC curve) was an independent marker of good functional outcome at 3 months (Table 2), after adjusting for the independent variables associated with poor functional outcome at that time-point (Supplementary Table 10).
Table 2.
Multivariate analysis
| OR not adjusted | 95% CI | P | OR adjusted | 95% CI | P | |
|---|---|---|---|---|---|---|
| Center (cat) | 0.45 | 0.26–0.78 | 0.005 | 0.84 | 0.25–1.98 | 0.682 |
| Age | 0.97 | 0.94–0.99 | 0.030 | 1.00 | 0.96–1.05 | 0.955 |
| Temperature in the first 24 h <37.5˚C (cat) | 3.86 | 2.15–6.92 | <0.0001 | 0.61 | 0.22–1.70 | 0.341 |
| Cardioembolic versus non-cardioembolic | 3.69 | 1.88–7.24 | <0.0001 | 2.05 | 0.75–5.58 | 0.162 |
| Previous Rankin | 0.33 | 0.22–0.51 | <0.0001 | 0.37 | 0.22–0.64 | <0.0001 |
| NIHSS on admission | 0.84 | 0.79–0.89 | <0.0001 | 0.87 | 0.81–0.95 | 0.001 |
| ΔRBM3 ≥10% | 18.87 | 9.45–37.68 | <0.0001 | 26.52 | 9.32–75.44 | <0.0001 |
Dependent variable: Clinical outcome at 3 months, not adjusted and adjusted by clinically relevant variables found in our study.
Cat = categorized; OR = odds ratio.
Blood RBM3 levels in cooled IS patients
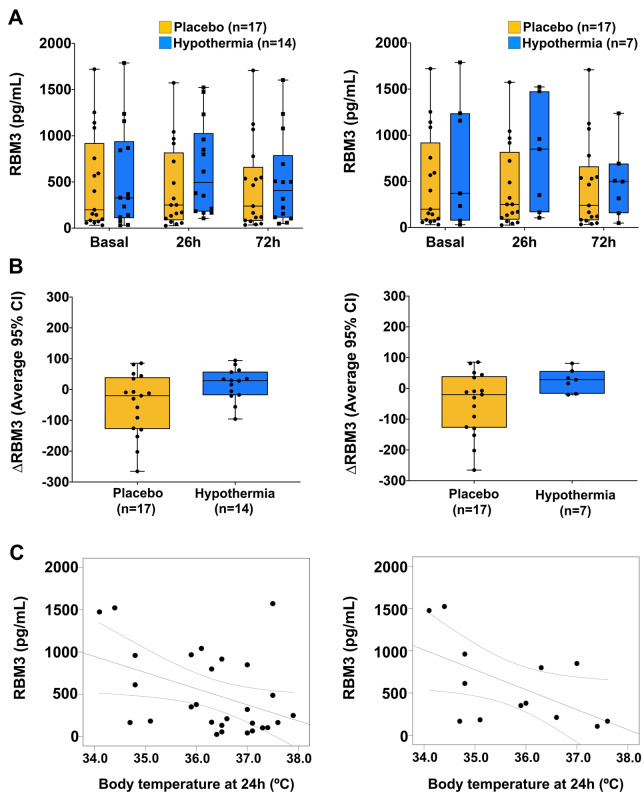
In the 31 patients from the EuroHYP-1 trial (van der Worp et al., 2014), we found no significant differences in RBM3 levels between those treated with hypothermia (n = 14) or placebo (n = 17), although rewarming levels tended to be higher in those that received active treatment (P = 0.131) (Fig. 6A, left). Results were similar (P = 0.153) when including only those patients meeting the pre-defined criteria for per-protocol analyses (Fig. 6A, right). We observed the same trend (P = 0.278) towards higher RBM3 levels in cooled patients when comparing ΔRBM3 values between baseline and rewarming (2 h after completing the 24-h treatment, i.e. at 26 h) with those in placebo patients (Fig. 6B, left). Results were again similar (P = 0.601) in patients meeting the pre-defined criteria for per-protocol analyses (Fig. 6B, right). No significant correlation was found between RBM3 levels and body temperature except for a negative correlation between RBM3 after rewarming (26 h) and temperature at 24 h (R = −0.414, P = 0.029) (Fig. 6C, left). Separate group analysis revealed similar results in the hypothermic group (R = −0.565, P = 0.44 for RBM3 after rewarming and body temperature at 24 h) (Fig. 6C, right). No such correlation at these or other time-points was found in the placebo group.
