Abstract
DNA lesions inflicted by activation-induced deaminase (AID) instrumentally initiate the processes reshaping immunoglobulin genes in mature B-cells, from local somatic hypermutation (SHM) to junctions of distant breaks during class switch recombination (CSR). It remains incompletely understood how these divergent outcomes of AID attacks are differentially and temporally focused, with CSR strictly occurring in the Ig heavy chain (IgH) locus while SHM concentrates on rearranged V(D)J regions in the IgH and Ig light chain loci. In the IgH locus, disruption of either the 3’Regulatory Region (3’RR) super-enhancer or of switch (S) regions preceding constant genes, profoundly affects CSR. Reciprocally, we now examined if these elements are sufficient to induce CSR in a synthetic locus based on the Igκ locus backbone. Addition of a surrogate “core 3’RR” (c3’RR) and of a pair of transcribed and spliced Switch regions, together with a reporter system for “κ-CSR” yielded a switchable Igκ locus. While the c3’RR stimulated SHM at S regions, it also lowered the local SHM threshold necessary for switch recombination to occur. The 3’RR thus both helps recruit AID to initiate DNA lesions, but then also promotes their resolution through long-distance synapses and recombination following double-strand breaks.
Author summary
Class switching allows B lymphocytes to replace expression of immunoglobin M with that of immunoglobulins G, A or E. The genetic support of class switching, is a unique and large deletion uniquely occuring within the immunoglobulin heavy chain (IgH) locus. This recombination is triggered after DNA lesions inflicted by the activation-induced deaminase (AID) enzyme. In immunoglobulin light chain loci, AID only stimulates somatic hypermutation. In such a non-IgH locus, we now show that the IgH 3’ superenhancer can promote junctions between distant DNA breaks and ectopic class switch recombination. This study identifies the minimal elements necessary for class-switch recombination to occur instead of hypermutation in a locus targeted by AID, i.e. transcribed (and spliced) target sites for AID in so-called S regions, and the 3’IgH superenhancer which both helps recruit AID for DNA lesions, and helps repair these lesions through distant gene synapsis and recombination.
Introduction
The Activation-Induced Deaminase (AID) enzyme has multiple roles in the B-cell lineage and their differential regulation remains to be fully characterized. In all species synthesizing Ig, AID primarily provides Ig variable (V) gene diversification through SHM or gene conversion (GCV) [1]. This ancestral repertoire broadening is even shared with an AID ortholog for the lamprey antigen receptors [2]. In frogs, birds and mammals, evolution endowed AID with the additional role to initiate CSR after DNA lesions affecting target S regions upstream of IgH constant (CH) genes [3].
In mouse B-cells, the 3’Regulatory Region super-enhancer (3’RR) is the master cis-regulatory element controlling the activities of AID within the IgH locus, either for SHM, CSR or locus suicide recombination (LSR) [4–7]. SHM and CSR follow B-cell activation. AID lesions in S regions initiate some low-level local SHM but more dramatically yield DNA double strand breaks (DSBs), followed by junctions at long distances [8]. The 3’RR modulates germline transcription of CH genes in activated B-cells, chromatin remodeling of S regions and AID recruitment to acceptor S regions [9]. Mammalian S regions consist of 1–10 kb-long highly repetitive G-rich DNA sequences containing clustered RGYW AID consensus motifs [10]. The mouse 3’RR includes 4 core enhancers: hs3a, hs1,2, hs3b and hs4. In addition to binding specific transcription factors, enhancers included in the 3’RR are transcribed into eRNA [7], and their function is regulated by a distantly transcribed long non-coding RNA (lncRNA) [11]. The first three enhancers are embedded within a ~25-kb dyad symmetry, while the fourth stands downstream [12, 13]. The four core enhancers combined into a short (2.1 kb) “core 3’RR” (c3’RR) show strong synergy and transcriptional activity, although not reaching that of the complete 30kb-long full-length 3’RR [13].
The specific contribution of the 3’RR in SHM, DNA breakage and joining of broken S regions remains elusive, since multiple knock-out experiments (KO) in the IgH locus all showed global defects and failed to uncouple the 3’RR functions in transcription or initiation of SHM and CSR [14]. While the 3’RR clearly promotes SHM and carries at least a dual role regarding both SHM and CSR, its influence on CSR is the best documented and most critical in the IgH locus. It is necessary for optimal germline transcription of acceptor S regions and its deletion almost abrogates accessibility of these S regions while preserving some accessibility to Sμ [4–6, 15]. S regions from 3’RR-deficient B-cells also show a loss of the chromatin marks H3K9ac and H3K4me3, for which they are normally enriched prior to CSR in activated B-cells [15–18]. Finally, the 3’RR likely helps synapsis of broken S regions, then defining chromosomal loops between S regions that stand on the same allele [19].
