Abstract
Background:
Adult-onset neurodegenerative diseases affect millions and negatively impact health care systems worldwide. Evidence suggests that air pollution may contribute to aggravation of neurodegeneration, but studies have been limited.
Objective:
We examined the potential association between long-term exposure to particulate matter in aerodynamic diameter [fine particulate matter ()] and disease aggravation in Alzheimer’s (AD) and Parkinson’s (PD) diseases and amyotrophic lateral sclerosis (ALS), using first hospitalization as a surrogate of clinical aggravation.
Methods:
We used data from the New York Department of Health Statewide Planning and Research Cooperative System (SPARCS 2000–2014) to construct annual county counts of first hospitalizations with a diagnosis of AD, PD, or ALS (total, urbanicity-, sex-, and age-stratified). We used annual concentrations estimated by a prediction model at a resolution, which we aggregated to population-weighted county averages to assign exposure to cases based on county of residence. We used outcome-specific mixed quasi-Poisson models with county-specific random intercepts to estimate rate ratios (RRs) for a 1-y exposure. We allowed for nonlinear exposure–outcome relationships using penalized splines and accounted for potential confounders.
Results:
We found a positive nonlinear association that plateaued above (, 95% CI: 1.04, 1.14 for a increase from 8.1 to ). We also found a linear positive association (, 95% CI: 1.01, 1.09 per increase), and suggestive evidence of an association with AD. We found effect modification by age for PD and ALS with a stronger positive association in patients of age but found insufficient evidence of effect modification by sex or urbanization level for any of the outcomes.
Conclusion:
Our findings suggest that annual increase in county-level concentrations may contribute to clinical aggravation of PD and ALS. Importantly, the average annual concentration in our study was , below the current American national standards, suggesting the standards may not adequately protect the aging population. https://doi.org/10.1289/EHP7425
Introduction
Alzheimer’s (AD) and Parkinson’s (PD) diseases are the most prevalent adult-onset neurodegenerative diseases worldwide, with an overall estimated annual incidence rate of 1,000–1,200 and 26.2–85.9 cases per 100,000 people, respectively (Lopez and Kuller 2019; Hirsch et al. 2016; Lix et al. 2010; Niu et al. 2017). Amyotrophic lateral sclerosis (ALS) is an adult-onset rare motor neuron degenerative disorder with an annual global incidence of 1–2.6 cases per 100,000 people (Talbott et al. 2016; Hirtz et al. 2007). AD, PD, and ALS are characterized by degeneration and loss of neuronal function in specific regions of the central or peripheral nervous systems that manifests as motor or cognitive deficits or both (Elbaz et al. 2016; Tysnes and Storstein 2017; Yegambaram et al. 2015; Al-Chalabi and Hardiman 2013). Proteinopathies [e.g., amyloid plaques in AD, aggregates in PD, or TAR DNA binding protein 43 (TDP-43) aggregates in ALS], oxidative stress, and neuroinflammation are pathophysiological processes commonly observed in patients with these conditions (Jellinger 1991; Selkoe 1991; Ince et al. 1998). Although clinical symptoms generally appear in late adulthood, neuronal degeneration and other cellular pathologies are known to begin years before clinical symptoms appear (van Zundert et al. 2012; Braak et al. 2004), which highlights not only the importance of earlier life events but also of long-term stressors. Furthermore, factors that aggravate neurodegeneration remain largely unknown. Disease aggravation is an important and relevant area of study because prognosis in these diseases is highly variable—even among those patients who share the same genetic variant(s) (Schrag et al. 2007; Wang et al. 2015; Paez-Colasante et al. 2015). Patients’ survival ranges from years to decades. Identification, therefore, of modifiable factors of disease aggravation could inform policies and interventions aiming to prolong the well-being of patients with neurodegenerative diseases.
Over the last decade various toxicological studies have linked exposure to air pollution and specifically to particles in aerodynamic diameter [fine particulate matter ()], with neuroinflammation, oxidative stress, and tau, amyloid, and proteinopathies (Calderón-Garcidueñas et al. 2004, 2008, 2010, 2012, 2016; Block and Calderón-Garcidueñas 2009; Jang et al. 2018; Cheng et al. 2016; Costa et al. 2014). exposure has also been consistently associated with systemic inflammation (Block and Calderón-Garcidueñas 2009; Genc et al. 2012; Calderón-Garcidueñas et al. 2008), which has been highlighted as a plausible biological mechanism by which can ultimately affect the nervous system (Feng et al. 2016; Block et al. 2007; Block and Calderón-Garcidueñas 2009). Furthermore, some epidemiological studies have identified long-term particle exposure as a risk factor for PD (Liu et al. 2016; Fu et al. 2019; Shin et al. 2018), AD (Fu et al. 2019), and ALS (Seelen et al. 2017). However, only a limited number of studies have examined the role exposure may play in disease aggravation in PD (Kioumourtzoglou et al. 2016; Lee et al. 2017; Shi et al. 2020) or AD (Kioumourtzoglou et al. 2016; Shi et al. 2020) and none in ALS. Moreover, all existing studies have focused on cohorts years of age (Medicare enrollees), leaving out a subpopulation of older adults (55–64 years of age) that is also affected by neurodegenerative diseases. Overall, little is known about the factors that determine or influence disease severity in neurodegenerative diseases and whether long-term exposure to can ultimately contribute to clinical disease aggravation.
In the present study, we have evaluated the potential contribution of annual exposure to AD, PD, and ALS clinical disease aggravation using patients’ first hospitalization as a surrogate. We focused our study on a 1-y exposure window based on existing evidence for a likely causal relationship between long-term exposure and nervous system effects (U.S. EPA 2019). Specifically, we examined whether annual county-wide concentrations in New York State (NYS) from 2000 to 2014 were associated with first hospitalization rates in each outcome. The goal of the present study was to assess whether year-long exposures to relatively medium-to-low concentrations of , such as the ones observed throughout NYS, contribute to clinical aggravation in these three neurodegenerative diseases. We hypothesized that if exposure aggravates clinical symptoms of disease, then years with higher concentrations will result in higher rates of first hospitalizations. We also examined sex and age as potential modifiers because aging is a major risk factor for neurodegenerative diseases and sex differences are observed in disease prevalence (Lopez and Kuller 2019; Hirsch et al. 2016; Lix et al. 2010; Niu et al. 2017). In addition, our study extended beyond previous studies by using a population that included both rural and urban residents, which allowed us to also evaluate effect modification by urbanization level.
