Abstract
Eosinophils are rare white blood cells that are recruited from circulation to accumulate in the lung in mouse models of allergic respiratory inflammation. In hematoxylin–eosin (HE) stained lungs, eosinophils may be difficult to detect despite their bright eosin staining in the secondary granules. For this reason, antibody-mediated detection of eosinophils is preferable for specific and clearer identification of these cells. Moreover, eosinophils may degranulate, releasing their granule proteins into surrounding tissue, and remnants of cytolysed cells cannot be detected by HE staining. The methods here demonstrate the use of eosinophil-specific anti-mouse antibodies to detect eosinophil granule proteins in formalin-fixed cells both in situ in paraffin-embedded lungs, as well as in cytospin preparations from the lung. These antibody staining techniques enable either colorimetric or fluorescence imaging of eosinophils or their granule proteins with the potential for additional antibodies to be added for detection of multiple molecules.
Keywords: Eosinophils, Immunohistochemistry, Lung, Eosinophil peroxidase, Major basic protein, Staining, Formalin-fixed, Granule proteins, Fluorescence
1. Introduction
Eosinophils are considered the hallmark cell that mediates destructive [1-3] and immune regulating [4-8] activities in asthma pathologies [9-11]. To analyze eosinophils in situ, lung sections are often used as a measure of their numbers and states of activation in allergic respiratory pathology. Allergen models of pulmonary inflammation induce many characteristics of human pathology including increased mucus secretion, smooth muscle thickening, airway inflammation, and eosinophilic infiltration [12, 13]. Although evidence of degranulation is controversial in most acute allergen models [14, 15], some chronic models develop significant release of granule proteins in the lungs [16], a feature found in human asthmatic lung biopsies and in lung injury [17]. These pathologic changes are often viewed with use of standard dyes that characterize inflammation (HE), mucus production and goblet cell metaplasia, periodic acid–Schiff (PAS) or collagen deposition as with Masson’s trichrome or picrosirius red.
In order to identify eosinophils in situ, the lungs of euthanized mice are often formalin-fixed, embedded in paraffin, and thinly sliced (5 μm) onto glass slides. These slides are then deparaffinized and stained with dyes meant to highlight eosinophils based on the unique nature of their granule proteins. The most common dyes are acidic and chosen due to their tendency to stain cationic eosinophil granule proteins. In short, eosinophils are eosin-philic (i.e., eosinloving), due to the acidic eosin dye accumulating on the highly positively charged and acidophilic granule proteins as discovered by Dr. Paul Ehrlich over a century ago [18]. Additional dyes that are used for identifying eosinophils include Congo red and Luna. With these dyes, however, distinction between neutrophils and eosinophils is challenging, and nonspecific staining is present. For these reasons, they tend to produce less specific staining than immunohistochemistry (IHC) or immunocytochemistry (ICC), which in contrast utilizes antibodies that recognize eosinophil-specific antigens [19]. Although eosinophils can be identified by dyes, they must be differentiated manually with a trained eye. These dyes are commercially available, have easily accessible instructions on their use, and will not be discussed in this chapter.
Immunostaining assays such as IHC, ICC, and immunofluorescence (IF) use antibodies that recognize specific proteins of interest [20]. Monoclonal antibodies are superior to polyclonal antibodies due to their specificity for unique epitopes (for reviews [21-23]). Secondary granule proteins in mouse eosinophils include eosinophil peroxidase (EPX), major basic protein (MBP-1), and the divergent homologs mouse ribonucleases (mEARs) that are related to human eosinophil-derived neurotoxin (hEDN) and eosinophil cationic protein (hECP) [24]. mEARS have been found in macrophages, and MBP-1 is a low-abundant protein in basophils. In humans, ECP and EDN are also found in neutrophils [25, 26]. An additional eosinophil-associated molecule that may be targeted with antibodies is Siglec-F [27], although this is found on alveolar macrophages and eosinophils [28]. Out of all the granule proteins identified so far in eosinophils, EPX is considered the most specific to this cell type based on mouse knockout studies, and antibodies targeting EPX can be used together with those that recognize MBP-1 for highly sensitive and specific detection of eosinophils in tissues [16, 29, 30]. For this reason, our laboratory has developed monoclonal antibodies that recognize mouse EPX [31] and MBP-1 [32, 33] to specifically target eosinophils for immunostaining of lung tissue and cytospins of bronchoalveolar lavage (BAL).
First, we describe how lungs are isolated and prepared for different immunostaining techniques. Lungs must be carefully inflated and removed to maintain resting lung architecture for proper analysis. Next, we describe five staining protocols: MBP IHC, EPX IHC, EPX fluorescent IHC, EPX indirect IF on lung slices, and dual EPX/MBP indirect IF on cytospin-prepped eosinophils. The techniques employed here have advantages depending on the desired end result and equipment available [34, 35]. Colorimetric IHC is highly stable and may be viewed/imaged repeatedly using brightfield microscopy. IF methods allow co-localization/multiplex imaging of antigens at once, yet photobleaching is problematic for repeated viewing/imaging. Conventional IHC utilizes an indirect approach where an enzyme-conjugated secondary antibody recognizes the primary antibody, and a signal is developed by chromogen deposition. Using secondary antibodies and enzyme development leads to high amplification of signal. This method is employed in MBP IHC, EPX IHC, and EPX fluorescent IHC. In brief, the most common enzyme protocols are peroxidase-based horseradish peroxidase (HRP)-conjugated secondary antibodies or phosphatase-based alkaline phosphatase (AP)-conjugated secondary antibodies that react with colorimetric dyes to form precipitate at the location of the antibody (i.e., in situ). The most common dye used is 3,3′-diaminobenzidine (DAB) that produces a brown/black color, although many alternatives are available that produce a range of blue, red, and purple colorimetric stains. Alternatively, fluorescent IHC or ICC can be performed by using tyramide signal amplification (TSA), which highly amplifies the fluorescence signal through HRP activation of the fluorophore-conjugated tyramide molecule [36]. This method allows very high spatial resolution in situ compared to colorimetric IHC. Fluorescent IHC/ICC or fluorophore-conjugated secondary antibody techniques permit fluorescence imaging up to three antigens/markers with most fluorescence microscopes [37]. The fluorescence method chosen may depend on availability of antibodies, as well as the auto-fluorescence intensity of the formalin-fixed tissue or cell. Additional methods are available for multiplex imaging of >30 antigens in situ in formalin-fixed tissues that often require cyclical staining, multispectral microscopes, and sophisticated imaging software [38-40]. Fluorescence imaging is superior to conventional chromogenic staining in that it is quantifiable and allows many more stains. This chapter will encompass standard IHC and up to 3-color IF imaging for eosinophils using standard laboratory equipment, microscopes, and imaging software.
2. Materials
All reagents should be prepared, stored, and used at room temperature unless otherwise indicated. Follow local waste disposal guidelines. Scale working solution volumes up or down dependent on your experiment.
2.1. Lung Collection for Fixation and Embedding
10-mL Luer-lock syringe.
Support stand with rod and clamp.
3-way stopcock.
Catheters: 20G, 30-mm length.
Butterfly needle infusion set: 21GA × 3/4 in. with a 12-in. tubing.
70% Ethanol: To make 200 mL, add 140 mL ethanol to 60 mL distilled water.
Surgical scissors.
Two surgical forceps.
10% Formalin.
50-mL Conical tubes or containers.
Paper towels.
4-0 Non-absorbable silk sutures: Cut into 5-in. lengths per mouse.
Euthanasia: pentobarbital or ketamine-xylazine.
2.2. Deparaffinization/Rehydration of Slides
Tissue-Tek® Staining Dish (see Note 2).
Incubator (55 °C).
Xylene (see Note 3).
50:50 Xylene/ethanol solution: To make 200 mL, add 100 mL of xylene to 100 mL of ethanol.
100% Ethanol: 200 proof (see Note 4).
95% Ethanol: To make 200 mL, add 190 mL of ethanol to 10 mL of distilled water.
75% Ethanol: To make 200 mL, add 150 mL of ethanol to 50 mL of distilled water.
Fig. 1.
Tissue-Tek® Slide Holder and Rack. (a) Tissue-Tek® Slide Holder that can hold up to 24 slides (grey) and staining lid and dish. The grey rack fits inside the holder. Slides are fully immersed in liquid when 200 mL of fluid is in the container. (b) Tissue-Tek® Rack is a convenient way to organize multiple staining dishes used for deparaffinization and rehydration of tissue sections
2.3. MBP IHC with a Red Alkaline Phosphatase Substrate as a Chromogen
Tissue-Tek® Staining Dish (see Note 2).
Shandon™ Sequenza™ Staining Rack and coverplates (see Note 5) (Fig. 2).
Wash buffer: 0.05 M Tris-HCl, 0.15 M NaCl, 0.05% Tween 20, pH 7.6. Alternatively, a 10× concentrate may be prepared and diluted to a 1:10 ratio before use by adding 10 mL of the concentrate to 90 mL of ultrapure water. Working solution can be stored at room temperature for 1 week.
Digest-All™ 3: ready-to-use pepsin solution. Store at 4 °C (see Note 6).
Antibody Diluent, Background Reducing (Agilent Dako): ready-to-use. Store at 4 °C.
Dual Endogenous Enzyme Blocker (Agilent Dako): ready-to-use. Store at 4 °C (see Note 7).
