Summary
Background
In the phase 3 SOLO2 trial (ENGOT Ov-21), maintenance therapy with olaparib tablets significantly prolonged progression-free survival (primary endpoint) compared with placebo in patients with a germline BRCA1 or BRCA2 (BRCA1/2) mutation and platinum-sensitive, relapsed ovarian cancer who had received two or more lines of previous chemotherapy. The most common subjective adverse effects included fatigue, nausea, and vomiting, which were typically low grade and self-limiting. Our a-priori hypothesis was that maintenance olaparib would not negatively affect health-related quality of life (HRQOL) and additionally that the prolongation of progression-free survival with olaparib would be underpinned by additional patient-centred benefits.
Methods
In SOLO2, 196 patients were randomly assigned to olaparib tablets (300 mg twice daily) and 99 to placebo. Randomisation was stratified by response to previous chemotherapy (complete vs partial) and length of platinum-free interval (>6–12 vs >12 months). The prespecified primary HRQOL analysis evaluated the change from baseline in the Trial Outcome Index (TOI) score during the first 12 months of the study. To be assessable, patients had to have an evaluable score at baseline and at least one evaluable follow-up form. Secondary planned quality-of-life (QOL) analyses included the duration of good quality of life (defined as time without significant symptoms of toxicity [TWiST] and quality-adjusted progression-free survival [QAPFS]). Efficacy and QOL outcomes were analysed in all randomly assigned patients (the full analysis set), and safety outcomes were analysed in all randomly assigned patients who received at least one dose of study drug. This ongoing study is registered with ClinicalTrials.gov, number NCT01874353, and is closed to new participants.
Findings
The adjusted average mean change from baseline over the first 12 months in TOI was −2·90 (95% CI −4·13 to −1·67) with olaparib and −2·87 (−4·64 to −1·10) with placebo (estimated difference −0·03; 95% CI −2·19 to 2·13; p=0·98). Mean QAPFS (13·96 [SD 10·96] vs 7·28 [5·22] months; difference 6·68, 95% CI 4·98–8·54) and mean duration of TWiST (15·03 [SD 12·79] vs 7·70 [6·42] months; difference 7·33, 95% CI 4·70–8·96) were significantly longer with olaparib than with placebo.
Interpretation
Olaparib maintenance therapy did not have a significant detrimental effect on HRQOL compared with placebo. There were clinically meaningful patient-centred benefits in both TWiST and QAPFS despite the adverse effects associated with olaparib. These patient-centred endpoints support the improvement in progression-free survival, the primary endpoint in SOLO2, and should be included in future trials of maintenance therapies.
Introduction
Olaparib is a potent oral inhibitor of poly(ADP-ribose) polymerase (PARP). It is approved in the European Union and in the USA (tablet formulation) for the maintenance treatment of patients with platinum-sensitive relapsed ovarian cancer who are in response to their most recent platinum regimen, regardless of BRCA1 or BRCA2 (BRCA1/2) mutation status.1,2
In 2017, findings from the phase 3 SOLO 2 trial showed that maintenance therapy with olaparib tablet formulation significantly prolonged progression-free survival in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation who had responded to platinum-based chemotherapy.3 In SOLO2, median investigator-assessed progression-free survival was 19·1 months with olaparib versus 5·5 months with placebo (hazard ratio [HR] 0·30, 95% CI 0·22–0·41; p<0·0001).3
There is general consensus to support selecting progression-free survival as the primary endpoint in clinical trials in patients with recurrent ovarian cancer. The Response Evaluation Criteria in Solid Tumors (RECIST) International Working Group developed standardised criteria for tumour progression, which is used to determine progression-free survival in patients enrolled in clinical trials.4 A significant prolongation in progression-free survival is assumed to be associated with patient benefits and to make treatment worthwhile. However, this assumption is rarely tested and, importantly, progression-free survival does not take into account the effect of treatment-related adverse effects on the health-related quality of life (HRQOL) of patients. This aspect is particularly relevant in the setting of maintenance therapy trials, because most patients are well after responding to chemotherapy, apart from residual adverse effects associated with previous chemotherapy. These patients do not have ovarian cancer-related symptoms and it is therefore not possible to improve HRQOL with maintenance therapy, although the adverse effects of treatment could negatively affect HRQOL and counterbalance the perceived benefits associated with a gain in progression-free survival. Maintenance therapies should therefore have an acceptable subjective toxicity profile to ensure that the delay in disease progression with treatment is also associated with a longer duration of good-quality life, and additional patient-centred benefits that matter to patients such as delaying the onset of symptoms of progression and the need for further chemotherapy. Notably, the 5th Ovarian Cancer Consensus Conference concluded that progression-free survival was an acceptable primary endpoint in trials of recurrent ovarian cancer only if supported by additional endpoints.5 For example, in cohorts of patients with expected median overall survival of more than 12 months, it was recognised that overall survival is heavily dependent on subsequent therapies and that progression-free survival should be supported by time to second subsequent therapy or death (TSST) and patient-reported outcomes.5
Research into patient-centred outcomes is informed by the perspectives, views, and values of patients; the results are used to support decision making, highlighting comparisons between treatments and outcomes that matter to patients.6 Although patient preferences were not assessed in SOLO2, it is worth noting that in a survey of women with recurrent ovarian cancer, most patients reported that they required at least a 5-month increase in progression-free survival to make treatment worthwhile.7 The adverse effects of treatment were also very important to patients and most would trade off a reduction in progression-free survival to avoid or reduce significant adverse effects, particularly when the treatment was not curative.7 This finding is supported by a study of patient preferences of chemotherapy for recurrent ovarian cancer in which the participants rated and ranked progression-free survival higher than all other attributes and, again, were willing to accept a shorter progression-free survival to avoid severe adverse effects, particularly nausea and vomiting.8 Ideally, a similar analysis should be considered in future trials of maintenance therapy with PARP inhibitors. Our a-priori hypothesis was that maintenance therapy with olaparib would not negatively affect HRQOL compared with placebo. Additionally, we hypothesised that olaparib would be associated with additional patient-centred benefits, such as time without symptoms of treatment toxicity (TWiST) and quality-adjusted progression-free survival (QAPFS), which were clinically meaningful and would support the expected prolongation of progression-free survival with olaparib, the primary endpoint of SOLO2.
