Abstract
Background: Indeterminate categories of thyroid cytopathology (categories B-III and B-IV of the Bethesda system) are integrated by a heterogeneous spectrum of cytological scenarios that are generally clustered for analysis and management recommendations. It has been suggested that aspirates exhibiting nuclear atypia have a higher risk of malignancy. This study aimed to assess whether cytologically indeterminate thyroid nodules with nuclear atypia have a significantly higher cancer risk than those without nuclear atypia.
Methods: On June 30, 2016, PubMed and EMBASE were searched for articles in English or Spanish using a search strategy developed by an endocrinologist and a librarian. Case reports were excluded, and no date limits were used. The references of all included studies were also screened for relevant missing studies. Studies were included if the prevalences of malignancy of cytologically indeterminate thyroid nodules with histological confirmation with and without nuclear atypia were reported. Studies were excluded if they had: (i) nodules suspicious for malignancy; (ii) nodules with non-indeterminate (B-III or B-IV) cytology on repeated biopsy, if performed; (iii) nodules not consecutively evaluated; or (iv) cohorts overlapping with another larger series. Two investigators independently assessed the eligibility and risk of bias of the studies. PRISMA and MOOSE guidelines were followed. Summary data were extracted from published reports by one investigator and independently reviewed by another. Data were pooled using a random-effects model. Heterogeneity was explored using subgroup analysis and mixed-effect model meta-regression. The odds ratio for malignancy of cytologically indeterminate thyroid nodules with nuclear atypia over cytologically indeterminate thyroid nodules without nuclear atypia was calculated.
Results: Of 2571 retrieved studies, 20 were eligible. The meta-analysis was conducted on summary data of 3532 cytologically indeterminate thyroid nodules: 1162 with and 2370 without nuclear atypia. The odds ratio for malignancy in cytologically indeterminate thyroid nodules with nuclear atypia was 3.63 [confidence interval 3.06–4.35]. There was no evidence of publication bias, and heterogeneity was insignificant (I2 < 0.01%, p = 0.40).
Conclusions: Nuclear atypia is a significant indicator of malignancy in cytologically indeterminate thyroid nodules and needs to be standardized and implemented into clinical practice.
Keywords: : atypia of undetermined significance or follicular lesion of undetermined significance, follicular neoplasm or Hürthle cell neoplasm, thyroid carcinoma, noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), thyroid cytology
Introduction
During the last decade, several classification systems have been developed to standardize the reporting of thyroid cytopathology, aiming to improve: (i) the cytology–histology correlation; (ii) communication between pathologists and clinicians; and (iii) sharing information between institutions for research purposes (1–4). In 2007, The Bethesda System For Reporting Thyroid Cytopathology (Bethesda) was developed in the United States, and its use has been recommended by the American Thyroid Association (ATA) since 2009 when the classification was published (1,5,6). This system classifies indeterminate cytology results into three categories: atypia/follicular lesion of undetermined significance (AUS/FLUS), follicular/Hürthle cell neoplasm (FN/HCN), and suspicious for malignancy (1). Indeterminate categories are integrated by a heterogeneous group of cytological scenarios, which are particularly diverse in the AUS/FLUS category, that are generally clustered for analysis and management recommendations (1). Moreover, there is significant inter- and intra-observer variability due to broad overlap in the diagnostic criteria of these categories (7–9).
It was recently found that the risk of cancer of cytologically indeterminate thyroid nodules (ITNs, AUS/FLUS, and FN/HCN) was better stratified when different cytological scenarios were analyzed separately (10). Among these, nodules exhibiting nuclear atypia had the highest risk of malignancy. This observation has been reported by other groups as well (11). However, nuclear atypia is not a generally accepted cancer risk factor. It was hypothesized that ITNs with nuclear atypia had a significantly higher risk of malignancy than other ITNs. A systematic review and meta-analysis were conducted on all observational studies in which the cancer risk of ITNs with and without nuclear atypia could be calculated. Therefore, the main objective of this study was to assess whether the presence of nuclear atypia in ITNs was associated with an increased cancer risk. The secondary aims were: (i) to analyze the risk of malignancy of nuclear atypia in AUS/FLUS and FN/HCN categories (or their equivalent in other classifications) separately; (ii) to calculate the prevalence of malignancy of ITNs with and without nuclear atypia, and within subcategories of ITNs without nuclear atypia: architectural atypia, oncocytic features, and other types of atypia (only in AUS/FLUS specimens); and (iii) to assess the association between the prevalence of malignancy of the indeterminate categories and the proportion of ITNs with nuclear atypia.
Patients and Methods
This systematic review and meta-analysis followed the PRISMA and MOOSE guidelines, and was not funded (12,13).
Search strategy and selection criteria
PubMed and EMBASE were systematically searched from inception up to and including June 30, 2016. The search strategy was developed by the investigators in consultation with a research librarian. Search terms included controlled vocabulary (MeSH) in various combinations with author-identified keywords, thyroid cytology terminology, and specified diagnostic terms (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy). The search results were limited to English and Spanish language publications. Additional filters regarding publication type were applied to the results from EMBASE (Supplementary Table S1). No date filters were applied. The references of the enrolled studies were manually screened. References that consisted of abstracts alone were not considered. Endnote X7 (Thomson Reuters) was used to compile and manage citations.