Blocking buffer: 5% normal goat serum in the wash buffer. Dilute to a 1:20 ratio by adding 10 μL of serum to 190 μL of the wash buffer (see Note 8).
Rat anti-MBP primary antibody: 1 mg/mL, Clone MT2-14.7.3 (Mayo Clinic, Arizona). Dilute to a 1:1000 ratio before use by adding 1 μL of the antibody to 999 μL of the antibody diluent to make a final concentration of 1 μg/mL. Use diluted antibody same day (see Notes 9 and 10).
Secondary antibody (ImmPRESS®-AP anti-rat polymer, Vector Labs): ready to use. Store at 4 °C (see Note 11).
Chromogen (ImmPACT® Vector® Red AP Substrate, Vector Labs): To prepare 2.5 mL of Vector Red working solution, add 1 drop of Reagent 1 and 1 drop of Reagent 2 to 2.5 mL of the diluent and mix well before use. Use immediately after preparation (see Note 12).
0.1% Methyl green: Add 200 mg of methyl green to 200 mL of ultrapure water.
Nonaqueous permanent mounting medium.
#1.5 Glass coverslip.
Fig. 2.
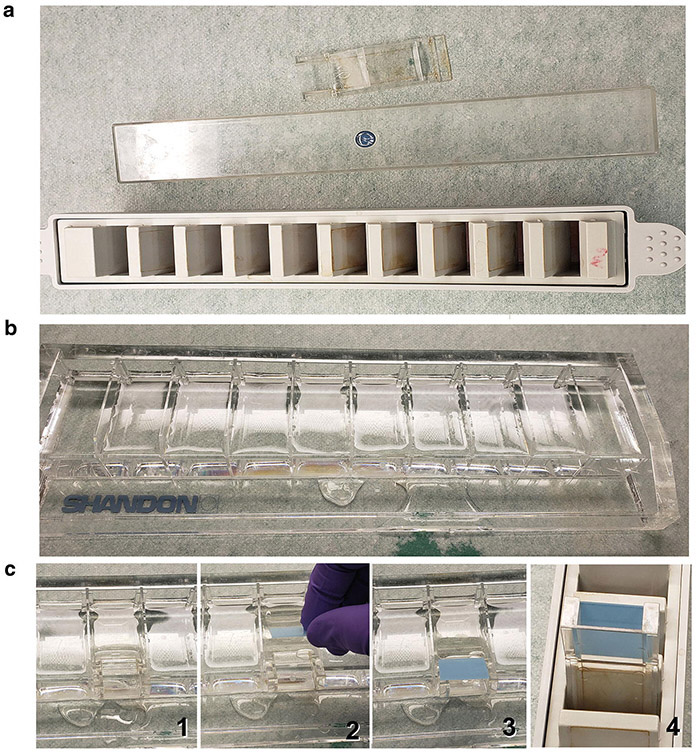
The use of Shandon™ Sequenza™ Staining Rack and coverplates allows for controlled flow of 200 μL of fluid over slides and for several slides to be processed at the same time. (a) Shandon™ Sequenza™ Staining Rack, lid, and coverplate. There is room for ten slides per rack. (b) Slide preparation rack filled with distilled water. (c) Instructions of how to load slides onto coverplate: (1) A container is filled with water; (2) Coverplate is submerged under water; (3) A slide is lowered onto the coverplate face-down, creating a small water filled void between the slide and coverplate; (4) Hold in place and then slide into the rack firmly
2.4. EPX IHC with DAB as a Chromogen
Items 1–6 from Subheading 2.3.
Rodent Decloaker Antigen Retrieval, 10× (Biocare Medical): Dilute the concentrate to a 1:10 ratio with ultrapure water before use (see Note 13).
Mouse anti-EPX primary antibody: 1 mg/mL, Clone MM25.82.2.1 (Mayo Clinic, AZ). Dilute to a 1:500 ratio before use by adding 1 μL of the antibody to 499 μL of the antibody diluent to a final concentration of 2 μg/mL. Use diluted antibody same day (see Notes 9 and 10).
Rodent Block M (Biocare Medical): ready-to-use. Store at 4 °C (see Note 15).
Goat anti-mouse IgG (H + L), HRP-conjugated secondary antibody: 0.4 mg/mL. Dilute to a 1:250 ratio before use by adding 1 μL of the antibody to 249 μL of the antibody diluent. Store the antibody at −20 °C. Use diluted antibody same day.
DAB chromogen: SignalStain® DAB Kit (Cell Signaling Technologies) or equivalent. To prepare the DAB working solution, add 1 drop (30 μL) SignalStain® DAB chromogen concentrate to 1 mL of SignalStain® DAB diluent and mix well before use. Working solutions are stable for up to 14 days when stored at 4 °C or up to 5 days when stored at room temperature (see Note 16).
Hematoxylin: Ready-to-use solution is commercially available.
Acid rinse solution: To prepare 200 mL, add 4 mL of glacial acetic acid to 196 mL of ultrapure water (see Note 17).
Bluing solution: To prepare 200 mL, add 3 mL of 30% ammonium hydroxide to 197 mL of 70% ethanol (see Note 18).
Nonaqueous permanent mounting medium.
#1.5 Glass coverslip.
Fig. 3.
Decloaking chamber (BioCare) with a plastic coplin jar. Water (500 mL) is placed inside the decloaker to distribute the heat around the coplin jar
2.5. EPX Fluorescent IHC with Tyramide Signal Amplification (TSA)
Items 1–6 from Subheading 2.3.
Rodent Decloaker Antigen Retrieval, 10× (Biocare Medical): Dilute the concentrate to a 1:10 ratio with ultrapure water before use (see Note 13).
Mouse anti-EPX primary antibody: 1 mg/mL, Clone MM25.82.2.1 (Mayo Clinic, AZ). Dilute to a 1:500 ratio before use by adding 1 μL of the antibody to 499 μL of the antibody diluent to a final concentration of 2 μg/mL (see Notes 9 and 10).
Rodent Block M (Biocare Medical): ready to use. Store at 4 °C (see Note 15).
Goat anti-mouse IgG (H + L) secondary antibody: 0.4 mg/mL, HRP-conjugated. Dilute to a 1:250 ratio before use by adding 1 μL of the antibody to 249 μL of the antibody diluent. Store the stock at −20 °C. Use diluted antibody same day.
TSA cyanine 3 (Cy3) kit: TSA™ Plus Cyanine 3 Kit or equivalent. Reconstitute TSA Plus stock with DMSO (HPLC-grade) according to manufacture recommendations. Dilute the stock solution to a 1:800 ratio before use by adding 1 μL of TSA dye to 799 μL of 1× Amplification Diluent to make TSA Plus working solution (see Note 19).
Phosphate-buffered saline (PBS): 1.5 mM KH2PO4, 155 mM NaCl, 2.7 mM Na2HPO4-7H2O, pH 7.4. To prepare 1 L, add 210 mg KH2PO4, 9 g NaCl, and 726 mg Na2HPO4-7H2O to 900 mL distilled water. Adjust pH and raise volume to 1 L with distilled water.
4′,6-Diamidino-2-phenylindole, dilactate (DAPI): 10.9 mM DAPI. Prepare a stock solution by dissolving 5 mg of DAPI in 1 mL ultrapure water. Aliquot and store the stock at −20 °C. To prepare working solution, dilute the stock to 1:5000 in PBS to 1 μg/mL. Store the working solution at 4 °C (see Note 20).
Mounting medium: ProLong™ Diamond Antifade Mountant (Invitrogen) or equivalent. Ready to use. Store at −20 °C (see Note 21).
#1.5 Glass coverslip.
2.6. EPX Indirect IF
Items 1–6 from Subheading 2.3.
Rodent Decloaker Antigen Retrieval, 10× (Biocare Medical): Dilute the concentrate to a 1:10 ratio with ultrapure water before use (see Note 13).
Mouse anti-EPX primary antibody: 1 mg/mL, Clone MM25.82.2.1 (Mayo Clinic, AZ). Dilute to a 1:100 ratio before use by adding 2 μL of the antibody to 198 μL of the antibody diluent to a final concentration of 10 μg/mL. Use diluted antibody same day (see Notes 9 and 10).
Rodent Block M (Biocare Medical): ready-to-use. Store at 4 °C (see Note 15).
Anti-mouse IgG secondary antibody: Alexa 594-conjugated. Dilute to a 1:500 ratio by adding 1 μL of the antibody to 499 μL the antibody diluent. Store stock at 4 °C. Use diluted antibody same day (see Note 22).
Phosphate-buffered saline (PBS): 1.5 mM KH2PO4, 155 mM NaCl, 2.7 mM Na2HPO4-7H2O, pH 7.4. To prepare 1 L, add 210 mg KH2PO4, 9 g NaCl, and 726 mg Na2HPO4-7H2O to 900 mL distilled water. Adjust pH and raise volume to 1 L with distilled water.
4′,6-Diamidino-2-phenylindole, dilactate (DAPI): 10.9 mM DAPI. Prepare a stock solution by dissolving 5 mg of DAPI in 1 mL of ultrapure water. Aliquot and store the stock at −20 °C. To prepare working solution, dilute stock 1:5000 in PBS to 1 μg/mL. Store working solution at 4 °C (see Note 20).
Mounting medium: ProLong™ Diamond Antifade Mountant (Invitrogen) or equivalent. Ready to use. Store at −20 °C (see Note 21).
