Abstract
Coronavirus disease 2019 (COVID-19), driven by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared a global pandemic in March 2020. Pathogenic T cells and inflammatory monocytes are regarded as the central drivers of the cytokine storm associated with the severity of COVID-19. In this study, we explored the characteristic peripheral cellular profiles of patients with COVID-19 in both acute and convalescent phases by single-cell mass cytometry (CyTOF). Using a combination of algorithm-guided data analyses, we identified peripheral immune cell subsets in COVID-19 and revealed CD4+ T-cell depletion, T-cell differentiation, plasma cell expansion, and the reduced antigen presentation capacity of innate immunity. Notably, COVID-19 induces a dysregulation in the balance of monocyte populations by the expansion of the monocyte subsets. Collectively, our results represent a high-dimensional, single-cell profile of the peripheral immune response to SARS-CoV-2 infection.
Keywords: acute phase, convalescent phase, COVID-19, CyTOF, peripheral blood mononuclear cell, SARS-CoV-2
INTRODUCTION
Coronavirus disease (COVID-19), a highly infectious disease characterized by viral pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has raised unprecedented global concern (64). Within 6 months, more than 7 million infections and more than 400,000 COVID-19-related deaths have been reported globally, stressing the health care systems (36). The genome of SARS-CoV-2 is ∼80% identical to that of SARS-CoV (72). The symptoms of COVID-19 mainly include fever, dry cough, dyspnea, fatigue, myalgia, and pneumonia, and clinical conditions range from asymptomatic to critically ill (20). Although most patients present mild or moderate symptoms and have a good prognosis, ∼20% of patients with COVID-19 develop severe pneumonia, acute respiratory distress syndrome, or multiple organ failure and require intensive care, and this patient subset shows an elevated rate of mortality (65). Given this situation, there is an ongoing interest in the identification of prognostic markers and immunological features from the easily accessible peripheral blood (11, 18, 42). The recent development of unbiased single-cell technologies with high accuracy and specificity has been employed in understanding the peripheral immune responses of COVID-19. These technologies allow scientists to obtain a comprehensive profile and develop new therapeutic strategies (4, 21, 29, 56, 60, 67, 76, 77). Peripheral immunity signature traits, including lymphocytes and myeloid cells along with their associated proinflammatory cytokines, have been identified as important indicators correlated with disease severity (20, 51, 58). Certain peripheral immunotype changes and cytokine secretion have also been reported to be associated with poor prognosis (26, 28), including a set of inflammatory cytokines (5, 31, 76), inflammatory monocytes (2, 14, 60), pathogenic T cells (9, 32, 69, 70, 75), and lymphopenia (40, 57, 74). Knowledge regarding the precise alterations in peripheral immunity in COVID-19 is still controversial (26, 62). The understanding of the immunopathology in SARS-CoV-2 infection is one that has major hurdles currently impeding COVID-19 treatment and vaccine design and evaluation.
In this study, we applied mass cytometry by time-of-flight (CyTOF) to comprehensively depict changes in the peripheral blood mononuclear cell profiles in nine patients infected with SARS-CoV-2, including five patients in the acute phase (AP) and four patients in the convalescent phase (CP). Using unsupervised automated clustering, we unveiled the peripheral blood immune cell landscape across different phases in response to SARS-CoV-2.
MATERIALS AND METHODS
Patients
Cryopreserved peripheral blood samples were obtained at Wuhan Hankou Hospital, China for mass cytometry from COVID-19 patients after obtaining written informed consent. Patients were divided into two groups according to the disease activity; the acute group was defined as an acute COVID-19-positive via real-time RT-PCR or via computed tomography findings that reported a pulmonary shadow with a low count of lymphocytes (<1.0 × 109/L). The convalescent group was identified via a negative real-time RT-PCR test. The 14 patients consisted of five men and six women and ranged from ages 41 to 79 yr old, with a median of 66 yr old. The clinical characteristic of the patients is provided in Supplemental Table S1 (all supplemental material is available at https://doi.org/10.6084/m9.figshare.12731072). The study was approved by the Ethics Committee of Wuhan Hankou Hospital, China.
Quantitative RT-PCR
The pharyngeal and nasal swab specimens were collected from patients at various time points after hospitalization. Viral RNAs were extracted from specimens using the QIAamp RNA viral kit (Qiagen, Heiden, Germany). The quantitative RT-PCR (qRT-PCR) was performed using the primers and probes targeting ORF1ab and N genes of SARS-CoV-2 according to the Chinese Center for Disease Control and Prevention guidance, and a commercial kit specific for SARS-CoV-2 detection (GeneoDX, Shanghai, China). A tentatively positive test was defined tentatively positive if qRT-PCR cycle threshold value (Ct value) ≤37 at any time-points during the hospitalization, which is recommended by the National Institute for Viral Disease Control and Prevention (China). Then the assay was repeated to confirm the positivity.
Antibodies.
Antibodies (Supplemental Table S2) were purchased preconjugated from Fluidigm or were purchased purified form and conjugated in-house using the Maxpar Direct Immune Profiling Assay kit (Fluidigm), according to the manufacturer’s instructions.
Live-Cell Barcoding
Cells from peripheral venous blood were labeled with a unique barcode by incubating with CD45-antibodies conjugated to distinct mental isotopes (162Dy,165Ho, 169Tm, and 175Lu). Samples were washed twice in the cell staining medium (CSM) and pooled into a single reaction vessel for further staining steps. Using this approach, we combined and processed up to five samples together, minimizing intersample staining variability, sample handling time, and antibody consumption.
Surface Staining
Barcoded cells were washed and incubated for 30 min at 4°C with metal-conjugated antibodies targeting surface antigens in 50 μl of surface-antibodies mixture (Table S1) in CSM. The pooled sample was stained for live-dead cell distinction with 0.5 μM cisplatin in PBS (Fluidigm) for 2 min at room temperature (RT), and incubated in a final concentration of 0.125 nM Ir intercalator (Fluidigm) diluted in PBS containing 1.6% formaldehyde, and stored at 4°C until acquisition.
Data Acquisition and Preprocessing
Immediately before data acquisition, the sample was washed once with PBS, once with deionized water, and resuspended at a concentration of 1.0 × 106 cells/mL in the 1/10 dilution of Eq. 4 Element Beads (Fluidigm) solution. The samples were acquired on a Helios (Fluidigm) at an event rate of 300–500 event/s with noise deduction. Before downstream analysis, barcodes were deconvoluted by manual Boolean gating in the case of CD45-barcoded samples using Cytobank (6). The data were gated to identify cell events (DNAhi) and exclusion of dead or dying cells (cisplatin+). The live cells were left for subsequent clustering and high dimensional analyses.
Dimensionality Reduction and Clustering
After preprocessing, all the FCS files were exported from Cytobank and read into R using flowCore R package (17). Preprocessed data were down-sampled to a maximum of 20,000 cells per sample and combined into a single data set for batch normalization. We then performed batch correction using Harmony R package (24) with default parameters. The data were arcsine normalized and filtered to keep the top genes based on variance across the aggregated data set. At last, Harmony batch correction was performed for each sample. We analyzed 100,000 cells in healthy control (HC) group, 100,000 cells in the AP group, 80,000 cells in the CP group.
We then used Seurat R package (3) for clustering, dimensionality reduction. We performed principal component analysis using variable genes, and the first 30 principal components (PCs) were used to perform t-stochastic neighbor embedding (t-SNE) analysis, a dimensionality-reducing visualization tool, to embed the data set into two dimensions. To construct a shared nearest-neighbor graph, the first 30 PCs were used. Next, we clustered the data set by a graph-based modularity-optimization algorithm of the Louvain method for cell detection. Clusters were manually annotated on the basis of canonical marker expression.
Statistical Analysis
Statistical analysis of the frequencies of immune cell subpopulations between groups were compared using the two-way ANOVA tests with Bonferroni’s post hoc correction with GraphPad Prism 8.0. Statistical analysis of the protein expression of each cell between groups was compared using two-tailed Wilcoxon rank-sum test with R (3.6.3). Two-sided P values of less than 0.05 were considered statistically significant.
Data Availability
Mass cytometry data analyzed in the article (Figs. 1–6) are available in a public repository at http://flowrepository.org/id/FR-FCM-Z2RC.
Figure 1.