Figure 6.
RBM3 levels in patients treated with hypothermia versus placebo. (A) RBM3 levels across the pre-specified collection time-points: basal (before cooling), 26 h (2 h after starting rewarming) and 72 h after stroke onset. Left panel includes all 31 patients and the right panel the SH group only, comprising patients fulfilling pre-defined criteria for per-protocol analyses (n = 7). (B) Boxplots represent the median (interquartile range) of ΔRBM3 values (%) between baseline and rewarming time-points in the SH and placebo arms (left, all cases; right, only patients fulfilling pre-defined criteria for per-protocol analyses). (C) Correlation between RBM3 levels at rewarming and body temperature at 24 h in all patients (left) and those treated with SH (right).
Discussion
RBM3 is an evolutionarily old protein whose relationship to cold stress remained undescribed until 1997 (Danno et al., 1997). Given its novelty and presumably complex role in the cell, its functions are still poorly understood. It has been reported to be involved in ribosome assembly, limitation of the unfolded protein response, interaction with microRNA regulators and cell-cycle regulation (Dresios et al., 2005; Matsuda et al., 2011; Pilotte et al., 2011; Wong et al., 2016; Zhu et al., 2016b). All knowledge to date concerning RBM3 and hypothermia has been obtained from preclinical in vitro and in vivo studies. Clinical relevance had not previously been investigated. Our study is the first to demonstrate an association between RBM3 and hypothermia not only in cerebrally lesioned adult rats but also in IS patients.
Our group had already established that both hypothermia protocols used in this study, focal and systemic hypothermia, reduce ischaemic lesion volume in animal models (Vieites-Prado et al., 2016). Focal hypothermia requires maintaining for 24 h to achieve the same protection as that afforded by 4 h of systemic cooling. But while focal hypothermia can be used in awake and freely moving animals, systemic hypothermia must be performed under anaesthesia to avoid hypothermic stress. Using magnetic resonance thermometry, we showed that focal hypothermia exerted a local cooling effect in the ischaemic region where the cooling device was located, whereas systemic hypothermia lowered whole body and brain temperature. The present study complements these earlier findings by showing that the decreases in brain temperature recorded by magnetic resonance thermometry during focal and systemic hypothermia are associated with increased levels of RBM3.
Selective RBM3 expression in the ipsilateral hemisphere where the focal hypothermia device is located appears a direct effect of temperature on cerebral tissue. Diffusion no doubt accounts for the slight increase in RBM3 protein levels observed in the contralateral hemisphere (Figs 1B and 2C).
Additionally, the increased RBM3 observed during systemic normothermia in both healthy and ischaemic animals could be caused by the sevoflurane used during the experimental procedure: sevoflurane mediates cell signalling pathways related to AKT, a protein impacting RBM3 activity and expression (Matchett et al., 2009; Wang et al., 2010). Supporting evidence is that RBM3 did not increase in non-anaesthetized normothermic focal control groups.
The viral infection strategy used in this study to evaluate the effect of enhanced or blocked RBM3 expression on cerebral ischaemic damage has been tested in other models. Thus, cerebral overexpression of RBM3 mediated by lentiviral infection restored structural synaptic plasticity and reversed neuronal deficit in an animal model of Alzheimer’s disease (Peretti et al., 2015). In the present study, the viral infection we implanted in the cortical region was successful (based on GFP gene reporter data) in enhancing and inhibiting local RBM3 expression. Although viral tissue infection was optimized in order to maximize GFP expression with minimal tissue damage caused by the needle injection, neither approach significantly impacted ischaemic infarct volume, possibly due to the wide ischaemic lesion compared with the reduced population of infected neurons. However, a trend in functional recovery was observed in animals with up-regulated RBM3.
The analysis of RBM3 levels in two independent cohorts of IS patients showed an association between increasing ΔRBM3 and good outcome at 3 months, reflecting a potentially protective role on neuronal tissue. The fact that the association with good prognosis was stronger than with temperature itself suggests the involvement of RBM3 in other neuroprotective mechanisms independent of body temperature. High RBM3 levels have been clinically associated with prolonged overall survival in intestinal-type gastric cancer (Ye et al., 2017), good prognosis in invasive breast cancer (Kang et al., 2018) and colon cancer (Jang et al., 2017), and improved response and survival in metastatic colorectal cancer (Siesing et al., 2017), none of which benefits appear temperature-related.