#1.5 Glass coverslip.
2.7. MBP and EPX Dual Fluorescent Immunocytochemistry (ICC)
Cells from peripheral blood or bronchoalveolar lavage in 5% BSA in PBS, stored at 4 °C.
ThermoScientific Cytospin™ 3 or 4 Cytocentrifuge and components, funnel filter paper, and clip.
Phosphate-buffered saline (PBS): 1.5 mM KH2PO4, 155 mM NaCl, 2.7 mM Na2HPO4-7H2O, pH 7.4. To prepare 1 L, add 210 mg KH2PO4, 9 g NaCl, and 726 mg Na2HPO4-7H2O to 900 mL distilled water. Adjust pH and raise volume to 1 L with distilled water.
5% (w/v) BSA: To make 100 mL, add 5 g of BSA to 100 mL of PBS. Store at 4 °C and use within 1 day (see Note 23).
Permeabilization buffer (PBT): PBS containing 0.2% (v/v) Triton™ X-100. First prepare a stock solution of 10% (v/v) Triton™ X-100 by adding 200 μL of Triton™ X-100 to 9.8 mL of PBS. To make working solution, dilute 10% stock to a 1:50 ratio by adding 10 μL of the stock to 490 μL of PBS (see Note 24).
Wash buffer (PBST): PBS containing 0.1% TWEEN® 20. First prepare a stock solution of 10% TWEEN® 20 by adding 100 μL of TWEEN® 20 to 9.9 mL of PBS. To make working solution, dilute 10% stock to 1:100 by adding 5 μL of the stock to 495 μL of PBS (see Note 24).
Antibody diluent: 1% BSA in PBST. To prepare 5 mL, add 1 mL of 5% BSA to 4 mL of PBST. Store at 4 °C.
Blocking buffer: 5% normal donkey serum in antibody diluent. To prepare 1 mL, add 50 μL of normal donkey serum to 950 μL of antibody diluent. Use on the same day (see Note 8).
Primary antibody mix: mouse anti-EPX [1 mg/mL] (Clone MM25.82.2.2, Mayo Clinic AZ) and rat anti-MBP [1 mg/mL] (Clone MT2-14.7.3, Mayo Clinic AZ). To prepare 1 mL, dilute the antibodies to 1:200 by adding 5 μL of anti-EPX and 5 μL of anti-MBP to 990 μL of antibody diluent (see Notes 9 and 10).
Secondary antibody mix: donkey anti-mouse Alexa 594 and donkey anti-rat Alexa 488. To prepare 1 mL, dilute the antibodies to 1:500 by adding 2 μL of anti-mouse Alexa 594 and 2 μL of anti-rat Alexa 488 to 996 μL of the antibody diluent (see Note 19).
4′,6-Diamidino-2-phenylindole, dilactate (DAPI): 10.9 mM DAPI. Prepare a stock solution by dissolving 5 mg DAPI in 1 mL ultrapure water. Aliquot and store stock at −20 °C. To prepare working solution, dilute stock 1:5000 in PBS to 1 μg/mL. Store working solution at 4 °C (see Note 20).
ProLong™ Diamond Antifade Mountant (Invitrogen): ready to use. Store at −20 °C (see Note 21).
#1.5 Glass coverslip.
3. Methods
3.1. Lung Collection for Fixation and Embedding
This protocol is optimized for BALB/c or C57BL/6 mice that are >6 weeks of age or 18–40 g in weight. Procedures must be approved by IACUC committee and under the assurances of the Office for Laboratory Animal Welfare. All incubations should be performed at room temperature unless noted otherwise. The mice used in these procedures have undergone a house dust mite allergen sensitization and challenge protocol [41].
Set up a syringe with a stopcock, a butterfly needle, and a catheter on the support stand (see Note 25) (Fig. 4).
Ensure the stopcock is off (perpendicular to syringe) and fill the syringe with the formalin solution (see Note 26). With the tip of the catheter placed into a disposable container, open the stopcock to let the formalin fill the length of the catheter and tubing. Make sure there are no air bubbles in the tubing line. Stop the flow by turning the stopcock to the off position.
Euthanize a mouse with a lethal dose of sodium pentobarbital or ketamine-xylazine (see Note 27), and lay the mouse on its back on top of paper towels to absorb excess fluids. Wet the fur around the throat and torso with 70% ethanol.
To remove the skin over the chest area, grab the skin under the jaw with forceps, creating a tent, and cut the skin with scissors from the length of the jaw to the bottom of the rib cage.
Lift up on the rib cage by grabbing bottom part of the sternum (the xiphoid process) with forceps and make an incision along the edge (beneath) of the rib cage from right to left to expose the diaphragm.
Cut the diaphragm away from the ribs (cutting left to right). Be careful not to poke or cut the lung. Any tears will lead to formalin leakage and lung deflation, altering the architecture.
While lifting the xiphoid process with forceps up away from the body, use the scissors to cut the rib cage on both sides about 2/3 distance to top of rib cage, approximately right below the clavicles. A final cut is made across the top of the ribcage to remove it and expose the heart and lungs.
The clavicles must be cut in order to remove the lungs from the mouse. Cut the clavicle on each side such that the section of bone remaining over the thymus and heart can be carefully removed from the mouse. This allows for total exposure of the trachea, heart, thymus, and lungs.
Expose the ventral side of the trachea by moving away the thyroid gland (pull apart, splitting the middle). Carefully cut the muscle layer over the trachea so as to expose the cartilage of the trachea.
Carefully loop the 5″ suture material underneath the trachea using forceps and then loosely form a knot immediately below the thyroid cartilage/voice box. Do not tighten.
Cut the trachea horizontally just enough to allow a 20G catheter insertion at the thyroid cartilage/voice box as this provides a solid and wide location to support this type of cut and provides a reference point (see Note 28).
Put the catheter into the trachea such that it is inserted only a few millimeters, past the loose knot, yet avoid going so far that there is resistance. Holding the catheter in place, tighten the knot until snug.
Open the stopcock and allow the lungs to fill. Turn off the stopcock once the lungs are fully inflated.
When the lung is fully inflated, at the same time, remove the catheter and tighten the knot completely, so no liquid escapes.
While holding trachea with forceps at the knot, cut above the forceps to sever trachea and cut any connective tissue holding the lungs in place.
Place the whole lung into a 50-mL conical filled with 30 mL formalin and store for 24 h (see Note 29).
Prepare for embedding and sectioning. This is beyond the scope of this chapter but is described elsewhere [42]. Sections stained in this protocol are 5-μm thick coronal slices of formalin-fixed and paraffin-embedded (FFPE) tissue.
Fig. 4.
Syringe and catheter setup to prepare formalin-inflated lungs. A 10-mL syringe is held in place by a clamp such that the 10-mL mark on the syringe is 20 cm above the benchtop. The blue stopcock controls the flow of formalin. The catheter is placed into the trachea during instillation of formalin
3.2. Deparaffinization/Rehydration FFPE Slides
Place slides in Tissue-Tek® Slide Holder and Tissue-Tek® Staining Dish (Fig. 1) and incubate the slides at 55 °C for 30 min with lid on the dish (see Note 30).
- In a fume hood, set up the indicated number of Tissue-Tek dishes with 200 mL of each solution, and place the slide holder into the staining dishes for the indicated times:
- Three dishes of xylene, 5 min each (see Note 31).
- One dish of 50:50 xylene/ethanol, 2 min.
- Two dishes of 100% ethanol, 2 min each.
- One dish of 95% ethanol, 2 min.
- One dish of 75% ethanol, 2 min.
Rinse the slides in running distilled water for 30 s. Store slides in water until next steps to keep hydrated.
3.3. MBP IHC with a Red AP Substrate as a Chromogen
After deparaffinization/rehydration of slides, load the slides into Shandon™ Sequenza™ Staining Rack with coverplates (see Note 32) (Fig. 2).
Add 200 μL of Digest-All™ 3 pepsin to the slides and incubate for 10 min.
Wash three times in wash buffer for 2 min each.
Add 200 μL of Dual Endogenous Enzyme Block to the slides and incubate for 10 min.
Wash three times in wash buffer for 2 min each.
Add 200 μL of the blocking buffer to the slides and incubate for 30 min (see Note 33).
Add 200 μL of the diluted anti-MBP antibody (1 μg/mL) to the slides and incubate overnight at 4 °C. For negative control slides, add diluent without the antibody (see Note 34).
Wash three times in wash buffer for 5 min each.
Add 200 μL of ImmPRESS Anti-Rat AP polymer to the slides and incubate for 30 min.
Wash three times in wash buffer for 5 min each.
Add 200 μL Vector Red chromogen to slides and incubate for 5 min (see Note 35).
Wash once with distilled water for 2 min, then transfer slides to a dish filled with distilled water to keep tissue hydrated.
To counterstain with methyl green, place slides in methyl green for 15 s (see Note 35), and wash slides in running distilled water until water is clear (about 10 s).
Dehydrate the slides (see Note 36) by placing them once in 95% ethanol for 1 min and twice in 100% ethanol for 1 min. Air-dry the slides.
Dip slides in xylene and coverslip with nonaqueous permanent mounting medium (Fig. 5).
Fig. 5.
