Abstract
Anthropogenic change is expected to alter environments at alarming rates. To predict the impact of modified environments on social behavior, we must study the relationship between environmental features and collective behavior in a genetically tractable model, zebrafish (Danio rerio). Here, we conducted a field study to examine the relationship between salient environmental features and collective behavior in four populations of zebrafish. We found zebrafish in flowing water formed volatile groups, whereas those in still water had more consistent membership and leadership. Groups in fast-flowing water were large (up to 2000 fish) and tightly knit with short nearest neighbor distances, whereas group sizes were smaller (11 fish/group) with more space between individual fish in still and slow-flowing water. These observations point to a possible profound role of water flow in influencing collective behavior in wild zebrafish.
Keywords: collective behavior, zebrafish, water flow, vegetation, environment, field study
Introduction
Anthropogenic change is on the rise, and expected to radically change landscapes, and consequently alter behavioral ecology.1,2 Physical environmental features can limit or facilitate social interactions by establishing barriers between group members, creating passages that connect disparate groups, or augment interindividual interactions with consequences for movement,3,4 huddling energetics,5 cohesion,6 polarity,7 aggression,8 group stability,9,10 information transmission,11 and evolution (e.g., allopatric speciation).12,13 Some of the environmental features that are most susceptible to anthropogenic change are water and vegetation.14,15 Hydrological shifts driven by human alteration of stream flow (e.g., dams and water diversions), agriculture activity, and natural and human-induced climate shifts result in changes in flow, water chemistry, pollution, and other physical properties.16–19 Vegetation is also changing owing to human activity such as deforestation and agriculture with effects on habitat cover and flow.20,21 Identifying which environmental features have the strongest effect on behavior is important for determining mechanisms, conducting risk assessments, and developing intervention plans. In this study, we document the relationship between environmental features and collective behavior across different zebrafish populations.
Flow has important impacts on grouping, positioning, and type of movement exhibited by animals. Water flow influences behavior in important ways imposing energetic constraints and facilitating movement.22–25 Fast-turbulent flows may lead to avoidance and dispersion, whereas more predictable flows may induce clustering and alignment where group members can minimize energetic output.26–30 Flow-dependent differences in aggregation are observed in guppies (Poecilia reticulate) and chub (Leuciscus cephalus) that form larger shoals in flowing than in still water.31,32 In response to different flow patterns, groups may adopt specific geometries such as bird flocks creating a V-formation,33 hill-shaped aggregations in barnacles (Semibalanus balanoides),34 fish forming long planar schools in fast-flowing water,35 or echelon formations in bowhead whales (Balaena mysticetus).36
To gain energetic advantages in flow conditions, group members may change positions depending on flow and vegetation cover,37 or adopt specific spatial positions depending on intrinsic qualities such as aerobic capacity,38 tail beat frequency,39 body size,40 or group properties.41 In this study, we assess how water flow relates to collective behavior in wild zebrafish, and we predict fish in flowing water will be more cohesive, and form larger groups as they aggregate to obtain hydrodynamic advantage, than fish in still water. Turbulence in faster flowing water may serve as a force that separates group members leading to greater fission–fusion than those groups occupying still and slower flowing conditions.
Physical obstructions and vegetation can also influence collective behavior by introducing structural complexity and turbulence. Environmentally complex habitats may support larger group sizes owing to more food availability,42 or animals may disperse setting up territories around landmarks to defend key resources.43 Enhanced environmental complexity may lead to frequent fission–fusion as individuals assess the need to disperse and take refuge in vegetation cover or remain in a school in the presence of predators,44 and deforestation may increase or decrease collective antipredatory responses because of changes in perceived risk.45 Such physical obstructions may have consequences for leadership and spatial position,46,47 as the presence of physical barriers leads to denser social networks,11,48 and manmade obstructions enhance reliance on socially transmitted information.11,49 Vegetation may buffer groups from the onslaught of fast flow, thereby alleviating the need for hydrodynamic formations, or vegetation can increase turbulence necessitating the adoption of energy saving group formations. Here, we assess how vegetation relates to collective behavior in wild zebrafish, and we predict that vegetation will exacerbate the effects of fast flow, increasing the volatility of groups, as they navigate around obstacles, but buffering groups from turbulence in slower flowing waters.
Wild zebrafish have been previously studied with some documentation of habitat measures,50–53 diet,54,55 and social behavior.56 In these field studies there was evidence of zebrafish habitat degradation, and a call to document natural zebrafish behavior in these habitats to inform the study of this important biomedical model. To test the effects of environmental features on collective behavior, we examined collective behavior in wild zebrafish at four sites in India that differ in water flow and vegetation. We compared the range of habitats, microhabitats, and the variation in leadership, nearest neighbor distance, fission–fusion, and group size at each of the sites.
Method
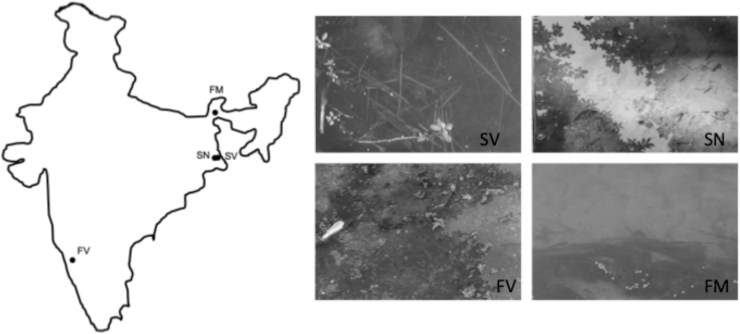
We conducted a field study in the postmonsoon season from November 2017 to January 2018, choosing four field sites representing different water flow and surface vegetation (Fig. 1). Microhabitat measures at these sites were previously described,56,57 and to give a representation of the sites during the present sampling period, we redescribe the microenvironments with additional physical parameters.
FIG. 1.
Map of study area (India) with sampling locations (black circles), and representative images and abbreviations for the study sites. FM, flowing, mixed vegetation; SN, still, nonvegetated; SV, still, vegetated; FV, flowing, vegetated. Color images can be seen at (https://youtu.be/HRF9E9ezE7Y).
We sampled zebrafish in two still water sites in West Bengal: a ditch adjacent to a rice-paddy field with mature in-water vegetation and no canopy cover allowing for full sun light and minimal manmade debris (SV: still, vegetated) and along a scantly vegetated, densely canopy-covered, low-light penetrated, highly littered irrigation canal (SN: still, nonvegetated). SV and SN were within 2–3 h walking distance.