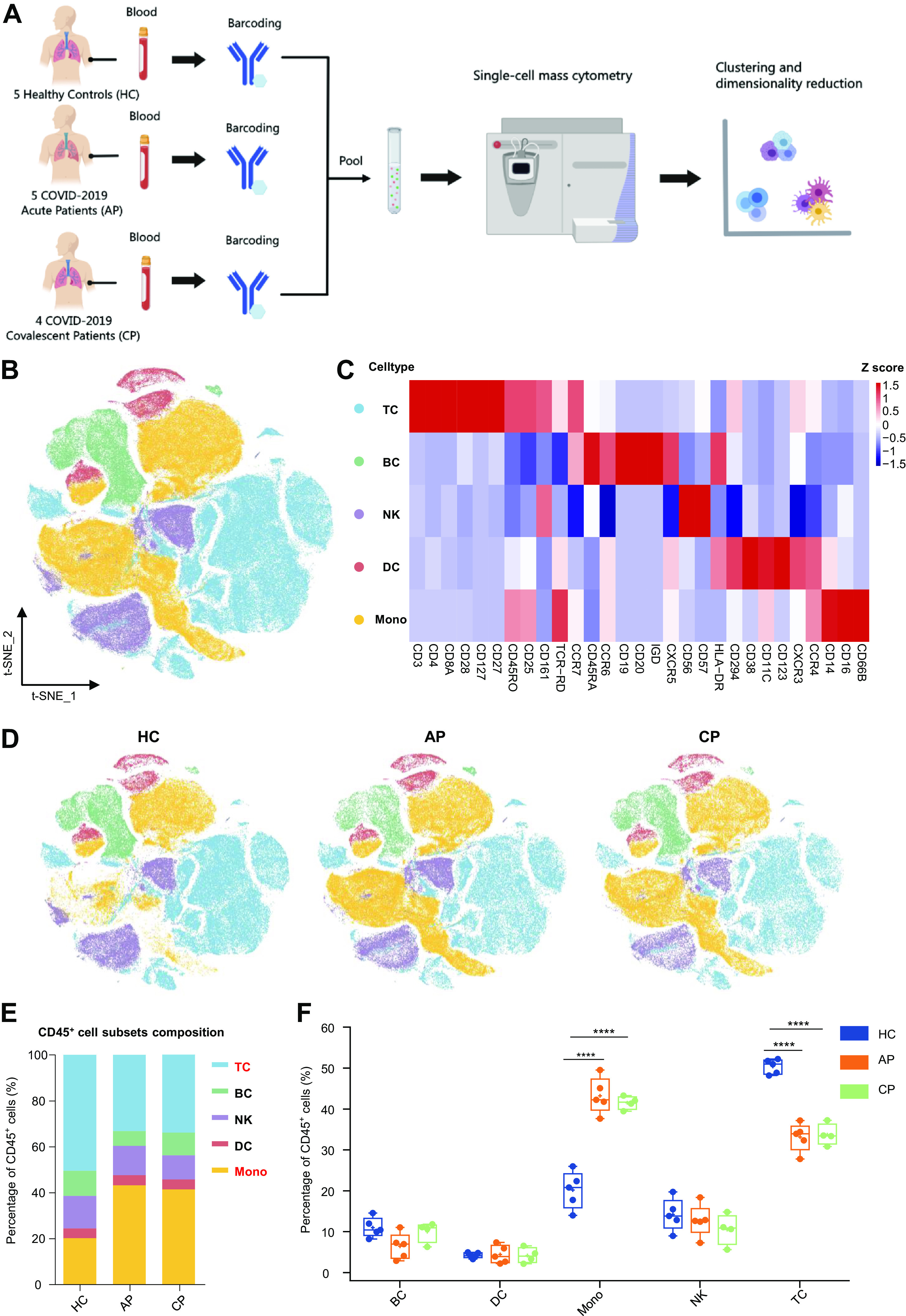
Experimental approach and characterization of blood CD45+ immune cells. A: experimental outline showing peripheral blood mononuclear cells (PBMC) collection and mass cytometry analysis. B: t-distributed stochastic neighbor embedding (t-SNE) plot of major immune cells in PBMCs. Cells are colored on the basis of cell types. C: heat map of major immune cells in PBMCs, clustered by their relative expression of the markers. D: t-SNE projections of major immune cells in PBMCs: 100,000, 100,000 and 80,000 cells from the healthy control (HC), acute phase (AP), and convalescent phase (CP) groups, respectively. E: relative fractions of major immune cells in blood CD45+-immune cells from the HC, AP, and CP group. F: percentage of major immune cells in blood CD45+ immune cells from the HC (n = 5), AP (n = 5), and CP group (n = 4). Adjusted P values are based on two-way ANOVA tests with Bonferroni’s post hoc correction between groups. ****Significant with adjusted P < 0.0001.
Fig. 6.
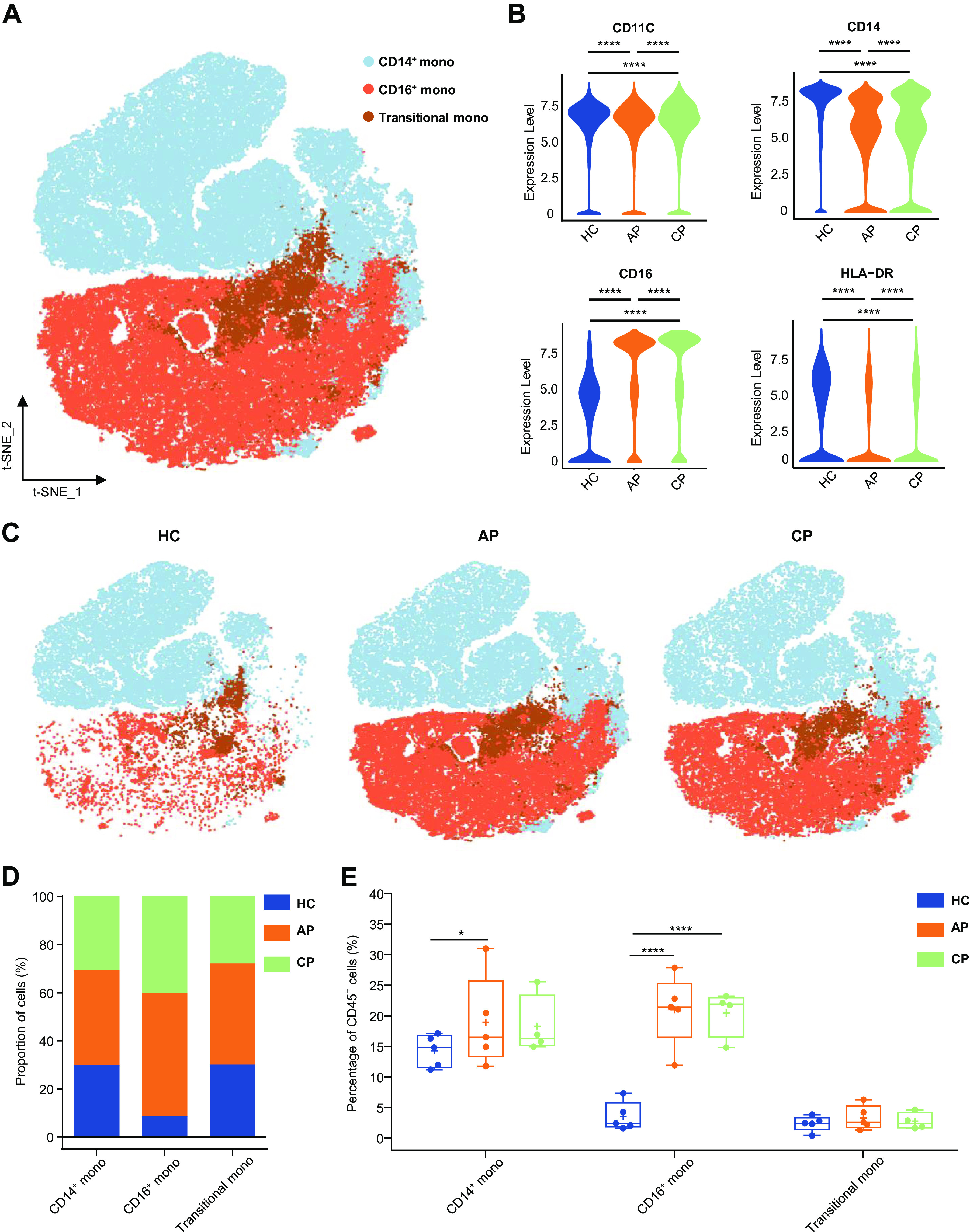
Characterization of single-cell monocytes from mass cytometry data. A: t-distributed stochastic neighbor embedding (t-SNE) plot of major monocyte subsets. Cells are colored on the basis of cell types. B: violin plot showing the expression of CD11C, CD14, CD16, HLA-DR in the monocytes cluster between (HC), acute phase (AP) and CP group. C: t-SNE projections of major monocyte subsets from the HC, AP, and CP group, respectively. D: bar plots highlighting cell abundances across monocyte subsets for the HC, AP, and CP group. E: percentage of major monocyte subsets in all blood CD45+ immune cells from the HC (n = 5), AP (n = 5), and CP group (n = 4). Adjusted P values are based on Wilcoxon rank-sum test (marker level) or two-way ANOVA tests with Bonferroni’s post hoc correction (parametric) between groups. ****Significant with adjusted P value < 0.0001. *Significant with adjusted P value < 0.05.
RESULTS
Single-Cell Mass Cytometry for the Analysis of Peripheral Immunity in COVID-19
To shape the immune cell landscape in peripheral circulation during SARS-CoV-2 infection, CyTOF was used to evaluate five healthy controls (HC) and nine patients with COVID-19 at different disease phases (AP, n = 5; CP, n = 4; Fig. 1A). The median age of patients was 66 yr; four of them were men and five were women (Supplemental Table S1). Among them, four patients had severe cases, three had moderate cases, and two had critical cases. Two patients received methylprednisolone in the hospital, both before sampling. Eight out of nine patients received antiviral treatment, such as remdesivir and oseltamivir at some point before sampling (Supplemental Table S1). After barcoding live cells, all samples were pooled, stained with distinct heavy metal-conjugated antibodies (Supplemental Table S2), and acquired using a mass cytometer. Following data preprocessing, we used the Seurat R package (3) to analyze the distribution of five major immune cell subsets (T cells, B cells, NK cells, DCs, and monocytes Fig. 1, B and C) and then visualized the high-dimensional data using the t-stochastic neighbor embedding (t-SNE) algorithm. To obtain a comprehensive view of immune changes, we also identified all immune cell subsets, according to the expression of canonical lineage markers (Supplemental Fig. S1A) and reclustered each cell type separately (Supplemental Fig. S1B).
Next, we explored whether the relative frequencies of CD45+ immune cell populations were altered in AP versus HC and CP (Fig. 1D). The composition of immune cells differed substantially between patients and HCs; both the AP and CP groups showed an obvious reduction in the frequency of T cells (HC vs. AP: P < 0.0001; HC vs. CP: P < 0.0001) and a significant elevation in monocytes (HC vs. AP; P < 0.0001; HC vs. CP; P < 0.0001), suggesting that T cells and monocytes are the most affected peripheral immune cell types by COVID-19 (Fig. 1, E and F). Immune cell frequencies were comparable in the AP and CP groups, which indicated that SARS-CoV-2 infection could lead to a profound immune dysfunction.