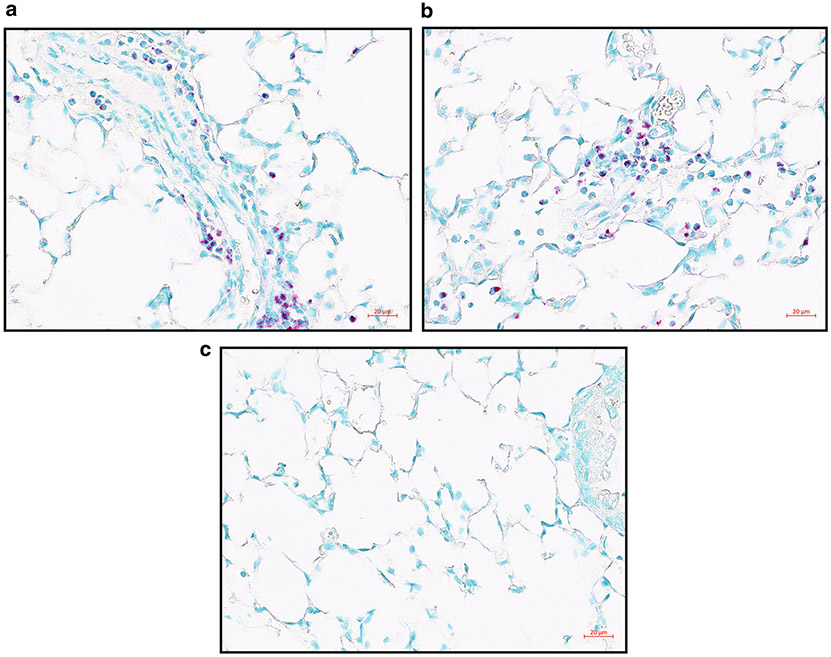
Allergen-challenged FFPE lung sections with MBP IHC with the red chromogen. (a, b) Two examples of MBP IHC in allergen-challenged lung FFPE slices. MBP is stained red showing the location of eosinophils, and methyl green counterstains nuclei green. (c) Negative control staining. Images were taken on Zeiss Imager.M2 with a ×40 objective
3.4. EPX IHC with DAB as a Chromogen
3.4.1. Antigen Retrieval
Add 500 mL distilled water to the Decloaker or equivalent.
Submerge deparaffinized and dehydrated slides into a staining jar with diluted antigen retrieval solution (see Note 37) and place them in the Decloaker.
Incubate the slides in Decloaker at 95 °C for 40 min, then 85 °C for 10 min. Remove the staining jar from the Decloaker, keeping the slides in the retrieval buffer, and allow to cool on benchtop for 20 min.
Rinse the slides in running distilled water until all the antigen retrieval solution is removed (see Note 38).
3.4.2. Antibody Incubation and Color Development
Load slides into Shandon™ Sequenza™ Staining Rack (see Note 32) (Fig. 2).
Add 200 μL of Digest-All™ 3 pepsin to the slides and incubate for 10 min.
Wash three times in wash buffer for 2 min each.
Add 200 μL Rodent M Block to slides and incubate for 30 min.
Wash three times in wash buffer for 2 min each.
Add 200 μL anti-EPX antibody (2 μg/mL) to the slides and incubate overnight at 4 °C. For negative control slides, add diluent without the antibody (see Note 34).
Wash three times in wash buffer for 5 min each.
Add 200 μL of anti-mouse HRP secondary to slides and incubate for 30 min.
Wash three times in wash buffer for 5 min each.
Add 200 μL of DAB chromogen to slides and incubate for 10 min (see Note 35).
Wash once with distilled water for 2 min, then transfer slides to a slide holder submerged in distilled water to keep tissue hydrated.
3.4.3. Hematoxylin Counterstaining
Incubate slides in hematoxylin for 5 min.
Wash slides in running distilled water until water is clear.
Immerse slides ten times into acid rinse solution.
Immerse slides ten times into distilled water.
Incubate slides for 1 min in bluing solution.
Immerse slides ten times into distilled water.
3.4.4. Dehydration and Coverslipping
Incubate slides in 75% ethanol for 1 min.
Incubate slides in one wash of 95% ethanol for 1 min each.
Incubate slides in two washes of 100% ethanol for 1 min each.
Air-dry slides.
Dip slides in xylene and coverslip with nonaqueous permanent mounting medium (Fig. 6).
Fig. 6.
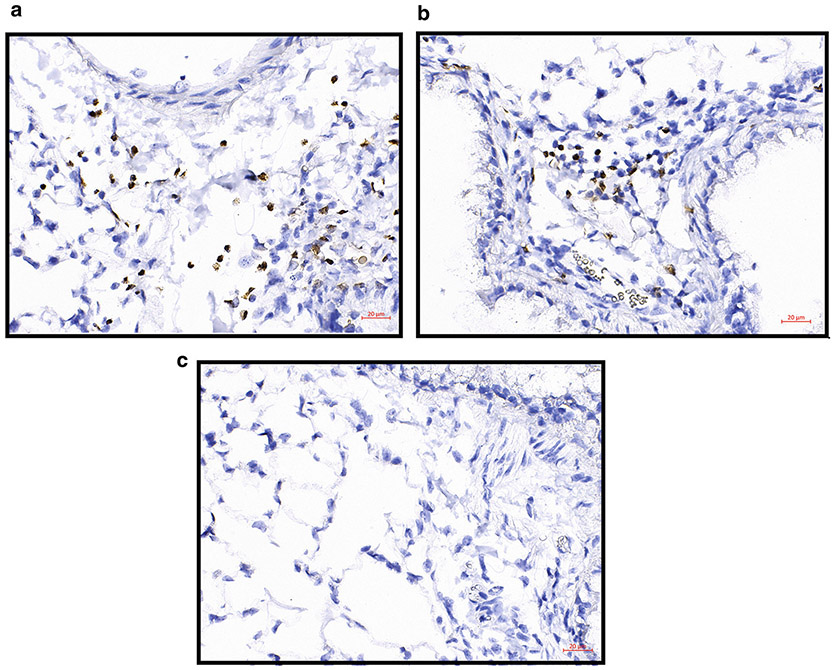
Allergen-challenged FFPE lung sections with EPX IHC with DAB as the chromogen. (a, b) Two examples of EPX IHC in allergen-challenged lung FFPE slices. EPX is stained brown showing the location of eosinophils, and hematoxylin counterstains nuclei blue/purple. (c) Negative control staining. Images were taken on Zeiss Imager.M2 with a ×40 objective
3.5. EPX Fluorescent IHC with TSA
Perform antigen retrieval as described in Subheading 3.4.1.
Pretreat and block the slides as described by Subheading 3.4.2, steps 1–5.
Add 200 μL of anti-EPX antibody [2 μg/mL] to slides and incubate overnight at 4 °C. For negative control slides, add diluent without the antibody (see Note 34).
Wash three times in wash buffer for 5 min each.
Add 200 μL of anti-mouse HRP secondary antibody to the slides and incubate for 1 h.
Wash three times in wash buffer for 5 min each.
Add 200 μL of TSA Cy3 dye solution to slides and incubate for 10 min protected from light. All following steps should be protected from light to reduce photobleaching.
Wash three times in wash buffer for 5 min each and rinse with PBS.
Counterstain nuclei by adding 200 μL of DAPI to the slides and incubate for 7 min.
Wash three times in PBS for 2 min each.
Remove one slide at a time from the rack and coverslip using ProLong™ Diamond Antifade mountant (see Note 39).
Lay slides flat and allow to dry overnight protected from light before imaging (see Note 40) (Fig. 7).
Fig. 7.
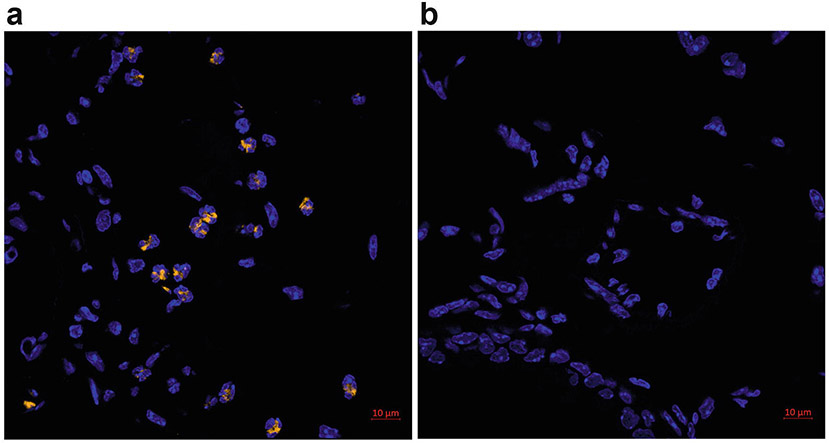
Allergen-challenged FFPE lung sections with EPX fluorescent IHC with TSA. (a) Eosinophils are stained for EPX with Cy3-conjugated tyramide substrate (orange). Nuclei are counterstained with DAPI (blue). (b) Negative control without the primary antibody. Image was acquired with a Plan-Apochromat ×63 objective on a Zeiss LSM 800 microscope
3.6. EPX Indirect IF
This FFPE lung staining method may be adapted for dual IF by adding an additional primary antibody such as a rat or rabbit antibody, combined with an appropriate fluorophore-conjugated secondary antibody (such as goat anti-rat or goat anti-rabbit Alexa 647) (see Note 41). Optimization of antigen retrieval and blocking agents will be required for additional primary antibodies.