In addition, we measured zebrafish in two flowing streams. One site (FM: flowing, mixed vegetation) was a fast-moving river in West Bengal, northeast India (a tributary of the Torsa River in Cooch Behar), where we measured zebrafish along a mix of vegetated and nonvegetated edges that were exposed to full sunlight with no visible human-made materials. The other site was a slow-flowing outcrop with vegetated banks, patches of vegetation in the water, medium light, and minimal human-made objects: the Thunga River at Sringeri, Karnataka in the Western Ghats, Southern India (FV: flow, vegetated). At the Western Ghats site (FV), we sampled three shoals in the main stream, two shoal in a canal connected to the mainstream, and one in a stagnant pool not connected to the main stream, but similar to the other sites in all other physical parameters. At all sites, the water was clear, so that we could conduct real-time observations and video filming of the fish.
At each site, we began by locating zebrafish groups—any aggregation of zebrafish that was separated by other zebrafish by at least 0.5 m. We then conducted observations at each of the identified sites. Zebrafish did not appear to respond to the observer, who in all cases took precautions not to disturb the fish (e.g., not making sudden movements). We collected behavioral data from a total of 57 zebrafish groups: 12 groups in SN, 7 in SV, 12 in FM, and 6 FV. For the groups at FM site, we obtained fission–fusion frequencies and group size measurements from 10 additional groups, and only group size measurements from 10 more groups. Most of the groups at the FM site (8 of them) were in areas that were free of algal patches, whereas only a small subset of the groups (n = 4) were in algal patches. Sample sizes for the measured variables differed because we were not always able to record data for every group accurately. We did not measure body sizes of zebrafish, because these have been reported previously,56 and can vary considerably with age and diet.58,59
For each group, we recorded estimates of Group Size, fission–fusion frequency, change in leadership frequency, and nearest neighbor distance. We sampled fission–fusion frequency for up to 1 min in real time, scoring fission–fusion frequency as the number of times a zebrafish strayed at least 5 body lengths from the group and then rejoined. We considered a change in leadership when the identity of the frontal fish changed. To obtain group size and nearest neighbor estimates, we counted fish both in real time and from videos, taken from above with a GoPro Hero 5 Session or Cannon Vixia HF R800 camera at a 1080 pixel resolution and optical zoom up to 57 × (e.g., Vid. https://youtu.be/4xDzelTpN_M).
For video-recorded groups that were larger than the field-of-view of the camera, we stitched still frames of the group together using Huggins software (2019.2.0.b690aa0334b5 built by Niklas Mischkulnig originally developed by Pablo D'Angelo © 2004–2019). By toggling the video back-and-forth, we could identify the position of the fish. Once we identified the position of a fish, we would place a point on the fish. We then counted those points to get an estimate of group size. In ImageJ 1.46q (Java 1.6.0_65, 32 bit), we measured a subset (at least three) of the fish and averaged them to get an average fish body length (in pixels) for the group. We then used this average body length to calibrate the distance between neighbors of a focal fish, which served as our two-dimensional nearest neighbor distance.
For real-time estimates of very large groups (the FM site at the Torsa River in West Bengal), we scanned the shoal identifying reference points to place four markers that indicate start and end of the shoal. We measured these markers to get shoal length and width and calculated the shoal surface area. From our video recordings, we obtained average fish densities for the shoals that we then extrapolated to calculate group size. Other field studies have used similar visual assessment methods to estimate fish group sizes accurately.56,60–62
Zebrafish in our samples remained near the surface of the water, such that our above-surface observations and two-dimensional photographs likely represent the spatial distribution of the group accurately. Zebrafish do move in three dimensions as seen in our underwater video (e.g., vid. https://youtu.be/YFjgoI47u_o), and that of another recent article,50 however; in laboratory settings, many studies assess zebrafish in shallow water to restrict their movement to two dimensions6,8 and assessing the three-dimensional movements of zebrafish accurately is technically challenging in the laboratory and field. These points led us to focus our sampling on groups that were in shallow, clear water. For real-time observations that required observers to assess distances between fish (e.g., NND, fission), we practiced estimating distances between objects in the water bodies, and then measuring them to calibrate our visual assessments, a proven method to enhance the consistency and accuracy of visual assessments of distance, fish body lengths, and nearest neighbor distances in the field.63,64
To ensure accuracy of our real-time behavioral assessments, all observers were previously trained until an interrater reliability of ∼90% was achieved. In addition, we compared a subset of the real-time observations with behavior coded from the video recordings to ensure there was congruency between these sampling approaches.
After each observation period, we also recorded microhabitat parameters at up to 12 locations at each site, representing the areas in which we recorded behavior of that zebrafish group. We assessed flow rate and vegetation cover measures using the techniques described previously.56 We also measured light using a LUTK-147 digital lux meter, and documented the presence or absence of canopy cover and human-made waste (or pollution). We did not get light and water chemistry measurements at the FV (Western Ghats) site, because of technical difficulties. These measurements allowed us to obtain a representation of the microhabitat of the zebrafish during the observation period. In addition, at each of the four sites (varying in water flow and vegetation), we sampled water quality at two to three representative locations. We used Tetra Test Strips™ to estimate ammonia, nitrate, nitrite, hardness, chlorine and Ph levels. We also used a HDE thermometer to measure temperature.
We used two-way analyses of variance (ANOVAs) with water flow (still or flowing) and vegetation (high or low/mixed) as factors to identify significant differences in behavior, microhabitat, and water quality across the four sites. We fit the ANOVA models using the “aov” function followed by “Anova” type III sum of squares functions in the “car” package65 for unbalanced ANOVAs in R,66 examining the residuals to determine whether log transformations were needed. When log transformations were not sufficient, we used Wilcoxon nonparametric tests to test the effects of vegetation and flow separately. We also used principal components analysis (with the Base R “prcomp” function and scaling the variables to account for unit variance) to examine relationships between behavioral and habitat measures.