Methods
Study Population
We obtained patient data from the New York Department of Health Statewide Planning and Research Cooperative System (SPARCS), a comprehensive data reporting system that collects information on hospital admissions and emergency department (ED) visits within NYS. SPARCS data include information on of all hospitalizations in nonfederal acute care facilities, regardless of insurance status. The data set also contains demographic data including age, sex, and patient residential address. At the first hospitalization, a unique identification number is assigned to the patient, which allows for patient tracking over time. We used SPARCS data on first hospitalizations and ED visits for AD, PD, and ALS from 2000 to 2014. Columbia University institutional review board approval was obtained to conduct the analysis. The same board waived the need for informed consent because of the public nature of the data.
Outcome Definition
We extracted all first hospitalizations and ED visits using the International Classification of Diseases Ninth Revision (ICD-9-CM) codes 331.0, 332.0, and 335.20 for AD, PD, and ALS, respectively. Each code used in this study is unique to each disease. We used both primary and secondary discharge codes to identify patients with each of these conditions and restricted our analyses to patients’ first hospitalization. Primary diagnoses capture health complications directly related to these outcomes (e.g., motor complications or cognitive and psychiatric impairments), whereas secondary diagnoses may capture health complications indirectly related or unrelated to these outcomes (e.g., falls, infections). By focusing on incidence of first hospitalization using both primary and secondary diagnoses, we are evaluating whether exposure is associated with cases developing—for the first time—clinical symptoms severe enough to require hospitalization. Our outcome definition thus captures the clinical crossing point to a more severe stage of the disease, a proxy for disease aggravation. This is supported by studies reporting higher rates of hospitalizations among patients with neurodegenerative diseases relative to older adults without neurodegenerative diseases (Alzheimer’s Association 2019; Lechtzin et al. 2001; Oguh and Videnovic 2012) and increases in number of hospitalizations as the disease progresses, with 50% of hospitalizations occurring in advanced stages of disease (Oguh and Videnovic 2012; Albert et al. 1999; Lechtzin et al. 2001). Previous studies have also used hospitalization data to evaluate disease aggravation (Lee et al. 2019; van Wijngaarden et al. 2021; Shi et al. 2020; Kioumourtzoglou et al. 2016) in neurodegenerative diseases. We had access to SPACRS data starting in 1995; thus, we were able to use data from between 1995 and 1999 to identify potentially prevalent cases and exclude any subjects with a primary or secondary diagnosis prior to 2000 from our analyses.
Air Pollution Data
Annual concentration estimates were predicted by a well-validated air pollution prediction model at a resolution (van Donkelaar et al. 2019). In summary, this model relates a combined total-column aerosol optical depth from multiple sources, primarily satellite retrievals, to near-surface concentrations using the spatiotemporally varying geophysical relationship predicted by a chemical transport model. Last, ground-based monitors are incorporated using a geographically weighted regression. The model performs well, with cross-validated . We used the annual concentrations predicted by this model to calculate annual population-weighted county averages of . First, we averaged the predicted annual concentrations over all grids within a county subdivision (minor civil county divisions, e.g., towns and townships). Then, we calculated population-weighted county mean concentrations by weighting more heavily the concentrations from subdivisions with larger populations within each county. We assigned exposures based on patients’ county of residence and year of first hospitalization.
Potential Confounders
For all analyses, we used aggregated counts of hospital data per county and year for each outcome. In this design, the unit of analysis is county-year; therefore, potential confounders can only be variables that vary from year to year and across counties, and covary both with the outcome (hospital admission counts) and the exposure ( concentrations). Individual-level variables, thus, cannot act as confounders.
To account for potential spatial confounding, we included county-specific socioeconomic status (SES) variables obtained from the U.S. Census Bureau and the American Community Survey for the years 2000 and 2004–2014. Data included median household income, percentage of residents below poverty, percentage of residents without a high school degree, and racial/ethnic distribution (White, Asian, African American, and Hispanic). In addition, to improve SES characterization, we included county-level smoking prevalence and percentage obesity data obtained from the Behavioral Risk Factor Surveillance System. For the above variables, we interpolated years without available data (i.e., 2001–2003) using a generalized additive model with a penalized spline for year to allow for nonlinear time trends. In addition to SES variables, we included a variable for county urbanization level to adjust for spatial confounding potentially arising from differences across rural and urban counties. We used the 2013 six-level urban–rural classification scheme for counties, developed by the National Center for Health Statistics (NCHS) (Ingram and Franco 2014). We condensed the six-level scheme into four levels by fusing together two urban levels and the two most rural levels. In summary, the most urban category in our analysis consists of “central metro” counties that contain the largest principal city of a metropolitan area, and the second most urban category, “fringe metro,” includes counties that do not include principal metropolitan cities. Both of these two levels have a population of . “Metro” counties are small- and medium-sized metropolitan areas with a population of . Last, the “rural” category consists of micropolitan or nonmetropolitan counties (Ingram and Franco 2014). Figure S1 presents a map of the urban–rural classification scheme for NYS counties.
To account for potential temporal confounding, we adjusted for long-term trends using calendar year (2000–2014) and summer and winter mean temperatures. We retrieved daily mean temperatures at a 1/8-degree grid from the North America Land Data Assimilation System (Mitchell et al. 2004). We calculated monthly mean temperatures at the county level from daily temperatures over all grids within a county, then averaged June–August and December–February estimates to obtain the summer and winter mean temperatures, respectively, for each year. We controlled for the mean summer and winter temperatures when estimating the association between and each of the outcomes.
Statistical Analysis
We ran separate models for each outcome of interest—AD, PD, and ALS—to assess whether year-to-year fluctuations in county-wide concentrations are associated with annual rates of first hospitalization. We used a log-linear model, a modification of the model described by Wang et al. (2016), to estimate the association between long-term exposure to and the three outcomes of interest. In summary, in this approach spatial and temporal confounding is controlled by the inclusion of county- and time-specific variables (described previously in the section “Potential Confounders”). Furthermore, there can be no confounding by person-specific factors that vary within years and counties because all persons in a county during a given year are assigned the same concentration. By including county-specific random intercepts and information on factors that vary across counties, such as SES and urbanicity, we control for confounding by factors varying across counties. Moreover, by nonlinearly adjusting for time trends (calendar year), we estimate whether year-to-year variation in , around its long-term trend, is associated with year-to-year variation in disease-specific first hospitalizations. The inclusion of calendar year in our models adjusts for long-term time trends, thus capturing changes in, for example, smoking prevalence and SES that may also vary in time. After adjustment for both spatial and temporal factors, we assume that any variation in levels is random with respect to other risk factors for the three outcomes of interest; under this assumption, our models should provide an unbiased estimate of the long-term effects.
The estimand of interest in this analysis is the ratio of the rate of disease-specific first hospitalizations across different levels of county-level concentrations. We estimated rate ratios (RRs) for the association between annual exposure and first hospitalization counts using outcome-specific generalized additive mixed models. Specifically, we used a quasi-Poisson regression, which allowed for potential overdispersion in the outcomes. We allowed for nonlinear exposure–outcome relationships and included both fixed and random effects. We used county-specific random intercepts to account for within-county clustering and to allow shared information across counties. We also included the natural log of the county population size as an offset term in the models to account for differences in populations size across counties.
