Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is a progressive and fatal lung disease with an unpredictable course and a median survival of three to four years. This timeline challenges providers to approach diagnosis, oxygen therapy, rehabilitation, transplantation, and end-of-life discussions in limited encounters. There is currently no widely accepted guideline for determining when IPF patients should be referred to palliative care (PC).
Objective: We sought to describe the patient and clinical factors associated with PC referral, as well as its impact on mortality and location of death. We also aimed to examine temporal trends in PC referral in this population.
Materials and Methods: Patient data were retrospectively extracted from the health system repository of our specialty referral center for all new IPF patients evaluated between 2000 and 2016 (n = 828). Exclusion criteria included transplant recipients and patients who did not have IPF.
Results: One hundred twelve (13.5%) IPF patients received formal PC referral. Recipients were older at diagnosis (72 years vs. 69 years, p < 0.001), had higher frequency of Charlson Comorbidity Index ≥1 (55% vs. 42%, p = 0.011), resided closer to our institution (16 miles vs. 54 miles, p < 0.001), and had a higher number of total outpatient visits (7 vs. 4, p < 0.001). PC was associated with less in-hospital death (44% vs. 60%, p = 0.006) and more in-home and hospice death (56% vs. 40%, p = 0.006).
Conclusions: IPF patients referred to PC were older with more severe comorbidities, resided closer to our specialty referral center, and had more outpatient follow-up. This was associated with more in-home and hospice deaths. The patient–provider relationship and frequency of follow-up visits likely play important roles in the introduction of end-of-life discussions.
Keywords: idiopathic pulmonary fibrosis, location of death, palliative care
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive and fatal lung disease with an unpredictable clinical course, ranging from long periods of clinical stability to fast periods of acute decompensation.1 Initial symptoms are nonspecific, including progressive dyspnea on exertion and nonproductive cough, which often delay initial diagnosis and management. Recent estimates of median survival range between three and four years from time of diagnosis, comparable with the clinical course of many malignancies.2–4 In addition, a previous study at our institution reported a median survival of one year from the first outpatient specialty referral center visit.5 This timeline often challenges providers to approach many aspects of patient care in limited encounters, from diagnosis to oxygen therapy to pulmonary rehabilitation to lung transplantation to advance care planning and end-of-life discussions.
The diagnosis of IPF has major and lasting impacts on patients by altering their activities of daily living as well as their interpersonal relationships with family and significant others.6,7 Patients and caregivers often experience tremendous stress and burden when faced with the many aspects of multidisciplinary care and management.8,9 This may result in the failure to recognize the disease course and its natural progression. Despite recent advancements in antifibrotic therapy, lung transplantation remains the only definitive cure for this population.10–13
Consequently, IPF patients are particularly well suited for, and may benefit from, early palliative care (PC) intervention, with focus on symptom management, quality of life, and advance care planning. We previously reported that only 13.7% of IPF decedents at our specialty referral center received formal PC referral between 2000 and 2012, with 71% of patients referred within 30 days of death.5 Many were evaluated in the intensive care unit during acute exacerbations with respiratory failure, limiting the full influence of PC on this population.
Previous studies demonstrated significant associations between early PC intervention and improved dyspnea, cough, fatigue, and comfort in patients with IPF.12,14 Recent literature has targeted multidisciplinary, collaborative care models to assist with achieving home deaths, as many IPF patients express preferences to die outside of the hospital in favor of more familiar environments.15 However, PC referral patterns continue to vary dramatically by institution, with reported ranges between 3% and 14%.16–19 Currently, the 2015 American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association (ATS/ERS/JRS/ALAT) clinical guidelines do not describe when IPF patients should be referred to PC.20
In this study, we aim to describe PC referral rates at our institution and investigate temporal trends since the initiation of an outpatient PC clinic affiliated with our specialty referral center. In addition, we aim to examine patient-specific characteristics associated with PC referral, as well as the impact of PC referral on patient outcomes, specifically mortality and death. We hypothesize that successful referral to PC services is dependent on the clinical characteristics of patients, such as age and baseline lung disease, and that the establishment of an outpatient PC clinic associated with our specialty referral center would increase referral rates.