While the 3’RR helps recruit AID, it thus clearly mediates additional effects that are important for CSR. An ideal way to evaluate those additional effects is to introduce the c3’RR into a locus that efficiently recruits AID beforehand for SHM.
In a previously developed model of ectopic CSR, a complete CSR substrate was designed, providing all the S sequences, transcription and splicing patterns known to be important for local recruitment of AID [20]. This substrate was introduced into the Igκ “KIKS” locus and underwent high transcription accompanied by efficient SHM and presence of localized internal S region deletions (ISD) [20]. Despite this efficient targeting by AID, “κ-CSR” events joining a pair of ectopic S regions remained exceptional and clearly much rarer than for “IgH-CSR”, with a defect likely affecting the occurrence of synapses between distant AID targets [20].
We hypothesized that these ectopic S regions inserted into an Igκ location exposed to AID, would provide an ideal model to explore the role of the 3’RR beyond AID recruitment and check whether this super-enhancer could be the missing piece of an Igκ switchable locus. We thus tried complementing the KIKS CSR substrate with a core “c3’RR” cassette, in order to facilitate tethering of two distant κ-S regions and check whether this could raise the level of κ-CSR closer to that of classical IgH-CSR.
Material and methods
Ethics statement
Procedures were reviewed and approved by the Ministère de l’Education Nationale de l’Enseignement Supérieur et Recherche autorisation APAFIS#16151-2018071716292105v3.
Mice
The strategy used to generate c3’RR-KIKS mice was identical to that previously described for KIKS mice [20], except for the inclusion of a c3’RR cassette downstream of the genomic fragment containing the Eiκ enhancer and Cκ gene. The c3’RR cassette included all four 3’RR enhancers in their normal palindromic layout, as described [13]. Knock-in was done in CK35 embryonic stem cells, which were injected into blastocysts. After germline transmission, mice were bred with a cre-expressing strain to delete the NeoR cassette. KIKS, c3’RR-KIKS and c3’RR-KIKS / Aicda-/- mice were used. All animal strains were bred and maintained in SOPF conditions.
ELISA assays
ELISAs for the presence of murine and human Igκ were performed on serum from KIKS, c3’RR-KIKS and c3’RR-KIKS / Aicda-/- as described [20].
Flow cytometry and cell sorting
Antibodies used for staining and sorting are detailed in S1 Table. Flow cytometry analyses were done on a BD Pharmingen LSRFortessa cytometer. Data were then analyzed with BD FACSDiva software (BD Biosciences, San Jose, CA). Gating strategy is shown in S2 Fig. Sorting was performed on a FACS ARIA 3 (BD Biosciences).
Immunization
Groups of 8-week-old mice were immunized by intraperitoneal injection of 200 μl SRBC and analyzed 8 days later.
SHM and switch junction analyses
SHM and switch junction analyses were performed on B220+/GL7+ and B220+/Igκ+ cells from Peyer’s patches, respectively, using previously described primers for Jκ5 3’ flanking intron and switch junction [20] and LVk4-63F: 5'- TGC AGA TTT TCA GCT TCC TG-3' and Sγ3R: 5'- CCT CAC CCA CCC TAG CTC A-3' for κ-Sγ3 region. PCR products (100ng) were fragmented using Ion Shear Plus reagents kit (Life Technologies), then barcodes and adaptors were ligated using Ion Xpress Plus Fragment (Life Technologies). Fragments around 200pb were selected using E-Gel Size Select 2% (Life Technologies) and sequenced on an Ion Proton System. Raw files were generated using Ion Torrent Suite (adapter- and barcode-trimmed), and junctions were analyzed using CSReport, together with mutation frequency [21]. Mutation frequency was analyzed using DeMinEr tool [22]. Sequences are deposited under the reference PRJEB34851.
Transcript analysis
Total RNA from Peyer’s patches was extracted with TRI Reagent (Ambion, Austin, TX), and 1 μg was used for cDNA synthesis using random primers (Applied Biosystems). Relative quantification of primary transcripts was performed with SYBR green Master Mix (Applied Biosystems) by reference to GADPH levels. QPCR primers and assays were as previously described [20].