Perform antigen retrieval as described in Subheading 3.4.1.
Pretreat and block the slides as described by Subheading 3.4.2, steps 1–5.
Add 200 μL of anti-EPX antibody [10 μg/mL] to the slides and incubate overnight at 4 °C. For negative controls, add diluent without the antibody (see Note 34).
Wash three times in wash buffer for 5 min each.
Add 200 μL of anti-mouse Alexa594 secondary antibody to the slides and incubate for 1 h protected from light. All following steps should be protected from light to prevent photobleaching.
Rinse, stain with DAPI, and coverslip the slides as described in Subheading 3.5, steps 8–12 (Fig. 8).
Fig. 8.
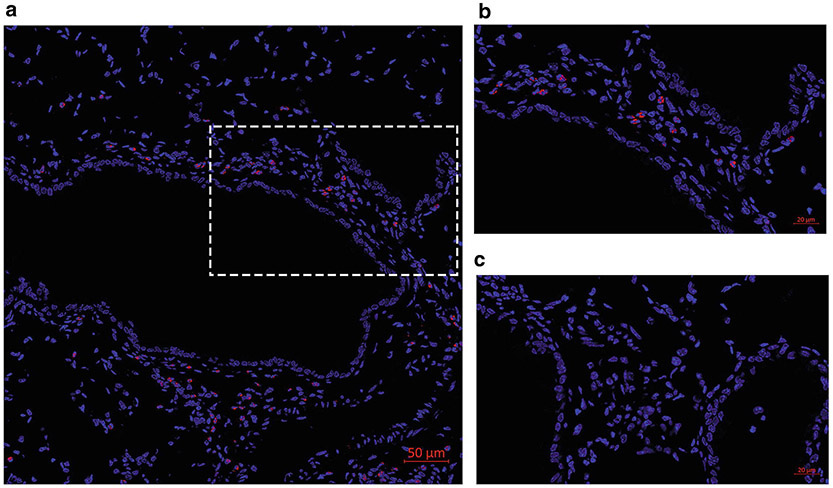
Allergen-challenged FFPE lung sections with EPX indirect IF. Eosinophils are stained for EPX (red) and nuclei are counterstained with DAPI (blue). (a) Tile (5 × 5) image was acquired with a Plan-Apochromat ×63 objective on a Zeiss LSM 800 microscope. (b) Zoomed in image of (a). (c) Negative control without the primary antibody
3.7. MBP and EPX Dual Fluorescent ICC.
Resuspend cells from peripheral blood or bronchoalveolar lavage at 1 × 106 cells/mL in cold 5% BSA/PBS (see Note 42).
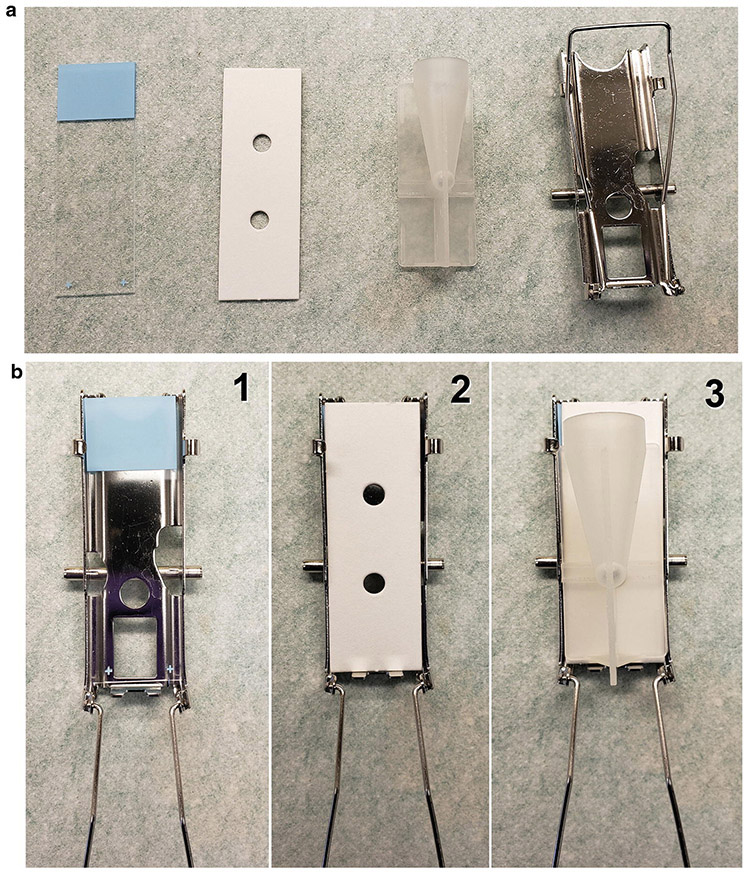
Set up cytospin cages with microscope slides and funnels. Load into a cytocentrifuge (Fig. 9).
Pre-wet the slides by adding 50 μL of 5% BSA to the funnels, bringing the cytocentrifuge up to 500 RPM (~28 × g) and stopping (see Note 43).
Add 50 μL of the cells to each funnel, then add 50 μL of 5% BSA.
Spin at 500 rpm, slow acceleration, for 5 min.
Remove the slides and immediately immerse in 4% formaldehyde for 15 min.
Wash three times in PBS for 5 min each.
Load the slides into Shandon™ Sequenza™ Staining Rack (see Note 32) (Fig. 2).
Rinse slides with PBS.
Add 200 μL of PBT to slides and incubate for 10 min.
Wash two times in wash buffer for 2 min each.
Add 200 μL of blocking buffer and incubate for 30 min at room temperature (see Note 33).
Add 200 μL of the primary antibody mixture and incubate overnight at 4 °C. For negative control slides, add the diluent without the antibodies (see Note 34).
Wash three times in wash buffer for 5 min each.
Add 200 μL of the secondary antibody mixture and incubate for 1 h protected from light. All following steps should be protected from light to reduce photobleaching.
Rinse, stain with DAPI for 2 min, and coverslip the slides as described in Subheading 3.5, steps 8–12 (Fig. 10).
Fig. 9.
Cytospin materials and slide preparation. (a) Materials include, from left to right, a labeled new clean slide, a filter card, a funnel, and a cage. (b) Setup sequence: (1) Place the slide in the cage; (2) Cover the slide with the filter paper, making sure to align its bottom edge flush with the bottom of the cage; (3) Place the funnel over the filter paper and slide such that the bottom of the funnel is directed toward the hole in the filter paper. Clamp shut and place in cytocentrifuge. The cell suspension is placed into the funnel, and the cells will be distributed onto the slide upon centrifugation
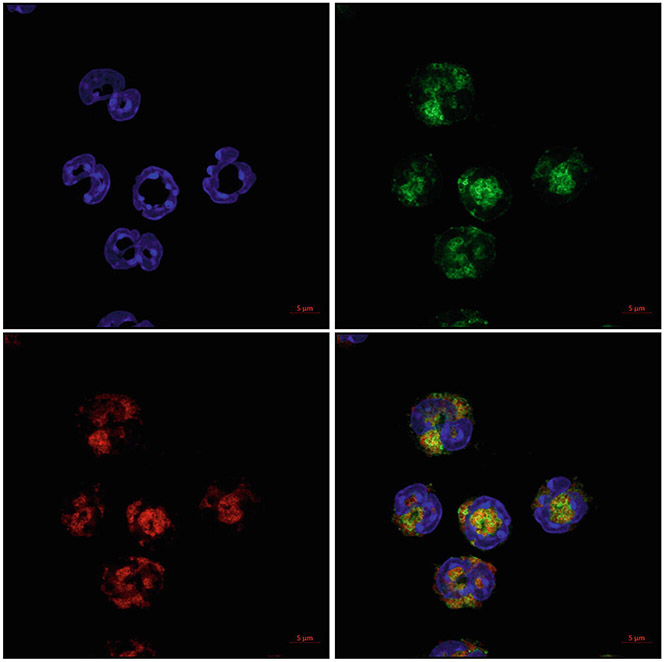
Fig. 10.
MBP and EPX dual fluorescent ICC. Cells were prepared by cytocentrifugation and then stained for both EPX and MBP. Eosinophils are stained for EPX (red) and MBP (green). Nuclei are counterstained with DAPI (blue). Image was acquired with a Plan-Apochromat ×63 objective using Airyscan on a Zeiss LSM 800 microscope
Acknowledgments
We would like to thank Dr. Matthew Rank, Dr. Benjamin Wright, Dr. Hirohito Kita, and Dr. Alfred Doyle for helping with discussions regarding this manuscript. Funding was supported by the NIH NIAID (AI132840-01A1 and AI145108-01) and Mayo Foundation.
Footnotes
Slide holders are not mandatory, but they are convenient for the deparaffinization/rehydration steps when working with multiple slides at once. These come in different sizes to meet your needs. Slide mailers are a cheap alternative that can be used to submerge slides into solutions.
Staining dishes are plastic, solvent resistant, and can tolerate the high temperature of a pressure cooker. They can handle rapid temperature changes and have a lid to reduce evaporation of solvents. Coplin jars or any solvent resistant container can be used as an alternative. These hold up to 24 slides, and we use a volume of 200 mL to submerge slides.
Xylene is highly flammable and should be kept under a fume hood in a closed container to avoid evaporation of fumes.
Ethanol is flammable and should be stored in a closed container to avoid evaporation.