Materials and Methods
Study design
This was a retrospective cohort study of IPF patients evaluated for the first time between January 2000 and December 2016 at the University of Pittsburgh Dorothy P. and Richard P. Simmons Center for Interstitial Lung Disease. This specialty referral center is affiliated with the University of Pittsburgh Medical Center, an academic tertiary referral center, and annually evaluates ∼250 new patients referred for possible IPF from local, regional, and distant practices. This study serves as a continuation of our previous report describing location of death in IPF decedents at our institution from 2000 to 2012.5 Patient data were further analyzed by subgroup cohorts, from 2000–2012 and 2013–2016, to evaluate PC referral trends over time since the initiation of a dedicated outpatient PC clinic at our institution in 2013. As this was a single-center study evaluating providers and their individual PC referral practice patterns, only deidentified provider frequencies of referral, rather than the total number of patients evaluated or the timeline of their practice at this institution, were included to preserve anonymity. This study was approved by the University of Pittsburgh Committee for Oversight and Research and Clinical Training Involving Decedents No. 780 and the University of Pittsburgh Institutional Review Board PRO17040191.
Patient population
A total of 828 patients with IPF, based on 2000 ATS/ERS and 2011 ATS/ERS/JRS/ALAT clinical diagnosis guidelines, were evaluated for the first time between 2000 and 2016.21,22 This comprised of 638 decedents and 190 living patients as of January 1, 2017. Exclusion criteria included lung transplant recipients, irrespective of their surgical timeline to PC referral, and patients who did not have IPF. Patients who underwent lung transplantation were not included as their postoperative clinical management and follow-up were dramatically different. In addition, previous reports cited considerable barriers in the acceptance of PC in this population.23
Measurements
Baseline demographic information was obtained from our university health system repository. This included age at diagnosis, age at death, sex, race, date of initial outpatient visit, distance of residence from our institution, number of outpatient visits, support group participation, and documented comorbidities at the initial visit. Baseline pulmonary characteristics were obtained within three months of the initial visit. This included percent predicted forced vital capacity (FVC%), percent predicted diffusing capacity of lungs for carbon monoxide (DLco%), GAP (gender, age, and physiology), Index, GAP Stage, Charlson Comorbidity Index (CCI), and oxygen requirement at the first visit.24,25 CCI scores were calculated using 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes for associated comorbidities. PC referral was defined as a formal request for consultation documented in the health system repository. Mortality and location of death were obtained by provider documentation in the health system repository and Internet searches for published death notices. Obituary information was confirmed with our specialty referral center's clinical database for patient identity verification. Location of death was categorized into hospital floor, academic intensive care unit, and home or hospice. Support group participation was defined as ≥1 meeting attendances. Patients evaluated by all deidentified providers were included for overall analyses, but only those providers who evaluated ≥10 IPF patients between 2000 and 2016 and took care of patients during both 2000 and 2012 and 2013–2016 time periods were included for analysis of individual practice patterns as detailed in Table 4.
Table 4.
Provider Palliative Care Referral
| 2000–2016 (%) | 2000–2012 (%) | 2013–2016 (%) | % Change | |
|---|---|---|---|---|
| Provider 1 | 15.5 | 14.5 | 18.2 | +3.7 |
| Provider 2 | 13.8 | 11.8 | 22.1 | +10.3 |
| Provider 3 | 19.1 | 12.5 | 23.1 | +10.6 |
| Provider 4 | 18.2 | 16.1 | 20.0 | +3.9 |
| Provider 5 | 10.0 | 10.0 | 22.2 | +12.2 |
| Total provider | 13.5 | 11.5 | 21.4 | +9.9 |
Deidentified providers demonstrated increased palliative care referral rates since 2013.