Cell culture
Splenocytes were collected, red blood cells were lysed and cells were CD43-depleted using CD43 microbeads (Miltenyi Biotec). Splenic B lymphocytes were cultured for 3 days in RPMI containing 10% FCS with LPS (1μg/mL, B4 Invivogen) or LPS + IL4 (20ng/mL, Peprotech). Cells and supernatants were recovered for LAM-HTGTS experiments and ELISA.
LAM-HTGTS
LAM-HTGTS was performed as previously described [23] using activated splenocytes or Peyer’s patches cells (enriched with the STEMCELL “B-cell isolation kit”). Jκ5-biotin (5’-TGT GTA AGA CAC AGG TTT TCA TGT) and Jκ5-nested (5’- CAG AAA ATC TTG AGA AAA TGG AGA-3’) primers and Sμ primers [24] were used to generate libraries. Data analysis of MiSeq sequencing reads was performed as previously described [25]. Graphical representation were performed as previously described [26]. All sequence alignments were done with the mouse mm10 genome (or its variant sequence corrected for our KIKS changes to the Igκ locus (Figs 1 and S1). Sequences are deposited under the reference PRJEB34781.
Fig 1. B-cell development.
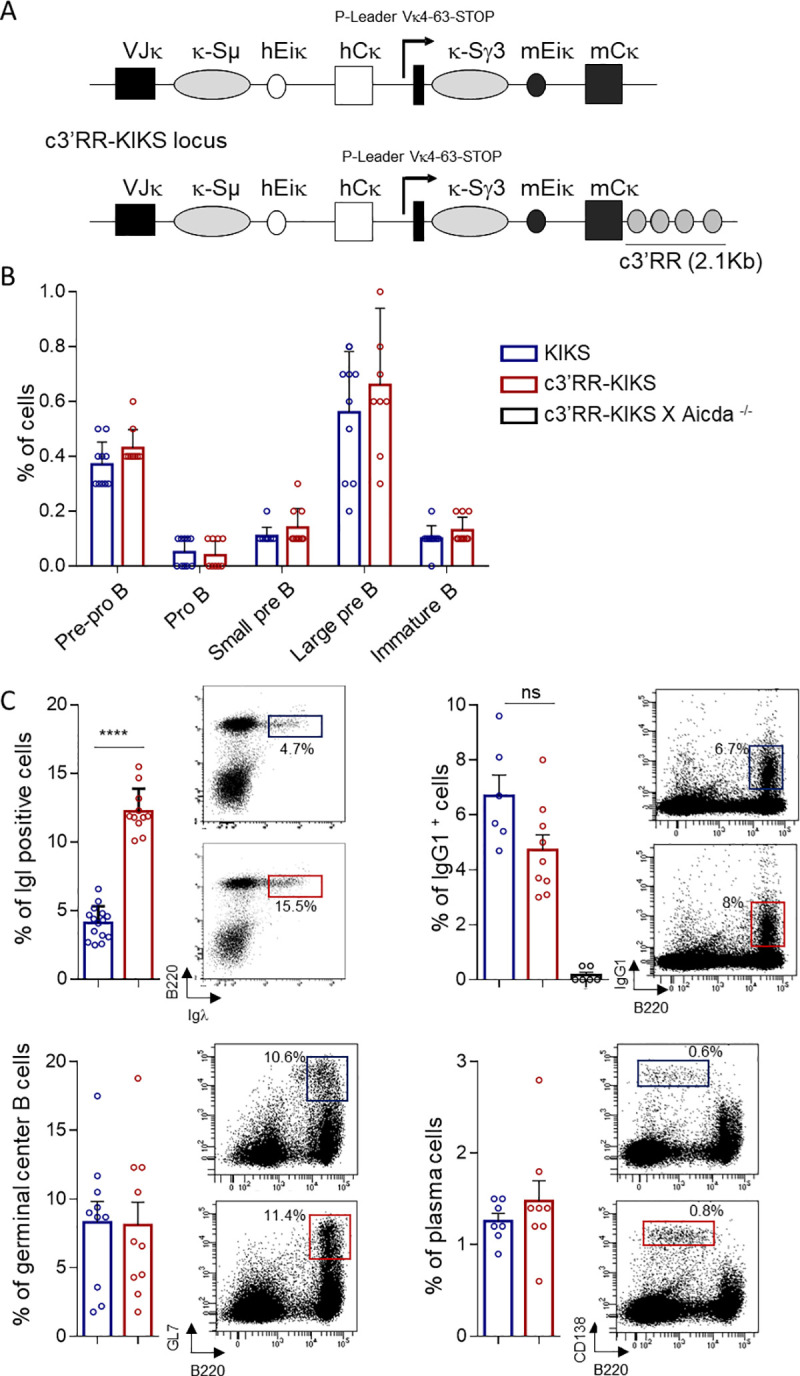
(A) Comparison between switchable Igκ loci. In c3’RR-KIKS mice, a c3’RR was inserted downstream of the locus. (B) Flow cytometry analysis of B-cell compartments in bone marrow. Percentages are given with mean+/- SEM as determined among total gated bone marrow lymphocytes. Data are representative of 10 animals of each genotype (left panel). (C) Flow cytometry analysis with representative dot plots, in spleen after SRBC immunization (D8), of switched B-cells (B220+/IgG1+), GC B-cells (B220+/GL7+) and plasma cells (B220low/CD138+) and mλ positive cells in non-immunized spleen. For switched B-cells, GC B cells and mλ the % are expressed on B220+ cells, and the % of plasma cells are expressed on total lymphocytes. Data are representative of 6 to 10 animals of each genotype. Percentages are given with mean+/- SEM. Mann-Whitney U-test for significance.