Methods
Study design and participants
The design of this randomised, double-blind, international, multicentre, phase 3 SOLO2 study (ENGOT Ov-21; NCT01874353) has been reported previously.3 In brief, SOLO2 was done across 123 sites in 16 countries (appendix pp 1-4) and eligible patients were aged 18 years or older with histologically confirmed, relapsed, high-grade serous ovarian cancer or endometrioid cancer, including primary peritoneal or fallopian tube cancer. Patients were required to have a germline BRCA1/2 mutation (confirmed by Myriad BRACAnalysis; Myriad Genetics, Salt Lake City, UT, USA) predicted or suspected to be deleterious, to have received at least two previous lines of platinum-based chemotherapy, to have a radiological (RECIST version 1.1) and CA125 response to their most recent platinum-based regimen and to have an Eastern Cooperative Oncology Group performance status of 0–1. Additional eligibility criteria are provided in the appendix (p 4).
The institutional review boards or independent ethics committees of all investigational sites approved the protocol. The study was performed in accordance with the Declaration of Helsinki, Good Clinical Practice, and the AstraZeneca policy on bioethics.9
Randomisation and masking
As previously reported,3 randomisation was done using a computer software program that generated random numbers (Global Randomisation System), with the randomisation scheme loaded into an interactive voice and web response system database. The method of randomisation was simple randomisation within each stratum level, the stratum levels being response to previous chemotherapy (complete vs partial) and length of platinum-free interval (>6–12 months vs >12 months). Investigators (or nominated assistants) contacted the centralised randomisation centre for allocation of randomised therapy. Masking was achieved using individual treatment codes assigned by the voice and web response system, with treatment assignment masked for patients, those giving the interventions, data collectors, and data analysers. Olaparib and placebo tablets (manufactured by AstraZeneca; Cambridge, UK) looked identical.
Procedures
Patients were randomly assigned (2:1) to receive maintenance therapy with olaparib tablets (300 mg twice daily) or matching placebo until disease progression or the investigator determined that they were no longer benefiting from treatment. As previously reported,3 toxicities could be managed by treatment interruptions and dose reductions, if required.
Outcomes
The primary efficacy endpoint in SOLO2 (investigator-assessed progression-free survival) and secondary safety and tolerability data (adverse events) have previously been reported.3
The HRQOL outcome reported here was the prespecified primary HRQOL analysis evaluated by the change from baseline in the Functional Assessment of Cancer Therapy—Ovarian Cancer (FACT-O) Trial Outcome Index (TOI) score during the first 12 months of the study. FACT-O is a reliable and well-validated instrument to assess QOL in women with ovarian cancer, including how they are affected by adverse effects associated with pharmacological treatments.10 The TOI is a summary index of physical and functional wellbeing and key ovarian cancer symptoms derived from the FACT-O questionnaire. TOI scores range from 0 to 100, with higher scores indicating better HRQOL. The FACT-O questionnaire was completed at baseline, week 5, week 13, and then every 12 weeks for 24 months, or until the date of data cutoff for the primary efficacy analysis (whichever came first). The FACT-O questionnaire was also completed at study treatment discontinuation and 30 days following the last dose of study drug, and then every 12 weeks during survival follow-up visits for patients with disease progression.
The patient-centred outcomes of QAPFS and TWiST were included as secondary endpoints in a planned QOL statistical analysis.
QAPFS was analysed to assess duration of good quality of life between the two treatment groups. QAPFS incorporates progression-free survival and health state into a single measure of net clinical benefit (appendix p 9). QAPFS is the product of the adjusted mean estimate of the EuroQol five-dimensions five-level (EQ-5D-5L) single-index utility score from randomisation to disease progression and the area under the Kaplan-Meier curve for time to progression (appendix p 4). The EQ-5D-5L questionnaire was completed at the same timepoints as the FACT-O questionnaire. The EQ-5D-5L asks patients to respond to five different dimensions covering mobility, self-care, usual activities, pain or discomfort, and anxiety or depression, as well as to rate how they feel on the day of assessment on a visual analogue scale.11 The five different dimensions are mapped to utilities using the established and validated values that are country-specific for each patient.
Quality-adjusted TWiST (QTWiST) is an attempt to integrate both the quantity and quality of survival. Survival is partitioned into three health states: toxicity (the period with clinically significant symptoms after randomisation and before protocol-defined disease progression); TWiST (the period without clinically significant symptoms after randomisation and before protocol-defined disease progression [or censoring for progression]); and relapse (the period between protocol-defined progression and death [or censoring for death]). The current analysis focused on TWiST, which was defined as the time without significant symptoms of toxicity (defined a priori as National Cancer Institute Common Terminology Criteria for Adverse Events [CTCAE] version 4.0 grade ≥2 nausea, vomiting, or fatigue) after randomisation and before protocol-defined disease progression (or censoring for progression). Toxicity data were obtained from the reported adverse events and the toxicity state included the total number of days spent after randomisation and before progression or, if no progression, to the last follow-up date, with grade 2 or worse nausea, vomiting, or fatigue, regardless of when the toxicity started or whether there were gaps between toxicities (appendix p 9). For each patient, this duration was summed; if a patient did not progress, the status of the toxicity variable was classified as being censored, otherwise the status was classified as an event. No overlapping periods of patients with adverse events were included on the summation. The duration of overlapping periods was counted from the start date of the first adverse event period until the stop date of the last period. Patients who experienced no qualifying adverse events before disease progression were censored at the day after randomisation and were assigned a duration of zero for the toxicity state. Dose reductions, which were triggered by episodes of toxicity, were also captured in the TWiST calculations. The model included only nausea, vomiting, and fatigue because they were the most common adverse effects reported in Study 1912 and were considered most likely to affect patient QOL. Additionally, we did a sensitivity analysis that included all adverse events of grade 2 or worse. The type, date of onset, and date of resolution of each toxicity were recorded prospectively. As it was possible for a patient to have more than one type of toxicity over time, overlapping toxicity intervals were not counted twice (eg, if a patient had grade 2 nausea that lasted from day 2 to day 5 and fatigue that lasted from day 2 to day 10, the number of days spent with toxicity was counted as 8 days).13
Changes over time in the FACT-O subscale scores for physical, social, emotional, and functional wellbeing and additional concerns (ovarian cancer subscale) were also assessed.