The blood samples from the IS patients that underwent hypothermic treatment in the EuroHYP-1 trial confirmed the association between RBM3 and cooling. Although the results were not statistically significant, this trend observed in just 17 patients can be considered remarkable. Although a larger sample size might achieve significance, the pool of eligible patients will remain limited until hypothermic treatment becomes established clinical practice. EuroHYP-1 was a pioneering phase III trial but access to multiple samples was limited by the low number of patients enrolled, incompatibility between hypothermic procedures and blood sampling, and the inability of many patients to complete the cooling protocol.
In conclusion, these preclinical and clinical results identify RBM3 as a molecular marker associated with the protective effect of hypothermia in IS and suggest its potential application as a promising protective target.
Funding
This study was partially supported by the Spanish Ministry of Economy and Competitiveness (SAF2017-84267-R), Xunta de Galicia (GRC2014/027, IN607A2018/3), Instituto de Salud Carlos III (RD16/0019 and PI17/00540; Miguel Servet contracts CPII17/00027 and CPII19/00020; Sara Borrell contract CD19/00033). EuroHYP-1 received funding from the European Union’s Seventh Framework Programme (FP7/2007–2013) under grant agreement n° 278709. European Union programme FEDER and the European Regional Development Fund – ERDF.
Competing interests
The authors report no competing interests.
Supplementary Material
Glossary
- AAV =
adeno-associated virus
- CIRP =
cold-inducible RNA-binding protein
- CSPs =
cold shock proteins
- FC =
focal control
- FH =
focal hypothermia
- GFP =
green fluorescent protein
- IS =
ischaemic stroke
- RBM3 =
RNA-binding motif protein 3
- SC =
Systemic normothermic control groups
- SH =
systemic hypothermia
- WB =
western blot
References
- Campos F, Pérez-Mato M, Agulla J, Blanco M, Barral D, Almeida Á, et al. Glutamate excitoxicity is the key molecular mechanism which is influenced by body temperature during the acute phase of brain stroke. PLoS One 2012; 7: e44191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J, Dávalos A, Marrugat J, Noya M.. Timing for fever-related brain damage in acute ischemic stroke. Stroke 1998; 29: 2455–60. [DOI] [PubMed] [Google Scholar]
- Danno S, Nishiyama H, Higashitsuji H, Yokoi H, Xue J-H, Itoh K, et al. Increased transcript level of RBM3, a member of the glycine-rich RNA-binding protein family, in human cells in response to cold stress. Biochem Biophys Res Commun 1997; 236: 804–7. [DOI] [PubMed] [Google Scholar]
- Darwazeh R, Yan Y.. Mild hypothermia as a treatment for central nervous system injuries: positive or negative effects. Neural Regen Res 2013; 8: 2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresios J, Aschrafi A, Owens GC, Vanderklish PW, Edelman GM, Mauro VP.. Cold stress-induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. Proc Natl Acad Sci U S A 2005; 102: 1865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Susavila H, Iglesias-Rey R, Dopico-Lopez A, Perez-Mato M, Sobrino T, Castillo J, et al. Inclusion criteria update for the rat intraluminal ischaemic model for preclinical studies. Dis Model Mech 2017; 10: 1433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts M, Petersson J, Brizzi M, Olsson-Hau S, Luijckx G-J, Algra A, et al. COOLIST (Cooling for Ischemic Stroke Trial): a multicenter, open, randomized, phase II. Clinical Trial 2017; 48: 219–21. [DOI] [PubMed] [Google Scholar]
- Han HS, Park J, Kim JH, Suk K.. Molecular and cellular pathways as a target of therapeutic hypothermia: pharmacological aspect. Curr Neuropharmacol 2012; 10: 80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HH, Lee HN, Kim SY, Hong S, Lee WS.. Expression of RNA-binding motif protein 3 (RBM3) and cold-inducible RNA-binding protein (CIRP) is associated with improved clinical outcome in patients with colon cancer. Anticancer Res 2017; 37: 1779–85. [DOI] [PubMed] [Google Scholar]
- Kang SH, Cho J, Jeong H, Kwon SY.. High RNA-binding motif protein 3 expression is associated with improved clinical outcomes in invasive breast cancer. J Breast Cancer 2018; 21: 288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott G. Neurodegeneration: Cold shock protects the brain. Nature 2015; 518: 177–8. [DOI] [PubMed] [Google Scholar]