Results
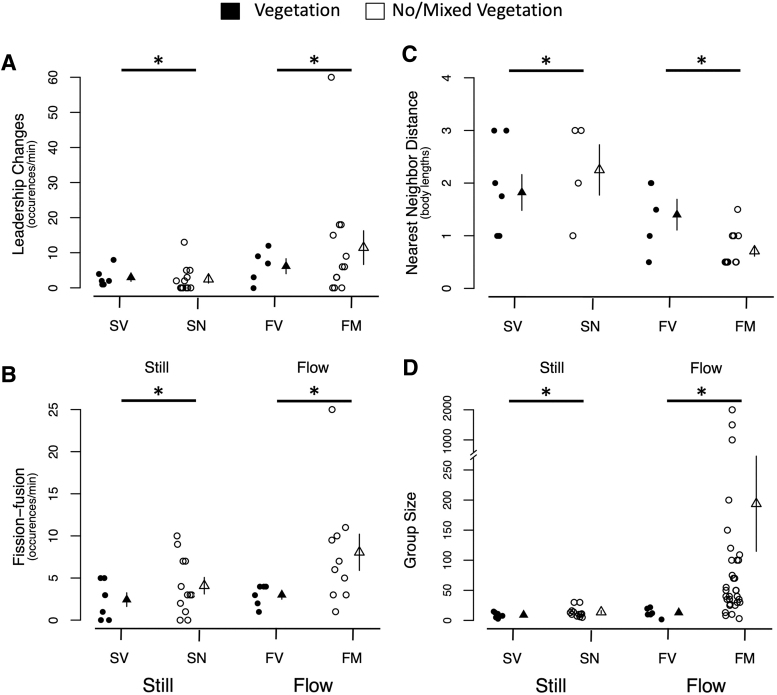
Zebrafish in flowing water exhibited two times more fission–fusions and two to three times more changes in leadership than did groups in still water (Fig. 2A, B). Zebrafish in still water formed groups that were relatively stable with 3.5 ± 0.70 fission–fusions per minute and 2.7 ± 0.80 frontal fish changes per minute. Zebrafish in faster flowing water (M = 8.0 ± 2.15 fission–fusions/min) were more volatile with greater than twice the fission–fusion frequency as populations in slower flowing water (M = 3.0 ± 0.52 fission–fusions/min; F1,31 = 4.6, p = 0.04; Fig. 2B). Similarly, zebrafish in faster flowing water (M = 11.5 ± 4.81 and 6.2 ± 2.13 leadership changes/min) experienced at least twice as many frontal position changes as populations in slower flowing water (M = 2.5 ± 1.10 and 3.0 ± 1.10; F1,31 = 4.5, p = 0.04). The pattern is the same when the highest value in changes/min is removed (F1,30 = 4.7, p = 0.04).
FIG. 2.
Water flow, but not vegetation is a predictor of collective behavior. (A) Groups in flowing water changed leadership more than groups in still water. We found no evidence of an interaction between vegetation and flow or a main effect of vegetation in our two-way ANOVA. (B) Groups in flowing water fission–fusion more than groups in still water. There was not a significant of main effect of vegetation or an interaction between vegetation and flow. (C) Groups in fast-flowing water with minimal vegetation (FM) were more cohesive than the other groups, which led to a significant vegetation by flow interaction, and main effect of flow. The main effect for vegetation did not reach significance. (D) Groups in fast-flowing, minimal-vegetation water are significantly larger than all other populations, which led to a main effect of flow. The interaction between vegetation and flow and the main effect of vegetation were not significant. Individual samples are represented by filled circles (●) for shoals in vegetated areas, and open circles (○) for shoals in nonvegetated or mixed habitats represent individual samples. Triangles group means are represented by filled triangles (▴) for shoals in vegetated areas, and open triangles (▵) for shoals in nonvegetated or mixed habitats. Error bars indicate one standard error. *P < 0.05. SV and SN were scored digitally and in real time, but data points from real-time observations are displayed. FV was scored in real-time. FM was scored in video and real time, and data from digital and real-time observations are presented. ANOVA, analysis of variance.
The presence of vegetation had little impact on fission–fusion or leadership changes. Neither the main effect of vegetation (fission–fusion: F1,31 = 0.6, p = 0.43; leadership changes: F1,31 = 0.01, p = 0.92) nor the interaction between vegetation and flow (fission–fusion: F1,31 = 1.2, p = 0.27; leadership changes: F1,31 = 0.6, p = 0.45) were statistically significant.
Zebrafish in fast-flowing water (FM) had nearest neighbor distances that were almost three times smaller than did groups in all other populations (Fig. 2C). Groups in slow-flowing and still water with and without vegetation had similarly loose groups. This difference led to a significant flow × plants interaction effect (F1, 25 = 6.7, p = 0.02). The large difference between the fast-flowing water with mixed vegetation and the other populations likely drives the main effect of flow (F1,25 = 13.2, p < 0.01).
Zebrafish in still water formed groups of 3–30 fish with an average of 11.7 ± 1.70 fish per group similar to groups in slower flowing water (M = 12.7 ± 3.0). Surprisingly, in the fast-flowing Torsa River tributary (FM site), we documented groups of up to 2000 fish. The larger groups sizes in flowing water led to a main effect of flow (F1,53 = 22.9, p < 0.01; Fig. 2D). Fish at sites with less vegetation also had larger group sizes than their more vegetated counterparts, but the flow × vegetation interaction effect (F1,53 = 3.4, p = 0.07) and the main effect of vegetation (F1,53 = 0.8, p = 0.43) did not reach significance. Zebrafish in these very large groups also moved together in a synchronized manner, exhibiting strong positive rheotaxis as fish oriented with their heads toward the flow–forming long, torpedo -shaped schools. The larger, synchronized schools of zebrafish moved in and out of vegetative patches, whereas the more loosely organized zebrafish groups of the FM site (Torsa river) were found in vegetative patches that were buffered from the faster flowing water. We did not observe rheotaxis or synchronized movement at any of the other three sites.
We explored relationships among behavioral and microhabitat measures with principal components analysis. We found that our results could be described sufficiently by three composite variables, which together explained 81% of the variation. About a third of the variation could be explained by shoals in fast-flowing water having large group sizes and short nearest neighbor distances, with PC1 explaining 34% of the variation and the major loadings being Flow Rate (0.47), Nearest Neighbor Distance (−0.46), and Group Size (0.41). A quarter of the variation was explained by shoals from sites with warm water and vegetation exploring less fish in other sites. That is, PC2 accounted for 25% of the variation with highest loadings on Vegetation Cover (0.54), Temperature (0.53), and Fission–Fusion (−0.52). Finally, PC3 explained 21% of the variation with heaviest loadings in Group Size (0.58) and Leadership Changes (0.76), as larger groups experienced a higher frequency of leadership changes.
Sites differed in flow, vegetation, temperature, and human debris, but had similar water quality
As expected, the sites differed in water flow. In the still-water sites (SV, SN) the flow was negligible. Flow was moderate (5.9 ± 0.82 cm/s) in the vegetated, slow-moving stream in Southern India (FV), and triple the speed (16.1 ± 8.12 cm/s) in the fast-flowing stream in Eastern India (FM). This difference in flow rate between still- and flowing-water sites was statistically significant (Wilcoxon test W = 24, p << 0.01). Similarly, surface vegetation cover averaged ∼21% in the vegetated sites (SV, FV), but was negligible in the nonvegetated still-water site (SN). In the fast-moving stream (FM), we observed zebrafish along both vegetated (28% surface vegetation in algal patches) and nonvegetated areas. This difference in percent vegetation cover between vegetated (SV and FV) and open water (SN and FM) sites was statistically significant (Wilcoxon test W = 28.5, p = 0.02).
The sites differed also in the presence of human debris. We documented plastic bottles, plates, and pots in SN and FV, but did not see any manmade waste in FM and SV. The sites also differed in vegetation that could provide shade and thus reduce the amount of available light. with the SN site having a dense canopy that reduced the light to 180 lux, whereas the vegetation at the SV provided less shade exposing the groups to more light 7732 lux. The north Indian site (FM) was free from tall, shading vegetation. The sites progressively decreased in temperature with latitude. The northern site (FM) was the warmest (24°C), followed by a 4°C drop in temperature in the mid-eastern sites (SN, SV), and even cooler water (18°C) in the southern most site. These differences led to a significant plant × flow interaction (F1, 33.58 = 18.1, p << 0.01).