Statistical analysis
As previously reported,3 SOLO2 was powered to detect differences in progression-free survival. The sample size was also sufficient to detect a difference of at least 6 points in TOI based on 90% power, 95% CI, and an SD of 15.
Efficacy and all QOL analyses were done based on the intention-to-treat principle in the full analysis set (comprising all randomly assigned patients), and safety outcomes were analysed in all randomly assigned patients who received at least one dose of study drug. To be assessable for HRQOL, patients were required to have an evaluable score at baseline and at least one evaluable follow-up form.
Change from baseline in TOI score (primary analysis) was analysed using a mixed model for repeated measures analysis of the change from baseline in TOI score for each visit until 12 months, regardless of the treatment they were receiving at 12 months (appendix p 4). A timepoint of 12 months was selected because we anticipated that most patients randomly assigned to placebo would have progressed after 12 months.
QAPFS for each treatment group was calculated using the product of the adjusted mean EQ-5D-5L utility from randomisation to progression and the area under the Kaplan-Meier curve for time to progression. The estimation of the mean EQ-5D single-index utility score from randomisation to disease progression was calculated using a mixed model for repeated measures analysis for each treatment group using restricted maximum likelihood estimation. The 95% CIs and p values for the between-group differences in QAPFS were calculated using bootstrap methods (appendix p 4).
TWiST duration (progression-free survival minus toxicity) was presented using Kaplan-Meier curves corresponding to toxicity and progression-free survival. The difference between the two treatment groups in mean TWiST was calculated and presented with a bootstrapped 95% CI and two-sided p value. Descriptive statistics were reported for the changes from baseline in FACT-O subscale scores. No imputations were made to correct for missing data because of the high compliance rate until end of treatment, which was the primary focus of the analysis.
All analyses were performed in SAS (version 9.3.1). The statistical analysis plan is available in the appendix. This study is registered with ClinicalTrials.gov, number NCT01874353, and is closed to new participants.
Role of the funding source
As previously reported,3 SOLO2 was a collaboration between Groupe d’Investigateurs National des Etudes des Cancers Ovariens et du sein (GINECO), the European Network for Gynaecological Oncological Trial groups (ENGOT), and the funder, AstraZeneca. This Article was written by the authors, with medical writing support paid for by the funder. All authors had full access to the raw data and had roles in data collection, analysis, and interpretation and manuscript writing. The decision to submit the manuscript for publication was made by all the authors. The corresponding author had full access to all the raw data and had final responsibility for the decision to submit for publication.
Results
As previously reported,3 295 patients were enrolled in SOLO2 between Sept 3, 2013, and Nov 21, 2014. The full analysis set included 196 patients randomly assigned to olaparib and 99 to placebo, of whom 195 received olaparib and 99 received placebo (one patient was randomised incorrectly and did not receive study treatment). The median total duration of treatment was 19·4 months (interquartile range [IQR] 8·2–25·5) for olaparib and 5.6 months (IQR 3·7–11·0) for placebo. Among patients who were still on study, compliance for both the FACT-O and EQ-5D-5L questionnaires was seen in 125 (91%) of 138 patients in the olaparib group and in 24 (92%) of 26 patients in the placebo group at week 49, and in 63 (68%) of 92 patients in the olaparib group and in 48 (68%) of 71 patients in the placebo group at the end of treatment (appendix pp 5-8, 10).
Patient demographics and baseline characteristics at study entry, which could potentially affect HRQOL, are presented in the table. At baseline, the majority of patients were not experiencing clinically significant adverse events.
Table:
Patient demographics and baseline characteristics
| Olaparib group (n=1196) | Placebo group (n=99) | |
|---|---|---|
| Median age, years (IQR) | 56 (51–63) | 56 (49–63) |
| ECOG performance status | ||
| 0 | 162 (83%) | 77 (78%) |
| 1 | 32 (16%) | 22 (22%) |
| Missing | 2 (1%) | 0 |
| Primary tumour location | ||
| Ovary | 164 (84%) | 86 (87%) |
| Fallopian tube or primary peritoneal | 31 (16%) | 13 (13%) |
| Missing | 1 (1%) | 0 |
| Response to previous platinum therapy | ||
| Complete response | 91 (46%) | 47 (47%) |
| Partial response | 105 (54%) | 52 (53%) |
| Previous platinum regimens | ||
| 2 | 110 (56%) | 62 (63%) |
| B | 60 (31%) | 20 (20%) |
| 4 | 18 (9%) | 12 (12%) |
| ≥5 | 7 (4%) | 5 (5%) |
| Time to disease progression in the penultimate platinum regimen | ||
| >6–12 months | 79 (40%) | 40 (40%) |
| >12 months | 117 (60%) | 59 (60%) |
| Median time from previous platinum regimen to randomisation, days (IQR) | 41.0 (32.0–48.0) | 38.0 (31.0–49.0) |
| Adverse events of interest at baseline* | ||
| Abdominal pain | 10 (5%) | 3 (3%) |
| Constipation | 21 (11%) | 14 (14%) |
| Nausea | 14 (7%) | 4 (4%) |
| Fatigue | 29 (15%) | 15 (15%) |
| Vomiting | 1 (1%) | 0 |
| Anaemia | 42 (21%) | 20 (20%) |
| Leucopenia | 8 (4%) | 2 (2%) |
| Mean TOI score (SD) | 75.26 (13.78) | 77.12 (11.35) |
| Mean total FACT-O score (SD) | 114.4 (19.31) | 116.6 (17.24) |
| Mean EQ-5D-5L score (SD) | 0.81(0.182) | 0.84 (0.120) |
Data are n (%), unless otherwise specified. ECOG=Eastern Cooperative Oncology Group. TOI=Trial Outcome Index. FACT-O=Functional Assessment of Cancer Therapy—Ovarian Cancer. EQ-5D-5L=EuroQol five-dimensions five-level. MedDRA=Medical Dictionary for Regulatory Activities.