Only studies in which groups of ITNs (AUS/FLUS or FN/HCN or equivalent categories) with and without mild/focal nuclear atypia were clearly defined and separable for the analysis were included in the meta-analysis. Studies were excluded if: (i) the prevalence of malignancy of ITNs with and without nuclear atypia, based on histological outcomes only, could not be abstracted with the information provided in the manuscript (authors of studies were not contacted); or if the study included: (ii) ITNs with cytology equivalent to the “suspicious for malignancy” category of the Bethesda system; (iii) ITNs with non-indeterminate (AUS/FLUS or FN/HCN or equivalent categories) cytology on repeated biopsy, if performed; (iv) ITNs not consecutively evaluated, or selected based on specific histological outcomes; or (v) ITNs included in another larger and/or more detailed series.
Data extraction and risk of bias assessment
Two investigators assessed study eligibility independently. Summary data were extracted by one investigator and reviewed independently by a second investigator. Disagreements at any time during the process were discussed and resolved by consensus. Data from the authors' institutional retrospective study was also included in the meta-analysis (10). Information was extracted for the following variables: study design (prospective/retrospective; period of study; population; cytology/histology reviewed/not reviewed); country and institution of the study; definition of nuclear atypia; number of patients (males/females) and mean age; number of nodules and mean size; number of cytological subcategories used and included; number of nodules (total/benign/malignant) with and without nuclear atypia (when available, this information was also extracted individually for AUS/FLUS and FN/HCN; and for nodules with architectural atypia, oncocytic features, and other types of atypia); and resection rates of each group.
The risk of bias of each of the enrolled studies was assessed by two investigators independently using the quality assessment tool for observational cohort and cross-sectional studies of the National Heart, Lung, and Blood Institute (14). All disagreements were resolved by consensus.
Data synthesis and analysis
All studies meeting the inclusion criteria were used to assess the risk of cancer of ITNs with over ITNs without nuclear atypia. All data analysis was done using R v3.3.1 (The R Foundation for Statistical Computing, Vienna, Austria) with the packages metaphor and forestplot downloaded from the Comprehensive R archive network (15,16). Odds ratios (OR), positive (LR+) and negative (LR–) likelihood ratios, and prevalence of malignancy were calculated for each study. Random-effects models (without moderators) using the inverse variance method of weighing were used to estimate overall OR and prevalence of malignancy. Furthermore, univariate mixed-effects models (with study level moderators) were employed in an attempt to explain the observed heterogeneity in the prevalence of malignancy in the groups of nodules with and without nuclear atypia. Measures of heterogeneity such as I2 and DerSimonian-Laird estimator for τ2 were calculated. Clopper–Pearson confidence intervals were calculated for all OR, LR+, LR–, and prevalence estimates. Weighed correlations were used to assess the association between prevalence of malignancy and proportion of nodules with nuclear atypia. The input data for the meta-analysis are described in the above section. For each study the number of patients, number of nodules (by nuclear atypia status and malignant status), and prevalence of malignancy (overall, with and without nuclear atypia) were coded as counts or proportions.
To determine if the observed heterogeneity in the prevalence of malignancy could be explained by characteristics of the included studies; a post hoc meta-regression analysis was conducted. Due to the small number of studies, only univariate meta-regression was considered using mixed-effects models, each with the moderator as a fixed effect. Graphical displays include forest plots, funnel plots, and scatterplots. Subgroup analyses were done for AUS/FLUS and FN/HCN, and for cytological subcategories of ITNs without nuclear atypia (architectural atypia, oncocytic features, and other types of atypia).
Results
Study characteristics and risk of bias assessment
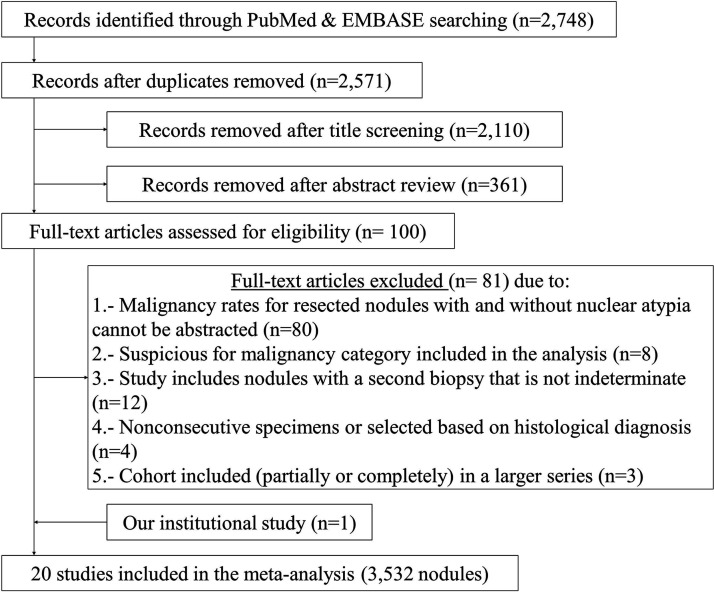
Of 2571 identified citations, after duplicates were removed through the literature search, 2471 were deemed ineligible following title and abstract screening. Of the 100 studies selected for full-text review, 19 met the inclusion criteria for the meta-analysis (Fig. 1). The exclusion criteria of the other 81 are detailed in Supplementary Table S2. Including the data from the authors' retrospective study, a total of 3532 ITNs were included in the meta-analysis (10,17–35). Only one study was prospective (27), and only one study was conducted on a pediatric population (Table 1) (26). Most studies (n = 12; 60%) had a fair or good (n = 7; 35%) quality rating in the risk of bias assessment, and only one (5%) was rated as poor (Supplementary Table S3). None of the studies provided sample size justification, power description, or variance and effects estimates (question #5), or evaluated key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure (nuclear atypia) and outcomes (cancer risk; question #14).