We followed a rigorous process to avoid any potential misspecification in the health outcome models. We first included all terms (exposure and potential confounders) linearly in the models. Then, we used penalized splines for all continuous covariates to allow for any potential nonlinear confounding. Once we identified the nonlinear confounders, we included a penalized spline for to comprehensively characterize the exposure–response relationship. If no deviations from linearity were detected, we included linear terms in the final model. For all penalized splines, we used generalized cross-validation to select the degrees of freedom (df) that optimally fit the data. When the association is linear, the estimated df is 1. Conversely, when the association is nonlinear, the estimated df are .
All linear associations are presented as RRs per increase in annual and 95% confidence intervals (CIs). In the cases of nonlinear associations, we present the entire exposure–response curve and the corresponding confidence bands. For nonlinear relationships, we also present RRs for two segments of the nonlinear curve: a) a standard deviation (SD) increase from the mean (), and b) an increase from a concentration 1 SD below the mean to the mean ().
Effect Modification
To assess potential effect modification by sex, age, and urbanization level, we ran sex-, age-, and urbanicity-stratified analyses. For age, we stratified the data into and years of age, and for urbanization, into the four urbanization levels described above (i.e., central metro, fringe metro, metro, and rural). Following the workflow described for the main analysis, we developed a health model for each subpopulation (two age models, two sex models, and four urbanization models) for each of the outcomes. The stratified health models also included the same covariates as in the main analysis, but with subpopulation-specific offsets in the case of the sex- and age-stratified models.
If one of the exposure--response associations in the stratified analyses was nonlinear, then we constructed stratum-specific RRs for two different segments of the association curve for both the linear and nonlinear associations. We estimated ratios of the expected rate at the mean of the exposure distribution () relative to the expected rates at 1 SD from the mean, that is, 8.1 relative to and 8.1 relative to . Subsequently, we assessed heterogeneity between strata separately for each curve segment—note that for linear associations, the RR in the two different curve segments would be the same. If all exposure--response associations within a given strata were linear, then we assessed heterogeneity using the RRs per increase in concentration. We evaluated evidence of effect modification in the multiplicative scale using Cochran’s -test (Kaufman and MacLehose 2013). The null hypothesis of the Cochran’s -test is that stratum-specific estimates are equal to the pooled estimate. Thus, for each stratified analysis (sex, age, and urbanicity) we obtained a single -value. For the stratified analyses in which at least one association was nonlinear, we report two -values because we performed two independent Cochran’s -test for the two different segments of the nonlinear curve (as describe in this section).
Sensitivity Analyses
To evaluate the accuracy of medical diagnosis in each outcome and to reduce the possibility of false positives influencing our results, we ran a sensitivity analysis in which we included only patients with at least two medical hospitalizations with a primary or secondary discharge code for each of the outcomes. The disease diagnosis in the second hospitalization served to confirm the diagnosis of the first hospitalization, but we still assigned exposure based on the year of the first hospitalization.
We also conducted a second sensitivity analysis to address disease aggravation misclassification (i.e., a hospitalization not representing disease aggravation), which could result from including first hospitalizations with a secondary diagnosis for the outcome in cases when the primary cause of admission was unrelated with aggravation. In this analysis, we included only first hospitalizations with a primary diagnosis for AD, PD, or ALS, that is, we removed first hospitalizations in which the outcome was not the primary reason of hospitalization.
Using the same calendar year as the first hospitalization (lag 0) to assign exposure may lead to error in exposure assessment for those individuals who had their first hospitalization earlier in the year. In this scenario, concentrations in the previous calendar year (lag 1) could better represent exposure. Thus, we ran a third sensitivity analysis in which we averaged lags 0 and 1 to assign exposure to cases. In this sensitivity analysis, we dropped the hospitalization data for the year 2000 because we predicted concentrations only from 2000 onward and were, thus, unable to construct the 1999–2000 average.
Finally, we assessed sensitivity of our results to the parameterization of time trends in the health models to adjust for potential confounding by long-term trends. In the main analysis, we used penalized splines to control for time trends (we detected deviations from linearity in all models). In this sensitivity analysis, we repeated the same models but used a) a linear term, b) a natural spline with 3 df, and c) a categorical variable for calendar year to adjust for time trends.
For all sensitivity analyses, we followed the same steps as in the main analysis to construct the models and also adjusted for the same set of confounders. All analyses (main, stratified, and sensitivity) were performed using R (version 3.6.1; R Development Core Team).
Results
Study Population Characteristics
We included data from all 62 NYS counties. The average number of first hospitalizations for each outcome per county-year, along with covariates, are presented in Table 1. The annual mean concentration per county was [interquartile range ]. Figure S2 presents temporal patterns in concentrations and disease rates across counties and on average. Across counties, the mean age (SD) at first hospitalization was 82 (0.7), 76 (1.0), and 65 (3.2) y for AD, PD, and ALS, respectively. The annual county mean counts for the age, sex, and urbanization strata are presented in Table 2 and total counts over the 14-y period in Table S1. We observed 264,075 AD, 114,514 PD, and 5,569 ALS first admissions (either as primary or secondary diagnoses) over the study period. The most common primary diagnosis category for AD and PD was diseases of the circulatory system (which includes cardiovascular diseases), 15.4% and 16.5% of total hospitalizations, respectively. Eleven percent of all AD first hospitalizations had a primary diagnosis of AD, and 9.5% of all PD first hospitalizations had a primary diagnosis of PD. For ALS, the most common primary diagnosis was ALS, accounting for 41.0% of the total hospitalizations (Figure S3).
Table 1.
Mean, standard deviation, and interquartile range for each outcome, concentrations, and covariates.
| Categories | Mean | SD | 25th percentile | Median | 75th percentile |
|---|---|---|---|---|---|
| Outcome () | |||||
| Alzheimer’s disease | 283.9 | 469.1 | 45.0 | 82.0 | 260.0 |
| Parkinson’s disease | 131.1 | 222.0 | 21.0 | 37.0 | 121.0 |
| Amyotrophic lateral sclerosis | 6.0 | 9.5 | 1.0 | 2.0 | 6.0 |
| Exposure | |||||
| () | 8.1 | 2.3 | 6.4 | 7.6 | 9.2 |
| Covariates | |||||
| Median income () | 49.1 | 12.6 | 41.3 | 45.7 | 52.4 |
| Below poverty (%) | 12.9 | 4.1 | 10.4 | 12.6 | 14.9 |
| Without high school (%) | 18.1 | 7.3 | 12.8 | 17.3 | 22.2 |
| Smoking prevalence (%) | 22.9 | 3.9 | 20.7 | 23.6 | 26.1 |
| Obesity (%) | 25.0 | 4.5 | 22.3 | 25.4 | 27.8 |
| Hispanic (%) | 6.5 | 8.6 | 1.9 | 2.9 | 6.2 |
| White not Hispanic (%) | 83.6 | 16.8 | 80.3 | 90.2 | 94.0 |
| Black not Hispanic (%) | 5.7 | 6.3 | 1.4 | 3.4 | 7.5 |
| Asian not Hispanic (%) | 2.2 | 3.4 | 0.5 | 0.9 | 2.2 |
| Summer mean temperature (°C) | 20.2 | 1.5 | 19.2 | 20.2 | 21.1 |
| Winter mean temperature (°C) | 2.5 | ||||
Note: The summary statistics show annual per county averages based in data from New York State from 2000–2014. , particulate matter in aerodynamic diameter; SD, standard deviation.