Investigator-recorded adverse events at baseline (all grades; MedDRA preferred term).
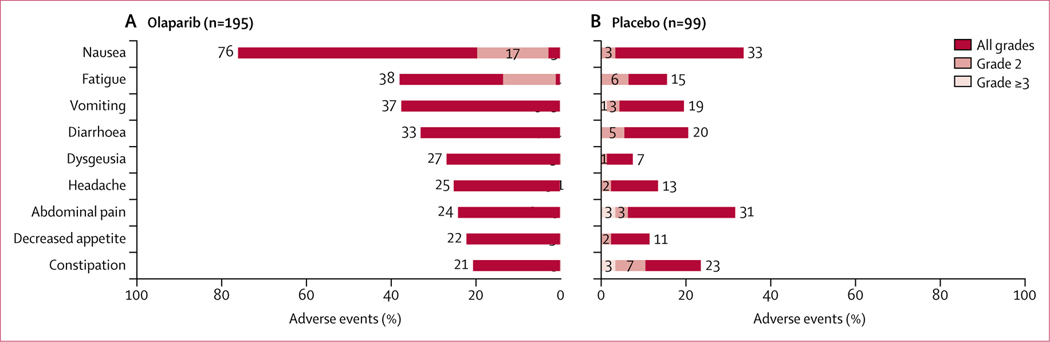
In SOLO2, the most commonly reported non-haematological adverse events (all grades; incidence >20%) in patients receiving olaparib included nausea, fatigue, vomiting, diarrhoea, dysgeusia, headache, abdominal pain, decreased appetite, and constipation (figure 1). Non-haematological adverse events of grade 2 or worse severity (incidence >5%) included nausea (38 [19%] olaparib recipients vs three [3%] placebo recipients), fatigue (26 [13%] vs six [6%]), abdominal pain (20 [10%] vs six [6%]), diarrhoea (16 [8%] vs five [5%]), and vomiting (15 [8%] vs four [4%]). Most of these grade 2 or worse adverse events were of grade 2 severity, as shown in figure 1. In addition to the non-haematological adverse events shown, fatigue and asthenia (grouped term) was reported in 128 (66%) patients in the olaparib group versus 39 (39%) patients in the placebo group for all grades, in 45 (23%) patients versus 15 (15%) patients for grade 2, and in eight (4%) patients versus two (2%) patients for grade 3 or worse.
Figure 1: Adverse events.
Figure shows all-grade, grade 2, and grade 3 or worse adverse events.
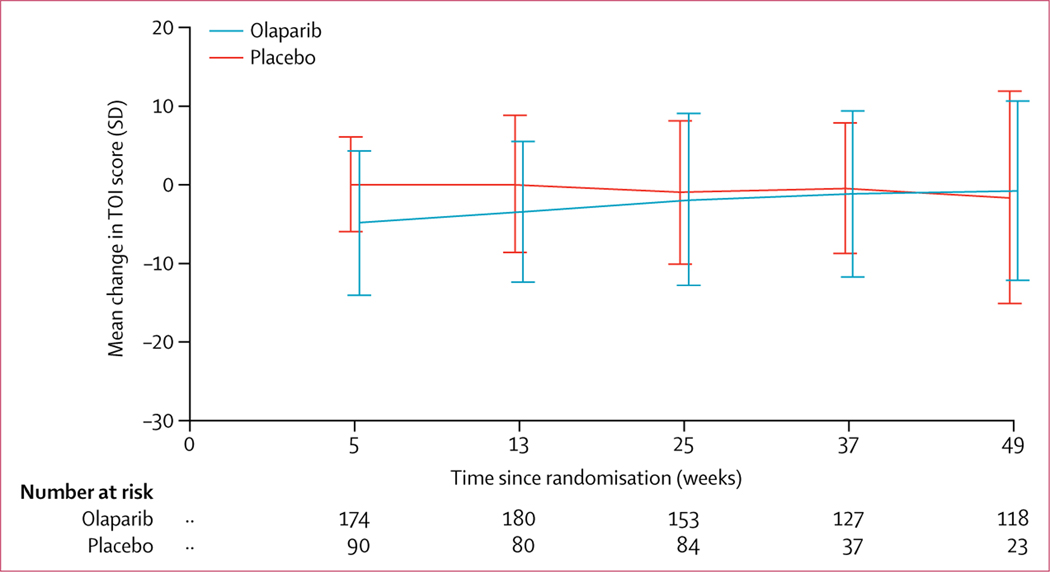
Mean TOI scores at baseline were 75·26 (SD 13·78) in patients randomly assigned to olaparib and 77·12 (11·35) in patients randomly assigned to placebo. Olaparib did not negatively affect mean TOI score over the first 12 months of the study (figure 2). The average adjusted mean change from baseline over the first 12 months in TOI was −2·90 (95% CI −4·13 to −1·67) with olaparib (n=185) and −2·87 (−4·64 to −1·10) with placebo (n=94), with an estimated difference of −0·03 (95% CI −2·19 to 2·13; p=0·98). 11 (6%) of 196 patients in the olaparib group and five (5%) of 99 patients in the placebo group were excluded because of noncompliance with the FACT-O questionnaire. Mean TOI scores over time are shown in the appendix (p 11).