FIG. 1.
Systematic review of the literature and study selection process.
Table 1.
Demographic and Design Characteristics of Studies Meeting Inclusion Criteria
| First author and year of publication | Country | Goal of study | Dates | Population included | N patients/nodules | N groupsa (NA, w/o NA; FC, OF, OTA) | Classification methodb | Resection ratec (NA, w/o NA) % |
|---|---|---|---|---|---|---|---|---|
| Rago 2007 (17) | Italy | Find predictors of malignancy of “cold” FN/HCNs | 2002–2005 | FN/HCNs | 505/505 | 4 (2,2; 1,1,0) | Not specified | n/a |
| Somma 2010 (18) | United States | Find cytomorphologic predictors of malignancy in ITNs | Before 2006 | ITNs (“atypical”) | 99/99 | 2 (1,1; 0,0,0) | Cytology review (blinded to histology) | 40 (n/a,n/a) |
| Renshaw 2010 (19) | United States | Subcategorization of B-III for risk stratification | 1996–2009 | B-III | 204/204 | 4 (1,3; 1,1,1) | Cytology review | 37 (44,34) |
| Luu 2011 (20) | United States | Subcategorization of B-III for risk stratification | 2004–2009d | B-III | 127/127 | 2 (1,1; 0,0,0) | Per report | 57 (71,51) |
| Chung 2011 (21) | South Korea | Subcategorization and sonographic features of B-III for risk stratification | 2005–2010 | B-IIIe | 100/100 | 7 (2,5; 1,1,3) | Cytology review | n/a |
| Horne 2012 (22) | United States | Subcategorization of ITNs for risk stratification | 2008–2009 | ITNs (∼B-III)e | 58/58 | 2 (1,1; 0,0,0) | Per report | 34 (52,23) |
| Acioglu 2012 (23) | Turkey | Diagnostic efficiency of FNA in dominant nodules of MNG; and risk stratification of ITNs | 2006–2009 | ITNs within MNGe | 73/73 | 4 (2,2; 1,1,0) | Cytology review | n/a |
| Chiu 2012 (24) | Canada | Evaluate surgical management of ITNs and identify predictors of malignancy | 2000–2008 | ITNse | 325/325 | 3 (1,2; 1,1,0) | Not specified | n/a |
| Kleiman 2013 (25) | United States | Determine association between cytologic features and BRAFV600E mutation in ITNs | 2003–2012 | ITNse | 274/274 | 2 (1,1; 0,0,0) | Per report | n/a |
| Smith 2013 (26) | United States | Histological correlation of ITNs in children | 2007–2011 | B-IIIe | 25/25 | 3 (1,2; 0,0,1) | Not specified | 58 (47,65) |
| Rosario 2014 (27) | Brazil | Find predictors of malignancy in B-III | 2009–2013 | B-III on RFNAe | 73/73 | 2 (1,1; 0,0,0) | Prospective | 100 (100) |
| Ryu 2014 (28) | South Korea | Histological outcomes and predictors of malignancy for B-III | 2008–2012 | B-III with histology | 116/116 | 2 (1,1; 0,0,0) | Not specified | n/a |
| Walts 2014 (29) | United States | Find cytomorphologic predictors of malignancy in B-III | 2008–2012 | B-III | 127/127 | 2 (1,1; 0,0,0) | Per report | n/a |
| Pagni 2014 (30) | Italy | Subcategorization of ITNs for risk stratification | 2009–2012 | Tir3 | 57/57 | 3 (1,2; 1,1,0) | Cytology review | 71 (63,75) |
| Ustun 2014 (31) | United States | Subcategorization of B-IV for risk stratification | 2008–2012 | B-IV | 380/399 | 3 (1,2; 1,1,0) | Cytology review | 54 (n/a,n/a) |
| Mathur 2014 (32) | United States | Subcategorization of B-III for risk stratification and reproducibility assessment | 2009–2013 | B-III after reviewe | n/a/255 | 4 (1,3; 1,1,1) | Per report | 31 (n/a,n/a) |
| Onder 2014 (33) | Turkey | Frequency and malignancy of ITNs and subcategorization of B-III for risk stratification | 2009–2012 | B-IIIe | 103/103 | 4 (1,3; 1,1,1) | Not specified | 24 (24,25) |
| Wu 2014 (34) | United States | Subcategorization of B-III for risk stratification | 2002–2008 | B-III | n/a/138 | 4 (1,3; 1,1,1) | Cytology review (blinded to histology) | 21 (n/a,n/a) |
| Macias 2015 (35) | United States | Development of a risk model to predict malignancy in ITNs | 2003–2012 | B-IV | 151/151 | 2 (1,1; 0,0,0) | Per report | n/a |
| Valderrabano 2017 (10) | United States | Subcategorization of ITNs for risk stratification | 2008–2015 | B-III and B-IV | 297/323 | 4 (1,3; 1,1,1) | Cytology review (blinded to histology) | 63 (65,62) |
Number of subcategories used in the study. In parentheses: the number of those groups that were analyzed in the nuclear atypia (NA) and without-nuclear atypia (w/o NA) groups, and with the architectural atypia (AA), oncocytic features (OF), and other types of atypia (OTA) groups.
Method used to allocate the subcategories in the study.