All sites were similar in ammonia, nitrates, nitrites, hardness, chlorine, alkalinity, and pH, such that our two-way ANOVAs and Wilcoxon tests did not detect significant effects of water flow or vegetation on these aspects (Table 1).
Table 1.
Mean Values (SE) of the Habit Measures Recorded at Four Indian Sites
| SV | SN | FV | FM | |
|---|---|---|---|---|
| At zebrafish groups | ||||
| Flow rate (cm/s) | 0 (0.0) | 0 (0.0) | 6 (0.8) | 16 (8.1) |
| Vegetation cover (%) | 21 (16.2) | 0 (0.0) | 23 (2.0) | 28 (4.0) |
| General measures | ||||
| Temperature | 20 (0.8) | 19 | 18 (0.0) | 24 (0.4) |
| Alkalinity (ppm) | 120 (0.0) | 80 (40.0) | – | 180 (0.0) |
| Ph | 7.8 (0.00) | 7.4 (0.15) | 7.3 (0.06) | 7.8 (0.00) |
| Ammonia (ppm) | 0 (0.0) | – | – | 0.5 (0.00) |
| Hardness (ppm) | 300 (0.0) | 300 (0.0) | – | 300 (0.0) |
| Nitrate (ppm) | 0 (0.0) | 0 (0.0) | – | 0 (0.0) |
| Nitrite (ppm) | 0 (0.0) | 0 (0.0) | – | 0.5 (0.00) |
| Chloride (ppm) | 0 (0.0) | 0 (0.0) | – | 0 (0.0) |
| Human-made debris | No | Yes | Yes | No |
| Light (lux) | 7731.7 (5462.63) | 180 | – | – |
| Substrate | Mud | Mud | Gravel, mud | Mud, Silt |
| Latitude (°N) | 21.6 | 21.6 | 13.4 | 41.4 |
A dash (–) indicates not measured.
FM, flowing, mixed vegetation; SN, still, nonvegetated; SV, still, vegetated; FV, flowing, vegetated.
Discussion
We found that zebrafish collective behavior in the field was predicted by water flow, but not vegetation. Groups in flowing water were more volatile showing a higher frequency of fission–fusion and changes in leadership, than those in still water. Group size paralleled water flow with groups in fast-flowing water having larger group sizes than those in slower flowing and still water. In a fast-flowing river in West Bengal, we found zebrafish in polarized schools with all fish aligned in the same direction facing into the current as previously reported.56 These groups were massive with up to 2000 fish per group, which surpasses previous group size reports56,67 and is more aligned with other recent reports.50 We did not find any evidence that vegetation exacerbated the impact of flow, but zebrafish were more cohesive in the fast-flowing, mixed vegetation environment, whereas zebrafish at all other populations were similarly cohesive. These findings suggest that some environmental features are more related to collective behavior than others, and that anthropogenic and seasonal changes in water flow may have important impacts on social behavior.
Flow has the ability to facilitate movement and impede collective motion by serving as an energetic constraint. We saw that groups in flowing water were more volatile, experiencing more fission–fusion and leadership changes than those in still water, suggesting that flow was imposing energetic constraints on the fish. The volatility of the group may result from individuals competing for more advantageous spatial positions,68 the turbulent forces actively separating group members, or individuals balancing between propulsive efficiency and group stability.69 Flow may wash out chemical cues that enable groups to establish stable social organizations70 resulting in less stable dominance hierarchies than those in still water.71
The impact of flow may depend on the flow speed, with faster flows having more disruptive effects on collective behavior or enhancing grouping as individuals create hydrodynamic formations. Flow-velocity-dependent behavior was observed here with groups in faster flowing water showing greater volatility, enhanced cohesion, and larger group sizes than those in the slower flowing tributaries of Thunga River. Others have reported linear relationships between flow velocity and energetics72 and group size73 sometimes in opposite directions.32
In the fast-flowing water, large zebrafish groups formed long planar formations facing into the flow possibly to reduce the energetic constraints of the flow or to enhance vigilance, as previous studies reported higher predation pressure in the fast-flowing Torsa tributaries than in the other sites.56 Planar formations in flow are observed in many species,33,35,36,74 and the shape that groups assume may depend on the energetics demands of moving in the environment,75 size of neighbors,32 learning,76 amount of available space6 and other abiotic factors.77,78 In our field expedition, faster flows corresponded with larger groups sizes, and consequently, with all measured behavior. Instead of flow, group size alone, or group size may interact with flow to modulate collective behavior. To disentangle the effects of group size and flow on zebrafish collective behavior, these variables should be systematically manipulated.
Plants may buffer the effects of flow for some collective behavior, but not others. We found that in flowing water with vegetation, the groups had nearest neighbor distances similar to those of groups in still water, but no other observable behavioral effects. This finding suggests that vegetation buffers cohesion, but not other behavior from flow effects. Vegetation was not a strong predictor of collective behavior in wild zebrafish in the field, which contrasts with findings in laboratory-tested wild zebrafish.8,79 These differences may result from a shift in selection pressures because of vegetation loss or seasonal changes, as the sites had upwards of 40% less vegetation in 2017 than when the sites were sampled in 2014,56 and the seasonal monsoons produce enough rain to radically change the landscape and microhabitat of zebrafish.80,81 Although others reported that environmental complexity involving vegetation and obstructions alter social behavior,11,82 the effects may depend on temperature,83,84 predation pressure,44 vegetation prevalence,79 exposure duration,85 and genetic background.79,86,87 For example, exposure to obstacles had duration-dependent effects on cognition,85 and vegetation had area-dependent effects on the prevalence of age classes in wild mouse populations.88 Future studies may want to identify which aspects of vegetation alter collective behavior.
Animals can cope with human-induced and seasonal changes to habitat by altering their behavior.89 In the laboratory, zebrafish groups respond to variable environments by altering aggression, activity, cohesion, feeding, and sensory systems.8,79,90,91 These adjustments to environmental change may depend on which environmental features are modified. Zebrafish in the laboratory modified their feeding and cohesion in response to flow, but not vegetation,8 and vegetation routinely interacted with water flow to affect activity and aggression.56,79 At the field sites, we observed decreases in vegetation, increases in human-made objects, and landscape shifts. The landscape surrounding Torsa river changed considerably from 2014 to 2017 sampling period with erosion of river embankments, lower river depth, and wider tributaries perhaps because of sand mining and flooding that destroyed several homes along the river (personal communication with residents).92,93 Some features of the environment may have greater impact on behavior than others as did water flow compared with vegetation in this study. Identifying the effect of the environmental features may permit conservationists to detect aberrant effects tied to environmental modification, and direct resources and policies to restore and protect influential environmental features.