Table 2.
Annual per-county mean first hospitalization counts, standard deviation, and interquartile range for all strata used in the effect modification analyses.
| Categories | Mean | SD | 25th percentile | Median | 75th percentile |
|---|---|---|---|---|---|
| Alzheimer’s disease | |||||
| Sex | |||||
| Female | 191.0 | 319.0 | 29.0 | 53.0 | 173.0 |
| Male | 93.6 | 151.0 | 16.0 | 30.0 | 87.7 |
| Age group (y) | |||||
| 19.9 | 35.5 | 3.0 | 6.0 | 17.0 | |
| 265.0 | 436.0 | 42.0 | 76.0 | 244.7 | |
| Urbanization | |||||
| Central metro | 1,237.6 | 624.6 | 656.0 | 1,277.0 | 1,755.0 |
| Fringe metro | 368.2 | 451.5 | 53.0 | 207.0 | 328.0 |
| Rural | 153.5 | 123.6 | 60.0 | 101.5 | 206.8 |
| Metro | 58.0 | 33.2 | 34.8 | 51.0 | 79.0 |
| Parkinson’s disease | |||||
| Sex | |||||
| Female | 57.8 | 98.8 | 9.0 | 16.0 | 52.0 |
| Male | 65.4 | 110.0 | 11.0 | 19.0 | 60.0 |
| Age group (y) | |||||
| 29.0 | 51.6 | 4.0 | 9.0 | 26.0 | |
| 94.9 | 160.0 | 15.0 | 26.0 | 86.0 | |
| Urbanization | |||||
| Central metro | 555.9 | 302.7 | 286.0 | 477.0 | 836.0 |
| Fringe metro | 192.9 | 234.0 | 29.0 | 106.0 | 179.5 |
| Rural | 62.6 | 47.4 | 27.0 | 47.0 | 89.0 |
| Metro | 25.3 | 14.4 | 15.0 | 22.5 | 34.0 |
| Amyotrophic lateral sclerosis | |||||
| Sex | |||||
| Female | 2.7 | 4.5 | 0.0 | 1.0 | 3.0 |
| Male | 3.3 | 5.3 | 0.0 | 1.0 | 3.0 |
| Age group (y) | |||||
| 4.2 | 6.5 | 1.0 | 2.0 | 4.0 | |
| 2.9 | 3.9 | 1.0 | 1.0 | 3.0 | |
| Urbanization | |||||
| Central metro | 22.4 | 11.3 | 15.0 | 21.0 | 29.0 |
| Fringe metro | 9.2 | 12.0 | 2.0 | 4.0 | 12.0 |
| Rural | 3.6 | 3.6 | 1.0 | 3.0 | 5.0 |
| Metro | 1.4 | 1.5 | 0.0 | 1.0 | 2.0 |
Note: Data are from all 62 counties in New York State from 2000–2014. SD, standard deviation.
Exposure–Response Relationship
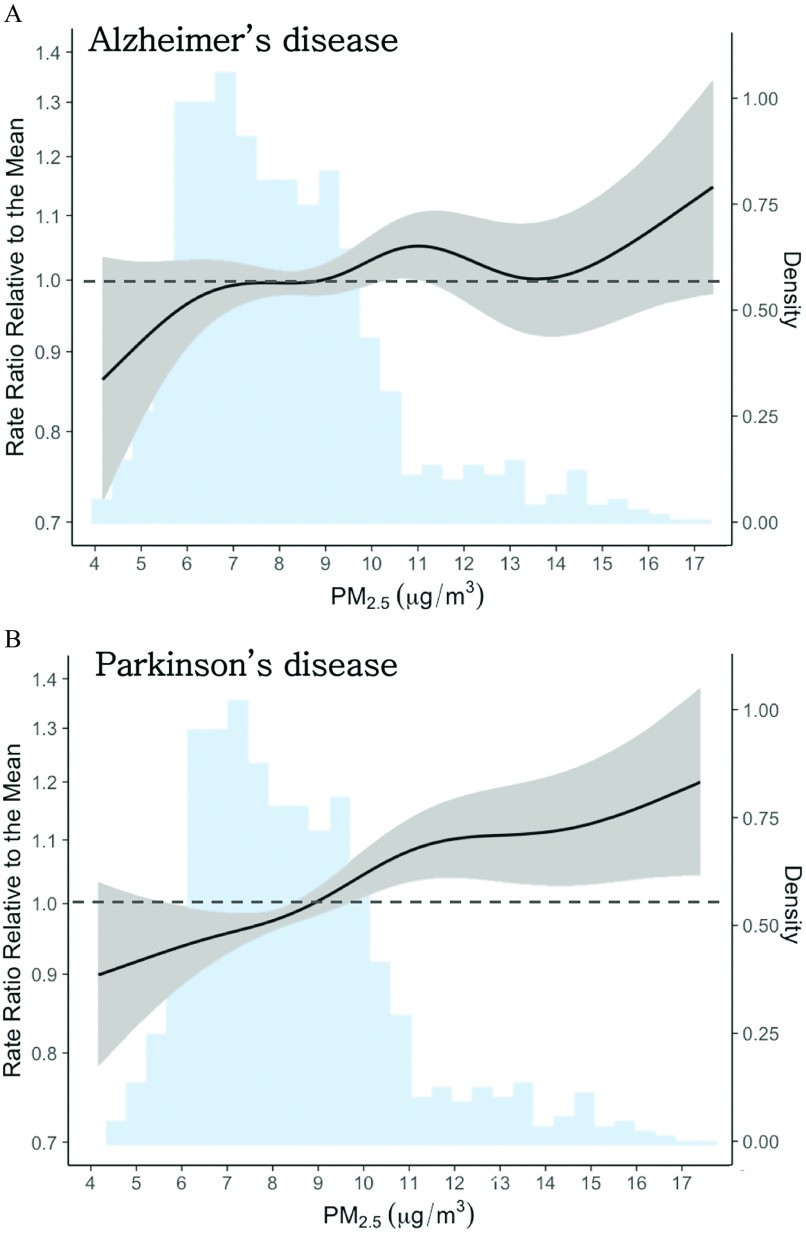
Figure 1 summarizes all findings from the main and stratified analyses, and Table S2 presents the detailed numeric estimates for all analyses. In the main analysis, we found a linear positive association with increase in concentrations (95% CI: 1.01, 1.09). We estimated a positive nonlinear association (, 95% CI: 1.04, 1.14 for a 1-SD increase from the mean, i.e., 8.1 to ), plateauing above concentrations of (Figure 2). For AD, we detected nonlinearity but inconclusive evidence of an association (Figure 2).