Figure 2: Change from baseline in TOI score over time.
Numbers given are numbers of patients with data available at each timepoint. TOI=Trial Outcome Index.
Mean total FACT-O and FACT-O HRQOL scores also remained stable over the course of treatment in patients receiving olaparib (appendix p 12). Changes over time in FACT-O subscale scores for physical, social, emotional, and functional wellbeing and additional concerns are shown in the appendix (pp 13-15).
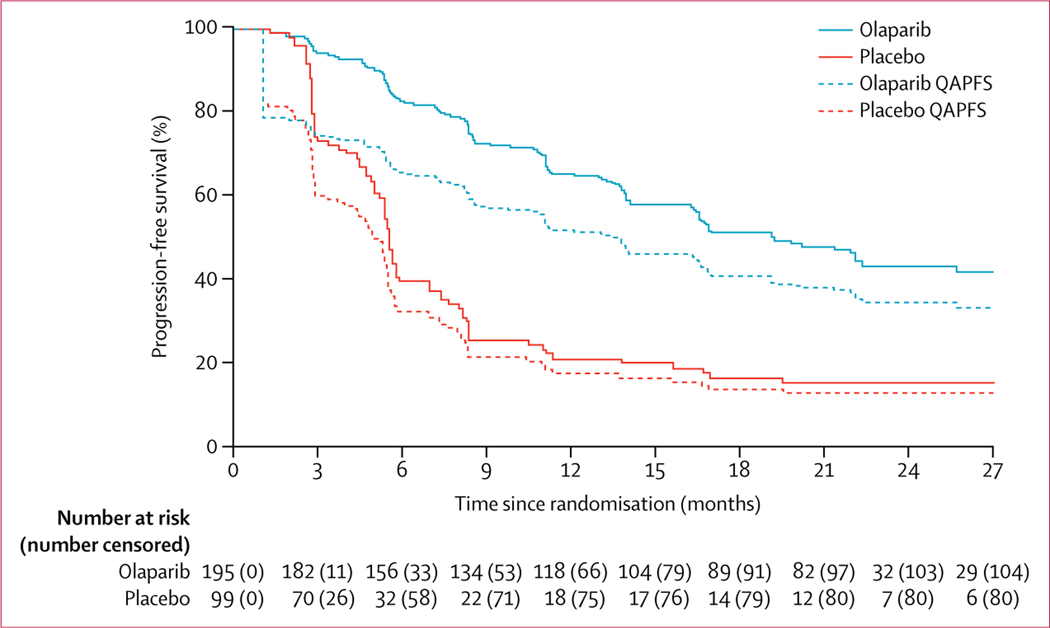
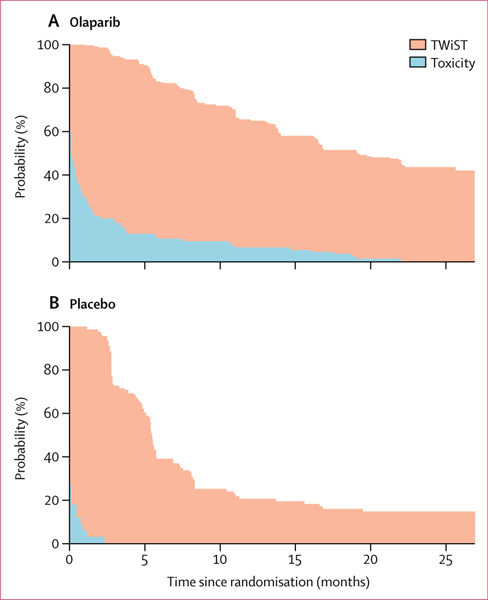
Olaparib was associated with significant patient-centred benefits based on QAPFS and TWiST, despite the toxicity experienced by patients receiving olaparib versus placebo. Mean QAPFS was longer with olaparib than with placebo (13·96 [SD 10·96] vs 7·28 [5·22] months; difference 6·68, 95% CI 4·98–8·54; p<0·0001; figure 3; appendix p 11). The mean duration of TWiST was also longer for patients receiving olaparib than for those receiving placebo (15·03 [SD 12·79] vs 7·70 [6·42] months; difference 7·33, 95% CI 4·70–8·96; p<0·0001; figure 4).
Figure 3: Progression-free survival and QAPFS for olaparib versus placebo.
QAPFS=quality-adjusted progression-free survival.
Figure 4: TWiST curves for olaparib (A) and placebo (B).
The horizontal axis is the duration of toxicity or the duration without toxicity or symptoms. The vertical axis is the probability of patients experiencing toxicity or being toxicity-free and symptom-free.19,20 TWiST=time without significant symptoms of toxicity.
Sensitivity analysis showed that the TWiST benefit for olaparib versus placebo remained when toxicity was defined as all adverse events of grade 2 or worse (13·70 vs 7·08 months; difference 6·62, 95% CI 4·08–8·25; appendix p 16).