Statistical analysis
Statistical tests used in the study are indicated in the figure legends and performed using GraphPad Prism (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
Results and discussion
c3’RR-KIKS locus description and B-cell development
To determine whether the 3’RR super-enhancer could by itself affect the output of AID-initiated single nucleotide lesions and increase the balance of distant recombination vs local alteration, we studied Igκ loci modified in order to support ectopic CSR. The KIKS Igκ variant locus (carrying only paired S regions) is known to show low-levels of κ-CSR junctions after AID-mediated DNA lesions [20]. We compared it to a c3’RR-KIKS variant carrying not only an κ-Sμ/κ-Sγ3 pair but also a surrogate c3’RR super-enhancer cassette (Figs 1A and S1). The c3’RR included the four core enhancers (hs3a, hs1.2, hs3a and hs4) of the IgH 3’RR. We have previously described that the insertion at the Igκ locus in the KIKS mice does not disturb early and late B cell development compared to wt mice. For this reason we only compared the two KI mouse models [20]. Normal early B-cell development occurred in both homozygous c3’RR-KIKS mice and KIKS mice, showing normal distribution of bone marrow pre-pro B, proB, large preB, small preB and immature B-cell populations (Figs 1B and S2). We only noticed an occasional increase of Ig lambda usage (Igλ) in some c3’RR-KIKS compared to KIKS mice, suggesting that LC rearrangements might even be accelerated by the knocked-in IgH elements, with Igλ then more frequently superseding the early rearranged Igκ. Late B-cell development also occurred normally, with no differences either regarding classical IgH locus CSR (IgG1+ cells in spleen), nor the accumulation of germinal center (GC) B-cells and plasma cells (Fig 1C).
The IgH 3’ super-enhancer cassette increases in vivo and in vitro ectopic κ-CSR in c3’RR-KIKS mice