SARS-CoV-2 Infection Results in Heterogeneous T-Cell Responses in Human Blood
Human T cells are among the main immune effectors against viral infection and play key roles in viral clearance in the host defense against acute respiratory infections, especially SARS-CoV-2 (50). It has been reported that the number and functional diversity of T cells decrease in patients with COVID-19 (40). We identified six cell subsets, according to the expression of canonical lineage markers and visualized the results using t-SNE (Fig. 2A; Supplemental Fig. S2A). Violin plots were generated to summarize the expression of four classical markers in the HC, AP, and CP groups (Fig. 2B). The expressions of CD4 and CD28 were more abundant in the HC group than in the AP and CP groups. In contrast, cells expressing CD27 and CD161 were more abundant in AP and CP than in HC. These findings indicated that infection with SARS-CoV-2 drives heterogeneous T-cell responses in peripheral blood. We compared the absolute number of T-cell subsets between the three groups to identify the effects of COVID-19 (Supplemental Fig. S2B). We found that there was a decline in the two most common T-cell types (CD4+ T cells and CD8+ T cells) in patients when compared with HC (Fig. 2, C–E). We further investigated the COVID-19-driven modulation of CD45+ immune cell proportion. The number of CD4+ T cells among CD45+ T cells decreased significantly (HC vs. AP; P < 0.0001; HC vs. CP; P < 0.0001; Fig. 2, C and D). Additionally, there was a significant decline in the frequency of CD8+ T cells in the AP group (HC vs. AP; P = 0.0329; HC versus CP; P = 0.0032; Fig. 2, C and D). The percentages of CD4+ and CD8+ cells were both lower in AP and CP (Fig. 2D), but the decline of CD4+ cells was so drastic that the relative percentage of CD8+ cells among total T cells appeared similar between the groups (Fig. 2E). We also examined rare phenotypically distinct cell types to fully depict the immune landscape, such as CD28+ CD161hi NKT cells (NKT) (23, 30), CD3+ TCRgD+ γδ T cells (GDT), CD4-CD8− double-negative T cells, and CD4−CD8− double-positive T cells (23) (Fig. 2, C and D, Supplemental Fig. S2B). Within the T-cell population, we analyzed the T-cell subsets composition among groups (Fig. 2E). In line with the findings for the changes in the lymphocyte populations in COVID-19, CD4+ T cells were significantly depleted among T cells in the AP group compared with the frequencies in the HC and CP groups and the ratio markedly elevated during convalescence (HC vs. AP; P = 0.009; AP vs. CP: P = 0.0152; Fig. 2E; Supplemental Fig. S2C). The heterogeneous T-cell response driven by SARS-CoV-2 improves our understanding of host adaptive immunity elicited by exogenous pathogens.
Figure 2.
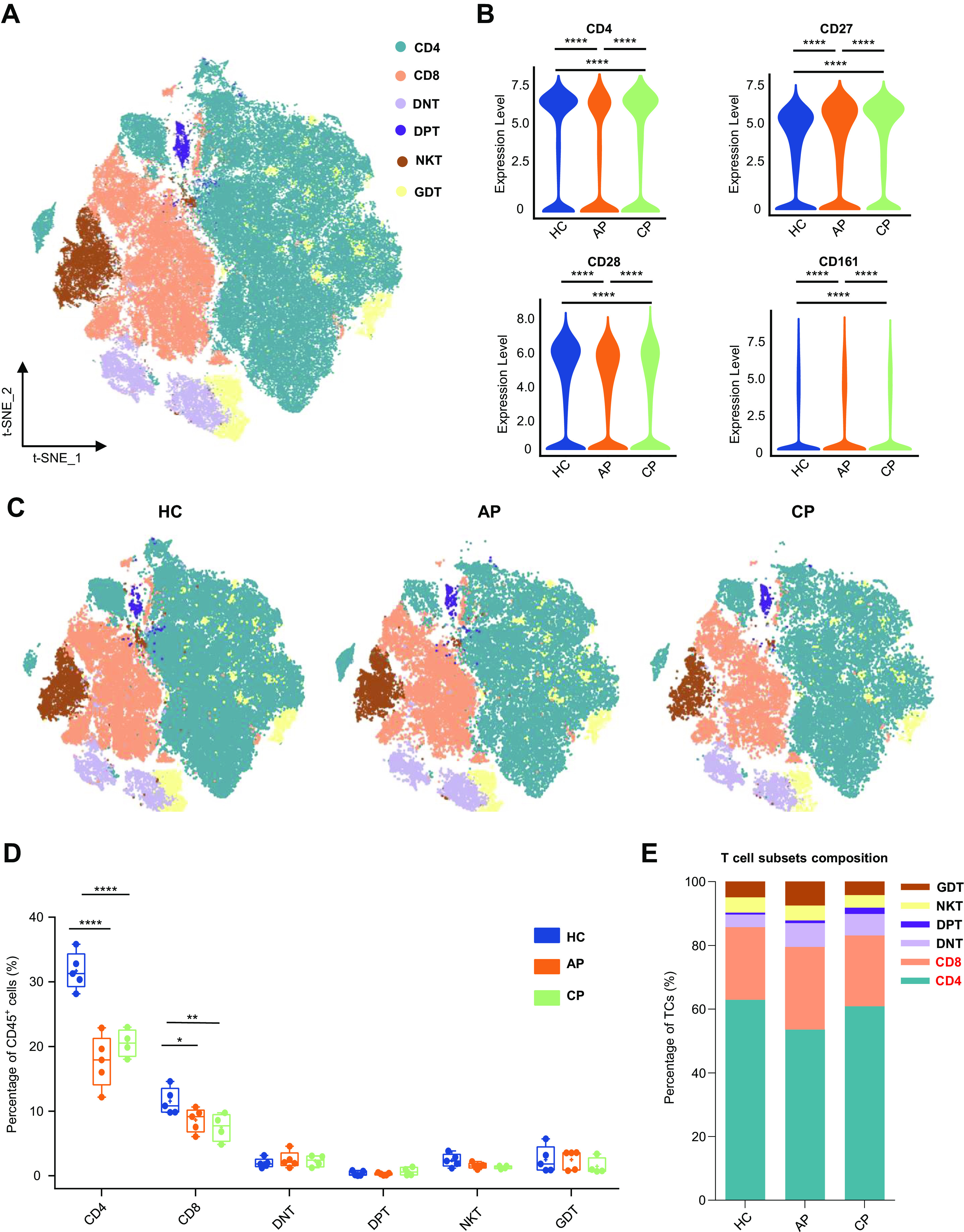
Characterization of single-cell T cells from mass cytometry data. A: t-distributed stochastic neighbor embedding (t-SNE) plot of major T-cell subsets. Cells are colored on the basis of cell types. B: violin plot showing the expression of CD4, CD28, CD27, and CD161 in the T-cells cluster between the healthy control (HC), acute phase (AP), and convalescent phase (CP) group. C: t-SNE projections of major T-cell subsets from the HC, AP, and CP group, respectively. D: percentage of major T-cell subsets in all blood CD45+ immune cells from HC (n = 5), AP (n = 5), and CP group (n = 4). E: relative fractions of major T-cell subsets in T cells from the HC, AP, and CP group. Adjusted P values are based on Wilcoxon rank-sum test (marker level) or two-way ANOVA tests with Bonferroni’s post hoc correction (parametric) between groups. ****Significant with adjusted P value < 0.0001. **Significant with adjusted P value < 0.01. *Significant with adjusted P value < 0.05.