Discussion
This study showed that the increase in progression-free survival with maintenance olaparib in SOLO2 is supported by clinically meaningful patient-centred benefits, including significant improvements in both TWiST and QAPFS, which take into consideration the adverse effects of olaparib. Additionally, as previously reported,3 time to first subsequent therapy or death (TFST) and TSST were also significantly longer with olaparib. In SOLO2, median progression-free survival with maintenance olaparib tablets versus placebo was 19·1 versus 5·5 months (HR 0·30, 95% CI 0·22–0·41; p<0·0001) after response to platinum-based chemotherapy in patients with a germline BRCA1/2 mutation and platinum-sensitive relapsed ovarian cancer.3 These findings confirmed the results of Study 19,12,14 a randomised phase 2 trial of maintenance olaparib capsules in patients with platinum-sensitive recurrent ovarian cancer that included both patients with and without BRCA mutations.
It is important to appreciate that most patients randomly assigned to olaparib or placebo in SOLO2 did not have cancer-related symptoms when they commenced maintenance therapy. They had responded to platinum-based chemotherapy and were well, apart from having residual effects of previous chemotherapy, including anaemia in roughly 20% of patients. The overarching aim of maintenance treatment is to delay the time to symptomatic progression and the need for further chemotherapy for as long as possible with acceptable toxicity and, importantly, without compromising the HRQOL of patients while on maintenance therapy. It is not possible to improve the HRQOL of patients who do not have cancer-related symptoms, although it is certainly possible that adverse effects of maintenance treatment could negatively affect HRQOL and potentially counterbalance any gain in progression-free survival. Therefore, a robust and meaningful evaluation of the effect of maintenance therapy on the HRQOL of patients is essential to conclude that treatment is worthwhile.
Although progression-free survival has become an important surrogate outcome to assess the efficacy of new drugs, its relationship with HRQOL is unclear. It is notable that, in this study, there was no statistically significant or clinically significant decline in TOI at the end of therapy or at the first post-progression visit, suggesting that most patients had few symptoms to affect HRQOL when they had RECIST progression. In an insightful commentary on progression-free survival as an endpoint, Booth and Eisenhauer15 questioned the relationship between progression-free survival and patient benefit and highlighted the paucity of studies addressing this important question. They concluded that it is time to take a hard look at progression-free survival and that there should be good evidence for its ability to predict improved HRQOL or overall survival to define new standards of care.15
We recognised that it was essential to underpin the progression-free survival primary endpoint of SOLO2 with additional patient-centred endpoints to determine whether there were benefits of maintenance olaparib to patients beyond the RECIST definition of progression as determined by CT. We therefore spent considerable time deliberating on the most relevant HRQOL and patient-centred endpoints that should be included. Our patient-reported outcomes hypotheses and analysis plan are consistent with the recommendations of the 5th Ovarian Cancer Consensus Conference5 and the Society of Gynecologic Onoclogy16 that progression-free survival alone is not an acceptable endpoint in clinical trials in patients with recurrent ovarian cancer.
Our a-priori HRQOL hypothesis was that maintenance olaparib would not negatively affect HRQOL compared with placebo and would also be associated with additional patient-centred benefits to support the primary endpoint of progression-free survival. The change from baseline to month 12 in TOI score was the primary HRQOL endpoint; it represents an established single index derived from the FACT-O questionnaire, is a well-validated instrument, evaluates functional and physical wellbeing, and includes the most relevant ovarian cancer symptoms. Compliance with the FACT-O and EQ-5D-5L questionnaires was high (more than 90% at 12 months), dropping to roughly 70% at the end of treatment. As expected, patients were relatively well at baseline (mean TOI score approximately 75). Importantly, maintenance therapy with olaparib did not have a significant detrimental effect on HRQOL in SOLO2 versus placebo. We noted no significant between-group difference in adjusted mean change in TOI score from baseline to month 12, which supports our primary hypothesis. In both olaparib and placebo recipients, the changes in TOI score were small and not considered clinically relevant.
We also selected additional patient-centred analyses, including QAPFS and TWiST as endpoints, in addition to TFST and TSST, which have previously been reported.3 TWiST and QAPFS are well developed methods of describing the duration of good quality of life (TWiST) and QAPFS in clinical trials in patients with a wide range of malignancies, as well as other health states and use validated measures.17–20 TWiST measures the clinical state of a patient’s experience (ie, whether they are experiencing symptoms or toxicity) regardless of their perception of how it affects their daily living. It is thus an objective measure of the effect of the physical and clinical symptoms. Conversely, QAPFS seeks to weight this duration against the importance that patients place on their clinical state by the utility (a measure of importance) of being in that state. It is worth noting that TWiST and QAPFS might not be improved in patients with a statistically significant increase in progressionfree survival when there is substantial toxicity. For example, in a trial21 of maintenance pazopanib following first-line chemotherapy for patients with ovarian cancer, a statistically significant 5-month increase in progression-free survival was reported with pazopanib. However, in a post-hoc exploratory analysis, QAPFS was 386 days (95% CI 366–404) in the pazopanib group and 359 days (95% CI 338–379 days) in the placebo group, which was not significant and raised questions about the patient-centred benefit of increased progression-free survival.22
We found that mean QAPFS was 13·96 months with olaparib versus 7·28 months with placebo. Other studies have used QAPFS as an outcome measure. For example, in the BOLERO-2 study17 in patients with hormone-receptor-positive metastatic breast cancer, QAPFS was 30·1 weeks (95% CI 27·60–32·58) for everolimus plus exemestane and 16·3 weeks (95% CI 14·07–18·46) for placebo plus exemestane. The investigators concluded that QAPFS as an outcome measure provided a complete picture of the benefits induced by the treatment groups in the BOLERO-2 trial.17
We aimed to assess the duration of good quality of life by analysing the difference in TWiST between the olaparib and placebo groups. Gelber and Goldhirsch18 initially developed the TWiST methodology for assessing adjuvant treatment in early breast cancer, whereby potentially toxic therapies are administered to patients who are asymptomatic. They reported that adjuvant chemotherapy and endocrine therapy were associated with a significant gain in TWiST in an era before the survival benefits of adjuvant therapy were clear, and later went on to develop QTWiST.19 We included QTWiST as a secondary endpoint in our analysis plan, but given that the survival data are immature, we have focused on TWiST as this more directly reflects the progression-free survival endpoint and, specifically, the potential effect of adverse effects and their duration on patients; it also complements QAPFS. We defined clinically significant symptoms after randomisation as any period of CTCAE grade 2 or worse nausea, vomiting, or fatigue, as these were the most common adverse effects experienced by patients in Study 19.12 For the toxicity period, the number of days during which these symptoms were maintained was totalled for each patient; those patients who did not experience these symptoms between randomisation and progressive disease were assigned a toxicity value of zero and censored on day 1 after randomisation. In SOLO2, the TWiST duration was 15·03 months with olaparib versus 7·70 months with placebo. The difference in TWiST benefit in favour of olaparib maintenance was maintained in the sensitivity analyses in which we analysed TWiST duration for all adverse events of grade 2 or worse (13·7 vs 7·1 months) as well as for nausea, vomiting, or fatigue of grade 1 or worse or grade 3 or worse (appendix p 5).