Data analysis
Categorical values were presented as numbers (n) and percentages (%). Continuous variables were presented as median values with interquartile ranges or mean values with standard deviation. Pairwise comparisons of baseline patient demographics and location of death were performed using Welch's t test or chi-squared test, when appropriate. Mortality estimates between cohorts were obtained using Kaplan–Meier survival curves and log-rank tests. Multiple logistic regression modeling, adjusting for confounders, was used to assess the impact of PC referral on patient location of death, specifically in-hospital mortality. In this model, we entered variables with p < 0.20 in univariate analyses and selected final confounders using stepwise backward approach. Cox multiple regression analysis was used to test the effect of PC referral on overall survival. Statistical significance was defined as p < 0.05. All analyses were performed using Stata 14.2 (StataCorp., College Station, TX).
Results
Baseline patient demographics
Descriptive baseline demographics from 2000 to 2016 are shown in Tables 1 and 2. Patients referred to PC were older at diagnosis (72 years vs. 69 years, p < 0.001), older at death (76 years vs. 73 years, p = 0.007), and had more severe comorbidities, as indicated by CCI ≥1 (55% vs. 42%, p = 0.011). This cohort resided closer to our specialty referral center (16 miles vs. 54 miles, p < 0.001), had more total outpatient visits (7 vs. 4, p < 0.001), and were more active support group participants (35% vs. 19%, p < 0.001). There were no differences in FVC%, DLco%, GAP Index, or GAP Stage at the first visit (Table 2).
Table 1.
Baseline Patient Demographics (2000–2016)
| All | No PC | PC | p | |||
|---|---|---|---|---|---|---|
| Results | N | Results | n | Results | ||
| Age at diagnosis (years) | 70 (64–75) | 707 | 69 (63–75) | 112 | 72 (67–78) | <0.001 |
| Age at death (years) | 74 (67–80) | 536 | 73 (67–79) | 100 | 76 (69–81) | 0.007 |
| Male, n (%) | 523 (63) | 716 | 458 (64) | 112 | 65 (58) | 0.23 |
| White non-Hispanic, n (%) | 802 (97) | 716 | 696 (97) | 112 | 106 (95) | 0.15 |
| Smoker, n (%) | 559 (68) | 714 | 491 (69) | 110 | 68 (62) | 0.041 |
| Tobacco (pack years), mean (SD) | 23.4 (27.3) | 673 | 22.9 (25.8) | 108 | 26.2 (35.4) | 0.36 |
| Initial oxygen requirement (L), mean (SD) | 1.1 (1.8) | 710 | 1.1 (1.8) | 111 | 1.2 (1.9) | 0.57 |
| Initial oxygen requirement >0 L, n (%) | 267 (33) | 710 | 228 (21) | 111 | 39 (35) | 0.53 |
| Diagnosis to first visit (days) | 199 (0–563) | 708 | 204 (0–569) | 112 | 115 (0–474) | 0.014 |
| Diagnosis to death (days) | 1150 (603–1980) | 535 | 1158 (593–1954) | 100 | 1094 (625–2076) | 0.95 |
| Distance from center (miles) | 47 (15–104) | 714 | 54 (18–113) | 111 | 16 (10–47) | <0.001 |
| Number of center visits | 4 (2–9) | 716 | 4 (2–8) | 111 | 7 (3–14) | <0.001 |
| Support group, n (%) | 178 (22) | 716 | 139 (19) | 112 | 39 (35) | <0.001 |
| Charlson Index, mean (SD) | 0.76 (1.08) | 716 | 0.73 (1.06) | 112 | 0.95 (1.16) | 0.07 |
| Charlson Index ≥1, n (%) | 366 (44) | 716 | 304 (42) | 112 | 62 (55) | 0.011 |
PC referral recipients were older, had higher Charlson Indices ≥1, resided closer to the outpatient center, and had more follow-up visits. Values are documented as median or percentages (IQR), except when marked.