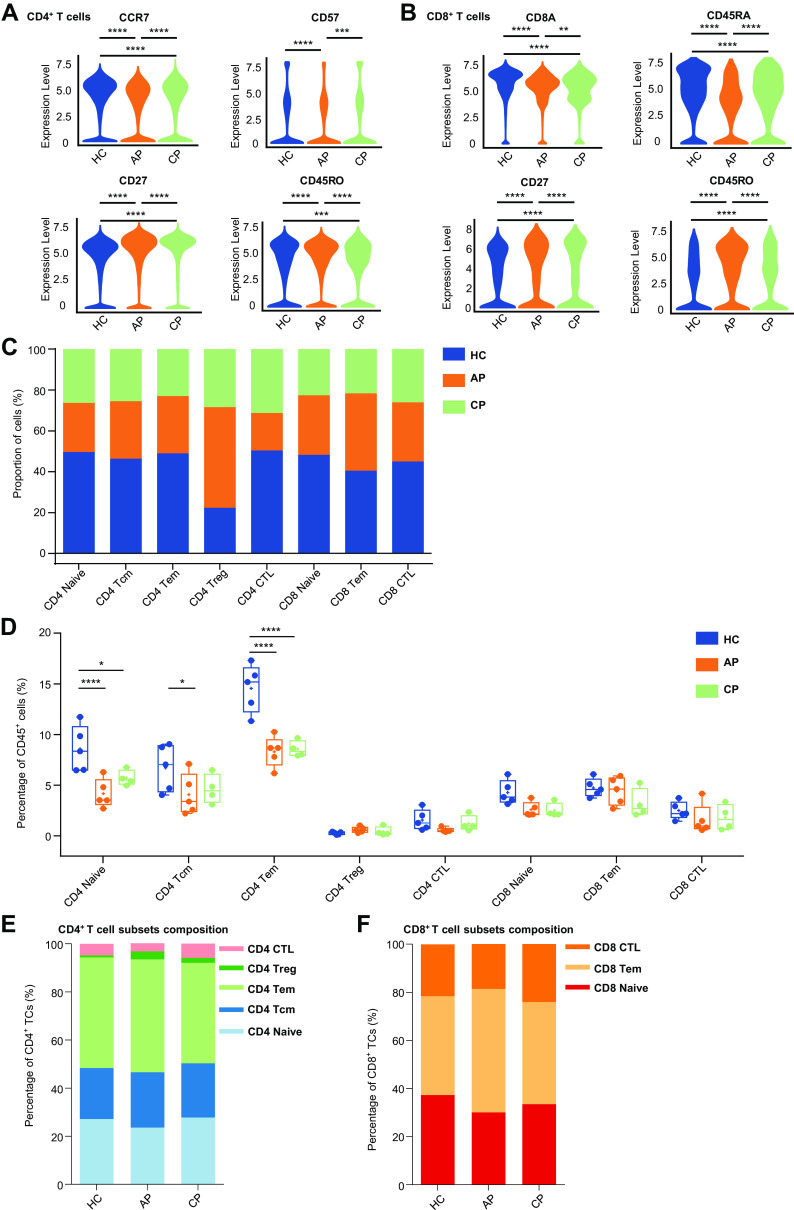
CD4+ T-Cell Subsets Constitute the Most Depleted Circulating Immune Cell Population in the Acute Phase of SARS-CoV-2 Infection
Following the findings that CD4+ T cells and CD8+ T cells are the most variable cell types in the peripheral blood, we next investigated CD4+ T cells and CD8+ T cells, respectively, to identify a specific immune phenotype. CD4+ T cells were identified on the basis of the expression of CD3 and CD4 and can be subdivided into five classes: CCR7+ CD45RA+ naive CD4+ T cells (CD4 Naive), CCR7+ CD45RO− central memory CD4+ T cells (CD4 Tcm); CCR7lo/− CD45RO+ CD27+ effector memory CD4+T cells (CD4 Tem); CD4+ CD25hi CD127lo/− regulatory T cells (CD4 Treg) CD57+ CD28- cytotoxic T cells (CD4 CTL) (Supplemental Fig. S2A and S3A). We found that both CCR7 and CD57 expression were decreased in CD4+ T cells in AP compared with those in HC (Fig. 3A). In addition, AP patients had more of CD27 and CD45RO expression, suggesting the increase of CD4+ T-cell differentiation and activation during acute SARS-CoV-2 infection (19, 22). The number of CD4+ T-cell subsets differed among groups (Fig. 3C). Among CD45+ immune cells, the percentage of T-cell subpopulation was decreased in patients, and several subsets were increased in the CP compared with that of AP group, especially the CD4+ T-cell subsets (Fig. 3D), which was further confirmed by the analysis of T-cell composition (Supplemental Fig. S3B). Notably, we identified that naive CD4+ T cells constituted one of the most depleted immune cell subsets during SARS-CoV-2 infection (HC vs. AP; P < 0.001; HC vs. CP: P = 0.0124; Fig. 3D). The ratios of CD4 Tcm and CD4 Tem were notably lower in the AP group compared with the HC group (CD4 Tcm: HC vs. AP; P = 0.0157; CD4 Tem: HC vs. AP; P < 0.001; Fig. 3D). Similarly, the frequency of CD4 Tem was lower in CP compared with that in the HC group (HC vs. CP: P < 0.001). We further examined the proportions of naive CD4+ T cells among T cells (Supplemental Fig. S3B). We found that CD4+ T cells could explain the decline in the T cells among CD45+ immune cells (Fig. 3B). There was a general loss of major CD4+ T-cell subsets in both AP and CP (Fig. 3D), but the ratios of these cells to the CD4+ T cells were unchanged (Fig. 3E; Supplemental Fig. S3C).
Figure 3.
Characterization of CD4+ T-cell subsets and CD8+ T-cell subsets. A: violin plot showing the expression of CCR7, CD57, CD27, and CD45RO in CD4+ T-cell cluster between the healthy control (HC), acute phase (AP) and convalescent plasma (CP) group. B: violin plot showing the expression of CD8A, CD45RA, CD27, and CD45RO in CD8+ T-cell cluster between the HC, AP, and CP group. C: bar plots highlighting cell abundances across CD4+ and CD8+ T cell subsets for the HC, AP, and CP group. D: percentage of CD4+ and CD8+ T-cell subsets in all blood CD45+ immune cells from the HC (n = 5), AP (n = 5), and CP group (n = 4). E: relative fractions of CD4+ T cell subsets in CD4+ T cells from the HC, AP, and CP group. F: relative fractions of CD8+ T-cell subsets in CD8+ T cells from the HC, AP, and CP group. Adjusted P values are based on Wilcoxon rank-sum test (marker level) or (parametric) two-way ANOVA tests with Bonferroni’s post hoc correction (parametric) between groups. ****Significant with adjusted P value < 0.0001. ***Significant with adjusted P value < 0.001. **Significant with adjusted P value < 0.01. *Significant with adjusted P value < 0.05.
CD8+ T cells expressed CD8A and CD8B and were subdivided into three classes: CCR7+ CD45RA+ naive CD8+ T cells (CD8 Naive), CCR7+ CD45RO+ effector memory CD8+ T cells (CD8 Tem), and CCR7lo/− CD27− cytotoxic CD8+ lymphocytes (CD8 CTL) (Supplemental Fig. S2A and S3A). For CD8+ T cells, the expressions of CD8A and CD45RA were more abundant in the HC group, whereas the expressions of CD27 and CD45RO were more abundant in the AP group (Fig. 3B). As with CD4+ T cells, CD8+ T cells expressed more effector markers in the acute phase. The frequencies of CD8+ T-cell subsets were comparable between groups (Fig. 3, D and 3F, Supplemental Fig. S3D). These results suggested that COVID-19 caused extensive lymphopenia, especially the CD4+ T-cell subsets.
COVID-19 Induces Strong Humoral Immune Responses
B cells are the dominant cell type responsible for the maintenance of humoral defense. Four major peripheral B-cell subsets can be measured by CyTOF in the human peripheral circulation (Fig. 4A and Supplemental Fig. S4A): CD56− CD14−CD19+ IGD+ CD27− naive B cells (naive BC); CD56− CD14− CD19+ CD27+ IGD− memory B cells (Memory BC); CD38+ CD20− antibody-secreting cells or plasma cells (plasma BC) expressing high levels of immunoglobulin genes. We identified a subset of CD19+ CD11C+ B cells defined as age-associated B cells (ABCs), which had attracted significant attention in recent years (37) (Fig. 4A, Supplemental Fig. S4A). These cells were isolated from aged donors and found to be closely associated with immune senescence; they were expected to provide a novel therapeutic avenue for viral infections (41). The AP group had higher CD27 and CD38 expression levels than those in the CP and HC groups, while IGD expression was decreased in the AP group. The three groups exhibited similarly low levels of CD11C (Fig. 4B). Among the B-cell subsets, subclustering identified that all subgroups, except for plasma cells, were reduced in patients (Fig. 4, C and D). Moreover, the percentage of naive B cells in the AP group was significantly decreased among CD45+ cells, which was restored in the CP group (HC vs. AP; P < 0.0001; AP vs. CP; P = 0.0005, Supplemental Fig. S4B). Next, we analyzed the composition of B cells and their subpopulations as it changed after acute infection (Supplemental Fig. S4C). We noted that plasma B-cell proportions were most elevated in AP and significantly decreased in CP (HC vs. AP; P = 0.0041; AP vs. CP; P = 0.0098; Fig. 4E), indicating that a strong humoral immune response was induced by SARS-CoV-2. Furthermore, an opposite trend was also found for the changes in naive B cells in both AP and CP groups (HC vs. AP; P < 0.0001; AP vs. CP; P = 0.0214; Fig. 4E). The frequencies of memory BC and ABC were similar among the three groups. In summary, strong humoral immunity is developed in COVID-19 patients in the acute stage.
Figure 4.
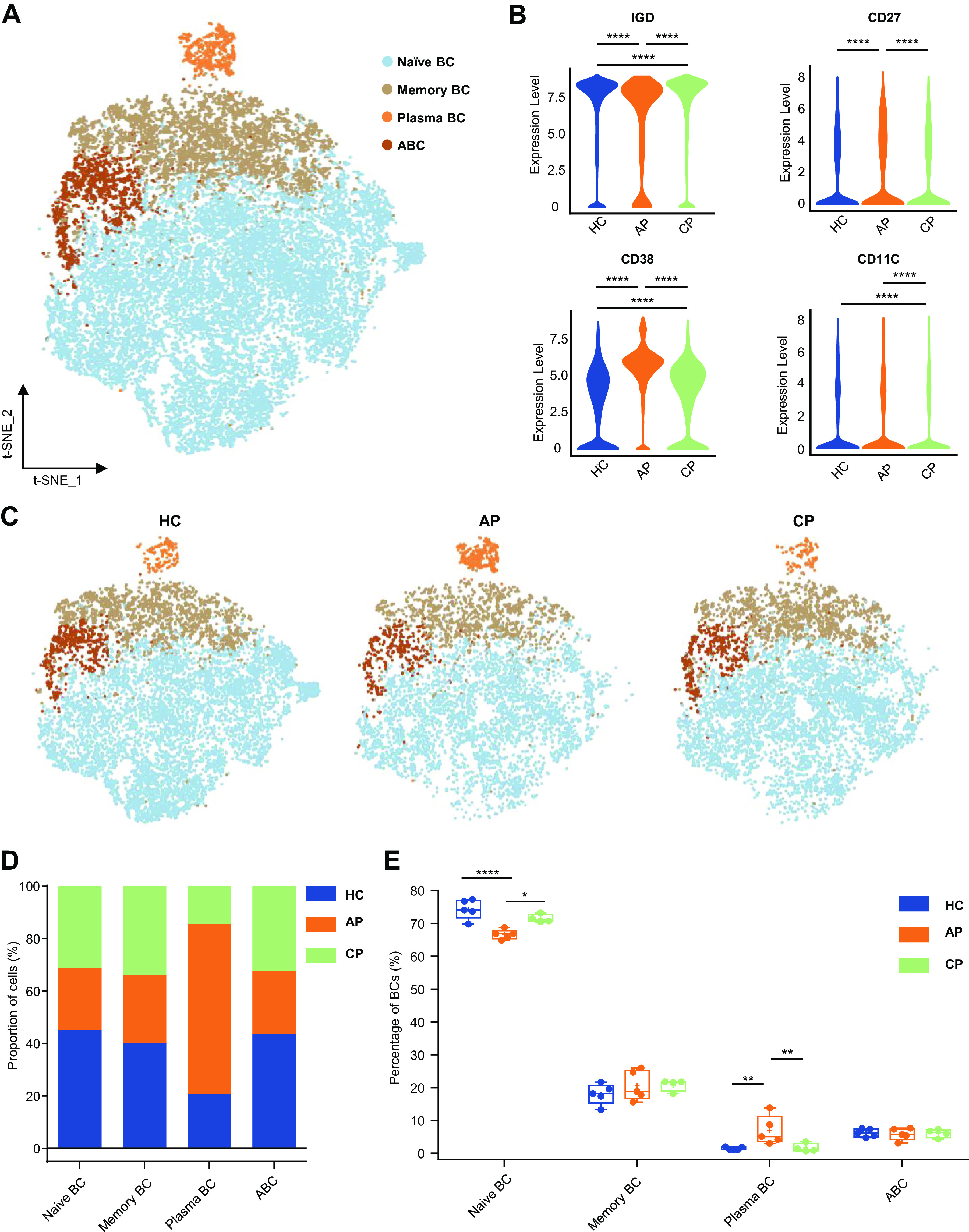
Characterization of single-cell B cells from mass cytometry data. A: t-distributed stochastic neighbor embedding (t-SNE) plot of major B-cell subsets. Cells are colored on the basis of cell types. B: violin plot showing the expression of IGD, CD27, CD38, and CD11C in the B cells cluster between healthy control (HC), acute phase (AP), and convalescent phase (CP) group. C: t-SNE projections of major B-cell subsets from the HC, AP and CP group. D: bar plots highlighting cell abundances across B cell subsets for the HC, AP, and CP group. E: percentage of major B cell subsets in B cells from the HC (n = 5), AP (n = 5) and CP group (n = 4). Adjusted P values are based on Wilcoxon rank-rum test (marker level) or two-way ANOVA tests with Bonferroni’s post hoc correction (parametric) between groups. ****Significant with adjusted P value < 0.0001; **Significant with adjusted P value < 0.01; *Significant with adjusted P value < 0.05.