Figure 1.
Rate ratio (RR) estimates for the association between 1-y exposure to and first hospitalizations in Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) in New York State (2000–2014). For linear associations (asterisk), we present the RR per increase in annual concentration. Because nonlinear relationships cannot be summarized with a single RR, for nonlinear associations, we present RR for two segments across the nonlinear curve using the mean concentration as the reference point () vs. 1 SD below the mean () and 1 SD above (). That is, in the nonlinear associations, RR are for the 5.8 to (solid circle) and the 8.1 to (open diamond) segments of the exposure–response curve. Error bars represent the 95% CI. Sample size information for each stratum can be found in Table S1 and the numeric values for the RRs and CIs in Table S2. Note: CI, confidence interval; , particulate matter in aerodynamic diameter (fine particulate matter); SD, standard deviation.
Figure 2.
Nonlinear association between 1-y exposure to and first hospitalization for (A) Alzheimer’s disease (AD) and (B) Parkinson’s disease (PD) in New York State (2000–2014). The solid black lines are the exposure–response curves, as rate ratios relative to the mean concentration (), and gray shaded areas are the 95% confidence bands—both correspond to the left y-axis. The blue shaded area in both plots is the density histogram for concentrations (right y-axis). Sample sizes: , . Note: , particulate matter in aerodynamic diameter (fine particulate matter).
Effect Modification
Figure 1 and Table S3 summarize results from the stratified analyses. Overall, we found limited evidence to indicate differential effects by sex or urbanization level. For AD, we also found insufficient evidence of effect modification by age at lower concentrations but some evidence of a difference at higher exposure concentrations with a pattern of a stronger association among those years of age (Table S2 and Figure S4). Similarly, the Cochran’s -test results indicated potential effect modification by age in PD and ALS, with higher effect estimates among those of age. For PD, we estimated a positive monotonically increasing nonlinear association in patients and a linear positive association in those of age (, 95% CI: 0.99, 1.06; Table S2 and Figure S5). Among ALS patients of age, we found a null association (, 95% CI: 0.93, 1.02) and among patients of age, a positive linear association (, 95% CI: 1.02, 1.11).
Sensitivity Analyses
We summarize results from the sensitivity analyses in Figure S6. In the sensitivity analysis that included only patients with at least two hospitalizations with a primary or secondary discharge code for each outcome, the association remained nonlinear and positive at concentrations above but in lower concentrations the association was null. The association remained nonlinear, but it became positive with a steeper slope at lower levels and then plateauing at concentrations above . For ALS, we found a null linear association.
In the sensitivity analysis that included only first hospitalizations with a primary diagnosis for the outcome, the association remained nonlinear and positive at concentrations . For AD, we found a null association for most of the exposure–response curve. In the case of ALS, we observed a linear positive association (, 95% CI: 1.00, 1.13).
When we averaged lag 0 and lag 1 concentrations for exposure assessment, we found a positive nonlinear association and a positive nonlinear association, with a pattern of stronger association in higher concentrations of the exposure–response curve. In this analysis, the association was null. This sensitivity analysis had smaller power compared with other analyses because we included one fewer year of data (14 vs. 15 y) and the average of concentrations across 2 y severely decreases exposure contrast.
Finally, we present results from the sensitivity analyses on the parameterization of the time term in the models for adjustment for long-term trends in Figure S7. The association with AD remained nonlinear with a pattern of a stronger association as concentration increases except in the model where the variable time was included as a categorical variable. For PD, the estimates from the models including time as a penalized or natural spline were similar; the model with the linear time term yielded a linear positive association; and the model with time as a categorical term yielded a null association. Finally, for ALS, the estimates from the models including time as a linear term or a natural spline were positive but attenuated, and the estimate from the model with categorical time was null. We note that including time as a categorical covariate can result in overadjustment given that this is the most flexible way to parameterize time trends and could explain some of the outcome variability that is due to the exposure. Figure S2 provides further support that time adjustment using a categorical variable may result in overadjustment: There were no strong time trends in any of the three outcomes.
Discussion
In this NYS-wide analysis, we estimated that exposure to higher levels of annual was associated with higher first hospitalization rates of PD and ALS, but found limited evidence to conclude an association with AD. We also did not find sufficient evidence to conclude that there was effect modification by sex or urbanization level for all outcomes. However, we found effect modification by age in PD and ALS, with a stronger association among patients that had their first hospitalization before the age of 70 y.
For PD, we observed a nonlinear association with a steeper slope at lower concentrations that flattened at higher concentrations. In the main analysis, the association was near-linear at concentrations below . The current National Ambient Air Quality Standard for annual levels is . The association we found, thus, was consistent at levels below the current national standards in all analyses.
The present study, to our knowledge, is the first to examine the association between and ALS aggravation. In the main analysis, we found a positive linear association. Because ALS is a rare outcome, we had limited power to detect associations despite being able to leverage information on all ALS patients hospitalized in NYS. Our sensitivity analyses resulted in exclusions in the ALS data included in the models, thus further reducing power, which in turn may have resulted in unstable estimates. The association was not consistent across all sensitivity analyses. Limiting the analysis to cases with at least two hospitalizations or averaging lags 0 and 1 to assign exposures attenuated the association to the null. Nonetheless, we found a positive association in the main analysis and in the sensitivity analysis that included only hospitalizations with a primary ALS diagnosis (41% of the cases in the main analysis). Future studies that leverage data from larger cohorts are crucial to confirm our findings for ALS.
Although in the main analysis we found only suggestive evidence of a association, in the sensitivity analysis where we included only cases with at least two hospitalizations for AD, we found a positive association. This sensitivity analysis had stricter inclusion criteria that likely eliminated a number of false positives—under ICD-9, other forms of dementia are frequently misclassified as AD (Pippenger et al. 2001). It is likely that our AD data included not only AD cases but a set of patients with various subtypes of dementia (misclassified as AD) of which not all may be aggravated by exposure. Complementary to this, it is also likely that a number of AD cases were misclassified as other forms of dementia, thus, not included in our study. The latter is supported by the much lower annual first hospitalization rate observed in our data relative to the annual incidence of AD (91.2 vs. 1,000 cases per 100,000). AD misclassification is not an issue unique to our study; in fact, this is a challenge faced by all studies that use administrative data to identify cases of dementia or dementia subtypes (Taylor et al. 2009). Thus, future studies that use cohorts that allow for more accurate diagnostic tools (e.g., brain scans) could provide valuable information to further the findings of this study. Studies evaluating the association between exposure and disease aggravation in various dementia subtypes would be also valuable.