Understanding the influence of environmental and genetic factors on behavior is crucial, because zebrafish are becoming a significant model system in biomedical research including studies of drug discovery,94,95 toxicology,96,97 brain disorders,98,99 autism,100 personalized psychiatry,101 and metabolic pathologies.102,103 Although our field study has emphasized the importance of environmental factors in collective behavior, some studies point to a genetic basis and consistent strain differences for many aspects of collective behavior104 and other social behavior such as leadership,105 collective movement,106–108 and aggression—boldness.87,109 Genetics may also account for the observed patterns in collective behavior as the SV and SF show little genetic distinction, and differ greatly from the populations in northern West Bengal and southern India.57 To explore the importance of gene by environment interactions in zebrafish collective behavior, transplant experiments may serve as invaluable tool.110 Furthermore, these studies may help to rectify the challenges with generalizing results between laboratory and field observations by identifying the mechanisms that modulate behavior.111
This study has implications for the laboratory study of zebrafish behavior. Laboratory zebrafish research hinges on proper husbandry and developing ecologically relevant assays,111 especially for behavioral phenotyping prevalent in toxicology,97 drug discovery,94 neuroscience,98 genetic screening,112 and psychiatric research.101 Much effort is devoted to ensuring relevant shoal sizes, densities, temperature, water quality, and enrichment to meet basic husbandry and experimental needs.113 The prowess of zebrafish is leveraged on the ability to develop high-throughput and high-content screens for the model. Such screens are sometimes slowed by the potentially false trade-off between speed and ecological accuracy of zebrafish social behavior assessments in two or three dimensions. Our field study shows that while zebrafish do move in three dimensions, there are many shoals in distinct wild populations that spend some of their time in shallow water occupying a largely two-dimensional space. These field observations provide ecological validity for the two-dimensional study of zebrafish behavior.
In summary, we found zebrafish inhabiting locations that had been previously sampled, despite apparent environmental change. Evidence of anthropogenic and seasonal changes with reduced vegetation cover, riverbank erosion, and increased presence of human-made objects at all sites, might speak to the vulnerability or resilience of the zebrafish. We found that water flow, but not vegetation was a strong predictor of collective behavior in multiple populations of wild zebrafish. We report that groups are larger, more volatile with shorter nearest neighbor distances in flowing water than groups in still water. In the fast-flowing water, we documented the largest group sizes of zebrafish in the literature surpassing previous reports. Future studies should carefully manipulate environmental features and group size, and perform transplant experiments to identify the influence of environmental, social, and genetic factors on collective behavior. Such laboratory, mesocosm or field studies could occur by observing the fish in shallow water that restricts their movement to two dimensions, as these experimental parameters are ecologically relevant to wild zebrafish. Further investigating the effect of these factors on group behavior will enable a better understanding of the evolutionary pressures that affect collective behavior, and help predict how zebrafish will respond to future environmental change.
Acknowledgments
The authors thank Rubina Mondal, Aditya Ghoshal, Tamal Roy, Bappi, and several local fishermen for their hospitality and field assistance. The authors also thank Jens Krause, David Noakes, Michael Simonich, and members of the Tanguay laboratory for helpful discussions on planning the field expedition and for comments on early versions of this article.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Science Foundation NSF Postdoc Fellowship 1611616 (to D.S.S.) and through Grant IOS-1257562 (to E.P.M.).
References
- 1. Bullock JM, Bonte D, Pufal G, da Silva Carvalho C, Chapman DS, García C, et al. Human-mediated dispersal and the rewiring of spatial networks. Trends Ecol Evol 2018;33:958–970 [DOI] [PubMed] [Google Scholar]
- 2. Foley JA, DeFries R, Asner GP, et al. Global consequences of land use. Science 2005;309:570–574 [DOI] [PubMed] [Google Scholar]
- 3. He P, Maldonado-Chaparro AA, Farine DR. The role of habitat configuration in shaping social structure: a gap in studies of animal social complexity. Behav Ecol Sociobiol 2019;73:9 [Google Scholar]
- 4. Tucker MA, Böhning-Gaese K, Fagan WF, Fryxell JM, Van Moorter B, Alberts SC, et al. Moving in the Anthropocene: global reductions in terrestrial mammalian movements. Science 2018;359:466–469 [DOI] [PubMed] [Google Scholar]
- 5. Shelton DS, Meyer PM, Ocasio KM. Environmental structure and energetic consequences in groups of young mice. Physiol Behav 2017;177:155–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shelton DS, Price BC, Ocasio KM, Martins EP. Density and group size influence shoal cohesion, but not coordination in zebrafish (Danio rerio). J Comp Psychol 2015;129:72–77 [DOI] [PubMed] [Google Scholar]
- 7. Imada H, Hoki M, Suehiro Y, Okuyama T, Kurabayashi D, Shimada A, et al. Coordinated and cohesive movement of two small conspecific fish induced by eliciting a simultaneous optomotor response. PLoS One 2010;5:e11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suriyampola PS, Sykes DJ, Khemka A, Shelton DS, Bhat A, Martins EP. Water flow impacts group behavior in zebrafish (Danio rerio). Behav Ecol 2016:arw138 [Google Scholar]
- 9. Ang TZ, Manica A. Aggression, segregation and stability in a dominance hierarchy. Proc R Soc B Biol Sci 2010;277:1337–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sueur C, Deneubourg J-L, Petit O, Couzin ID. Group size, grooming and fission in primates: a modeling approach based on group structure. J Theor Biol 2011;273:156–166 [DOI] [PubMed] [Google Scholar]
- 11. Webster MM, Atton N, Hoppitt WJE, Laland KN. Environmental complexity influences association network structure and network-based diffusion of foraging information in fish shoals. Am Nat 2013;181:235–244 [DOI] [PubMed] [Google Scholar]
- 12. Butlin RK, Smadja CM. Coupling, reinforcement, and speciation. Am Nat 2017;191:155–172 [DOI] [PubMed] [Google Scholar]