Rate of resection of the indeterminate categories included in the meta-analysis. In parentheses: rate of resection of nodules with and without nuclear atypia. The overall rate of resection among the studies with this information available was 41%; 49% for nodules with nuclear atypia and 46% for nodules without nuclear atypia.
Period of study at University of Massachusetts Memorial Medical Center; the period of study at Rhode Island Hospital was from 2005 to 2008.
Only a fraction of the study cohort met inclusion criteria. Data provided in the table represent that fraction.
B-III, atypia/follicular lesion of undetermined significance (category III of the Bethesda system); B-IV, follicular/Hurthle cell neoplasm (category IV of the Bethesda system); AA, architectural atypia group; FN/HCN, follicular neoplasm/Hürthle cell neoplasm; ITNs, cytologically indeterminate thyroid nodules (includes categories equivalent to AUS/FLUS and FN/HCN of the Bethesda system only); MNG, multinodular goiter; NA, nuclear atypia group; n/a, not available; RFNA, repeat fine-needle aspiration biopsy; OF, oncocytic features group; OTA, other type of atypia group; Tir3, category 3 of the Italian consensus for the classification and reporting of thyroid cytology (equivalent to AUS/FLUS and FN/HCN categories of the Bethesda system); w/o NA, without nuclear atypia group.
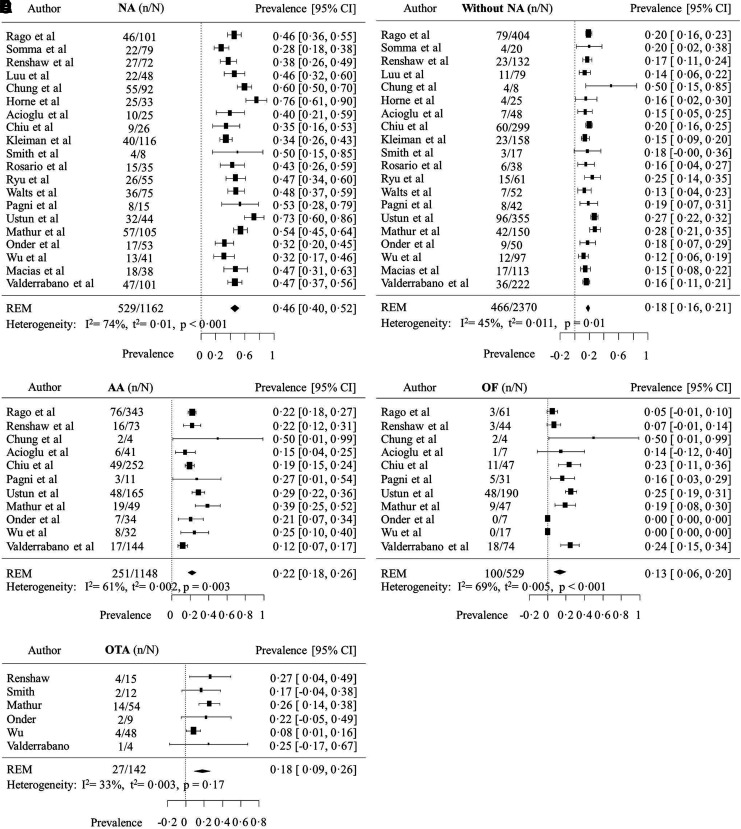
Most studies included in the meta-analysis were conducted in the United States (n = 12), although there were studies conducted in other North and South American institutions (n = 2), Europe (n = 2), and Asia (n = 4). Only one study was published before the Bethesda system was developed (17). Information was available for the subgroup analyses in 12 studies (1452 nodules) for AUS/FLUS; four studies (1252 nodules) for FN/HCN; 11 studies (1148 nodules) for architectural atypia; 11 studies (544 nodules) for oncocytic features; and six studies (142 nodules) for other types of atypia. One third (n = 1162) of all nodules included in the meta-analysis had nuclear atypia reported, whereas the other 67% (n = 2370) did not. Using a random-effects model, the expected prevalence of nuclear atypia among ITNs was calculated to be 40% overall [confidence interval (CI) 30–51%], 47% among AUS/FLUS specimens [CI 34–60%], and 21% among FN/HCN specimens [CI 13–29%]. Differences in the overall rate of resection of ITNs with and without nuclear atypia were not significant (49% vs. 46%; p = 0.22), but this information was not available in the majority of the studies.
Prevalence and risk of malignancy of ITNs
The overall risk of malignancy (OR) of ITNs with nuclear atypia over ITNs without nuclear atypia was 3.63 ([CI 3.06–4.35]; p < 0.0001). Similar results were found when the risk was calculated for AUS/FLUS (OR = 3.63; [CI 2.82–4.68]; p < 0.001) and FN/HCN specimens separately (OR = 4.38 [CI 3.19–5.19]; p < 0.001; Fig. 2). The heterogeneity of this risk measure was not significant (I2 < 0.01%; p = 0.40), and there was no evidence of publication bias based on Egger's test (p = 0.76). The overall LR+ was 2.13 [CI 1.86–2.44], and the overall LR– was 0.62 [CI 0.55–0.71]. Similar results were found when AUS/FLUS—LR +1.98 [CI 1.64–2.39] and LR– 0.48 [CI 0.39–0.59]—and FN/HCN specimens—LR +2.84 [CI 2.15–3.77] and LR– 0.73 [CI 0.6–0.89]—were analyzed separately.
FIG. 2.