In the stratified analyses, we found insufficient evidence to conclude that there was effect modification by sex or urbanization level for all outcomes. Previous studies have reported similar findings on sex effect modification (Shin et al. 2018; Kioumourtzoglou et al. 2016). However, a recent nationwide study using information on Medicare enrollees, reported higher estimates in women for both AD and PD (Shi et al. 2020), in agreement with other studies that also reported stronger associations of exposure with risk (Liu et al. 2016) and aggravation (Lee et al. 2017) of PD among females relative to males. Conversely, a study in a cohort of female nurses reported a null association between and PD (Palacios et al. 2014). Overall, effect modification by sex is inconclusive and requires further study, particularly given the higher prevalence of PD and ALS among males relative to females (Lopez and Kuller 2019; Hirsch et al. 2016; Lix et al. 2010; Niu et al. 2017). Regarding urbanization, previous studies have focused mainly on urban centers largely because of the limited data available outside metropolitan areas. Our findings do not indicate differences across urbanization levels, in contrast to the recent nationwide Medicare study that found higher estimates for AD and PD with increasing population density (Shi et al. 2020).
We estimated a positive association between long-term exposure and PD and ALS first hospitalization in patients but not among those years of age. Based on our results, patients with a first hospitalization before 70 years of age may be particularly vulnerable to exposure. In contrast, patients who have their first hospitalization at a later age ( years of age) may have an overall better health status and, thus, be less affected by exposure. Given that we used primary and secondary diagnoses, patients with a first hospitalization at a younger age may have stronger genetic predisposition or coexisting medical conditions that render patients with higher sensitivity to exposure. Importantly, we do not identify the -years-of-age period as a vulnerable window of exposure during which exposure can aggravate clinical symptoms of the disease; instead, patients within this age group may share underlying characteristics that increase their vulnerability to exposure. Further, older patients are more likely to experience competing events. Shi et al. (2020) assessed age modification stratifying at 80 y for AD and PD and related dementias and found no differences between those below vs. above 80 years of age, despite massive power. However, that study was restricted to Medicare enrollees of age, thus excluding patients with earlier first hospitalization for neurodegenerative diseases (i.e., before age 65 y). Our data, conversely, allowed us to also evaluate the association with among adults of age. Our results indicate that exposure likely has differential impacts across patients of different ages, requiring further exploration of age-specific impacts of on neurodegenerative disease aggravation.
Last, in the present study we were interested in evaluating the association between air pollution and disease aggravation and used as an indicator of the air pollution mixture. Future studies to examine the association between specific pollutants or pollution sources are important to help better understand the effects of air pollution on neurodegenerative diseases.
Strengths
This study has several strengths. Disease prognosis, upon clinical diagnosis, varies greatly among AD, PD, and ALS patients and the nature for such variation is still largely unknown. The present study is one of the few epidemiological studies to address this knowledge gap. Here, we evaluated long-term exposure to as a potential contributor to clinical aggravation of disease. Moreover, this study covered a geographical area that included both urban and rural locations and a diverse population with a broad range of ages, allowing us to evaluate age subpopulations and effect modification by age and urbanicity. Other studies using hospitalizations to examine this association in the United States have leveraged the Medicare population, which only includes enrollees of age (Shi et al. 2020; Kioumourtzoglou et al. 2016; Lee et al. 2019); SPARCS includes information on hospitalizations of all ages. Furthermore, health data for the years 1995–1999 allowed us to exclude any potential prevalent cases. Finally, we used flexible models to characterize the exposure– response relationships.
Limitations
However, our findings should be interpreted in light of our limitations. Because SPARCS only includes information on hospitalizations, we did not have data on noncases to perform an individual-level time-to-event analysis. Furthermore, due to small numbers—especially for ALS—we aggregated analyses at the county level. We used predicted concentrations to assign county-level exposures. Although the prediction model has excellent predictive accuracy (van Donkelaar et al. 2019) and is highly spatially resolved to capture population-averaged county-level exposures, some exposure measurement error is still expected. Any resulting bias, however, is expected to be toward the null (Kioumourtzoglou et al. 2014; Hart et al. 2015; Wu et al. 2019) given that the model we used has been shown to perform well even at rural locations in NYS (Jin et al. 2019) and, thus, any error is likely nondifferential. First hospitalization data are likely to miss a number of cases because patients may not be hospitalized even as disease symptoms worsen. Nonetheless, hospitalization data still capture a significant number of cases entering a severe stage of the disease (Oguh and Videnovic 2012; Albert et al. 1999; Lechtzin et al. 2001). Outcome misclassification resulting from including patients who do not experience disease aggravation, but are hospitalized due to unrelated health issues, may also occur because we included hospitalizations with a secondary diagnosis for each disease. However, our findings were robust to sensitivity analyses addressing potential outcome misclassification. Hospitalization is not a perfect surrogate for disease aggravation, but there is a paucity of data on clinical aggravation of disease, with specific biomarkers or scores, from large cohorts living over large areas to allow for adequate exposure contrasts. Finally, we cannot exclude the possibility of residual confounding. Nonetheless, we adjusted for multiple factors that varied across counties and in time to account for potential confounding by spatially and temporally varying factors. We do not expect, therefore, that our results can be fully explained by residual confounding.
Conclusions
In the present study, we estimated the effect of long-term exposure on AD, PD, and ALS clinical disease aggravation. Our findings indicate that 1-y exposure to in levels permissible by the current national standards potentially contribute to clinical disease aggravation in PD and ALS. Moreover, our findings indicate that certain patient subpopulations are likely to present higher vulnerability to exposure.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health/National Institute of Environmental Health Sciences through grants R21 ES028472, R01 ES028805, R01 ES030616, P30 ES000002, P30 ES009089, and NIA R01 AG066793 and training grants T32 ES007322, and T32 ES007142.