- 13. Pyron RA, Costa GC, Patten MA, Burbrink FT. Phylogenetic niche conservatism and the evolutionary basis of ecological speciation. Biol Rev 2015;90:1248–1262 [DOI] [PubMed] [Google Scholar]
- 14. Fischer J, Lindenmayer DB. Landscape modification and habitat fragmentation: a synthesis. Glob Ecol Biogeogr 2007;16:265–280 [Google Scholar]
- 15. McDonald RI, Green P, Balk D, Fekete BM, Revenga C, Todd M, et al. Urban growth, climate change, and freshwater availability. Proc Natl Acad Sci USA 2011;108:6312–6317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Landrigan PJ, Fuller R, Acosta NJ, et al. The lancet commission on pollution and health. Lancet 2017;391:462–512 [DOI] [PubMed] [Google Scholar]
- 17. Barnett TP, Pierce DW, Hidalgo HG, Bonfils C, Santer BD, Das T, et al. Human-induced changes in the hydrology of the western United States. Science 2008;319:1080–1083 [DOI] [PubMed] [Google Scholar]
- 18. Brown AR, Gunnarsson L, Kristiansson E, Tyler CR. Assessing variation in the potential susceptibility of fish to pharmaceuticals, considering evolutionary differences in their physiology and ecology. Phil Trans R Soc B 2014;369:20130576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vörösmarty CJ, Pahl-Wostl C, Bunn SE, Lawford R. Global water, the anthropocene and the transformation of a science. Curr Opin Environ Sustain 2013;5:539–550 [Google Scholar]
- 20. Asbjornsen H, Goldsmith GR, Alvarado-Barrientos MS, et al. Ecohydrological advances and applications in plant–water relations research: a review. J Plant Ecol 2011;4:3–22 [Google Scholar]
- 21. Dosskey MG, Vidon P, Gurwick NP, Allan CJ, Duval TP, Lowrance R. The role of riparian vegetation in protecting and improving chemical water quality in streams. J Am Water Resour Assoc 2010;46:261–277 [Google Scholar]
- 22. Filella A, Nadal F, Sire C, Kanso E, Eloy C. Model of collective fish behavior with hydrodynamic interactions. Phys Rev Lett 2018;120:198101. [DOI] [PubMed] [Google Scholar]
- 23. Flierl GR, Grünbaum D, Levins S, Olson D. From individuals to aggregations: the interplay between behavior and physics. J Theor Biol 1999;196:397–454 [DOI] [PubMed] [Google Scholar]
- 24. Martin AP Phytoplankton patchiness: the role of lateral stirring and mixing. Prog Oceanogr 2003;57:125–174 [Google Scholar]
- 25. Petroff A, Libchaber A. Hydrodynamics and collective behavior of the tethered bacterium Thiovulum majus. Proc Natl Acad Sci USA 2014;111:E537–E545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flierl GR, Woods NW. Copepod aggregations: influences of physics and collective behavior. J Stat Phys 2015;158:665–698 [Google Scholar]
- 27. Gaylord B, Gaines SD. Temperature or transport? range limits in marine species mediated solely by flow. Am Nat 2000;155:769–789 [DOI] [PubMed] [Google Scholar]
- 28. Liao JC A review of fish swimming mechanics and behaviour in altered flows. Philos Trans R Soc B Biol Sci 2007;362:1973–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liao JC, Beal DN, Lauder GV, Triantafyllou MS. Fish exploiting vortices decrease muscle activity. Science 2003;302:1566–1569 [DOI] [PubMed] [Google Scholar]
- 30. McWhirter JL, Noguchi H, Gompper G. Flow-induced clustering and alignment of vesicles and red blood cells in microcapillaries. Proc Natl Acad Sci USA 2009;106:6039–6043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Allouche S, Gaudin P. Effects of avian predation threat, water flow and cover on growth and habitat use by chub, Leuciscus cephalus, in an experimental stream. Oikos 2001;94:481–492 [Google Scholar]
- 32. Hockley FA, Wilson CAME, Graham N, Cable J. Combined effects of flow condition and parasitism on shoaling behaviour of female guppies Poecilia reticulata. Behav Ecol Sociobiol 2014;68:1513–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hummel D Aerodynamic aspects of formation flight in birds. J Theor Biol 1983;104:321–347 [Google Scholar]
- 34. Pullen J, LaBarbera M. Modes of feeding in aggregations of barnacles and the shape of aggregations. Biol Bull 1991;181:442–452 [DOI] [PubMed] [Google Scholar]
- 35. Abrahams M, Colgan P. Fish schools and their hydrodynamic function: a reanalysis. Environ Biol Fishes 1987;20:79–80 [Google Scholar]
- 36. Fish FE, Goetz KT, Rugh DJ, Brattström LV. Hydrodynamic patterns associated with echelon formation swimming by feeding bowhead whales (Balaena mysticetus). Mar Mammal Sci 2013;29:E498–E507 [Google Scholar]
- 37. Svendsen JC, Eskesen AO, Aarestrup K, Koed A, Jordan AD. Evidence for non-random spatial positioning of migrating smolts (Salmonidae) in a small lowland stream. Freshw Biol 2007;52:1147–1158 [Google Scholar]
- 38. Killen SS, Marras S, Steffensen JF, McKenzie DJ. Aerobic capacity influences the spatial position of individuals within fish schools. Proc R Soc B Biol Sci 2012;279:357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Svendsen JC, Skov J, Bildsoe M, Steffensen JF. Intra-school positional preference and reduced tail beat frequency in trailing positions in schooling roach under experimental conditions. J Fish Biol 2003;62:834–846 [Google Scholar]
- 40. Ward Ashley J W., Schaerf Timothy M., Herbert-Read James E., Morrell Lesley, Sumpter David J. T., Webster Mike M. Local interactions and global properties of wild, free-ranging stickleback shoals. R Soc Open Sci 2017;4:170043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cattelan S, Griggio M. Within-shoal phenotypic homogeneity affects shoaling preference in a killifish. Biol Lett 2018;14:20180293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johnson DDP, Kays R, Blackwell PG, Macdonald DW. Does the resource dispersion hypothesis explain group living? Trends Ecol Evol 2002;17:563–570 [Google Scholar]
- 43. Suriyampola PS, Eason PK. The effects of landmarks on territorial behavior in a Convict Cichlid, Amatitlania siquia. Ethology 2015;121:785–792 [Google Scholar]
- 44. Orpwood JE, Magurran AE, Armstrong JD, Griffiths SW. Minnows and the selfish herd: effects of predation risk on shoaling behaviour are dependent on habitat complexity. Anim Behav 2008;76:143–152 [Google Scholar]
- 45. Hua F, Sieving KE. Understory avifauna exhibits altered mobbing behavior in tropical forest degraded by selective logging. Oecologia 2016;182:743–754 [DOI] [PubMed] [Google Scholar]
- 46. Kemp PS, Gessel MH, Williams JG. Seaward migrating subyearling chinook salmon avoid overhead cover. J Fish Biol 2005;67:1381–1391 [Google Scholar]
- 47. Kemp PS, Armstrong JD, Gilvear DJ. Behavioural responses of juvenile Atlantic salmon (Salmo salar) to presence of boulders. River Res Appl 2005;21:1053–1060 [Google Scholar]