Risk of malignancy associated with the presence of nuclear atypia. (A) Risk of malignancy of indeterminate thyroid nodules (ITNs) with nuclear atypia over ITNs without nuclear atypia. (B) Risk of malignancy of atypia/follicular lesion of undetermined significance (AUS/FLUS) specimens with nuclear atypia over AUS/FLUS specimens without nuclear atypia. (C) Risk of malignancy of follicular/Hürthle cell neoplasm (FN/HCN) specimens with nuclear atypia over FN/HCN specimens without nuclear atypia. (D) Funnel plots for publication bias analysis for (from left to right) all ITNs, AUS/FLUS specimens, and FN/HCN specimens. Note that the Bethesda system was not used in all the studies. For subgroup analysis, only those studies using Bethesda or equivalent categories were included. CI, confidence interval; NA, nuclear atypia; n, malignant cases; N, group size; OR, odds ratio; LR+, positive likelihood ratio; LR–, negative likelihood ratio; REM, random-effects model.
The adjusted overall prevalence of malignancy was 29% [CI 26–33%], and it was significantly higher (p < 0.0001) in ITNs with nuclear atypia (46% [CI 40–52%]) than in ITNs without nuclear atypia (18% [CI 16–21%]; Fig. 3). There was substantial heterogeneity in the prevalence of malignancy of these two groups between studies, particularly among nodules with nuclear atypia (I2 = 74%; p < 0.001). The overall prevalence of malignancy was not significantly different (p = 0.33) between cytological subcategories of ITNs without nuclear atypia: 22% [CI 18–26%]; 13% [CI 7–20%]; and 18% [CI 9–26%] for nodules with architectural atypia, oncocytic features, or other types of atypia, respectively.
FIG. 3.
Prevalence of malignancy of ITNs with and without nuclear atypia. Prevalence of malignancy of ITNs (A) with NA; (B) without NA; (C) with architectural atypia (AA); (D) oncocytic features (OF); and (E) other types of atypia (OTA).
Sources of heterogeneity
The study evaluated whether differences in the proportion of ITNs with nuclear atypia could explain the known inter-institutional variability in the prevalence of malignancy of the indeterminate categories of thyroid cytopathology. A direct association was found between the proportion of ITNs with nuclear atypia and the prevalence of malignancy in the study cohort (r = 0.61 [CI 0.22–0.98]; p = 0.007; Supplementary Fig. S1). This association was stronger for studies included in the AUS/FLUS subgroup analysis (r = 0.84 [CI 0.49–1.00]; p = 0.001) but was lost in the studies included in the FN/HCN subgroup analysis (r = −0.30 [CI −1.00 to 0.40]; p = 0.35).
The study also evaluated whether differences in the diagnostic threshold for nuclear atypia were responsible for the differences in the proportion of ITNs with nuclear atypia. If that was the case, one would expect to find an inverse association between the proportion of ITNs with nuclear atypia and their prevalence of malignancy. No correlation was found between prevalence of malignancy within ITNs with nuclear atypia and the proportion of ITNs with nuclear atypia in the cohort (r = 0.01 [CI −0.48 to 0.49]; p > 0.99). These two variables were not significantly associated in either the AUS/FLUS (r = 0.49 [CI −0.08 to 1.00]; p = 0.12) or FN/HCN (r = −0.65 [CI −1.00 to 0.99]; p = 0.70) subgroup analysis.
Three separate dichotomous variables were tested in univariate models. These moderators are the country in which the study was conducted (United States vs. other), study population (AUS/FLUS only study vs. other), and cytology classification method (through slide review vs. per report or not specified). None of the three possible moderators significantly influenced the prevalence of malignancy, and only a maximum of 4% of the observed heterogeneity could be accounted for in both the nuclear atypia and non-nuclear atypia subgroups.
Discussion
In this systematic review and meta-analysis, the risk of cancer was 2.6 times greater in ITNs with nuclear atypia than in ITNs without nuclear atypia. This finding was consistent and persisted when AUS/FLUS and FN/HCN nodules were analyzed individually, despite significant heterogeneity in the prevalence of malignancy across the studies. It was also found that the proportion of nodules with nuclear atypia among indeterminate categories of thyroid cytology explains, at least in part, the differences observed in the prevalence of malignancy between institutions, particularly for the AUS/FLUS category.
Strengths and limitations
The literature review was restricted to manuscripts in English and Spanish, which means that relevant articles published in other languages were omitted. Sixty percent of the studies included in the meta-analysis were conducted in the United States. Results could be different in other underrepresented geographical areas. All but one of the studies were retrospective, and the meta-analysis was conducted on study-level data, which limits the quality of the results. However, there was no evidence of publication bias.
All studies, except that of the authors, were published before the change in nomenclature for the “encapsulated non-invasive follicular variant of papillary thyroid carcinoma,” now termed “non-invasive follicular thyroid neoplasm with papillary like nuclear features” (NIFTP) (36). For the purpose of consistency, NIFTPs in that series were also considered to be malignant to calculate the rates of malignancy (10). NIFTPs are currently not considered benign or malignant, but rather precursor lesions of invasive encapsulated follicular variant of papillary thyroid carcinomas, and hence need to be surgically removed (36). Thus, even if the rate of malignancy might be significantly lower in the nuclear and architectural atypia groups, they still reflect the percentage of nodules for which surgery is currently indicated (10). The prevalence of malignancy could also be overestimated because it was calculated based on resected nodules only, which might be more likely associated with other cancer risk factors such as suspicious sonographic features. This limitation, however, should have similarly modified the calculation for all subgroups without modifying the difference in risk between them because the rates of resection of ITNs with and without nuclear atypia were not significantly different in studies that reported this information. Unfortunately, this information was lacking in many studies.