References
- Albert SM, Costa R, Merchant C, Small S, Jenders RA, Stern Y. 1999. Hospitalization and Alzheimer’s disease: results from a community-based study. J Gerontol A Biol Sci Med Sci 54(5):M267–M271, PMID: 10362011, 10.1093/gerona/54.5.M267. [DOI] [PubMed] [Google Scholar]
- Al-Chalabi A, Hardiman O. 2013. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol 9(11):617–628, PMID: 24126629, 10.1038/nrneurol.2013.203. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. 2019. Alzheimer’s disease facts and figures. Alzheimers Dement 15(3):321–387, 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- Block ML, Calderón-Garcidueñas L. 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32(9):506–516, PMID: 19716187, 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69, PMID: 17180163, 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. 2004. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318(1):121–134, PMID: 15338272, 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Avila-Ramírez J, Calderón-Garcidueñas A, González-Heredia T, Acuña-Ayala H, Chao CK, et al. 2016. Cerebrospinal fluid biomarkers in highly exposed PM2.5 urbanites: the risk of Alzheimer’s and Parkinson’s diseases in young Mexico City residents. J Alzheimers Dis 54(2):597–613, PMID: 27567860, 10.3233/JAD-160472. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Franco-Lira M, Henríquez-Roldán C, Osnaya N, González-Maciel A, Reynoso-Robles R, et al. 2010. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Exp Toxicol Pathol 62(1):91–102, PMID: 19297138, 10.1016/j.etp.2009.02.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Kavanaugh M, Block M, D’Angiulli A, Delgado-Chávez R, Torres-Jardón R, et al. 2012. Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J Alzheimers Dis 28(1):93–107, PMID: 21955814, 10.3233/JAD-2011-110722. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Reed W, Maronpot RR, Henríquez-Roldán C, Delgado-Chavez R, Calderón-Garcidueñas A, et al. 2004. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol 32(6):650–658, PMID: 15513908, 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, et al. 2008. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid β-42 and α-synuclein in children and young adults. Toxicol Pathol 36(2):289–310, PMID: 18349428, 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2013. International Classification of Diseases, Ninth Revision, Clinical Modification(ICD-9-CM). http:// www.cdc.gov/nchs/icd/icd9cm.htm [accessed 8 January 2019].
- Cheng H, Saffari A, Sioutas C, Forman HJ, Morgan TE, Finch CE. 2016. Nanoscale particulate matter from urban traffic rapidly induces oxidative stress and inflammation in olfactory epithelium with concomitant effects on brain. Environ Health Perspect 124(10):1537–1546, PMID: 27187980, 10.1289/EHP134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P. 2014. Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. BioMed Res Int 2014:736385, PMID: 24524086, 10.1155/2014/736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A, Carcaillon L, Kab S, Moisan F. 2016. Epidemiology of Parkinson’s disease. Rev Neurol (Paris) 172(1):14–26, PMID: 26718594, 10.1016/j.neurol.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Feng S, Gao D, Liao F, Zhou F, Wang X. 2016. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol Environ Saf 128:67–74, PMID: 26896893, 10.1016/j.ecoenv.2016.01.030. [DOI] [PubMed] [Google Scholar]
- Fu P, Guo X, Cheung FMH, Yung KKL. 2019. The association between PM2.5 exposure and neurological disorders: a systematic review and meta-analysis. Sci Total Environ 655:1240–1248, PMID: 30577116, 10.1016/j.scitotenv.2018.11.218. [DOI] [PubMed] [Google Scholar]
- Genc S, Zadeoglulari Z, Fuss SH, Genc K. 2012. The adverse effects of air pollution on the nervous system. J Toxicol 2012:782462, PMID: 22523490, 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JE, Liao X, Hong B, Puett RC, Yanosky JD, Suh H, et al. 2015. The association of long-term exposure to PM2.5 on all-cause mortality in the Nurses’ Health Study and the impact of measurement-error correction. Environ Health 14(1):38, PMID: 25926123, 10.1186/s12940-015-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T. 2016. The incidence of Parkinson’s disease: a systematic review and meta-analysis. Neuroepidemiology 46(4):292–300, PMID: 27105081, 10.1159/000445751. [DOI] [PubMed] [Google Scholar]
- Hirtz D, Thurman D, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. 2007. How common are the “common” neurologic disorders? Neurology 68(5):326–337, PMID: 17261678, 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- Ince PG, Lowe J, Shaw PJ. 1998. Amyotrophic lateral sclerosis: current issues in classification, pathogenesis and molecular pathology. Neuropathol Appl Neurobiol 24(2):104–117, PMID: 9634206, 10.1046/j.1365-2990.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- Ingram DD, Franco SJ. 2014. 2013 NCHS urban-rural classification scheme for counties. Vital Health Stat 2 166:1–73, PMID: 24776070. [PubMed] [Google Scholar]
- Jang S, Kim EW, Zhang Y, Lee J, Cho SY, Ha J, et al. 2018. Particulate matter increases beta-amyloid and activated glial cells in hippocampal tissues of transgenic Alzheimer’s mouse: involvement of PARP-1. Biochem Biophys Res Commun 500(2):333–338, PMID: 29654761, 10.1016/j.bbrc.2018.04.068. [DOI] [PubMed] [Google Scholar]
- Jellinger KA 1991. Pathology of Parkinson’s disease. Mol Chem Neuropathol 14(3):153–197, PMID: 1958262, 10.1007/BF03159935. [DOI] [PubMed] [Google Scholar]
- Jin X, Fiore AM, Civerolo K, Bi J, Liu Y, van Donkelaar A, et al. 2019. Comparison of multiple PM2.5 exposure products for estimating health benefits of emission controls over New York State, USA. Environ Res Lett 14(8):084023, 10.1088/1748-9326/ab2dcb. [DOI] [Google Scholar]
- Kaufman JS, MacLehose RF. 2013. Which of these things is not like the others? Cancer 119(24):4216–4222, PMID: 24022386, 10.1002/cncr.28359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou MA, Schwartz JD, Weisskopf MG, Melly SJ, Wang Y, Dominici F, et al. 2016. Long-term PM2.5 exposure and neurological hospital admissions in the northeastern United States. Environ Health Perspect 124(1):23–29, PMID: 25978701, 10.1289/ehp.1408973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou MA, Spiegelman D, Szpiro AA, Sheppard L, Kaufman JD, Yanosky JD, et al. 2014. Exposure measurement error in PM2.5 health effects studies: a pooled analysis of eight personal exposure validation studies. Environ Health 13(1):2, PMID: 24410940, 10.1186/1476-069X-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtzin N, Wiener CM, Clawson L, Chaudhry V, Diette GB. 2001. Hospitalization in amyotrophic lateral sclerosis: causes, costs, and outcomes. Neurology 56(6):753–757, PMID: 11274310, 10.1212/WNL.56.6.753. [DOI] [PubMed] [Google Scholar]