NK cells and DCs Were Altered Heterogeneously by COVID-19
We next analyzed NK lymphocytes and DCs in COVID-19 samples. NK lymphocytes were frequently CD123- CD45RA+ and CD45+ CD56+ and were divided into three subsets according to CD16 and CD57 expression (Fig. 5A, Supplemental Fig. S5A). CD16+ CD56+ cells were defined as early NKs (NK1), CD16+ CD57- cells were cytotoxic NKs (NK2), and CD16+ CD57+ cells were defined as late NKs (NK3) (Fig. 5A, Supplemental Fig. S5A) (34, 66). The AP group showed higher levels of CD16, CD57, and CD38 than those in the CP and HC groups, suggesting higher cytotoxic activity (Fig. 5B). The number and frequency of NK subsets among CD45+ cells were comparable among groups (Fig. 5C, Supplemental Fig. S5B). In addition, we observed a higher ratio of NK2 in the AP and a reversal of the decline of NK2 in CP within the NK subset compared with those in the HC group (Fig. 5D, Supplemental Fig. S5C). NK2 is known to eliminate viral infections by direct cytotoxicity (54), and we noted a moderate, but not statistically significant, increase in NK2 ratio in AP. There was no significant difference in NK1 and NK3 within the NK subset (Fig. 5D, Supplemental Fig. S5C). This is not surprising, given that previous studies also reported heterogeneous responses of NK cells under the influence of COVID-19 (32, 58).
Figure 5.
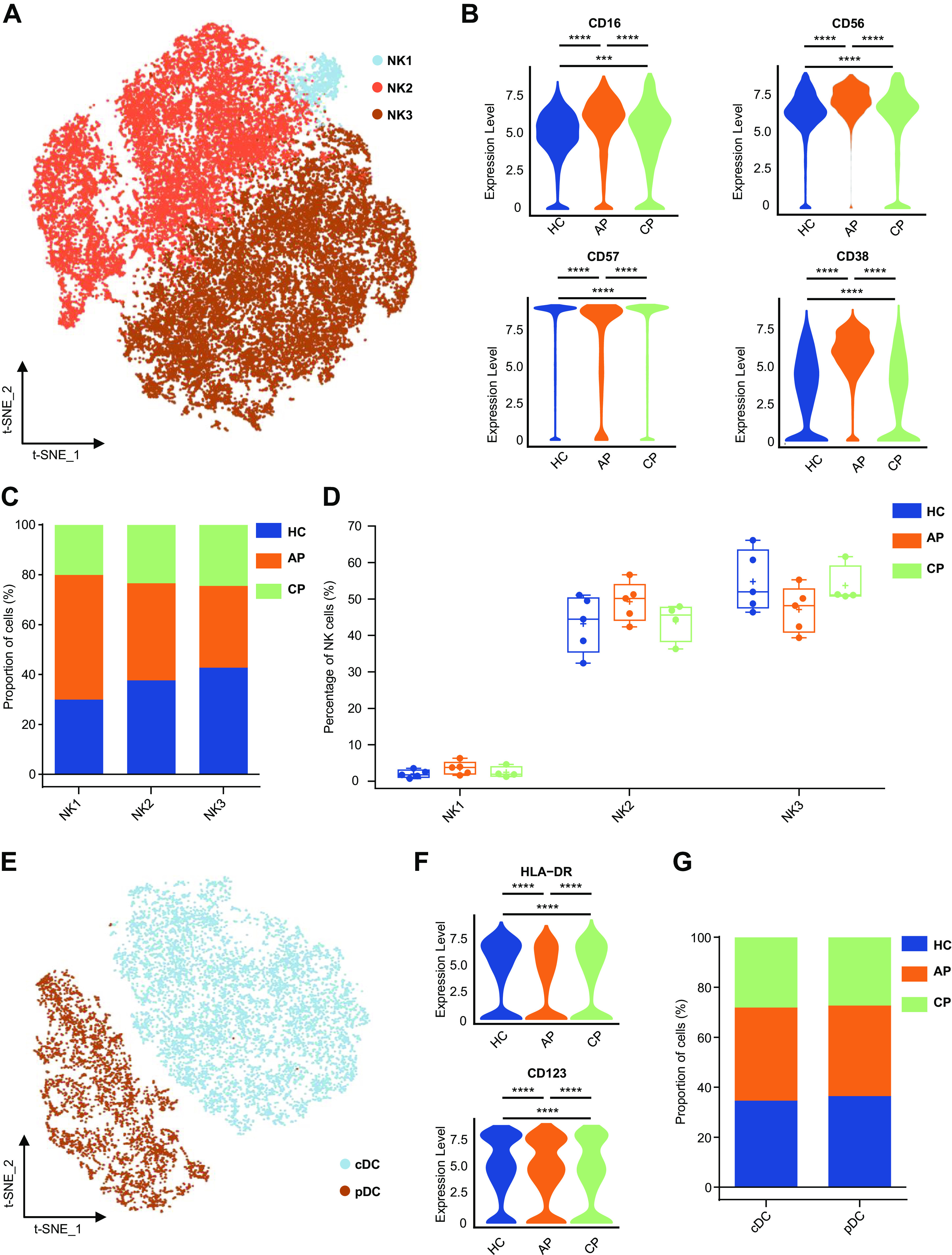
Characterization of single-cell natural killer (NK) cells and dendritic cells (DCs) from mass cytometry data. A: t-SNE plot of major NK cell subsets. Cells are colored on the basis of cell types. B: violin plot showing the expression of CD16, CD56, CD57, and CD38 in the NK-cell cluster between the healthy control (HC), acute phase (AP), and convalescent phase (CP) group. C: bar plots highlighting cell abundances across NK cell subsets for the HC, AP, and CP group. D: percentage of major NK-cell subsets in NK cells from the HC (n = 5), AP (n = 5), and CP group (n = 4). E: t-SNE plot of major DC subsets. Cells are colored on the basis of based on cell types. F: violin plot showing the expression of HLA-DR and CD123 in the DC cluster between HC, AP, and CP group. G: bar plots highlighting cell abundances across DC subsets for the HC, AP, and CP group. Adjusted P values are based on Wilcoxon rank-sum test (marker level) or two-way ANOVA tests with Bonferroni’s post hoc correction (parametric)between groups. ****Significant difference with adjusted P value < 0.0001.
DCs are essential for the activation of CD4+ and CD8+ T-cell adaptive immune responses and are key components in the immune response against invading viruses (35). We reclustered the DCs to characterize the phenotypic alterations in COVID-19 (Fig. 5E, Supplemental Fig. S5C). Conventional DCs (cDCs), the most common form of DCs, were defined on the basis of the expression of surface markers CD14− CD16− CD11C+ HLA-DR+. Plasmacytoid DCs (pDCs), derived from lymphoid cells, express CD123+ CD38+, and are best known for their ability to produce large amounts of type 1 interferons (1). Violin plots were generated to summarize the expression of DC markers among groups. Compared with HC, the AP group had decreased HLA-DR expression (Fig. 5F), suggesting that SARS-CoV-2 attenuated the capacity of DC antigen presentation. The increase of CD123 expression of DCs in the AP group demonstrated that pDC may play an important role in the regulation of SARS-CoV-2 infection (Fig. 5F). Our analysis of DCs showed that the percentage of cDC in CD45+ immune cells in AP and CP varied greatly among individuals, which may reflect the heterogeneity of COVID-19 patients in DCs changes (Supplemental Fig. S5E). cDC and pDC were similar among groups (Fig. 5G; Supplemental Fig. S5F).