Significant differences in the prevalence of malignancy between subgroups of nodules without nuclear atypia (architectural atypia, oncocytic atypia, and other types of atypia) were not identified, although this might be due to smaller sample sizes of the subgroups. Furthermore, the subgroup analysis was performed on all ITNs, whereas the authors' institutional experience suggests that differences between architectural atypia and oncocytic features were significant for AUS/FLUS but not for FN/HCN specimens, unless NIFTPs were considered benign tumors (10). The lack of standardization of the subgroups between studies could have significantly increased the heterogeneity of groups, further reducing the chances to find significant differences.
Sources of heterogeneity
The overall prevalence of malignancy found in the meta-analysis is very similar to that calculated in a recent meta-analysis for ITNs with AUS/FLUS and FN/HCN cytology, suggesting that the data were not contaminated with higher-risk cytology categories (37). The variability in the prevalence of malignancy of ITNs between institutions is well recognized (37,38). The results show that this is, at least in part, due to differences in the proportion of ITNs with nuclear atypia within the cohort. However, significant heterogeneity was also observed in the prevalence of malignancy of ITNs with and without nuclear atypia across the studies. This is likely due to heterogeneity in the diagnostic criteria used to define the presence of nuclear atypia (Supplementary Table S4), as well as to differences in the diagnostic threshold for nuclear atypia, which is also well recognized (36,39,40). However, this analysis suggests that the risk of malignancy of ITNs with nuclear atypia over ITNs without nuclear atypia is independent from that diagnostic heterogeneity, perhaps because it is applied similarly to cytological and histological specimens.
Some nuclear features might be better predictors of malignancy than others. Several studies have suggested that pseudoinclusions and grooves may carry an increased risk of malignancy compared to other nuclear features, although a definitive diagnosis of papillary thyroid carcinoma cannot be established in the absence of other nuclear changes (21,25,29). It is also likely that the degree of nuclear atypia (number of features present and/or number of cells exhibiting the features) is correlated to the risk of cancer (41,42). These issues, however, require further investigation.
Implications for diagnostic molecular marker tests
The performance of diagnostic molecular marker tests is significantly influenced by the pretest probability of malignancy (43). Given the significant differences in the risk of malignancy between different cytological scenarios, it might be necessary to calculate the institutional prevalence within those scenarios and not only within the diagnostic categories, as is currently recommended by the ATA, to optimize their use (6). Among ITNs with nuclear atypia, the prevalence of malignancy could be too high for molecular markers to provide useful information: a negative result might be unreliable, whereas a positive result is unlikely to change management because the elevated pretest risk of malignancy already indicated surgery as the preferred option. Molecular marker tests might perform better on ITNs without nuclear atypia because of the lower pretest risk of malignancy. However, their performance seems impaired in some other cytological scenarios, for example in ITNs with oncocytic features (44–46). Future studies will need to validate the performance of molecular marker tests in specific cytological scenarios.
Conclusions
The risk of malignancy of ITNs exhibiting nuclear atypia was two to three times higher than that of ITNs without nuclear atypia, a finding that was very consistent in the literature. There was significant variability, however, in the prevalence of malignancy of ITNs with and without nuclear atypia between studies, which might be due to differences in the definition and interpretation of nuclear atypia. Incorporating the presence or absence of nuclear atypia in reporting systems of thyroid cytology is likely to improve (i) the cytological–histological correlation, (ii) communication between pathologists and clinicians, and (iii) sharing information between institutions for research purposes. The interpretation and reporting of nuclear atypia, however, need to be standardized, and prospective studies are necessary to validate these results.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cibas ES, Ali SZ. 2009. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 19:1159–1165 [DOI] [PubMed] [Google Scholar]
- 2.The Royal College of Pathologists. Guidance on the reporting of thyroid cytology specimens. Available at: https://www.rcpath.org/resourceLibrary/g089-guidancereportingthyroidcytology-jan16.html (accessed December12, 2017)
- 3.Nardi F, Basolo F, Crescenzi A, Fadda G, Frasoldati A, Orlandi F, Palombini L, Papini E, Zini M, Pontecorvi A, Vitti P. 2014. Italian consensus for the classification and reporting of thyroid cytology. J Endocrinol Invest 37:593–599 [DOI] [PubMed] [Google Scholar]