The reporter system included in the KIKS and c3’RR-KIKS loci was designed in order to monitor κ-CSR loci as a switch from expression of human κ (hCκ) to mouse κ-LC (mCκ) (Figs 1A and S1). In vivo, both KIKS and c3’RR-KIKS mice developed small numbers of “κ-switched” mCκ-expressing B-cells in spleen. We quantified IgH-switched B cells in spleens from nonimmunized and SRBC-immunized mice by quantifying B220+/IgM- cells in which IgG1-switched B cells represented a majority of IgH-switched B cells (Figs 2A and S3A). We first verified that spleen resting B-cells don’t not express mCκ (S3B Fig). In nonimmunized spleens, where IgH-switched (B220+, IgM-) B-cells were only present in low numbers in both models (Fig 2A left panel), κ-CSR was detectable in low amounts and significantly increased under the influence of the c3’RR (0.7% in c3’RR-KIKS vs 0.28% in KIKS, p = 0.0027) (Fig 2A right panel and S2 Table). After SRBC immunization (boosting classical IgH-CSR in spleens), κ-CSR was also boosted in both models but the trend towards a higher level in c3’RR-KIKS mice did not reach statistical significance by comparison to that in KIKS mice (Fig 2A right panel and S2 Table). Peyer’s patches represent a chronically inflamed lymphoid tissue, where classical IgH-CSR was higher than in spleens in both models (Fig 2B left panel). κ-CSR was also increased in Peyer’s patches when considering total B-cells (again with a trend to higher κ-CSR in c3’RR-KIKS vs KIKS mice but not reaching significance at p = 0.08) (Fig 2B middle panel and S2 Table). Gated GC B-cells from Peyer’s patches (i.e. activated GL7+ B-cells), showed the clearest difference between both models when focusing on cells where CSR was ongoing, then revealing a significant increase driven by the c3’RR (3.2% vs 1.5% mCκ+ cells, respectively in c3’RR-KIKS and KIKS mice, p = 0.003) (Fig 2B right panel and S2 Table). The mCκ+ cells were detected only in cells that have switched at IgH locus. In both the KIKS and the c3’RR-KIKS mice, we found an almost undetectable level of κCSR (below 0.1%) when the analysis was focused on cells with an unswitched IgH locus (gated as IgM+) (S3C Fig). Although κ-CSR then remained 10 to 15 fold below the parallel IgH-CSR, this confirmed that it could be positively stimulated by integrating an IgH super-enhancer cassette into the modified Igκ locus. AID-dependence of the κ-CSR process was also confirmed by its abrogation in AID-deficient c3’RR-KIKS mice (Fig 2A and 2B). This increased number of mouse Igκ+ B-cells at the BCR expression level also