The 5th Ovarian Cancer Consensus Conference5 recommended that progression-free survival should be supported by TSST, patient-reported outcomes, or both in patients with expected overall survival greater than 12 months. TFST and TSST are intermediate time-dependent endpoints that can also be used to determine whether there are additional demonstrable benefits to patients of prolonging progression-free survival. These endpoints provide information on the effect of maintenance therapy on delay in time to subsequent chemotherapy for symptomatic progression; in this study, the TFST and TSST findings suggested that olaparib maintenance therapy did not reduce the likelihood of response and benefit of subsequent therapy after progression. Most patients with a germline BRCA1/2 mutation and platinum-sensitive, relapsed ovarian cancer will receive multiple lines of treatment after progression, and it is also very likely that many patients in the placebo group will also be treated with a PARP inhibitor. All these treatments can affect post-progression survival and could obscure or dilute any overall survival advantage associated with maintenance olaparib. Hence, it is important to also include intermediate endpoints such as TFST and TSST in maintenance trials. We have previously reported that the median TFST was 27·9 months with olaparib versus 7·1 months with placebo (HR 0·28, 95% CI 0·21–0·38; p<0·0001), which was a significant result.3 The delay in starting chemotherapy was longer than the median progression-free survival, which suggests that many patients were symptom-free at the time of RECIST-defined progression and reflects clinical practice in which palliative chemotherapy is withheld until patients have clinically significant symptoms rather than immediately starting chemotherapy upon progression determined by CT. Median TSST was 18·2 months in the placebo group and had not yet been reached at 3 years in the olaparib group (HR 0·37, 95% CI 0·26–0·53; p<0·0001),3 indicating that progression after maintenance olaparib did not negatively affect subsequent response to chemotherapy.
A limitation of this study is that patient preferences were not assessed in SOLO2 and could have provided information on the value patients place on progressionfree survival and whether the adverse effects associated with olaparib are offset by the prolongation in progression-free survival and TFST. Although these factors would not have changed the results of this study, it would be informative to have also had collected data on patient preferences, and this should be considered in future studies of maintenance therapy.
Olaparib did not have a detrimental effect on HRQOL compared with placebo and there were additional significant patient-centred benefits in terms of TWiST and QAPFS, as well as in TFST and TSST. All these predefined endpoints support the benefit to patients of a prolongation of progression-free survival, which is the primary endpoint in maintenance trials in ovarian cancer, and should be routinely included in future trials.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed and the databases of the American Society of Clinical Oncology, European Cancer Organisation, European Society of Gynaecological Oncology, European Society for Medical Oncology, and Society of Gynaecological Oncology for articles and conference abstracts published between Jan 1, 2016, and Jan 1, 2018, including the search terms “poly(ADP-ribose) polymerase inhibitor” or “PARP inhibitor”, “ovarian cancer”, and “quality of life”, using no language restrictions. The start date of Jan 1, 2016, reflects the fact that no phase 3 trials of PARP inhibitor maintenance therapy were reported before this date. In a previous phase 2 trial (Study 19) in patients with platinum-sensitive, relapsed ovarian cancer, maintenance monotherapy with the oral PARP inhibitor olaparib did not have a significant detrimental effect on HRQOL compared with placebo.
Added value of this study
To our knowledge, SOLO2 is the first trial to report the impact of maintenance therapy with a PARP inhibitor on predefined HRQOL and patient-centred endpoints to help interpret the benefits to patients with platinum-sensitive, relapsed ovarian cancer of prolongation of progression-free survival. This focus is particularly important in maintenance therapy trials given that most patients do not have symptoms associated with ovarian cancer at randomisation. In the prespecified primary HRQOL analysis, the mean change from baseline in the Functional Assessment of Cancer Therapy—Ovarian Cancer Treatment Outcomes Index score during the first 12 months of the study did not significantly differ between olaparib and placebo groups. Additionally, the secondary planned QOL analyses that we report showed longer quality-adjusted progression-free survival (QAPFS) and time without significant symptoms of toxicity (TWiST) in patients randomly assigned to olaparib compared with placebo. These results support the primary outcome of SOLO2 and indicate that the significant prolongation of progression-free survival with olaparib in this patient population was achieved with no appreciable detrimental effect on patients’ QOL and supported by additional patient-centred benefits.
Implications of all of the available evidence
Maintenance olaparib was associated with clinically meaningful patient-centred benefits, including a significant prolongation in QAPFS and TWiST, which are novel endpoints in maintenance trials in ovarian cancer. These results show the significant benefit of maintenance olaparib to patients beyond the RECIST definition of progression, the primary endpoint of SOLO2, and highlight the importance of including patient-centred outcomes in addition to HRQOL in trials of maintenance therapy, in line with the recommendations of the 5th Ovarian Cancer Consensus Conference.