- 4.Kakudo K, Kameyama K, Miyauchi A, Nakamura H. 2014. Introducing the reporting system for thyroid fine-needle aspiration cytology according to the new guidelines of the Japan Thyroid Association. Endocr J 61:539–552 [DOI] [PubMed] [Google Scholar]
- 5.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 6.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson MT, Boonyaarunnate T, Aragon Han P, Umbricht CB, Ali SZ, Zeiger MA. 2013. A tertiary center's experience with second review of 3885 thyroid cytopathology specimens. J Clin Endocrinol Metab 98:1450–1457 [DOI] [PubMed] [Google Scholar]
- 8.Bajaj J, Morgenstern N, Sugrue C, Wasserman J, Wasserman P. 2012. Clinical impact of second opinion in thyroid fine needle aspiration cytology (FNAC): a study of 922 interinstitutional consultations. Diagn Cytopathol 40:422–429 [DOI] [PubMed] [Google Scholar]
- 9.Cibas ES, Baloch ZW, Fellegara G, LiVolsi VA, Raab SS, Rosai J, Diggans J, Friedman L, Kennedy GC, Kloos RT, Lanman RB, Mandel SJ, Sindy N, Steward DL, Zeiger MA, Haugen BR, Alexander EK. 2013. A prospective assessment defining the limitations of thyroid nodule pathologic evaluation. Ann Intern Med 159:325–332 [DOI] [PubMed] [Google Scholar]
- 10.Valderrabano P, Khazai L, Thompson ZJ, Leon ME, Otto KJ, Hallanger-Johnson JE, Wadsworth JT, Wenig BM, Chung CH, Centeno BA, McIver B. 2017. Cancer risk stratification of indeterminate thyroid nodules: a cytological approach. Thyroid 27:1277–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishino M, Wang HH. 2014. Should the thyroid AUS/FLUS category be further stratified by malignancy risk? Cancer Cytopathol 122:481–483 [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269, w264. [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. 2000. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 14.National Heart, Lung, and Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies. Availabel at: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort (accessed May19, 2017)
- 15.Viechtbauer B. 2010. Conducting meta-analyses in R with the metafor package. J Stat Softw 36:1–48 [Google Scholar]
- 16.Gordon M, Lumley T. 2017. Forestplot: advanced forest plot using “grid” graphics. R package v1.7. Available at: https://CRAN.R-project.org/package=forestplot (accessed December12, 2017)
- 17.Rago T, Di Coscio G, Basolo F, Scutari M, Elisei R, Berti P, Miccoli P, Romani R, Faviana P, Pinchera A, Vitti P. 2007. Combined clinical, thyroid ultrasound and cytological features help to predict thyroid malignancy in follicular and Hupsilonrthle cell thyroid lesions: results from a series of 505 consecutive patients. Clin Endocrinol 66:13–20 [DOI] [PubMed] [Google Scholar]
- 18.Somma J, Schlecht NF, Fink D, Khader SN, Smith RV, Cajigas A. 2010. Thyroid fine needle aspiration cytology: follicular lesions and the gray zone. Acta Cytol 54:123–131 [DOI] [PubMed] [Google Scholar]
- 19.Renshaw AA. 2010. Should “atypical follicular cells” in thyroid fine-needle aspirates be subclassified? Cancer Cytopathol 118:186–189 [DOI] [PubMed] [Google Scholar]
- 20.Luu MH, Fischer AH, Stockl TJ, Pisharodi L, Owens CL. 2011. Atypical follicular cells with equivocal features of papillary thyroid carcinoma is not a low-risk cytologic diagnosis. Acta Cytol 55:526–530 [DOI] [PubMed] [Google Scholar]
- 21.Chung YS, Yoo C, Jung JH, Choi HJ, Suh YJ. 2011. Review of atypical cytology of thyroid nodule according to the Bethesda system and its beneficial effect in the surgical treatment of papillary carcinoma. J Korean Surg Soc 81:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horne MJ, Chhieng DC, Theoharis C, Schofield K, Kowalski D, Prasad ML, Hammers L, Udelsman R, Adeniran AJ. 2012. Thyroid follicular lesion of undetermined significance: Evaluation of the risk of malignancy using the two-tier sub-classification. Diagn Cytopathol 40:410–415 [DOI] [PubMed] [Google Scholar]
- 23.Acioglu E, Yigit O, Seden N, Huq GE. 2012. The predictive value of dominant nodules and the management of indeterminate group in multinodular goiter. Eur Arch Otorhinolaryngol 269:283–287 [DOI] [PubMed] [Google Scholar]
- 24.Chiu CG, Yao R, Chan SK, Strugnell SS, Bugis S, Irvine R, Anderson D, Walker B, Jones SJ, Wiseman SM. 2012. Hemithyroidectomy is the preferred initial operative approach for an indeterminate fine needle aspiration biopsy diagnosis. Can J Surg 55:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleiman DA, Sporn MJ, Beninato T, Crowley MJ, Nguyen A, Uccelli A, Scognamiglio T, Zarnegar R, Fahey TJ. 3rd 2013. Preoperative BRAF(V600E) mutation screening is unlikely to alter initial surgical treatment of patients with indeterminate thyroid nodules: a prospective case series of 960 patients. Cancer 119:1495–1502 [DOI] [PubMed] [Google Scholar]
- 26.Smith M, Pantanowitz L, Khalbuss WE, Benkovich VA, Monaco SE. 2013. Indeterminate pediatric thyroid fine needle aspirations: a study of 68 cases. Acta Cytol 57:341–348 [DOI] [PubMed] [Google Scholar]
- 27.Rosario PW. 2014. Thyroid nodules with atypia or follicular lesions of undetermined significance (Bethesda category III): importance of ultrasonography and cytological subcategory. Thyroid 24:1115–1120 [DOI] [PubMed] [Google Scholar]