IQR, interquartile range; PC, palliative care; SD, standard deviation.
Table 2.
Pulmonary Characteristics (2000–2016)
| All | No PC | PC | p | |||
|---|---|---|---|---|---|---|
| Results | n | Results | n | Results | ||
| Initial FVC% | 64 (52–78) | 676 | 65 (52–78) | 109 | 62 (52–76) | 0.77 |
| Initial DLco% | 43 (31–58) | 596 | 43 (33–58) | 94 | 47 (33–58) | 0.77 |
| Last FVC% | 59 (46–72) | 438 | 60 (47–74) | 91 | 55 (42–68) | 0.125 |
| Last DLco% | 38 (28–55) | 338 | 39 (29–56) | 70 | 36 (25–50) | 0.037 |
| Initial GAP Index, mean (SD) | 4.1 (1.5) | 594 | 4.1 (1.5) | 94 | 4.1 (1.3) | 0.82 |
| Initial GAP Stage ≥2, n (%) | 461 (57) | 594 | 402 (68) | 94 | 59 (63) | 0.35 |
There were no differences in initial pulmonary function. Values are documented as median or percentages (IQR), except when marked.
DLco, diffusing capacity of lungs for carbon monoxide; FVC, forced vital capacity; GAP, gender, age, and physiology; PC, palliative care.
PC and location of death
Descriptions of location of death from 2000 to 2016 are shown in Table 3. PC referral recipients had less in-hospital death (44% vs. 60%, p = 0.006) and more in-home and hospice death (56% vs. 40%, p = 0.006). Similar trends were observed in subgroup analyses of patient cohorts from 2000–2012 and 2013–2016 (Supplementary Tables S1 and S2; Supplementary Data are available online at www.liebertpub.com/jpm). In a logistic regression analysis for the entire study period, after adjusting for age, initial comorbidities, race, and time of diagnosis to first visit, PC referral was associated with lower risk of in-hospital mortality (odds ratio 0.52, 95% confidence interval 0.31–0.86, p = 0.010).
Table 3.
Location of Death (2000–2016)
| All | No PC | PC | p | |||
|---|---|---|---|---|---|---|
| Results | N | Results | n | Results | ||
| Death, n (%) | 519 (83) | 542 | 447 (83) | 80 | 72 (90) | 0.09 |
| Death, n (%) | 347 | 85 | 0.006 | |||
| Hospital | 245 (57) | 208 (60) | 37 (44) | |||
| Home or hospice | 187 (44) | 139 (40) | 48 (56) | |||
| Death: academic ICU, n (%) | 125 (51) | 207 | 107 (52) | 36 | 18 (50) | 0.85 |
PC referral recipients have less in-hospital death. Values are documented as median or percentages (IQR), except when marked *indicating mean (SD). ICU, intensive care unit.