The Shandon™ Sequenza™ Staining Rack requires a minimum 200 μL of solution per slide. The staining rack is convenient as all staining and washing steps are performed in a portable rack. Once the slides are loaded, there is no need to move them until the very end of the protocol. The coverplate/rack system also keeps the slides uniformly hydrated, preventing issues associated with cell or tissue dehydration. Alternatively, staining can be performed using traditional methods (hydrophobic pen/incubation in humidified chamber). However, it is important to keep the tissue wet throughout staining. Traditional methods require lower reagent volumes to be applied to each slide, which is a benefit over the rack method.
This protocol has been optimized using commercially available Digest-All™ 3 pepsin solution. Other pepsin solutions would require further optimization.
Dual Endogenous Enzyme Blocker (Agilent Dako) reagent helps to block endogenous peroxidases and phosphatases that may react with the chromogen and develop nonspecific background staining [43-45]. It is compatible with both HRP-based and AP-based detection protocols.
This step helps to block nonspecific binding of the primary antibody, as well as the secondary. The species of the serum may match the species in which the secondary antibody was raised, although goat serum is a common serum used for many protocols and sufficient with monoclonal primary rat and mouse antibodies. Normal sera can be stored short term at 4 °C, while long-term storage can be done at −20 °C. Centrifuge stock serum at 13,000 × g for 5 min before use to remove precipitates.
Rat anti-MBP (clone: MT2-14.7.3) [33] and mouse anti-EPX (clone: MM25-82.2.1) [31] are only available through Mayo Clinic at this time and can be obtained by contacting the senior author of this chapter and as described here [32]. These antibodies are highly purified by IgG column purification and prepared without sodium azide for storage. Stocks are validated in-house before shipment. Antibodies are aliquoted and shipped as 50 μg lyophilized samples that are stable for many years at −80 °C. Lyophilized antibodies are reconstituted with molecular grade water to generate 1 mg/mL antibody solution. Reconstituted antibodies are stable for greater than 6 months at 4 °C.
Antibody dilutions may require adjustment per tissue stained or fixation methods.
We have had great success using this specific secondary antibody, but this may be substituted for another AP-polymer secondary antibody system. Alternatively, as the dual enzyme block is used in this protocol, the AP detection system can be swapped for an HRP-based system with an appropriate chromogen (i.e., DAB). Various substrates with different colors and properties are available for both enzymes, so one might choose one enzyme over the other based on the substrate of interest [46, 47].
This chromogen is also fluorescent and can be viewed using Texas red filter (600–650 λ).
This specific retrieval buffer is important in blocking endogenous mouse IgG, which can cross-react with the secondary antibody, and be a source of background staining. This buffer also inactivates endogenous peroxidases, serving as an enzyme block and reducing background staining in HRP-based detection systems.
During fixation, epitopes are masked and heat-induced antigen retrieval helps to unmask these epitopes, so the primary antibody can bind the antigen of interest [20, 48, 49]. We prefer to use the Decloaker (Biocare) because of its precise control of temperature and time. This protocol does not call for high temperature/pressure, so any incubator that can reach 95 °C may be used.
This commercial blocking reagent helps to block endogenous mouse IgG and reduce nonspecific background staining in mouse tissues. When performing a mouse-on-mouse protocol, the secondary antibody cannot distinguish between the primary antibody and any endogenous IgG found within the tissue. If this endogenous IgG is not blocked sufficiently, it becomes a cause for high background staining.
Alternative DAB kits or HRP substrates can be used in place of this kit, but incubation times may require adjustment. Endogenous phosphatases might not be effectively blocked, so we do not recommend using an AP detection system with this protocol.
The acid rinse helps to remove nonspecific hematoxylin staining.
Hematoxylin will stain nuclei a reddish-purple, and this reagent changes it to a bluish-purple.
Cyanine 3 dye in the TSA kit is light-sensitive and requires protection from light. Working solution can be stored at 4 °C for up to 1 month. The concentration of the dye can be adjusted to increase the staining intensity, but EPX is a very abundant protein, and we have found that 1:800 gives a good signal-to-noise ratio. Too high concentrations of the dye can lead to increased background and signal developing outside the cell. Not only does TSA highly amplify the fluorescence signal, it is compatible with highly multiplexed techniques (reviewed here [36]) because the dye is covalently attached to the tissue.
DAPI is light-sensitive, so protect all solutions from light. DAPI is also a suspected carcinogen, so handle with proper personal protection equipment. We have found that DAPI containing mounting media causes background and prefer to do a separate staining step prior to mounting. Stock solution is stable for at least 6 months. The dilactate formulation is more water soluble than the dihydrochloride.
ProLong™ Diamond is a hardening reagent whose refractive index is highest once fully cured. Slides can be imaged immediately after coverslipping, but for optimal imaging allow reagent to cure. There is no need to seal the slide edges.
Protect fluorophore-conjugated antibodies from light. Centrifuge the antibody solution briefly to pellet aggregates—only use the supernatant. The fluorochrome(s) can be changed depending on the experiment and microscope setup. Alexa-based fluorophores are more stable than original fluorophores, such as FITC or rhodamine, when exposed to ambient light [50]. Autofluorescence in formalin-fixed samples can be seen at all visible wavelengths, but the intensity is the highest around the blue-green region (475–525 λ), so we prefer to use red-shifted colors (>525 λ) [34]. Various immunostaining methods to reduce FFPE autofluorescence in lung tissues are described elsewhere [51].
BSA takes a while to dissolve, and it is best to prepare ahead of time. After adding BSA to PBS, incubate at room temperature until fully dissolved (about 45 min for 5 g). To remove BSA stuck to the side of the container, gently swirl the solution but be careful not to over agitate, which will cause it to foam. For long-term storage at 4 °C, filter solution through 0.2 μm flask filter and maintain aseptic techniques.
Stock detergent solutions are very viscous. Aspirate and dispense slowly. We have found that swirling the pipette while dispensing into PBS helps to get the detergent into solution faster. 10% solution is not as viscous and is easier to pipette.
The syringe holding formalin needs to be 20–25 cm above the table to ensure proper pressure to inflate lungs to 25 cm H2O. This height results in approximately 70% of the air lung capacity being, providing optimal structural integrity for imaging, rather than complete lung collapse. By the time of embedding and slide preparation, though, the volume of the lung after dehydration and processing is not equivalent to a live viable lung [52, 53].
The most commonly used fixative is 10% neutral-buffered formalin (pH 7.0). Depending on the epitope and antibody parameters, many fixatives, such as zinc-formalin or glutaraldehyde-formalin (http://www.ihcworld.com/_protocols/histology/fixatives.htm), provide unique advantages but should be optimized before use as these fixatives may alter the antigenicity of the epitope of interest. Cryofixation and sectioning avoid the covalent crosslinking of these fixatives, as well as processing-induced removal of lipid-based compounds from tissues. However, these methods are beyond the scope of this chapter. The eosinophil antibody protocols listed here all use phosphate-free neutral-buffered formalin (ThermoFisher), which is the equivalent of a 4% (v/v) formaldehyde solution.
Although carbon dioxide (CO2) exposure is a common method of euthanasia, we recommend either ketamine-xylazine or sodium pentobarbital-based euthanasia method as CO2 may result in hemorrhaging of the lung [54, 55]. Depending on the physiological kinetics of the molecules being studied, other considerations may be taken into account when selecting euthanasia methods [56]. Please review AVMA (American Veterinary Medical Association) Guidelines for the Euthanasia of Animals (https://www.avma.org/kb/policies/documents/euthanasia.pdf) or appropriate guidelines for animal use at your institution.
Before the lung is filled with formalin, the lungs may be manipulated for additional usages. For example, one may perform a bronchoalveolar lavage (BAL) by inserting an 18G catheter with a syringe filled with 1 mL of PBS at a tracheotomy site [57]. However, this may lead to some structural changes in lung architecture due to the pressure changes to obtain BAL. If perfusion is needed to clear the circulatory system of blood, this may be performed once the heart is exposed soon after euthanasia to avoid clotting. If only one lobe of the lungs is needed for IHC, suture material may be used to tie off the right or left lobe and cut off the main bronchus of that lobe distal to the knot and trachea. The separated lobe may be used for flow cytometry or other measures. The knot creates a closure so that the lobe left behind is still filled with formalin without leakage and may be used for histology.
Fixation time and temperature can alter the extent of covalent bonds and therefore the epitope availability for IHC [49, 58, 59]. For long-term storage, formalin-fixed samples may be dehydrated and stored in 100% ethanol (200 proof).
This step softens paraffin prior to deparaffinization. Slide should be kept upright and incubated for a minimum of 15 min and up to 1 h. We have found 30 min to be optimal. If problematic, the incubation can be done immersed in xylene so long as ventilation is good, and lid remains sealed on container.
Xylene is used to dissolve paraffin wax. During all washes, agitate slides once every minute by lifting them up and down.
To avoid trapping air bubbles, load slides onto coverplates submerged in distilled water (Fig. 2). After loading onto the rack, add water to slides to ensure the flow is slow and consistent. Rapid draining is indicative of an incorrect setup. In this case, try to reload the coverplate and slide and repeat the drain test. Make sure all incubations are done with the lid of the rack on to maintain humidity. This reduces evaporation of reagents on slides. If not using a rack, make sure slides are kept wet in a humid enclosure.