COVID-19 Induced the Dysregulation of Monocyte Composition and Proportion
We further quantitatively evaluated COVID-19-driven changes in monocytes. Human peripheral blood myeloid cells, including monocytes, which promote antigen presentation and inflammatory activity (49), were isolated from AP and CP individuals into three transcriptionally distinct subsets via t-SNE: classical monocytes (CD14+ mono), intermediate monocytes (transitional mono), and nonclassical monocytes (CD16+ mono) (63) (Fig. 6A and Supplemental Fig. S6A). Classical monocytes share high CD14 expression (CD38+ CD14hi) and are the most abundant monocyte subset. A minor population is intermediate monocytes with CD14 expression and low or negative CD16 expression (CD38lo/− CD14int), whereas nonclassical monocytes show low or negative CD14 expression and high CD16 expression (CD38− CD14−). AP and CP groups showed higher levels of CD11C, CD14, CD16, and HLA-DR than those in the HC group (Fig. 6B), indicating an inflammatory state in patients with COVID-19. Within monocyte clusters, we found distinct manifestations by comparing monocyte clusters in the t-SNE map (Fig. 6C). Monocyte expansion was very prominent, including both nonclassical and classical monocytes (Fig. 6, D and E). This included a significant increase in CD16+ mono among CD45+ immune cells in the AP groups (HC vs. AP; P < 0.0001; HC vs. CP; P < 0.0001; Fig. 6E). We also observed that human monocytes from patients in the acute phase had significantly increased levels of CD14+ mono (HC vs. AP; P = 0.0394; Fig. 6E). Furthermore, COVID-19 patients had a greater percentage of CD16+ monocytes among other types of monocytes (Supplemental Fig. S6, B and C). Together, these data showed a dysregulation in the balance of monocyte populations in COVID-19 patients, as manifested by a substantial increase in monocyte subsets. The compositions of monocyte subsets in the AP and CP groups were largely similar, suggesting that immunological changes persisted during convalescence (51, 60).
DISCUSSION
Only a limited number of studies have characterized the SARS-CoV-2-specific immune cell responses using high-throughput CyTOF technology (16, 25, 32, 58, 71), and even fewer studies have put the spotlight on convalescing patients. More importantly, it is unclear whether patients from different geographical regions respond differently to SARS-CoV-2. Second, while it is well documented that COVID-19 patients are characterized by lymphopenia and increased numbers of neutrophils (20, 25, 62), little is known about the dysregulated inflammatory response among different T cells (7), and there have been inconsistent findings regarding the roles of different monocyte subsets (14, 25, 32, 58, 60, 67, 71, 75). In our data set, we have revealed peripheral immune cell subsets in COVID-19 and identified CD4+ T-cell depletion, T-cell differentiation and activation, plasma cell expansion, and the reduced antigen presentation capacity of innate immunity. Finally, we have identified several new results, including the finding that COVID-19 promoted T-cell polarization from naive to memory and effector cells with higher CD27 and CD45RO expression, and that COVID-19 induced a dysregulation in the balance of monocyte subpopulations (e.g., a significant increase of CD16+ nonclassical monocytes).
In our study, the capacity of antigen-presenting function in DCs was impaired in COVID-19 acute infection. A previous study also noticed DCs maturation during acute SARS-CoV-2 infection (73). The dysfunction DCs, with the lack of cytokine production, may be one of the reasons for immune evasion during SARS-CoV-2 infection (27). Apart from this, NK cells are also essential parts of the early immune response against viral infections, particularly by clearing virus-infected cells (61). Although the expression levels of cytotoxic genes were elevated in the AP group with increased NK2, the frequency of NK cells decreased during SARS-CoV-2 infection. We found that NK cells were depleted in COVID-19 patients, potentially resulting from immune suppression, apoptosis, or the downregulation of cytotoxicity by the virus (53). DCs and NK cells were both decreased significantly in critically severe patients who had been supplied with ventilator (62). The exhaustion of both DCs and NK cells may be part of the innate antiviral immunity activation during the acute phase of infection (38, 39).
We found that the frequency of CD3+ T cells was reduced after infection, in agreement with previous studies (20, 25, 31, 40, 57). We found that lymphopenia was preferential for CD4+ T cells. However, some have suggested that lymphopenia in COVID-19 affected CD4+T cells less than that for CD8+ T cells (32, 57). Nevertheless, Hadjadj et al. (16) showed that the frequencies of CD8+ T-cell subsets were not significantly decreased. The discrepancy in results may be due to the differences in clinical characteristics, including ages, sex, and ethnicity. We also observed that SARS-CoV-2 promoted T-cell polarization from naive to memory and effector cells with higher CD27 and CD45RO expression. It is important to note that the convalescent patients remained in a fragile state with T-cell depletion in the peripheral blood.
Our study also suggested that SARS-CoV-2 induced strong humoral immunity, consistent with previous reports (14, 25, 32, 68, 76). Hospitals are starting to investigate the efficacy of passive antibody therapies for COVID-19, using convalescent plasma as a source of therapeutic polyclonal antibodies for treatment. Early reports suggest a positive impact on clinical status (10, 46). Long-term immune protection is achieved by the induction of long-lived plasma cells and memory B cells. In this study, the ratios of memory B cells and plasma B cells were slightly adjusted during convalescence. The lifespan of B-cell memory responses to SARS-CoV-2 is still under investigation (52).
Previous studies suggested that monocytes could be one of the major contributors to the mortality during COVID-19 infection during the inflammatory storms (33, 55), especially via CD14+ classical monocytes secretion (14, 44, 45, 60). The significant expansion of monocytes in circulation might indicate an inflammatory state during SARS-CoV-2 infection (14). Our CyTOF study is in line with the previous study on single-cell transcription level that circulating CD14+ monocytes increase to exacerbate inflammation in COVID-19 (43, 47, 60). Our data also show a significant increase in CD16+ nonclassical monocytes. Consistently, an aging-related COVID-19 study also reported the expansion of CD16+ monocytes (71). A gene ontology study based on single-cell transcriptomics also showed that both CD14+ monocytes and CD16+ monocytes were significantly upregulated in the acute SARS-CoV-2 infection (67). Further studies with a larger sample size are needed to verify the changes of CD16+ monocytes and its function in COVID-19 (8, 15).
Owing to technical limitations and constraints on the availability of COVID-19 blood samples, this study addressed alterations in the circulating immune system but did not directly assess the tissue-infiltrating immune cell compartment in the lung or proinflammatory cytokines and chemokines produced by immune effector cells. Furthermore, our sample size was small, and patients varied in the timing of clinical manifestations, which could influence the immune landscape. Some of the patients were treated by antiviral remdesivir (12, 13, 48, 59), which targets the viral RNA-dependent RNA polymerase, and its direct immunomodulatory effect is still unknown. Finally, owing to our inability to directly handle the SARS-CoV-2 virus, and the lack of patients’ blood samples, we were unable to perform in vitro and in vivo experiments to validate our results.
In summary, our high-throughput single-cell analysis approach provides a comprehensive account of the immunological signature in patients with COVID-19 in the acute phase and convalescence. This study contributes to our understanding of peripheral immune responses in COVID-19 and highlights new directions for studies of the mechanisms underlying COVID-19 and treatment approaches.
GRANTS
This study was funded by Local Innovative and Research Teams Project of Guangdong Pearl River Talents Programme; Clinical Innovation Research Programme of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR0201001); CAMS Innovation Fund for Medical Sciences (2019-I2M-5-005); Construction Project of High-Level Hospitals in Guangdong Province (303020107; 303010303058); Program of Shanghai Academic Research Leader (no. 20XD1423300); and the State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University.
DISCLAIMERS
The funding body had no role in study design, collection, management, analysis and interpretation of data, writing of the manuscript, and the decision to submit the manuscript for publication.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.S., H.T., Y.Z., and Y.L. conceived and designed research; W.L., J.Y., and W.W. performed experiments; W.S., X.L., P.M., and J.Y. analyzed data; W.S., X.L., and P.M. interpreted results of experiments; W.S. and X.L. prepared figures; W.S. and X.L. drafted manuscript; W.S., Q.C., P.M., L.X., W.W., H.T., and Y.Z. edited and revised manuscript; W.S., Q.C., W.L., W.W., H.T., Y.Z., and Y.L. approved final version of manuscript.