PC and mortality
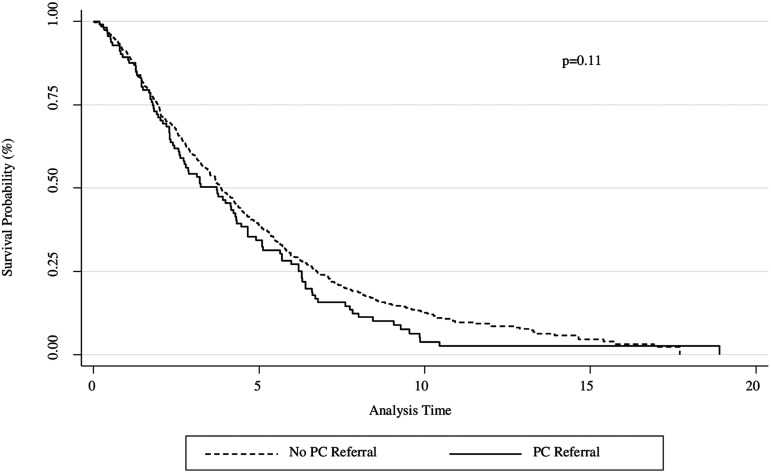
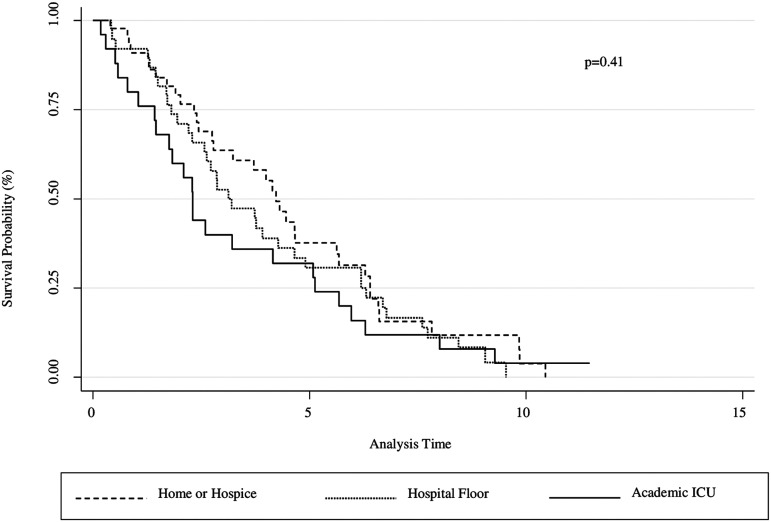
Kaplan–Meier survival curves are shown in Figures 1 and 2. There were no mortality differences between the presence and absence of PC referral for the entire study population from 2000 to 2016 (Fig. 1). There were no mortality differences when comparing location of PC referral between outpatient, hospital floor, and academic intensive care unit settings (Fig. 2). In subgroup analyses, PC referral recipients had lower survival (p = 0.034) from 2013 to 2016 (Supplementary Fig. S1); however, there was no difference when adjusted for baseline FVC% and initial oxygen requirement using an adjusted Cox model (Supplementary Fig. S2). These covariates were chosen as they have been used as prognostic markers of mortality in IPF; recent studies have associated lower FVC% and higher titrated supplemental oxygen requirement to maintain resting saturation >96% with worse survival outcomes in this population.3,26
FIG. 1.
Kaplan–Meier Survival Curve (2000–2016). Mortality estimates for patients who received PC (solid) compared with those who did not receive PC (dashed). There were no survival differences between groups. PC, palliative care.
FIG. 2.
Kaplan–Meier Survival Curve (2000–2016). Mortality estimates for patients who received PC based on location, including outpatient (dashed), hospital floor (dotted), and academic ICU (solid). There were no survival differences between groups. ICU, intensive care unit.
PC referral trends
Descriptive baseline demographics of patient subgroup cohorts from 2000–2012 and 2013–2016 are shown in Supplementary Tables S3–S6. Since 2013, IPF patients referred to PC services had more advanced baseline lung disease, as measured by lower FVC% (54% vs. 69%, p < 0.001), lower DLco% (38% vs. 50%, p = 0.002), higher mean GAP Index (4.4 vs. 3.8, p = 0.010), higher frequency of GAP Stage ≥2 (76% vs. 52%, p = 0.012), and higher frequency of any supplemental oxygen utilization at the first visit (47% vs. 27%, p = 0.033). These clinical characteristics were not observed in the 2000–2012 patient subgroup cohort.
Provider variability
Frequencies of provider referral to PC are shown in Table 4. The overall frequency of PC referral for the entire study population from 2000 to 2016 was 13.5%. In subgroup analyses, there was an increase from 11.5% between 2000 and 2012 to 21.4% between 2013 and 2016. All deidentified providers who evaluated IPF patients during both timeline cohorts demonstrated higher PC referral frequencies over time, with absolute increases ranging from 3.7% to 12.2%.