Allow at least 30-min incubation to efficiently block the tissue at room temperature. Incubations can be extended without any detrimental effects. Overnight incubations at 4 °C are often acceptable as well. Do not wash off blocking buffer before adding primary antibodies. The staining rack will drain excess blocking buffer when antibodies are added. If not using a staining rack, remove blocking buffer by tapping side of slide on a paper towel before adding antibodies.
This is to control for nonspecific binding of the secondary antibody. IgG isotype antibody can also be used to control for nonspecific binding of the primary antibody. Always run a negative control slide (not containing primary antibody) with experiments and, if possible, have the negative control be a serial section of the sample or at minimum the same tissue origin and conditions.
Increase or decrease incubation time to optimize staining intensity.
While the slides can be air-dried overnight, the dehydration allows the slides to be coverslipped within 10 min.
Depending on the number of slides being stained, we use a plastic coplin jar (<5 slides) or staining dish (6–24 slides) to hold our slides during antigen retrieval.
Gently run water into the container until all foam/bubbles are gone. Ensure the water stream is not directly on the sections to avoid damaging tissues.
Try to remove as much buffer as possible without letting specimen dry by gently tapping the slide on a paper towel. ProLong™ is a viscous reagent. If using a micropipette to dispense the reagent, ensure to pipette slowly to prevent bubbles. We usually load a pipette tip with the mountant before removing slides from the staining rack to prevent excessive drying of the tissue. If bubbles form on specimens, use a 10-μL micropipette tip to pop or aspirate bubbles. If bubbles form in the stock reagent, transfer to a microcentrifuge tube and centrifuge at 10,000 × g for 2 min. Protect ProLong™ from light for it is light sensitive.
ProLong™ Diamond is a hardening reagent whose refractive index is highest once fully cured. Slides can be imaged immediately after coverslipping, but for the best images, wait for the reagent to cure. There is no need to seal the slide. Caution must be taken when handling/imaging slides that have not been cured as the coverslip can slide around.
The lung FFPE EPX IF and TSA protocols can be adapted for multiplex staining by the addition of other primary antibodies and their corresponding secondary antibodies. This will require optimization of antigen retrieval, blocking steps, and antibody dilutions similar to as described above and in literature [60, 61].
Techniques for peripheral blood isolation or brochoalveolar isolation are described elsewhere [57, 62]. Make sure the cells stay cold on ice to maintain viability. Cell numbers can be modified to fit experimental needs, but this density of cell suspension yields a nice uncrowded distribution of cells.
Pre-wetting the slides with BSA helps the cells stick to the slides. Set up as many cytospin cages as needed for your experiment. Make sure to always have an even number of cages to counterbalance the centrifuge. The majority of cytospin centrifuges have their speed setting in RPM, which is equivalent of approximately 28 × g.
References
- 1.Walsh GM, Al-Rabia M, Blaylock MG, Sexton DW, Duncan CJ, Lawrie A (2005) Control of eosinophil toxicity in the lung. Curr Drug Targets Inflamm Allergy 4(4):481–486 [DOI] [PubMed] [Google Scholar]
- 2.Aleman F, Lim HF, Nair P (2016) Eosinophilic endotype of asthma. Immunol Allergy Clin N Am 36(3):559–568. 10.1016/j.iac.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 3.Kariyawasam HH, Robinson DS (2006) The eosinophil: the cell and its weapons, the cytokines, its locations. Semin Respir Crit Care Med 27(2):117–127. 10.1055/s-2006-939514 [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen EA, Lee NA, Lee JJ (2014) Re-defining the unique roles for eosinophils in allergic respiratory inflammation. Clin Exp Allergy 44(9):1119–1136. 10.1111/cea.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochner BS (2018) The eosinophil: for better or worse, in sickness and in health. Ann Allergy Asthma Immunol 121(2):150–155. 10.1016/j.anai.2018.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weller PF, Spencer LA (2017) Functions of tissue-resident eosinophils. Nat Rev Immunol 17(12):746–760. 10.1038/nri.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg HF, Dyer KD, Foster PS (2013) Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 13(1):9–22. 10.1038/nri3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA (2010) Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy 40(4):563–575. 10.1111/j.1365-2222.2010.03484.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busse WW, Lemanske RF Jr (2001) Asthma. N Engl J Med 344(5):350–362. 10.1056/NEJM200102013440507 [DOI] [PubMed] [Google Scholar]
- 10.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME (2008) Eosinophils: biological properties and role in health and disease. Clin Exp Allergy 38(5):709–750. 10.1111/j.1365-2222.2008.02958.x [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen EA, Helmers RA, Lee JJ, Lee NA (2012) The expanding role(s) of eosinophils in health and disease. Blood 120 (19):3882–3890. 10.1182/blood-2012-06-330845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer-Martin H, Reuter S, Taube C (2014) Mouse models of allergic airway disease. Methods Mol Biol 1193:127–141. 10.1007/978-1-4939-1212-4_13 [DOI] [PubMed] [Google Scholar]
- 13.Marques-Garcia F, Marcos-Vadillo E (2016) Review of mouse models applied to the study of asthma. Methods Mol Biol 1434:213–222. 10.1007/978-1-4939-3652-6_15 [DOI] [PubMed] [Google Scholar]
- 14.Denzler KL, Borchers MT, Crosby JR, Cieslewicz G, Hines EM, Justice JP, Cormier SA, Lindenberger KA, Song W, Wu W, Hazen SL, Gleich GJ, Lee JJ, Lee NA (2001) Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J Immunol 167 (3):1672–1682. 10.4049/jimmunol.167.3.1672 [DOI] [PubMed] [Google Scholar]
- 15.Willetts L, Felix LC, Jacobsen EA, Puttagunta L, Condjella RM, Zellner KR, Ochkur SI, Kim JD, Luo H, Lee NA, Lee JJ, Moqbel R, Lacy P (2018) Vesicle-associated membrane protein 7-mediated eosinophil degranulation promotes allergic airway inflammation in mice. Commun Biol 1:83 10.1038/s42003-018-0081-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobsen EA, Ochkur SI, Doyle AD, LeSuer WE, Li W, Protheroe CA, Colbert D, Zellner KR, Shen HH, Irvin CG, Lee JJ, Lee NA (2017) Lung pathologies in a chronic inflammation mouse model are independent of eosinophil degranulation. Am J Respir Crit Care Med 195(10):1321–1332. 10.1164/rccm.201606-1129OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willetts L, Parker K, Wesselius LJ, Protheroe CA, Jaben E, Graziano P, Moqbel R, Leslie KO, Lee NA, Lee JJ (2011) Immunodetection of occult eosinophils in lung tissue biopsies may help predict survival in acute lung injury. Respir Res 12:116 10.1186/1465-9921-12-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kay AB (2016) Paul Ehrlich and the early history of granulocytes. Microbiol Spectr 4(4). 10.1128/microbiolspec.MCHD-0032-2016 [DOI] [PubMed] [Google Scholar]
- 19.Meyerholz DK, Griffin MA, Castilow EM, Varga SM (2009) Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicol Pathol 37(2):249–255. 10.1177/0192623308329342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magaki S, Hojat SA, Wei B, So A, Yong WH (2019) An introduction to the performance of immunohistochemistry. Methods Mol Biol 1897:289–298. 10.1007/978-1-4939-8935-5_25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivell R, Teerds K, Hoffman GE (2014) Proper application of antibodies for immunohistochemical detection: antibody crimes and how to prevent them. Endocrinology 155 (3):676–687. 10.1210/en.2013-1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saper CB (2009) A guide to the perplexed on the specificity of antibodies. J Histochem Cytochem 57(1):1–5. 10.1369/jhc.2008.952770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alkan SS (2004) Monoclonal antibodies: the story of a discovery that revolutionized science and medicine. Nat Rev Immunol 4 (2):153–156. 10.1038/nri1265 [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg HF (2015) Eosinophil-derived neurotoxin (EDN/RNase 2) and the mouse eosinophil-associated RNases (mEars): expanding roles in promoting host defense. Int J Mol Sci 16(7):15442–15455. 10.3390/ijms160715442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acharya KR, Ackerman SJ (2014) Eosinophil granule proteins: form and function. J Biol Chem 289(25):17406–17415. 10.1074/jbc.R113.546218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochkur SI, Kim JD, Protheroe CA, Colbert D, Condjella RM, Bersoux S, Helmers RA, Moqbel R, Lacy P, Kelly EA, Jarjour NN, Kern R, Peters A, Schleimer RP, Furuta GT, Nair P, Lee JJ, Lee NA (2012) A sensitive high throughput ELISA for human eosinophil peroxidase: a specific assay to quantify eosinophil degranulation from patient-derived sources. J Immunol Methods 384(1–2):10–20. 10.1016/j.jim.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bochner BS (2009) Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy 39(3):317–324. 10.1111/j.1365-2222.2008.03173.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng YH, Mao H (2012) Expression and preliminary functional analysis of Siglec-F on mouse macrophages. J Zhejiang Univ Sci B 13(5):386–394. 10.1631/jzus.B1100218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA (2004) Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305(5691):1773–1776. 10.1126/science.1099472 [DOI] [PubMed] [Google Scholar]