- 28.Ryu YJ, Jung YS, Yoon HC, Hwang MJ, Shin SH, Cho JS, Lee JS, Kim HK, Kang HC, Lim HS, Yoon JH, Park MH. 2014. Atypia of undetermined significance on thyroid fine needle aspiration: surgical outcome and risk factors for malignancy. Ann Surg Treat Res 86:109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walts AE, Mirocha J, Bose S. 2014. Follicular lesion of undetermined significance in thyroid FNA revisited. Diagn Cytopathol 42:18–22 [DOI] [PubMed] [Google Scholar]
- 30.Pagni F, Prada M, Goffredo P, Isimbaldi G, Crippa S, Di Bella C, Leone BE. 2014. “Indeterminate for malignancy” (Tir3/Thy3 in the Italian and British systems for classification) thyroid fine needle aspiration (FNA) cytology reporting: morphological criteria and clinical impact. Cytopathology 25:170–176 [DOI] [PubMed] [Google Scholar]
- 31.Ustun B, Chhieng D, Van Dyke A, Carling T, Holt E, Udelsman R, Adeniran AJ. 2014. Risk stratification in follicular neoplasm: a cytological assessment using the modified Bethesda classification. Cancer Cytopathol 122:536–545 [DOI] [PubMed] [Google Scholar]
- 32.Mathur A, Najafian A, Schneider EB, Zeiger MA, Olson MT. 2014. Malignancy risk and reproducibility associated with atypia of undetermined significance on thyroid cytology. Surgery 156:1471–1476 [DOI] [PubMed] [Google Scholar]
- 33.Onder S, Firat P, Ates D. 2014. The Bethesda System For Reporting Thyroid Cytopathology: an institutional experience of the outcome of indeterminate categories. Cytopathology 25:177–184 [DOI] [PubMed] [Google Scholar]
- 34.Wu HH, Inman A, Cramer HM. 2014. Subclassification of “atypia of undetermined significance” in thyroid fine-needle aspirates. Diagn Cytopathol 42:23–29 [DOI] [PubMed] [Google Scholar]
- 35.Macias CA, Arumugam D, Arlow RL, Eng OS, Lu SE, Javidian P, Davidov T, Trooskin SZ. 2015. A risk model to determine surgical treatment in patients with thyroid nodules with indeterminate cytology. Ann Surg Oncol 22:1527–1532 [DOI] [PubMed] [Google Scholar]
- 36.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, Barletta JA, Wenig BM, Al Ghuzlan A, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, El-Naggar AK, Gooding WE, Hodak SP, Lloyd RV, Maytal G, Mete O, Nikiforova MN, Nose V, Papotti M, Poller DN, Sadow PM, Tischler AS, Tuttle RM, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA. 2016. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2:1023–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Straccia P, Rossi ED, Bizzarro T, Brunelli C, Cianfrini F, Damiani D, Fadda G. 2015. A meta-analytic review of The Bethesda System For Reporting Thyroid Cytopathology: has the rate of malignancy in indeterminate lesions been underestimated? Cancer Cytopathol 123:713–722 [DOI] [PubMed] [Google Scholar]
- 38.Wang CC, Friedman L, Kennedy GC, Wang H, Kebebew E, Steward DL, Zeiger MA, Westra WH, Wang Y, Khanafshar E, Fellegara G, Rosai J, Livolsi V, Lanman RB. 2011. A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid 21:243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirokawa M, Carney JA, Goellner JR, DeLellis RA, Heffess CS, Katoh R, Tsujimoto M, Kakudo K. 2002. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol 26:1508–1514 [DOI] [PubMed] [Google Scholar]
- 40.Lloyd RV, Erickson LA, Casey MB, Lam KY, Lohse CM, Asa SL, Chan JK, DeLellis RA, Harach HR, Kakudo K, LiVolsi VA, Rosai J, Sebo TJ, Sobrinho-Simoes M, Wenig BM, Lae ME. 2004. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol 28:1336–1340 [DOI] [PubMed] [Google Scholar]
- 41.Chen JC, Pace SC, Khiyami A, McHenry CR. 2014. Should atypia of undetermined significance be subclassified to better estimate risk of thyroid cancer? Am J Surg 207:331–336; discussion 335–336 [DOI] [PubMed] [Google Scholar]
- 42.Hong IK, Kim JH, Cho YU, Park SY, Kim SJ. 2016. Clinicopathological factors increased the risk of malignancy in thyroid nodules with atypical or follicular lesions of undetermined significance (AUS/FLUS) risk factor of malignancy in thyroid nodule with AUS/FLUS. Ann Surg Treat Res 90:201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferris RL, Baloch Z, Bernet V, Chen A, Fahey TJ, 3rd, Ganly I, Hodak SP, Kebebew E, Patel KN, Shaha A, Steward DL, Tufano RP, Wiseman SM, Carty SE; American Thyroid Association Surgical Affairs Committee 2015. American Thyroid Association statement on surgical application of molecular profiling for thyroid nodules: current impact on perioperative decision making. Thyroid 25:760–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrell RM, Bimston DN. 2014. Surgical utility of afirma: effects of high cancer prevalence and oncocytic cell types in patients with indeterminate thyroid cytology. Endocr Pract 20:364–369 [DOI] [PubMed] [Google Scholar]
- 45.Brauner E, Holmes BJ, Krane JF, Nishino M, Zurakowski D, Hennessey JV, Faquin WC, Parangi S. 2015. Performance of the Afirma gene expression classifier in Hurthle cell thyroid nodules differs from other indeterminate thyroid nodules. Thyroid 25:789–796 [DOI] [PubMed] [Google Scholar]
- 46.Valderrabano P, Leon ME, Centeno BA, Otto KJ, Khazai L, McCaffrey JC, Russell JS, McIver B. 2016. Institutional prevalence of malignancy of indeterminate thyroid cytology is necessary but insufficient to accurately interpret molecular marker tests. Eur J Endocrinol 174:621–629 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.