Discussion
Our findings indicate that IPF patients referred to PC were older with more severe comorbidities, resided closer to our institution, and had more outpatient follow-up. PC referral was associated with less in-hospital death. Since 2013, there had been an increase in the overall rate of PC referral at our specialty referral center, with particular emphasis on more severe baseline pulmonary disease. To the best of our knowledge, this is the largest single-center cohort study describing patient, clinical, and provider factors associated with PC referral on patients with IPF and the impact of PC referral on location of death.
Due to the relentless progression and unpredictable time course of IPF, patients with this chronic illness would be predicted to benefit from early PC referral, as measured by patient–provider experiences. Current literature cites tremendous variability in PC referral rates for chronic illnesses, most of which are significantly lower than referral rates for malignancies.27,28 Based on the experiences from our single-center study, many patients who did not receive PC resided significant distances from our institution, which likely limited their continuity of care. As a result, those who may have benefited from early PC intervention were lost to follow-up. Despite recent emphasis on advance care planning and end-of-life discussions in this population, there remain no guidelines for determining when to refer IPF patients to PC.
When assessing all IPF patients evaluated at our specialty referral center since its institution in 2000, PC referral recipients were older at diagnosis and had more severe comorbidities at the initial visit. These results were reflected in prior reports citing similar clinical characteristics as predictors of worse prognosis in this population.3,14,24,29 In addition, PC referral recipients resided closer to our specialty referral center, had higher frequency of outpatient visits, and were more active support group participants. These findings confirm the importance of the patient–provider relationship in the introduction of PC and end-of-life discussions, as patients and caregivers who are more engaged and readily participate in educational and support groups may have improved disease awareness and become more open to advance care planning and PC conversations.8,30,31 In addition to the patient–provider relationship, a factor that may explain these correlations is access to health care, such as language and distance barriers. In this study, we utilized geographic distance as a proxy marker for other barriers, but this may not take into consideration those who lived too far to present for evaluation due to their supplemental oxygen requirements or lacked adequate modes of transportation. It is noteworthy that all patients required insurance at our specialty referral center.
PC referral was associated with lower frequencies of in-hospital death and higher frequency of in-home and hospice death. These results are congruent with recent studies describing patient and family preferences about end-of-life location of death and comfort with familiar environments.15 There were no survival differences for the entire study cohort. While PC referral recipients had lower survival between 2013 and 2016, this difference disappeared when adjusted for baseline FVC% and initial oxygen requirement. A similar duration from diagnosis to death, irrespective of PC referral, further argues against an association between PC and mortality in this patient subgroup cohort.
We observed changes in PC referral trends since 2013. This timeline coincided with our specialty referral center's focus on more severe baseline lung disease, as indicated by lower FVC% and DLco%, and higher GAP Index and GAP Stage. The absence of these pulmonary differences in the 2000–2012 subgroup cohort may reflect the result of a previously random, unstructured PC referral pattern that shifted in focus to worse pulmonary disease beginning in 2013, which also corresponded to the advent of the GAP Index in late 2012.24
This is the first report of provider influences on PC referral in the IPF population. Since the creation of an outpatient PC clinic with a focus on interstitial lung diseases in 2013 at our tertiary referral center, modeled after similar institutional oncologic services, we report significant increases in the cumulative PC referral frequencies by providers from 11.5% through 2012 to 21.4% since 2013. Similar trends were reflected in each deidentified provider patient panel.
There are several limitations of this study worth mentioning. First, this was a single-center retrospective cohort study at a specialty referral center affiliated with an academic tertiary referral center, which restricts its applicability to the general population. Second, the data are dependent on its documentation in the health system repository and complete provider notes. Third, the exclusion of all lung transplant recipients may preclude patients who were regularly followed up by providers at our specialty referral center before surgery; these patients were not included for the purposes of this study as the timeline separating appropriateness of PC and lung transplant referrals remains unclear. Fourth, the introduction of prognostication scores, such as the advent of the GAP Index, and revised treatment protocols have shifted toward patient-centered focus with subsequent changes in management that may not be fully reflected in this retrospective cohort study spanning 16 years. Fifth, it is unclear whether all PC referrals resulted in actual PC consultation as this information was not readily accessible for review. Finally, it is possible that providers held end-of-life discussions with patients and caregivers without official PC referral, thereby under-reporting its incidence in this population.