REFERENCES
- 1.Ali S, Mann-Nüttel R, Schulze A, Richter L, Alferink J, Scheu S. Sources of type I interferons in infectious immunity: plasmacytoid dendritic cells not always in the driver’s seat. Front Immunol 10: 778, 2019. doi: 10.3389/fimmu.2019.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbalat R, Lau L, Locksley RM, Barton GMJ. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol 10: 1200–1207, 2009. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36: 411–420, 2018. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y, Su B, Guo X, Sun W, Deng Y, Bao L, Zhu Q, Zhang X, Zheng Y, Geng C, Chai X, He R, Li X, Lv Q, Zhu H, Deng W, Xu Y, Wang Y, Qiao L, Tan Y, Song L, Wang G, Du X, Gao N, Liu J, Xiao J, Su XD, Du Z, Feng Y, Qin C, Qin C, Jin R, Xie XS. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell 182: 73–84.e16, 2020. doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130: 2620–2629, 2020. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen TJ, Kotecha N. Cytobank: providing an analytics platform for community cytometry data analysis and collaboration. Curr Top Microbiol Immunol 377: 127–157, 2014. doi: 10.1007/82_2014_364. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol 20: 529–536, 2020. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33: 375–386, 2010. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Liu Y, Wang G, Yuan Z, Feng Z, Zhang Y, Wu Y, Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol 11: 827, 2020. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y, Peng C, Yuan M, Huang J, Wang Z, Yu J, Gao X, Wang D, Yu X, Li L, Zhang J, Wu X, Li B, Xu Y, Chen W, Peng Y, Hu Y, Lin L, Liu X, Huang S, Zhou Z, Zhang L, Wang Y, Zhang Z, Deng K, Xia Z, Gong Q, Zhang W, Zheng X, Liu Y, Yang H, Zhou D, Yu D, Hou J, Shi Z, Chen S, Chen Z, Zhang X, Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA 117: 9490–9496, 2020. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir ED, Amadori L, Khan NS, Wong CK, Shamailova R, Hill CA, Wang Z, Remark R, Li JR, Pina C, Faries C, Awad AJ, Moss N, Bjorkegren JLM, Kim-Schulze S, Gnjatic S, Ma’ayan A, Mocco J, Faries P, Merad M, Giannarelli C. Single-cell immune landscape of human atherosclerotic plaques. Nat Med 25: 1576–1588, 2019. doi: 10.1038/s41591-019-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem 295: 4773–4779, 2020. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, Gotte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem 295: 6785–6797, 2020. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo C, Li B, Ma H, Wang X, Cai P, Yu Q, Zhu L, Jin L, Jiang C, Fang J, Liu Q, Zong D, Zhang W, Lu Y, Li K, Gao X, Fu B, Liu L, Ma X, Weng J, Wei H, Jin T, Lin J, Qu K. Single-cell analysis of two severe COVID-19 patients reveals a monocyte-associated and tocilizumab-responding cytokine storm. Nat Commun 11: 3924, 2020. doi: 10.1038/s41467-020-17834-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta M, Mahanty S, Ahmed R, Rollin PEJV. Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with ebola virus secrete MIP-1α and TNF-α and inhibit poly-IC-induced IFN-α in vitro. Virology 284: 20–25, 2001. doi: 10.1006/viro.2001.0836. [DOI] [PubMed] [Google Scholar]
- 16.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, Péré H, Charbit B, Bondet V, Chenevier-Gobeaux C, Breillat P, Carlier N, Gauzit R, Morbieu C, Pène F, Marin N, Roche N, Szwebel TA, Merkling SH, Treluyer JM, Veyer D, Mouthon L, Blanc C, Tharaux PL, Rozenberg F, Fischer A, Duffy D, Rieux-Laucat F, Kernéis S, Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369: 718–724, 2020. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahne F, LeMeur N, Brinkman RR, Ellis B, Haaland P, Sarkar D, Spidlen J, Strain E, Gentleman R. flowCore: a Bioconductor package for high throughput flow cytometry. BMC Bioinformatics 10: 106, 2009. doi: 10.1186/1471-2105-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartmann FJ, Bernard-Valnet R, Quériault C, Mrdjen D, Weber LM, Galli E, Krieg C, Robinson MD, Nguyen XH, Dauvilliers Y, Liblau RS, Becher B. High-dimensional single-cell analysis reveals the immune signature of narcolepsy. J Exp Med 213: 2621–2633, 2016. doi: 10.1084/jem.20160897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendriks J, Gravestein LA, Tesselaar K, van Lier RAW, Schumacher TNM, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol 1: 433–440, 2000. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 20.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L, Shi Y, Gong B, Jiang L, Liu X, Yang J, Tang J, You C, Jiang Q, Long BJ. Blood single cell immune profiling reveals the interferon-MAPK pathway mediated adaptive immune response for COVID-19 (Preprint). medRxiv 2020. doi: 10.1101/2020.03.15.20033472. [DOI]
- 22.Johannisson A, Festin R. Phenotype transition of CD4+ T cells from CD45RA to CD45R0 is accompanied by cell activation and proliferation. Cytometry 19: 343–352, 1995. doi: 10.1002/cyto.990190409. [DOI] [PubMed] [Google Scholar]
- 23.Koh YI, Shim JU, Wi J, Kwon YE. The role of natural killer T cells in the pathogenesis of acute exacerbation of human asthma. Int Arch Allergy Immunol 158: 131–141, 2012. doi: 10.1159/000330908. [DOI] [PubMed] [Google Scholar]
- 24.Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, Baglaenko Y, Brenner M, Loh PR, Raychaudhuri S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat Methods 16: 1289–1296, 2019. doi: 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner CAG, Weisman AR, Agyekum RS, Mathew D, Baxter AE, Vella LA, Kuthuru O, Apostolidis SA, Bershaw L, Dougherty J, Greenplate AR, Pattekar A, Kim J, Han N, Gouma S, Weirick ME, Arevalo CP, Bolton MJ, Goodwin EC, Anderson EM, Hensley SE, Jones TK, Mangalmurti NS, Luning Prak ET, Wherry EJ, Meyer NJ, Betts MR. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol 5: eabd7114, 2020. doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, Munoz-Ruiz M, McKenzie DR, Hayday TS, Francos-Quijorna I, Kamdar S, Joseph M, Davies D, Davis R, Jennings A, Zlatareva I, Vantourout P, Wu Y, Sofra V, Cano F, Greco M, Theodoridis E, Freedman J, Gee S, Chan JNE, Ryan S, Bugallo-Blanco E, Peterson P, Kisand K, Haljasmagi L, Chadli L, Moingeon P, Martinez L, Merrick B, Bisnauthsing K, Brooks K, Ibrahim MAA, Mason J, Lopez Gomez F, Babalola K, Abdul-Jawad S, Cason J, Mant C, Seow J, Graham C, Doores KJ, Di Rosa F, Edgeworth J, Shankar-Hari M, Hayday AC. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med 26: 1623–1635, 2020. [Erratum in Nat Med 26: 1663, 2020]. doi: 10.1038/s41591-020-1038-6. [DOI] [PubMed] [Google Scholar]
- 27.Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, Nicholls JM, Peiris JS, Lau YL. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood 106: 2366–2374, 2005. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q, Wu J. Coronavirus infections and immune responses. J Med Virol 92: 424–432, 2020. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, Liu L, Amit I, Zhang S, Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 26: 842–844, 2020. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y, Roberts TJ, Wang CR, Cho S, Brutkiewicz RR. Long-term loss of canonical NKT cells following an acute virus infection. Eur J Immunol 35: 879–889, 2005. doi: 10.1002/eji.200425495. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 55: 102763, 2020. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, Alanio C, Kuri-Cervantes L, Pampena MB, D’Andrea K, Manne S, Chen Z, Huang YJ, Reilly JP, Weisman AR, Ittner CAG, Kuthuru O, Dougherty J, Nzingha K, Han N, Kim J, Pattekar A, Goodwin EC, Anderson EM, Weirick ME, Gouma S, Arevalo CP, Bolton MJ, Chen F, Lacey SF, Ramage H, Cherry S, Hensley SE, Apostolidis SA, Huang AC, Vella LA, Betts MR, Meyer NJ, Wherry EJ; UPenn COVID Processing Unit . Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 369: eabc8511, 2020. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 20: 355–362, 2020. [Erratum in Nat Rev Immunol 20: 448, 2020]. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen CM, White MJ, Goodier MR, Riley EM. Functional significance of CD57 expression on human NK cells and relevance to disease. Front Immunol 4: 422, 2013. doi: 10.3389/fimmu.2013.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Keeffe M, Mok WH, Radford KJ. Human dendritic cell subsets and function in health and disease. Cell Mol Life Sci 72: 4309–4325, 2015. doi: 10.1007/s00018-015-2005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization Coronavirus disease 2019 (COVID-19): situation report – 144. 2020. https://www.who.int/docs/default-sourcecoronaviruse/situation-reports/20200612-covid-19-sitrep-144.pdf?sfvrsn=66ff9f4f_4.