- Lee H, Myung W, Kim DK, Kim SE, Kim CT, Kim H. 2017. Short-term air pollution exposure aggravates Parkinson’s disease in a population-based cohort. Sci Rep 7:44741, PMID: 28300224, 10.1038/srep44741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Schwartz J, Wang Y, Dominici F, Zanobetti A. 2019. Long-term effect of fine particulate matter on hospitalization with dementia. Environ Pollut 254(pt A):112926, PMID: 31404729, 10.1016/j.envpol.2019.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Young MT, Chen JC, Kaufman JD, Chen H. 2016. Ambient air pollution exposures and risk of Parkinson disease. Environ Health Perspect 124(11):1759–1765, PMID: 27285422, 10.1289/EHP135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lix LM, Hobson DE, Azimaee M, Leslie WD, Burchill C, Hobson S. 2010. Socioeconomic variations in the prevalence and incidence of Parkinson’s disease: a population-based analysis. J Epidemiol Community Health 64(4):335–340, PMID: 19679711, 10.1136/jech.2008.084954. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Kuller LH. 2019. Epidemiology of aging and associated cognitive disorders: prevalence and incidence of Alzheimer’s disease and other dementias. Handb Clin Neurol 167:139–148, PMID: 31753130, 10.1016/B978-0-12-804766-8.00009-1. [DOI] [PubMed] [Google Scholar]
- Mitchell KE, Lohmann D, Houser PR, Wood EF, Schaake JC, Robock A, et al. 2004. The multi-institution North American Land Data Assimilation System (NLDAS): utilizing multiple GCIP products and partners in a continental distributed hydrological modeling system. J Geophys Res 109(D7):D07S90, 10.1029/2003JD003823. [DOI] [Google Scholar]
- Niu H, Álvarez-Álvarez I, Guillén-Grima F, Aguinaga-Ontoso I. 2017. Prevalence and incidence of Alzheimer’s disease in Europe: a meta-analysis. Neurologıa 32(8):523–532, PMID: 27130306, 10.1016/j.nrleng.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Oguh O, Videnovic A. 2012. Inpatient management of Parkinson disease: current challenges and future directions. Neurohospitalist 2(1):28–35, PMID: 23983860, 10.1177/1941874411427734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Colasante X, Figueroa-Romero C, Sakowski SA, Goutman SA, Feldman EL. 2015. Amyotrophic lateral sclerosis: mechanisms and therapeutics in the epigenomic era. Nat Rev Neurol 11(5):266–279, PMID: 25896087, 10.1038/nrneurol.2015.57. [DOI] [PubMed] [Google Scholar]
- Palacios N, Fitzgerald KC, Hart JE, Weisskopf MG, Schwarzschild MA, Ascherio A, et al. 2014. Particulate matter and risk of Parkinson disease in a large prospective study of women. Environ Health 13(1):80, PMID: 25294559, 10.1186/1476-069X-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippenger M, Holloway RG, Vickrey BG. 2001. Neurologists’ use of ICD-9CM codes for dementia. Neurology 56(9):1206–1209, PMID: 11342688, 10.1212/wnl.56.9.1206. [DOI] [PubMed] [Google Scholar]
- Schrag A, Dodel R, Spottke A, Bornschein B, Siebert U, Quinn NP. 2007. Rate of clinical progression in Parkinson’s disease. a prospective study. Mov Disord 22(7):938–945, PMID: 17415791, 10.1002/mds.21429. [DOI] [PubMed] [Google Scholar]
- Seelen M, Toro Campos RA, Veldink JH, Visser AE, Hoek G, Brunekreef B, et al. 2017. Long-term air pollution exposure and amyotrophic lateral sclerosis in Netherlands: a population-based case–control study. Environ Health Perspect 125(9):097023, PMID: 29989551, 10.1289/EHP1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ 1991. The molecular pathology of Alzheimer’s disease. Neuron 6(4):487–498, PMID: 1673054, 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Shi L, Wu X, Danesh Yazdi M, Braun D, Abu Awad Y, Wei Y, et al. 2020. Long-term effects of PM2.5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet Health 4(12):e557–e565, PMID: 33091388, 10.1016/S2542-5196(20)30227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Brook JR, et al. 2018. Effects of ambient air pollution on incident Parkinson’s disease in Ontario, 2001 to 2013: a population-based cohort study. Int J Epidemiol 47(6):2038–2048, PMID: 30124852, 10.1093/ije/dyy172. [DOI] [PubMed] [Google Scholar]
- Talbott EO, Malek AM, Lacomis D. 2016. The epidemiology of amyotrophic lateral sclerosis. Handb Clin Neurol 138:225–238, PMID: 27637961, 10.1016/B978-0-12-802973-2.00013-6. [DOI] [PubMed] [Google Scholar]
- Taylor DH Jr, Østbye T, Langa KM, Weir D, Plassman BL. 2009. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis 17(4):807–815, PMID: 19542620, 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysnes OB, Storstein A. 2017. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 124(8):901–905, PMID: 28150045, 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2019. Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2019). EPA/600/R-19/188. Washington, DC: U.S. EPA; http://ofmpub.epa.gov/eims/eimscomm.getfile?p_download_id=539935 [accessed 22 January 2021]. [Google Scholar]
- van Donkelaar A, Martin RV, Li C, Burnett RT. 2019. Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ Sci Technol 53(5):2595–2611, PMID: 30698001, 10.1021/acs.est.8b06392. [DOI] [PubMed] [Google Scholar]
- van Wijngaarden E, Rich DQ, Zhang W, Thurston SW, Lin S, Croft DP, et al. 2021. Neurodegenerative hospital admissions and long-term exposure to ambient fine particle air pollution. Ann Epidemiol 54:79–86.e4, PMID: 33010415, 10.1016/j.annepidem.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert B, Izaurieta P, Fritz E, Alvarez FJ. 2012. Early pathogenesis in the adult-onset neurodegenerative disease amyotrophic lateral sclerosis. J Cell Biochem 113(11):3301–3312, PMID: 22740507, 10.1002/jcb.24234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lopez OL, Sweet RA, Becker JT, DeKosky ST, Barmada MM, et al. 2015. Genetic determinants of disease progression in Alzheimer’s disease. J Alzheimers Dis 43(2):649–655, PMID: 25114068, 10.3233/JAD-140729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kloog I, Coull BA, Kosheleva A, Zanobetti A, Schwartz JD. 2016. Estimating causal effects of long-term PM2.5 exposure on mortality in New Jersey. Environ Health Perspect 124(8):1182–1188, PMID: 27082965, 10.1289/ehp.1409671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Braun D, Kioumourtzoglou MA, Choirat C, Di Q, Dominici F. 2019. Causal inference in the context of an error prone exposure: air pollution and mortality. Ann Appl Stat 13(1):520–547, PMID: 31649797, 10.1214/18-AOAS1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegambaram M, Manivannan B, Beach TG, Halden RU. 2015. Role of environmental contaminants in the etiology of Alzheimer’s disease: a review. Curr Alzheimer Res 12(2):116–146, PMID: 25654508, 10.2174/1567205012666150204121719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.