Future directions will aim to assess the impact of PC on health-related quality of life from patient and provider perspectives. We will examine the role of PC in health care cost differences between recurrent hospital admissions and home or hospice care during the last year of life. We will evaluate clinical outcomes in a randomized, prospective assessment of PC intervention compared with the current standard of care.
Supplementary Material
Acknowledgments
This study was supported by the University of Pittsburgh Dorothy P. and Richard P. Simmons Center for Interstitial Lung Disease at the University of Pittsburgh Medical Center.
Prior Abstract Publication/Presentation: 2018 American Thoracic Society International Meeting, San Diego, CA; 2017 Pulmonary Fibrosis Foundation Summit, Nashville, TN.
Author Disclosure Statement
D.J.K. reports collaborative research funding from Regeneron Pharmaceuticals. K.F.G. serves as a consultant for Bayer. N.K. reports personal fees from Biogen Idec, Boehringer Ingelheim, Third Rock, Pliant, Samumed, NuMedii, Indalo, as a consultant and nonfinancial support from miRagen, all outside the submitted work; he also holds patents on New Therapies in Pulmonary Fibrosis with royalties paid by Biotech and Peripheral Blood Gene Expression. The remaining authors have no potential conflicts of interest with any companies or organizations whose products or services may be discussed in this article.
The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of this article.
References
- 1. Lederer DJ, Martinez FJ: Idiopathic pulmonary fibrosis. N Engl J Med 2018;378:1811–1823 [DOI] [PubMed] [Google Scholar]
- 2. Nathan SD, Shlobin OA, Weir N, et al. : Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest 2011;140:221–229 [DOI] [PubMed] [Google Scholar]
- 3. Ley B, Collard HR, King TE: Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431–440 [DOI] [PubMed] [Google Scholar]
- 4. Fujimoto H, Kobayashi T, Azuma A: Idiopathic pulmonary fibrosis: Treatment and prognosis. Clin Med Insights Circ Respir Pulm Med 2015;9(Suppl. 1):179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lindell KO, Liang Z, Hoffman LA, et al. : Palliative care and location of death in decedents with idiopathic pulmonary fibrosis. Chest 2015;147:423–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bajwah S, Higginson IJ, Ross JR, et al. : The palliative care needs for fibrotic interstitial lung disease: A qualitative study of patients, informal caregivers and health professionals. Palliat Med 2013;27:869–876 [DOI] [PubMed] [Google Scholar]
- 7. Rajala K, Lehto JT, Saarinen M, et al. : End-of-life care of patients with idiopathic pulmonary fibrosis. BMC Palliat Care 2016;15:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Belkin A, Albright K, Swigris JJ: A qualitative study of informal caregivers' perspectives on the effects of idiopathic pulmonary fibrosis. BMJ Open Resp Res 2014:1:e000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindell KO, Kavaliertos D, Gibson KF, et al. : The palliative care needs of patients with idiopathic pulmonary fibrosis: A qualitative study of patients and family caregivers. Heart Lung 2017;46:24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richeldi L, du Bois RM, Raghu G, et al. : Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071–2082 [DOI] [PubMed] [Google Scholar]
- 11. Taniguchi H, Ebina M, Kondoh Y, et al. : Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:821–829 [DOI] [PubMed] [Google Scholar]
- 12. King TE, Bradford WZ, Castro-Bernardini S, et al. : A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083–2092 [DOI] [PubMed] [Google Scholar]