- 48. Leu ST, Farine DR, Wey TW, Sih A, Bull CM. Environment modulates population social structure: experimental evidence from replicated social networks of wild lizards. Anim Behav 2016;111:23–31 [Google Scholar]
- 49. Jones TB, Aplin LM, Devost I, Morand-Ferron J. Individual and ecological determinants of social information transmission in the wild. Anim Behav 2017;129:93–101 [Google Scholar]
- 50. Sundin J, Morgan R, Finnøen MH, Dey A, Sarkar K, Jutfelt F. On the Observation of Wild Zebrafish (Danio rerio) in India. Zebrafish 2019;16:546–553 [DOI] [PubMed] [Google Scholar]
- 51. Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish 2007;4:21–40 [DOI] [PubMed] [Google Scholar]
- 52. Spence R, Fatema MK, Reichard M, et al. The distribution and habitat preferences of the zebrafish in Bangladesh. J Fish Biol 2006;69:1435–1448 [Google Scholar]
- 53. Bhat A Patterns in the distribution of freshwater fishes in rivers of Central Western Ghats, India and their associations with environmental gradients. Hydrobiologia 2004;529:83–97 [Google Scholar]
- 54. Spence R, Fatema MK, Ellis S, Ahmed ZF, Smith C. Diet, growth and recruitment of wild zebrafish in Bangladesh. J Fish Biol 2007;71:304–309 [Google Scholar]
- 55. McClure MM, McIntyre PB, McCune AR. Notes on the natural diet and habitat of eight danionin fishes, including the zebrafish Danio rerio. J Fish Biol 2006;69:553–570 [Google Scholar]
- 56. Suriyampola PS, Shelton DS, Shukla R, Roy T, Bhat A, Martins EP. Zebrafish social behavior in the wild. Zebrafish 2016;13:1–8 [DOI] [PubMed] [Google Scholar]
- 57. Whiteley AR, Bhat A, Martins EP, et al. Population genomics of wild and laboratory zebrafish (Danio rerio). Mol Ecol 2011;20:4259–4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Siccardi AJ, Garris HW, Jones WT, Moseley DB, D'Abramo LR, Watts SA. Growth and survival of zebrafish (Danio rerio) fed different commercial and laboratory diets. Zebrafish 2009;6:275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Castranova D, Lawton A, Lawrence C, Baumann DP, Best J, Coscolla J, et al. The effect of stocking densities on reproductive performance in laboratory zebrafish (Danio rerio). Zebrafish 2011;8:141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hankin DG, Reeves GH. Estimating total fish abundance and total habitat area in small streams based on visual estimation methods. Can J Fish Aquat Sci 1988;45:834–844 [Google Scholar]
- 61. Heggenes J, Brabrand Åg, Saltveit S. Comparison of three methods for studies of stream habitat use by young brown trout and Atlantic salmon. Trans Am Fish Soc 1990;119:101–111 [Google Scholar]
- 62. Isaak DJ, Ver Hoef JM, Peterson EE, Horan DL, Nagel DE. Scalable population estimates using spatial-stream-network (SSN) models, fish density surveys, and national geospatial database frameworks for streams. Can J Fish Aquat Sci 2016;74:147–156 [Google Scholar]
- 63. Dugatkin LA, Godin J-GJ. Predator inspection, shoaling and foraging under predation hazard in the Trinidadian guppy, Poecilia reticulata. Environ Biol Fishes 1992;34:265–276 [Google Scholar]
- 64. Yulianto I, Hammer C, Wiryawan B, Pardede ST, Kartawijaya T, Palm HW. Improvement of fish length estimates for underwater visual census of reef fish biomass. J Appl Ichthyol 2015;31:308–314 [Google Scholar]
- 65.Fox J, Weisberg S, Adler D, et al. 2012. Package ‘car.’ Vienna R Found Stat Comput. [Google Scholar]
- 66. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2015. www.R-project.org/ (Accessed September23, 2014) [Google Scholar]
- 67. Engeszer RE, Alberici Da Barbiano L, Ryan MJ, Parichy DM. Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Anim Behav 2007;74:1269–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Herskin J, Steffensen JF. Energy savings in sea bass swimming in a school: measurements of tail beat frequency and oxygen consumption at different swimming speeds. J Fish Biol 1998;53:366–376 [Google Scholar]
- 69. Li G, Kolomenskiy D, Liu H, Thiria B, Godoy-Diana R. On the energetics and stability of a minimal fish school. PLoS One 2019;14:e0215265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gonçalves-de-Freitas E, Teresa FB, Gomes FS, Giaquinto PC. Effect of water renewal on dominance hierarchy of juvenile Nile tilapia. Appl Anim Behav Sci 2008;112:187–195 [Google Scholar]
- 71. Sneddon LU, Hawkesworth S, Braithwaite VA, Yerbury J. Impact of environmental disturbance on the stability and benefits of individual status within dominance hierarchies. Ethology 2006;112:437–447 [Google Scholar]
- 72. Halsey LG, Wright S, Racz A, Metcalfe JD, Killen SS. How does school size affect tail beat frequency in turbulent water? Comp Biochem Physiol A Mol Integr Physiol 2018;218:63–69 [DOI] [PubMed] [Google Scholar]
- 73. Rieucau G, Fernö A, Ioannou CC, Handegard NO. Towards of a firmer explanation of large shoal formation, maintenance and collective reactions in marine fish. Rev Fish Biol Fish 2015;25:21–37 [Google Scholar]
- 74. Weihs D Hydromechanics of fish schooling. Nature 1973;241:290–291 [Google Scholar]
- 75. Ashraf I, Bradshaw H, Ha T-T, Halloy J, Godoy-Diana R, Thiria B. Simple phalanx pattern leads to energy saving in cohesive fish schooling. Proc Natl Acad Sci USA 2017;114:9599–9604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Verma S, Novati G, Koumoutsakos P. Efficient collective swimming by harnessing vortices through deep reinforcement learning. Proc Natl Acad Sci USA 2018;115:5849–5854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dai Longzhen, He Guowei, Zhang Xiang, Zhang Xing. Stable formations of self-propelled fish-like swimmers induced by hydrodynamic interactions. J R Soc Interface 2018;15:20180490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Murphy DW, Olsen D, Kanagawa M, King R, Kawaguchi S, Osborn J, et al. The three dimensional spatial structure of Antarctic krill schools in the laboratory. Sci Rep 2019;9:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bhat A, Greulich MM, Martins EP. Behavioral plasticity in response to environmental manipulation among Zebrafish (Danio rerio) populations. PLoS One 2015;10:e0125097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dubey VK, Sarkar UK, Pandey A, Sani R, Lakra WS. The influence of habitat on the spatial variation in fish assemblage composition in an unimpacted tropical River of Ganga basin, India. Aquat Ecol 2012;46:165–174 [Google Scholar]
- 81. Shukla R, Bhat A. Environmental drivers of α-diversity patterns in monsoonal tropical stream fish assemblages: a case study from tributaries of Narmada basin, India. Environ Biol Fishes 2017;100:749–761 [Google Scholar]