translated into a change in amounts of secreted Ig in sera, with secreted mouse κ-LCs significantly increased in both non-immunized (18.8μg/mL vs 6.1μg/mL, p = 0.0002) and SRBC-immunized (68μg/mL vs 27.6μg/mL, p = 0.016) c3’RR-KIKS vs KIKS mice (Fig 2C). We next monitored κ-CSR onset in vitro after LPS or LPS/IL4 stimulation. In vitro κ-CSR occurred at very low levels but, as in vivo, we observed a significant increase (p = 0.033 and p = 0.028 for LPS and LPS/IL4 stimulation, respectively) in mCκ+ cells in c3’RR-KIKS mice by flow cytometry (Fig 3A and 3B and S2 Table) confirming the role of the c3’RR in κ-CSR regulation.
Fig 2. 3’RR core enhancers ectopically promote CSR in vivo.
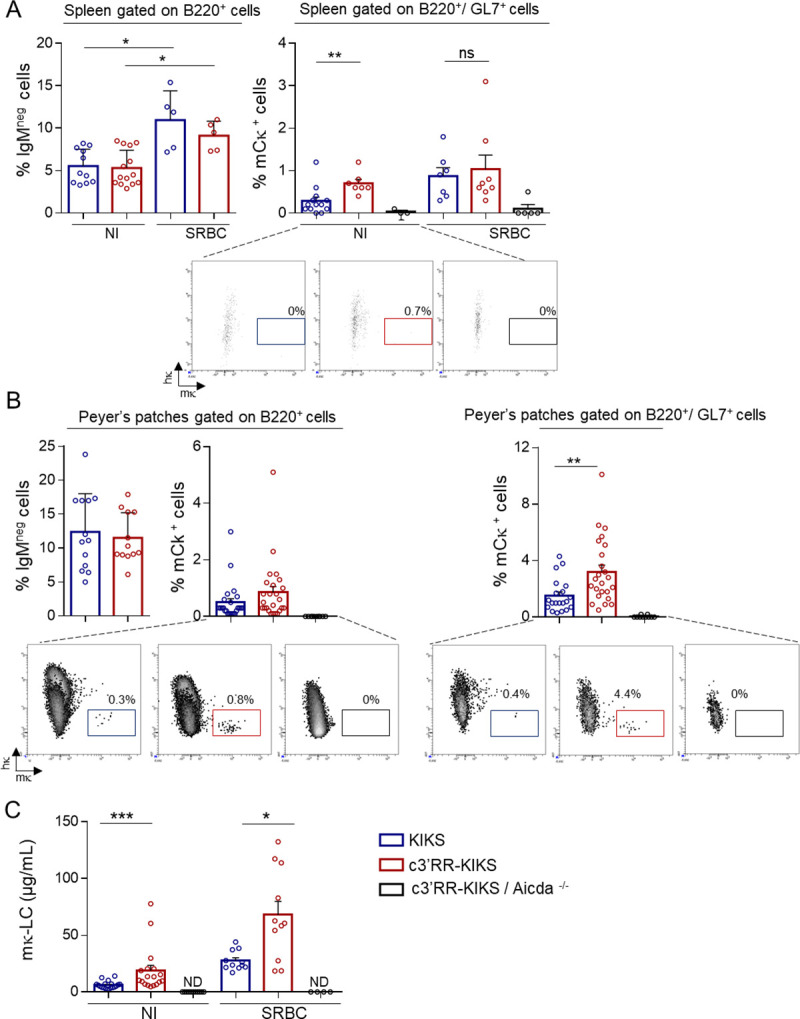
(A) Left: Evaluation of the amount of cells switching at the IgH locus (B220+, IgMneg cells) in spleens from NI or SRBC immunized mutant mice (day 8) Right: Comparison of κ-CSR efficiency as evaluated by counting mCκ+ spleen B-cells (gated on B220+/GL7+ cells) by flow cytometry, in spleens from non-immunized (NI) or SRBC immunized (day 8) mice from KIKS, c3’RR-KIKS and c3’RR-KIKS / Aicda-/- mice. Percentages +/- SEM are representative of 5 to 13 animals of each genotype (B) Left: Evaluation of the amount of cells switching at the IgH locus (B220+, IgMneg cells) in Peyer’s patches from KIKS, c3’RR-KIKS mice. Middle and right: Comparison of κ-CSR efficiency as evaluated by counting mCκ+ spleen B-cells either gated on B220 positive cells (middle) or on B220+/GL7+(right) by flow cytometry, in Peyer’s patches from KIKS, c3’RR-KIKS and c3’RR-KIKS / Aicda-/- mice. Percentages +/- SEM are representative of 10 to 25 animals of each genotype. (C) ELISA quantification of serum mκ-LC in NI and SRBC-immunized mice (Day 8). Percentages +/- SEM are representative of 11 to 19 animals of each genotype.
Fig 3. 3’RR core enhancers ectopically promote CSR in vitro.
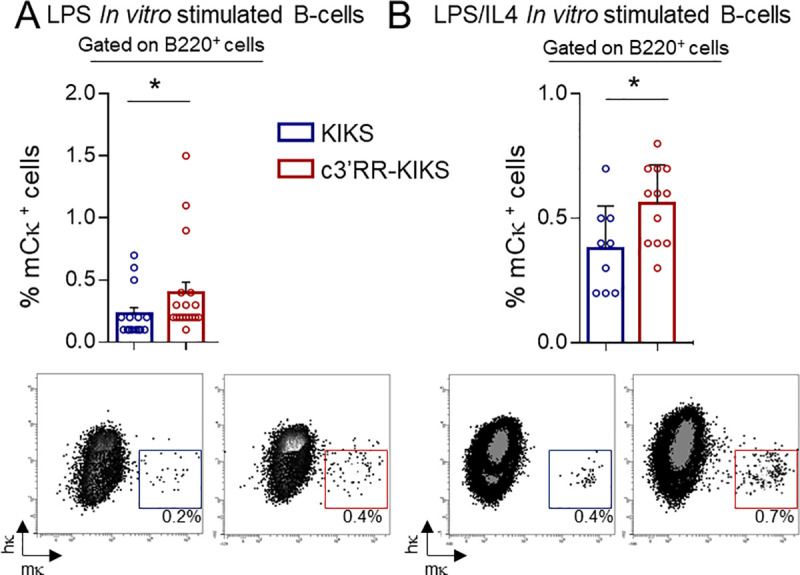
(A) Comparison of κ-CSR efficiency as evaluated by counting LPS in vitro stimulated mCκ+ spleen B-cells (gated on B220+ cells) by flow cytometry from KIKS and c3’RR-KIKS mice.(B) as in (A) for LPS/IL4 in vitro stimulation. Percentages are given with mean+/- SEM. Mann-Whitney test for significance.