Acknowledgments
We thank the patients and their families for their participation in this study, as well as all investigators and on-site personnel. The study was sponsored by AstraZeneca. We thank Gillian Keating (Mudskipper Business) who provided medical writing assistance funded by AstraZeneca.
Funding AstraZeneca.
MF reports personal fees from AstraZeneca during the conduct of the study and personal fees from MSA outside the submitted work.
RB reports full-time employment with AstraZeneca during the conduct of the study and AstraZeneca stock ownership. FH reports personal fees from Roche, AstraZeneca, PharmaMar, MSD, Medac, and Novartis, and non-financial support from Roche, AstraZeneca, and PharmaMar, outside the submitted work. DE reports full-time employment with AstraZeneca during the conduct of the study. MR reports personal fees and non-financial support from AstraZeneca, and grants from Bristol-Myers Squibb and Merck, outside the submitted work. AC reports grants from AstraZeneca during the conduct of the study, and grants and personal fees from AstraZeneca and grants from Clovis Oncology outside the submitted work. RTP reports personal fees from AstraZeneca during the conduct of this study. CS reports personal fees and non-financial support from AstraZeneca during the conduct of the study; grants, personal fees, and non-financial support from Clovis Oncology and Roche outside the submitted work; and other support from Clovis Oncology, Eisai Australia, and Beigene outside the submitted work. FJ reports other support from Roche, Tesaro and AstraZeneca, outside the submitted work. EP-L reports personal fees from AstraZeneca, Roche, Pfizer, Tesaro, and Clovis, and non-financial support from AstraZeneca, Roche, and Clovis, outside the submitted work.
Footnotes
Declaration of interests
All other authors declare no competing interests.
References
- 1.European Medicines Agency. Lynparza: summary of product characteristics. 2018. http://www.ema.europa.eu/docs/en_GB/documenLlibrary/EPAR__ProducLInformation/human/003726/WC500180151.pdf (accessed July 2, 2018).
- 2.FDA . Lynparza: highlights of prescribing information. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208558s000lbl.pdf (accessed April 19, 2018).
- 3.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18: 1274–84. [DOI] [PubMed] [Google Scholar]
- 4.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–7 [DOI] [PubMed] [Google Scholar]
- 5.Wilson MK, Pujade-Lauraine E, Aoki D, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recurrent disease. Ann Oncol 2017; 28: 727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank L, Basch E, Selby JV. The PCORI perspective on patient-centered outcomes research. JAMA 2014; 312: 1513–14. [DOI] [PubMed] [Google Scholar]
- 7.Minion LE, Coleman RL, Alvarez RD, Herzog TJ. Endpoints in clinical trials: what do patients consider important? A survey of the Ovarian Cancer National Alliance. Gynecol Oncol 2016; 140: 193–98. [DOI] [PubMed] [Google Scholar]
- 8.Havrilesky LJ, Alvarez SA, Ehrisman JA, et al. Patient preferences in advanced or recurrent ovarian cancer. Cancer 2014; 120: 3651–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AstraZeneca. Global policy: bioethics. 2016. https://www.astrazeneca.com/content/dam/az/PDF/Bioethics_policy.pdf (accessed April 19, 2018).
- 10.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the functional assessment of cancer therapy—ovarian. J Clin Oncol 2001; 19: 1809–17 [DOI] [PubMed] [Google Scholar]
- 11.Oppe M, Devlin NJ, van Hout B, Krabbe PF, de Charro F. A program of methodological research to arrive at the new international EQ-5D-5L valuation protocol. Value Health 2014; 17: 445–53. [DOI] [PubMed] [Google Scholar]
- 12.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012; 366: 1382–92. [DOI] [PubMed] [Google Scholar]
- 13.Beaumont JL, Salsman JM, Diaz J, et al. Quality-adjusted time without symptoms or toxicity analysis of pazopanib versus sunitinib in patients with renal cell carcinoma. Cancer 2016; 122: 1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014; 15: 852–61. [DOI] [PubMed] [Google Scholar]
- 15.Booth CM, Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol 2012; 30: 1030–33. [DOI] [PubMed] [Google Scholar]
- 16.Herzog TJ, Armstrong DK, Brady MF, et al. Ovarian cancer clinical trial endpoints: Society of Gynecologic Oncology white paper. Gynecol Oncol 2014; 132: 8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaby V, Adunlin G, Ali AA, Tawk R. Using quality-adjusted progression-free survival as an outcome measure to assess the benefits of cancer drugs in randomized-controlled trials: case of the BOLERO-2 trial. Breast Cancer Res Treat 2014; 146: 669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelber RD, Goldhirsch A. A new endpoint for the assessment of adjuvant therapy in postmenopausal women with operable breast cancer. J Clin Oncol 1986; 4: 1772–79. [DOI] [PubMed] [Google Scholar]
- 19.Gelber RD, Goldhirsch A, Cavalli F. Quality-of-life-adjusted evaluation of adjuvant therapies for operable breast cancer. Ann Intern Med 1991; 114: 621–28. [DOI] [PubMed] [Google Scholar]
- 20.Glasziou PP, Cole BF, Gelber RD, Hilden J, Simes RJ. Quality adjusted survival analysis with repeated quality of life measures. Stat Med 1998; 17: 1215–29. [DOI] [PubMed] [Google Scholar]
- 21.du Bois A, Floquet A, Kim JW, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol 2014; 32: 3374–82. [DOI] [PubMed] [Google Scholar]
- 22.Friedlander M, Rau J, Lee CK, et al. Quality of life in patients with advanced epithelial ovarian cancer (EOC) randomized to maintenance pazopanib or placebo after first-line chemotherapy in the AGO-OVAR 16 trial. Measuring what matters-patient-centered end points in trials of maintenance therapy. Ann Oncol 2018; 29: 737–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.