- 82. Murray GPD, Stillman RA, Britton JR. Habitat complexity and food item size modify the foraging behaviour of a freshwater fish. Hydrobiologia 2016;766:321–332 [Google Scholar]
- 83. Perez AU, Schmitter-Soto JJ, Adams AJ, Herrera-Pavón RL. Influence of environmental variables on abundance and movement of bonefish (Albula vulpes) in the Caribbean Sea and a tropical estuary of Belize and Mexico. Environ Biol Fishes 2019;102:1421–1434 [Google Scholar]
- 84. Martin DJ, Wasserman LJ, Dale VH. Influence of riparian vegetation on posteruption survival of coho salmon fingerlings on the west-side streams of Mount St. Helens, Washington. North Am J Fish Manag 1986;6:1–8 [Google Scholar]
- 85. Leger M, Paizanis E, Dzahini K, Quiedeville A, Bouet V, Cassel JC, et al. Environmental enrichment duration differentially affects behavior and neuroplasticity in adult mice. Cereb Cortex 2015;25:4048–4061 [DOI] [PubMed] [Google Scholar]
- 86. Martins EP, Bhat A. Population-level personalities in zebrafish: aggression-boldness across but not within populations. Behav Ecol 2014;25:368–373 [Google Scholar]
- 87. Wright D, Rimmer LB, Pritchard VL, Butlin RK, Krause J. Inter and intra-population variation in shoaling and boldness in the zebrafish (Danio rerio). J Fish Biol 2003;63:258–259 [DOI] [PubMed] [Google Scholar]
- 88. Nupp TE, Swihart RK. Effect of forest patch area on population attributes of white-footed mice (Peromyscus leucopus) in fragmented landscapes. Can J Zool 1996;74:467–472 [Google Scholar]
- 89. Wong BBM, Candolin U. Behavioral responses to changing environments. Behav Ecol 2015;26:665–673 [Google Scholar]
- 90. Suriyampola PS, Cacéres J, Martins EP. Effects of short-term turbidity on sensory preference and behaviour of adult fish. Anim Behav 2018;146:105–111 [Google Scholar]
- 91. Sykes DJ, Suriyampola PS, Martins EP. Recent experience impacts social behavior in a novel context by adult zebrafish (Danio rerio). PLoS One 2018;13:e0204994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Padmalal D, Maya K, Sreebha S, Sreeja R. Environmental effects of river sand mining: a case from the river catchments of Vembanad lake, Southwest coast of India. Environ Geol 2008;54:879–889 [Google Scholar]
- 93. Saviour MN Environmental impact of soil and sand mining: a review. Int J Sci Environ Technol 2012;1:125–134 [Google Scholar]
- 94. Lam P-Y, Peterson RT. Developing zebrafish disease models for in vivo small molecule screens. Curr Opin Chem Biol 2019;50:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Peterson EK, Buchwalter DB, Kerby JL, LeFauve MK, Varian-Ramos CW, Swaddle JP. Integrative behavioral ecotoxicology: bringing together fields to establish new insight to behavioral ecology, toxicology, and conservation. Curr Zool 2017;63:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dai Y-J, Jia Y-F, Chen N, Bian WP, Li QK, Ma YB, et al. Zebrafish as a model system to study toxicology. Environ Toxicol Chem 2014;33:11–17 [DOI] [PubMed] [Google Scholar]
- 97. Tanguay RL The rise of zebrafish as a model for toxicology. Toxicol Sci 2018;163:3–4 [DOI] [PubMed] [Google Scholar]
- 98. Kalueff AV, Stewart AM, Gerlai R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci 2014;35:63–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Stewart AM, Braubach O, Spitsbergen J, Gerlai R, Kalueff AV. Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci 2014;37:264–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Meshalkina DA, N. Kizlyk M, V. Kysil E, Collier AD, Echevarria DJ, Abreu MS, et al. Zebrafish models of autism spectrum disorder. Exp Neurol 2018;299:207–216 [DOI] [PubMed] [Google Scholar]
- 101. Volgin AD, Yakovlev OA, Demin KA, de Abreu MS, Alekseeva PA, Friend AJ, et al. Zebrafish models for personalized psychiatry: insights from individual, strain and sex differences, and modeling gene x environment interactions. J Neurosci Res 2019;97:402–413 [DOI] [PubMed] [Google Scholar]
- 102. Gut P, Reischauer S, Stainier DYR, Arnaout R. Little fish, big data: zebrafish as a model for cardiovascular and metabolic disease. Physiol Rev 2017;97:889–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Seth A, Stemple DL, Barroso I. The emerging use of zebrafish to model metabolic disease. Dis Model Mech 2013;6:1080–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Séguret Axel, Collignon Bertrand, Halloy José. Strain differences in the collective behaviour of zebrafish (Danio rerio) in heterogeneous environment. R Soc Open Sci 2016;3:160451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Judge TA, Bono JE, Ilies R, Gerhardt MW. Personality and leadership: a qualitative and quantitative review. J Appl Psychol 2002;87:765. [DOI] [PubMed] [Google Scholar]
- 106. Greenwood AK, Mills MG, Wark AR, Archambeault SL, Peichel CL. Evolution of schooling behavior in threespine sticklebacks is shaped by the Eda gene. Genetics 2016;203:677–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Robison BD, Rowland W. A potential model system for studying the genetics of domestication: behavioral variation among wild and domesticated strains of zebra danio (Danio rerio). Can J Fish Aquat Sci 2005;62:2046–2054 [Google Scholar]
- 108. Wright D, Nakamichi R, Krause J, Butlin RK. QTL analysis of behavioral and morphological differentiation between wild and laboratory zebrafish (Danio rerio). Behav Genet 2006;36:271–284 [DOI] [PubMed] [Google Scholar]
- 109. Moretz JA, Martins EP, Robison BD. Behavioral syndromes and the evolution of correlated behavior in zebrafish. Behav Ecol 2007;18:556–562 [Google Scholar]
- 110. Nooten SS, Andrew NR: Transplant experiments–a powerful method to study climate change impacts. In: Global Climate Change and Terrestrial Invertebrates. Johnson SN and Jones TH (eds), pp. 46–67, John Wiley & Sons, Ltd., New York City, NY, 2016 [Google Scholar]
- 111. Parichy DM Advancing biology through a deeper understanding of zebrafish ecology and evolution. eLife 2015;4:e05635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gerlai R, Poshusta TL, Rampersad M, Fernandes Y, Greenwood TM, Cousin MA, et al. Forward genetic screening using behavioral tests in zebrafish: a proof of concept analysis of mutants. Behav Genet 2017;47:125–139 [DOI] [PubMed] [Google Scholar]
- 113. Aleström P, D'Angelo L, Midtlyng PJ, Schorderet DF, Schulte-Merker S, Sohm F, et al. Zebrafish: housing and husbandry recommendations. Lab Anim 2019. 10.1177/0023677219869037 [DOI] [PMC free article] [PubMed]