- 37.Pillai S Now you know your ABCs. Blood 118: 1187–1188, 2011. doi: 10.1182/blood-2011-06-355131. [DOI] [PubMed] [Google Scholar]
- 38.Poehlmann H, Schefold JC, Zuckermann-Becker H, Volk HD, Meisel C. Phenotype changes and impaired function of dendritic cell subsets in patients with sepsis: a prospective observational analysis. Crit Care 13: R119, 2009. doi: 10.1186/cc7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollara G, Kwan A, Newton PJ, Handley ME, Chain BM, Katz DR. Dendritic cells in viral pathogenesis: protective or defective? Int J Exp Pathol 86: 187–204, 2005. doi: 10.1111/j.0959-9673.2005.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 71: 762–768, 2020. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubtsova K, Rubtsov AV, Cancro MP, Marrack P. Age-associated B cells: a T-bet-dependent effector with roles in protective and pathogenic immunity. J Immunol 195: 1933–1937, 2015. doi: 10.4049/jimmunol.1501209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryon JJ, Moss WJ, Monze M, Griffin DE. Functional and phenotypic changes in circulating lymphocytes from hospitalized Zambian children with measles. Clin Diagn Lab Immunol 9: 994–1003, 2002. doi: 10.1128/CDLI.9.5.994-1003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez-Cerrillo I, Landete P, Aldave B, Sanchez-Alonso S, Sanchez-Azofra A, Marcos-Jimenez A, Avalos E, Alcaraz-Serna A, de Los Santos I, Mateu-Albero T, Esparcia L, Lopez-Sanz C, Martinez-Fleta P, Gabrie L, Del Campo Guerola L, Calzada MJ, Gonzalez-Alvaro I, Alfranca A, Sanchez-Madrid F, Munoz-Calleja C, Soriano JB, Ancochea J, Martin-Gayo E. Differential redistribution of activated monocyte and dendritic cell subsets to the lung associates with severity of COVID-19 (Preprint). medRxiv 2020. doi: 10.1101/2020.05.13.20100925. [DOI]
- 44.Serbina NV, Cherny M, Shi C, Bleau SA, Collins NH, Young JW, Pamer EG. Distinct responses of human monocyte subsets to Aspergillus fumigatus conidia. J Immunol 183: 2678–2687, 2009. doi: 10.4049/jimmunol.0803398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serbina NV, Jia T, Hohl TM, Pamer EGJARI. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 26: 421–452, 2008. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, Wei J, Xiao H, Yang Y, Qu J, Qing L, Chen L, Xu Z, Peng L, Li Y, Zheng H, Chen F, Huang K, Jiang Y, Liu D, Zhang Z, Liu Y, Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 323: 1582–1589, 2020. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11: 762–774, 2011. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, Neville S, Carra E, Lew W, Ross B, Wang Q, Wolfe L, Jordan R, Soloveva V, Knox J, Perry J, Perron M, Stray KM, Barauskas O, Feng JY, Xu Y, Lee G, Rheingold AL, Ray AS, Bannister R, Strickley R, Swaminathan S, Lee WA, Bavari S, Cihlar T, Lo MK, Warren TK, Mackman RL. Discovery and synthesis of a phosphoramidate prodrug of a Pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J Med Chem 60: 1648–1661, 2017. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 49.Ströher U, West E, Bugany H, Klenk H-D, Schnittler H-J, Feldmann H. Infection and activation of monocytes by Marburg and Ebola viruses. J Virol 75: 11025–11033, 2001. doi: 10.1128/JVI.75.22.11025-11033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20: 363–374, 2020. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thevarajan I, Nguyen THO, Koutsakos M, Druce J, Caly L, van de Sandt CE, Jia X, Nicholson S, Catton M, Cowie B, Tong SYC, Lewin SR, Kedzierska K. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med 26: 453–455, 2020. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, Levantovsky R, Malle L, Moreira A, Park MD, Pia L, Risson E, Saffern M, Salomé B, Esai Selvan M, Spindler MP, Tan J, van der Heide V, Gregory JK, Alexandropoulos K, Bhardwaj N, Brown BD, Greenbaum B, Gümüş ZH, Homann D, Horowitz A, Kamphorst AO, Curotto de Lafaille MA, Mehandru S, Merad M, Samstein RM; Sinai Immunology Review Project . Immunology of COVID-19: current state of the science. Immunity 52: 910–941, 2020. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Erp EA, van Kampen MR, van Kasteren PB, de Wit J. Viral infection of human natural killer cells. Viruses 11: 243, 2019. doi: 10.3390/v11030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 9: 503–510, 2008. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 55.Wang C, Xie J, Zhao L, Fei X, Zhang H, Tan Y, Nie X, Zhou L, Liu Z, Ren Y, Yuan L, Zhang Y, Zhang J, Liang L, Chen X, Liu X, Wang P, Han X, Weng X, Chen Y, Yu T, Zhang X, Cai J, Chen R, Shi ZL, Bian XW. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine 57: 102833, 2020. doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang D, Bodovitz S. Single cell analysis: the new frontier in 'omics'. Trends Biotechnol 28: 281–290, 2010. doi: 10.1016/j.tibtech.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, Song S, Ma Z, Mo P, Zhang Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis 221: 1762–1769, 2020. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang W, Su B, Pang L, Qiao L, Feng Y, Ouyang Y, Guo X, Shi H, Wei F, Su X, Yin J, Jin R, Chen D. High-dimensional immune profiling by mass cytometry revealed immunosuppression and dysfunction of immunity in COVID-19 patients. Cell Mol Immunol 17: 650–652, 2020. doi: 10.1038/s41423-020-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395: 1569–1578, 2020. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wen W, Su W, Tang H, Le W, Zhang X, Zheng Y, Liu X, Xie L, Li J, Ye J, Dong L, Cui X, Miao Y, Wang D, Dong J, Xiao C, Chen W, Wang H. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov 6: 31, 2020. [Erratum in Cell Discov 6: 41, 2020]. doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilk AJ, Blish CA. Diversification of human NK cells: lessons from deep profiling. J Leukoc Biol 103: 629–641, 2018. doi: 10.1002/JLB.6RI0917-390R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martínez-Colón GJ, McKechnie JL, Ivison GT, Ranganath T, Vergara R, Hollis T, Simpson LJ, Grant P, Subramanian A, Rogers AJ, Blish CA. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med 26: 1070–1076, 2020. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong KL, Tai JJ-Y, Wong W-C, Han H, Sem X, Yeap W-H, Kourilsky P, Wong S-C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 118: e16–e31, 2011. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 64.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. A new coronavirus associated with human respiratory disease in China. Nature 579: 265–269, 2020. [Erratum in Nature 580: E7, 2020]. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323: 1239–1242, 2020. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 66.Yang C, Siebert JR, Burns R, Gerbec ZJ, Bonacci B, Rymaszewski A, Rau M, Riese MJ, Rao S, Carlson KS, Routes JM, Verbsky JW, Thakar MS, Malarkannan S. Heterogeneity of human bone marrow and blood natural killer cells defined by single-cell transcriptome. Nat Commun 10: 3931, 2019. doi: 10.1038/s41467-019-11947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang JY, Wang XM, Xing X, Xu Z, Zhang C, Song JW, Fan X, Xia P, Fu JL, Wang SY, Xu RN, Dai XP, Shi L, Huang L, Jiang TJ, Shi M, Zhang Y, Zumla A, Maeurer M, Bai F, Wang FS. Single-cell landscape of immunological responses in patients with COVID-19. Nat Immunol 21: 1107–1118, 2020. doi: 10.1038/s41590-020-0762-x. [DOI] [PubMed] [Google Scholar]
- 68.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis ciaa344, 2020. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng HY, Zhang M, Yang CX, Zhang N, Wang XC, Yang XP, Dong XQ, Zheng YT. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol 17: 541–543, 2020. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, Xu Y, Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 17: 533–535, 2020. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng Y, Liu X, Le W, Xie L, Li H, Wen W, Wang S, Ma S, Huang Z, Ye J, Shi W, Ye Y, Liu Z, Song M, Zhang W, Han JJ, Belmonte JCI, Xiao C, Qu J, Wang H, Liu GH, Su W. A human circulating immune cell landscape in aging and COVID-19. Protein Cell 11: 740–770, 2020. doi: 10.1007/s13238-020-00762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273, 2020. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou R, To KK, Wong YC, Liu L, Zhou B, Li X, Huang H, Mo Y, Luk TY, Lau TT, Yeung P, Chan WM, Wu AK, Lung KC, Tsang OT, Leung WS, Hung IF, Yuen KY, Chen Z. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity 53: 864–877.e5, 2020. doi: 10.1016/j.immuni.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou T, Su TT, Mudianto T, Wang J. Immune asynchrony in COVID-19 pathogenesis and potential immunotherapies. J Exp Med 217: e20200674. 2020. doi: 10.1084/jem.20200674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, Sun R, Tian Z, Xu X, Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev 7: 998–1002, 2020. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu L, Yang P, Zhao Y, Zhuang Z, Wang Z, Song R, Zhang J, Liu C, Gao Q, Xu Q, Wei X, Sun HX, Ye B, Wu Y, Zhang N, Lei G, Yu L, Yan J, Diao G, Meng F, Bai C, Mao P, Yu Y, Wang M, Yuan Y, Deng Q, Li Z, Huang Y, Hu G, Liu Y, Wang X, Xu Z, Liu P, Bi Y, Shi Y, Zhang S, Chen Z, Wang J, Xu X, Wu G, Wang FS, Gao GF, Liu L, Liu WJ. Single-cell sequencing of peripheral blood mononuclear cells reveals distinct immune response landscapes of COVID-19 and influenza patients. Immunity 53: 685–696.e3, 2020. doi: 10.1016/j.immuni.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BMJC, Feldman J, Muus C, Wadsworth MH II, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J, Banovich N, Barbry P, Brazma A, Desai T, Duong TE, Eickelberg O, Falk C, Farzan M, Glass I, Haniffa M, Horvath P, Hung D, Kaminski N, Krasnow M, Kropski JA, Kuhnemund M, Lafyatis R, Lee H, Leroy S, Linnarson S, Lundeberg J, Meyer K, Misharin A, Nawijn M, Nikolic MZ, Ordovas-Montanes J, Pe’er D, Powell J, Quake S, Rajagopal J, Tata PR, Rawlins EL, Regev A, Reyfman PA, Rojas M, Rosen O, Saeb-Parsy K, Samakovlis C, Schiller H, Schultze JL, Seibold MA, Shalek AK, Shepherd D, Spence J, Spira A, Sun X, Teichmann S, Theis F, Tsankov A, van den Berge M, von Papen M, Whitsett J, Xavier R, Xu Y, Zaragosi L-E, Zhang K; HCA Lung Biological Network . SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181: 1016–1035.e19, 2020. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Mass cytometry data analyzed in the article (Figs. 1–6) are available in a public repository at http://flowrepository.org/id/FR-FCM-Z2RC.
Figure 1.

Experimental approach and characterization of blood CD45+ immune cells. A: experimental outline showing peripheral blood mononuclear cells (PBMC) collection and mass cytometry analysis. B: t-distributed stochastic neighbor embedding (t-SNE) plot of major immune cells in PBMCs. Cells are colored on the basis of cell types. C: heat map of major immune cells in PBMCs, clustered by their relative expression of the markers. D: t-SNE projections of major immune cells in PBMCs: 100,000, 100,000 and 80,000 cells from the healthy control (HC), acute phase (AP), and convalescent phase (CP) groups, respectively. E: relative fractions of major immune cells in blood CD45+-immune cells from the HC, AP, and CP group. F: percentage of major immune cells in blood CD45+ immune cells from the HC (n = 5), AP (n = 5), and CP group (n = 4). Adjusted P values are based on two-way ANOVA tests with Bonferroni’s post hoc correction between groups. ****Significant with adjusted P < 0.0001.
Fig. 6.

Characterization of single-cell monocytes from mass cytometry data. A: t-distributed stochastic neighbor embedding (t-SNE) plot of major monocyte subsets. Cells are colored on the basis of cell types. B: violin plot showing the expression of CD11C, CD14, CD16, HLA-DR in the monocytes cluster between (HC), acute phase (AP) and CP group. C: t-SNE projections of major monocyte subsets from the HC, AP, and CP group, respectively. D: bar plots highlighting cell abundances across monocyte subsets for the HC, AP, and CP group. E: percentage of major monocyte subsets in all blood CD45+ immune cells from the HC (n = 5), AP (n = 5), and CP group (n = 4). Adjusted P values are based on Wilcoxon rank-sum test (marker level) or two-way ANOVA tests with Bonferroni’s post hoc correction (parametric) between groups. ****Significant with adjusted P value < 0.0001. *Significant with adjusted P value < 0.05.