- 13. Kistler KD, Nalysnyk L, Rotella P, Esser D: Lung transplantation in idiopathic pulmonary fibrosis: A systemic review of the literature. BMC Pulm Med 2014;14:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim JH, Lee JH, Ryu YJ, Chang JH: Clinical predictors of survival in idiopathic pulmonary fibrosis. Tuberc Respir Dis (Seoul) 2012;73:162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalluri M, Richman-Eisenstat J: Early and integrated palliative care to achieve a home death in idiopathic pulmonary fibrosis. J Pain Symptom Manage 2017;53:1111–1115 [DOI] [PubMed] [Google Scholar]
- 16. Ahmadi Z, Wysham NG, Lundstrom S, et al. : End-of-life care in oxygen-dependent ILD compared with lung cancer: A national population-based study. Thorax 2016;71:510–516 [DOI] [PubMed] [Google Scholar]
- 17. Sharp C, Lamb H, Jordan N, et al. : Development of tools to facilitate palliative and supportive care referral for patients with idiopathic pulmonary fibrosis. BMJ Support Palliat Care 2018;8:340–346 [DOI] [PubMed] [Google Scholar]
- 18. Liang Z, Hoffman LA, Nouraie M, et al. : Referral to palliative care infrequent in patients with idiopathic pulmonary fibrosis admitted to an intensive care unit. J Palliat Med 2017;20:134–140 [DOI] [PubMed] [Google Scholar]
- 19. Rush B, Berger L, Celi LA: Access to palliative care for patients undergoing mechanical ventilation with idiopathic pulmonary fibrosis in the United States. Am J Hosp Palliat Care 2018;35:492–496 [DOI] [PubMed] [Google Scholar]
- 20. Raghu G, Bochwerg B, Zhang Y, et al. : An official ATS/ERS/JRS/ALAT clinical practice guideline: Treatment of idiopathic pulmonary fibrosis. An update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 2015;192:e3–e19 [DOI] [PubMed] [Google Scholar]
- 21. American Thoracic Society; European Respiratory Society. Idiopathic pulmonary fibrosis: Diagnosis and treatment: International Consensus Statement. Am J Respir Crit Care Med 2000;161:646–664 [DOI] [PubMed] [Google Scholar]
- 22. Raghu G, Collard HR, Egan JJ, et al. : An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colman RE, Curtis JR, Nelson JE, et al. : Barriers to optimal palliative care of lung transplant candidates. Chest 2013;143:736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ley B, Ryerson CJ, Vittinghoff E, et al. : A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 2012;156:684–691 [DOI] [PubMed] [Google Scholar]
- 25. Charlson ME, Pompei P, Ales KL, MacKinzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–83 [DOI] [PubMed] [Google Scholar]
- 26. Hook JL, Arcasoy SM, Zemmel D, et al. : Titrated oxygen requirement and prognostication in idiopathic pulmonary fibrosis. Eur Respir J 2012;39:359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beernaert K, Cohen J, Deliens L, et al. : Referral to palliative care in COPD and other chronic diseases: A population-based study. Respir Med 2013;107:1731–1739 [DOI] [PubMed] [Google Scholar]
- 28. Traue DC, Ross JR: Palliative care in non-malignant diseases. J R Soc Med 2005;98:503–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raghu G, Amatto VC, Behr J, Stowasser S: Comorbidities in idiopathic pulmonary fibrosis patients: A systematic literature review. Eur Respir J 2015;46:1113–1130 [DOI] [PubMed] [Google Scholar]
- 30. Magnani D, Lenoci G, Balduzzi S, et al. : Effectiveness of support groups to improve the quality of life of people with idiopathic pulmonary fibrosis a pre-post test pilot study. Acta Biomed 2017;88:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lindell KO, Nouraie M, Klesen MJ, et al. : Randomised clinical trial of an early palliative care intervention (SUPPORT) for patients with idiopathic pulmonary fibrosis (IPF) and their caregivers: Protocol and key design considerations. BMJ Open Respir Res 2018;5:e000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.