- Matchett GA, Allard MW, Martin RD, Zhang JH.. Neuroprotective effect of volatile anesthetic agents: molecular mechanisms. Neurol Res 2009; 31: 128–34. [DOI] [PubMed] [Google Scholar]
- Matsuda A, Ogawa M, Yanai H, Naka D, Goto A, Ao T, et al. Generation of mice deficient in RNA-binding motif protein 3 (RBM3) and characterization of its role in innate immune responses and cell growth. Biochem Biophys Res Commun 2011; 411: 7–13. [DOI] [PubMed] [Google Scholar]
- Peretti D, Bastide A, Radford H, Verity N, Molloy C, Martin MG, et al. RBM3 mediates structural plasticity and protective effects of cooling in neurodegeneration. Nature 2015; 518: 236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotte J, Dupont-Versteegden EE, Vanderklish PW.. Widespread regulation of miRNA biogenesis at the Dicer step by the cold-inducible RNA-binding protein, RBM3. PLoS One 2011; 6: e28446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesing C, Sorbye H, Dragomir A, Pfeiffer P, Qvortrup C, Ponten F, et al. High RBM3 expression is associated with an improved survival and oxaliplatin response in patients with metastatic colorectal cancer. PLoS One 2017; 12: e0182512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Worp HB, Macleod MR, Bath PM, Demotes J, Durand-Zaleski I, Gebhardt B, et al. EuroHYP-1: European multicenter, randomized, phase III clinical trial of therapeutic hypothermia plus best medical treatment vs. best medical treatment alone for acute ischemic stroke. Int J Stroke 2014; 9: 642–5. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR.. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 2007; 130: 3063–74. [DOI] [PubMed] [Google Scholar]
- Vieites-Prado A, Iglesias-Rey R, Fernandez-Susavila H, da Silva-Candal A, Rodriguez-Castro E, Grohn OH, et al. Protective effects and magnetic resonance imaging temperature mapping of systemic and focal hypothermia in cerebral ischemia. Stroke 2016; 47: 2386–96. [DOI] [PubMed] [Google Scholar]
- Wang JK, Yu LN, Zhang FJ, Yang MJ, Yu J, Yan M, et al. Postconditioning with sevoflurane protects against focal cerebral ischemia and reperfusion injury via PI3K/Akt pathway. Brain Res 2010; 1357: 142–51. [DOI] [PubMed] [Google Scholar]
- Wellmann S, Truss M, Bruder E, Tornillo L, Zelmer A, Seeger K, et al. The RNA-binding protein RBM3 is required for cell proliferation and protects against serum deprivation-induced cell death. Pediatr Res 2010; 67: 35–41. [DOI] [PubMed] [Google Scholar]
- Wong JJ, Au AY, Gao D, Pinello N, Kwok CT, Thoeng A, et al. RBM3 regulates temperature sensitive miR-142-5p and miR-143 (thermomiRs), which target immune genes and control fever. Nucleic Acids Res 2016; 44: 2888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. Neurodegenerative disease: RBM3, a protein upregulated during hibernation, provides new insights into neurodegeneration. Nat Rev Neurol 2015; 11: 124. [DOI] [PubMed] [Google Scholar]
- Ye FP, Jin PS, Cai XN, Cai PP, An HM.. High RNA-binding motif protein 3 (RBM3) expression is independently associated with prolonged overall survival in intestinal-type gastric cancer. Med Sci Monit 2017; 23: 6033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Yang WL, Ji Y, Qiang X, Wang P.. Cold-inducible RNA-binding protein mediates neuroinflammation in cerebral ischemia. Biochim Biophys Acta 2014; 1840: 2253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Buhrer C, Wellmann S.. Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold. Cell Mol Life Sci 2016. a; 73: 3839–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Yan J, Bregere C, Zelmer A, Goerne T, Kapfhammer JP, et al. RBM3 promotes neurogenesis in a niche-dependent manner via IMP2-IGF2 signaling pathway after hypoxic-ischemic brain injury. Nat Commun 2019; 10: 3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zelmer A, Kapfhammer JP, Wellmann S.. Cold-inducible RBM3 inhibits PERK phosphorylation through cooperation with NF90 to protect cells from endoplasmic reticulum stress. FASEB J 2016. b; 30: 624–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material.