Transcription and hypermutation of the knocked-in S regions prior to κ-CSR
AID targeting requires transcription for both SHM and CSR, and we thus assessed the amount of κ-S region primary transcripts in KIKS and c3’RR-KIKS Peyer’s patches (Fig 4A). Both the κ-Sμ (p<0.0001) and κ-Sγ3 (p = 0.0007) regions proved significantly more transcribed in c3’RR-KIKS mice (Fig 4B and S3 Table). As expected upon transcription of a locus that is naturally accessible to AID and before any κ-CSR event, local hypermutation was clearly detectable within unrearranged κ-S regions and within the Jκ5 3’ flanking intron from Peyer’s patch GC B-cells. For un-switched κ-Sμ, we analyzed 570 bp downstream of Jκ5, (i.e. 220 bp from the 3’ Jκ5 flanking intron and 350 bp from the inserted κ-Sμ). We also analyzed the first 200 bp of un-switched κ-Sγ3 (Fig 4A). To correct the substitution frequency at each nucleotide position along the sequenced region we used the DeMinEr tool [22], which uses deep sequencing data from mutated (KIKS and c3’RR-KIKS) and unmutated samples (c3’RR-KIKS X Aicda -/-). Using NGS, mutation frequencies in the [3’Jκ5 intron—κ-Sμ] region were scored with reference to the Jκ rearrangement status (Jκ1 or Jκ5). Confirming previous analyzes in KIKS mice [20], a high mutation rate at long distances from the promoter was observed throughout the [3’Jκ5 intron—κ-Sμ] region (Fig 4C and S4 Table). In c3’RR-KIKS mice, this mutation rate tended to be higher than in KIKS mice (4.6 mut/kb vs 3.1 mut/kb for Jκ1 rearrangement and 12.1 mut/kb vs 9.6 mut/kb for Jκ5 rearrangement) (Fig 4C). By contrast, the mutation load of the acceptor κ-Sγ3 region did not significantly differ (Fig 4C right panel and S4 Table). Thus, inclusion of the c3’RR globally increased transcription of κ-S regions and increased SHM around the donor κ-Sμ region in unswitched cells. This confirms the Igκ locus as a privileged non-IgH location strongly exposing knocked-in S regions to AID, but still with possible modulation by the inclusion of an additional Ig enhancer. Since the increased κ-CSR competence brought by the surrogate c3’RR seems to correlate with both higher transcription and with equivalent or higher AID-induced SHM prior to κ-CSR (in c3’RR-KIKS vs KIKS mice), we next wanted to precisely compare the structures of recombined switch junctions in both models.
Fig 4. 3’RR core enhancers enhance transcription and SHM.
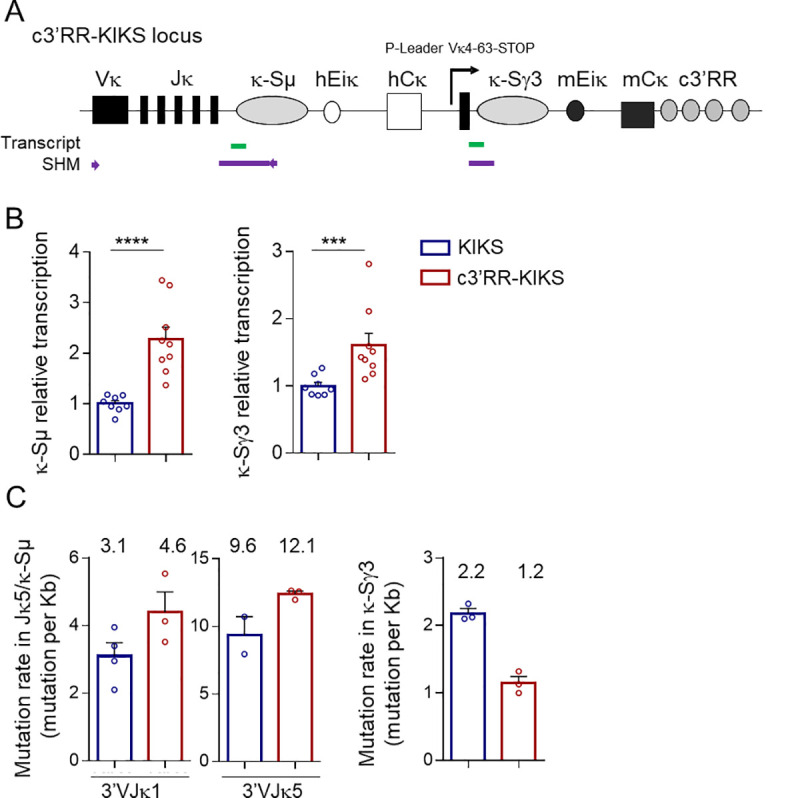
(A) c3’RR-KIKS locus with the location of regions (thick lines) that were tested either for transcription (green) or for SHM (purple). Arrows represent primers used for SHM PCR amplification. (B) Relative κ-Sμ and κ-Sγ3 region transcripts analyzed in Peyer’s patch GC B-cells (GC) (n = 8 to 9 mice) from KIKS and c3’RR-KIKS mice. (C) Mutation rate in the [3’Jκ5 intron—κ-Sμ] region according to the Jκ rearrangement (Jκ1 or Jκ5) (left and middle panels) and mutation rates in the κ-Sγ3 region (right panel) (n = 2 to 4 individual mice) in GC B cells from KIKS, c3’RR-KIKS and c3’RR-KIKS / Aicda-/- mice. The mutation frequency (mutations per Kb) is indicated over the bar graphs. Data are mean ± SEM, Mann-Whitney test for significance.