- 30.Ochkur SI, Doyle AD, Jacobsen EA, LeSuer WE, Li W, Protheroe CA, Zellner KR, Colbert D, Shen HH, Irvin CG, Lee JJ, Lee NA (2017) Frontline science: eosinophil-deficient MBP-1 and EPX double-knockout mice link pulmonary remodeling and airway dysfunction with type 2 inflammation. J Leukoc Biol 102(3):589–599. 10.1189/jlb.3HI1116-488RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochkur SI, Kim JD, Protheroe CA, Colbert D, Moqbel R, Lacy P, Lee JJ, Lee NA (2012) The development of a sensitive and specific ELISA for mouse eosinophil peroxidase: assessment of eosinophil degranulation ex vivo and in models of human disease. J Immunol Methods 375 (1–2):138–147. 10.1016/j.jim.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoury P, Akuthota P, Ackerman SJ, Arron JR, Bochner BS, Collins MH, Kahn JE, Fulkerson PC, Gleich GJ, Gopal-Srivastava R, Jacobsen EA, Leiferman KM, Francesca LS, Mathur SK, Minnicozzi M, Prussin C, Rothenberg ME, Roufosse F, Sable K, Simon D, Simon HU, Spencer LA, Steinfeld J, Wardlaw AJ, Wechsler ME, Weller PF, Klion AD (2018) Revisiting the NIH taskforce on the Research needs of Eosinophil-Associated Diseases (RE-TREAD). J Leukoc Biol 104(1):69–83. 10.1002/JLB.5MR0118-028R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denzler KL, Farmer SC, Crosby JR, Borchers M, Cieslewicz G, Larson KA, Cormier-Regard S, Lee NA, Lee JJ (2000) Eosinophil major basic protein-1 does not contribute to allergen-induced airway pathologies in mouse models of asthma. J Immunol 165 (10):5509–5517. 10.4049/jimmunol.165.10.5509 [DOI] [PubMed] [Google Scholar]
- 34.O’Hurley G, Sjostedt E, Rahman A, Li B, Kampf C, Ponten F, Gallagher WM, Lindskog C (2014) Garbage in, garbage out: a critical evaluation of strategies used for validation of immunohistochemical biomarkers. Mol Oncol 8(4):783–798. 10.1016/j.molonc.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prost S, Kishen RE, Kluth DC, Bellamy CO (2016) Choice of illumination system & fluorophore for multiplex immunofluorescence on FFPE tissue sections. PLoS One 11(9): e0162419 10.1371/journal.pone.0162419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stack EC, Wang C, Roman KA, Hoyt CC (2014) Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 70(1):46–58. 10.1016/j.ymeth.2014.08.016 [DOI] [PubMed] [Google Scholar]
- 37.Donaldson JG (2015) Immunofluorescence staining. Curr Protoc Cell Biol 69:4.3.1–4.3.7. 10.1002/0471143030.cb0403s69 [DOI] [PubMed] [Google Scholar]
- 38.Dixon AR, Bathany C, Tsuei M, White J, Barald KF, Takayama S (2015) Recent developments in multiplexing techniques for immunohistochemistry. Expert Rev Mol Diagn 15(9):1171–1186. 10.1586/14737159.2015.1069182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koh J, Kwak Y, Kim J, Kim WH (2020) High-throughput multiplex immunohistochemical imaging of the tumor and its microenvironment. Cancer Res Treat 52(1):98–108. 10.4143/crt.2019.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anyaegbu CC, Lee-Pullen TF, Miller TJ, Abel TN, Platell CF, McCoy MJ (2019) Optimisation of multiplex immunofluorescence for a non-spectral fluorescence scanning system. J Immunol Methods 472:25–34. 10.1016/j.jim.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 41.Jacobsen EA, Lesuer WE, Willetts L, Zellner KR, Mazzolini K, Antonios N, Beck B, Protheroe C, Ochkur SI, Colbert D, Lacy P, Moqbel R, Appleton J, Lee NA, Lee JJ (2014) Eosinophil activities modulate the immune/inflammatory character of allergic respiratory responses in mice. Allergy 69(3):315–327. 10.1111/all.12321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morton J, Snider TA (2017) Guidelines for collection and processing of lungs from aged mice for histological studies. Pathobiol Aging Age Relat Dis 7(1):1313676 10.1080/20010001.2017.1313676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radulescu RT, Boenisch T (2007) Blocking endogenous peroxidases: a cautionary note for immunohistochemistry. J Cell Mol Med 11 (6):1419 10.1111/jY582-4934.2007.00185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garba MT, Marie PJ (1986) Alkaline phosphatase inhibition by levamisole prevents 1,25-dihydroxyvitamin D3-stimulated bone mineralization in the mouse. Calcif Tissue Int 38(5) :296–302. 10.1007/bf02556610 [DOI] [PubMed] [Google Scholar]
- 45.Kim SW, Roh J, Park CS (2016) Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J Pathol Transl Med 50 (6) :411–418. 10.4132/jptm.2016.08.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petersen KH, Lohse J, Ramsgaard L (2018) Automated sequential chromogenic IHC double staining with two HRP substrates. PLoS One 13(11):e0207867 10.1371/journal.pone.0207867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osman TA, Oijordsbakken G, Costea DE, Johannessen AC (2013) Successful triple immunoenzymatic method employing primary antibodies from same species and same immunoglobulin subclass. Eur J Histochem 57(3): e22 10.4081/ejh.2013.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi SR, Shi Y, Taylor CR (2011) Antigen retrieval immunohistochemistry: review and future prospects in research and diagnosis over two decades. J Histochem Cytochem 59 (1):13–32. 10.1369/jhc.2010.957191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi SR, Key ME, Kalra KL (1991) Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39(6):741–748. 10.1177/39.6.1709656 [DOI] [PubMed] [Google Scholar]
- 50.Mahmoudian J, Hadavi R, Jeddi-Tehrani M, Mahmoudi AR, Bayat AA, Shaban E, Vafakhah M, Darzi M, Tarahomi M, Ghods R (2011) Comparison of the photobleaching and photostability traits of Alexa Fluor 568- and fluorescein isothiocyanate-conjugated antibody. Cell J 13(3):169–172 [PMC free article] [PubMed] [Google Scholar]
- 51.Davis AS, Richter A, Becker S, Moyer JE, Sandouk A, Skinner J, Taubenberger JK (2014) Characterizing and diminishing auto-fluorescence in formalin-fixed paraffin-embedded human respiratory tissue. J Histochem Cytochem 62(6):405–423. 10.1369/0022155414531549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lum H, Mitzner W (1985) Effects of 10% formalin fixation on fixed lung volume and lung tissue shrinkage. A comparison of eleven laboratory species. Am Rev Respir Dis 132 (5):1078–1083. 10.1164/arrd.1985.132.5.1078 [DOI] [PubMed] [Google Scholar]
- 53.Limjunyawong N, Mock J, Mitzner W (2015) Instillation and fixation methods useful in mouse lung cancer research. J Vis Exp 102: e52964 10.3791/52964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boivin GP, Hickman DL, Creamer-Hente MA, Pritchett-Corning KR, Bratcher NA (2017) Review of CO(2) as a euthanasia agent for laboratory rats and mice. J Am Assoc Lab Anim Sci 56(5):491–499 [PMC free article] [PubMed] [Google Scholar]
- 55.Fisher S, Burgess WL, Hines KD, Mason GL, Owiny JR (2016) Interstrain differences in CO2-induced pulmonary hemorrhage in mice. J Am Assoc Lab Anim Sci 55(6):811–815 [PMC free article] [PubMed] [Google Scholar]
- 56.Schoell AR, Heyde BR, Weir DE, Chiang PC, Hu Y, Tung DK(2009) Euthanasia method for mice in rapid time-course pulmonary pharmacokinetic studies. J Am Assoc Lab Anim Sci 48 (5):506–511 [PMC free article] [PubMed] [Google Scholar]
- 57.Van Hoecke L, Job ER, Saelens X, Roose K (2017) Bronchoalveolar lavage of murine lungs to analyze inflammatory cell infiltration. J Vis Exp 123 10.3791/55398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bass BP, Engel KB, Greytak SR, Moore HM (2014) A review of preanalytical factors affecting molecular, protein, and morphological analysis of formalin-fixed, paraffin-embedded (FFPE) tissue: how well do you know your FFPE specimen? Arch Pathol Lab Med 138 (11):1520–1530. 10.5858/arpa.2013-0691-RA [DOI] [PubMed] [Google Scholar]
- 59.Bogen SA, Vani K, Sompuram SR (2009) Molecular mechanisms of antigen retrieval: antigen retrieval reverses steric interference caused by formalin-induced cross-links. Bio-tech Histochem 84(5):207–215. 10.3109/10520290903039078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kajimura J, Ito R, Manley NR, Hale LP (2016) Optimization of single- and dual-color immunofluorescence protocols for formalin-fixed, paraffin-embedded archival tissues. J Histochem Cytochem 64(2):112–124. 10.1369/0022155415610792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isidro RA, Isidro AA, Cruz ML, Hernandez S, Appleyard CB (2015) Double immunofluorescent staining of rat macrophages in formalin-fixed paraffin-embedded tissue using two monoclonal mouse antibodies. Histochem Cell Biol 144(6):613–621. 10.1007/s00418-015-1364-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reichman H, Rozenberg P, Munitz A (2017) Mouse eosinophils: identification, isolation, and functional analysis. Curr Protoc Immunol 119:14.43.11–14.43.22. 10.1002/cpim.35 [DOI] [PubMed] [Google Scholar]