Molecular analysis of SHM within switch junctions
We analyzed the total mutation load in hybrid κ-S regions from B220+/mk+ cells from Peyers’ patches by specifically amplifying the rearranged κ-Sμ/κ-Sγ3 regions by nested PCR (Fig 5A). As expected, in both Igκ knock-in configurations, this rate was globally higher for rearranged κ-Sμ/Sγ3 junctions than for unrearranged κ-S regions (Figs 5B and 4C). However, the respective ranking of both models for SHM occurrence then appeared unexpectedly inversed: less SHM was present in rearranged κ-S sequences from c3’RR-KIKS (12.5 mut/kb: 93 mutations/7414 pb analyzed) than from KIKS B-cells (15.6 mut/kb: 1140 mutations/92472 pb analyzed) (p = 0.029) (Fig 5B). This suggests that in the Igκ locus, presence of the c3’RR facilitates / accelerates either the occurrence of DSBs or their repair through synapsis of paired targets. The 3’RR thus impacts the outcome of AID-initiated lesions and locally tends to increase the recombination vs mutation ratio.
Fig 5. κ-CSR junction analysis.
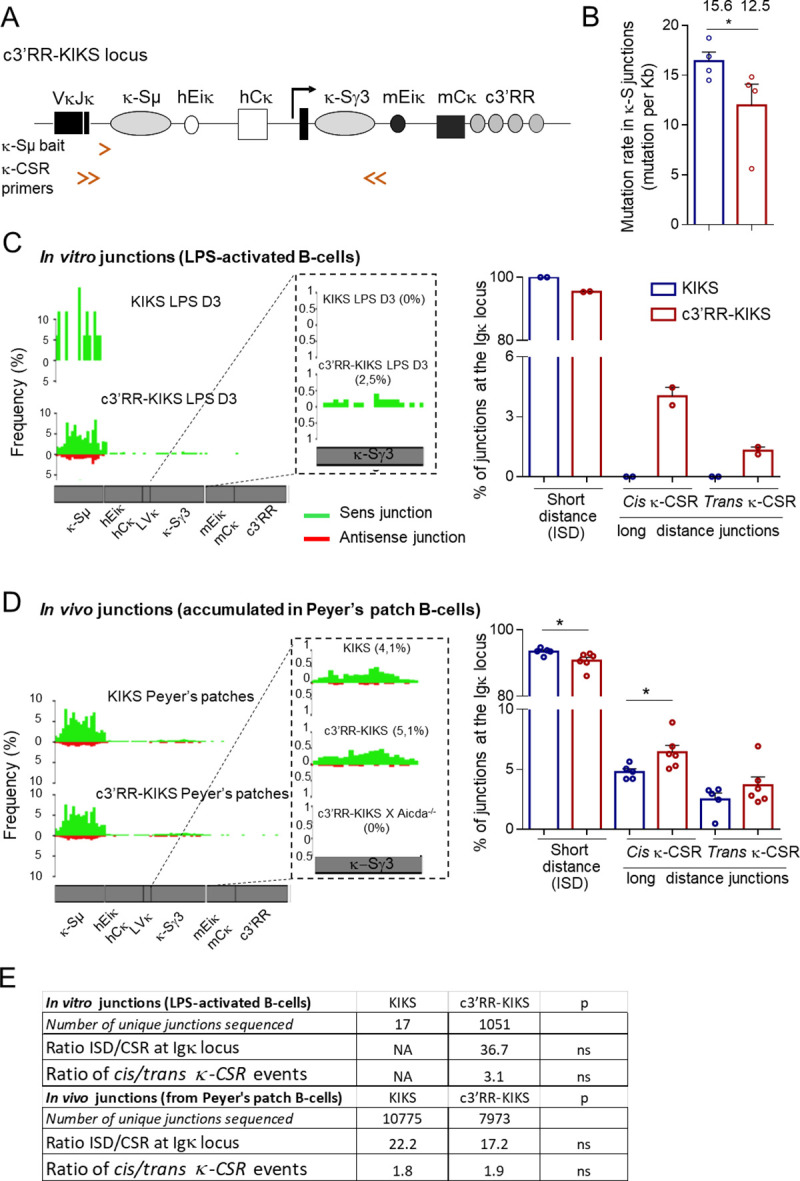
(A) c3’RR-KIKS locus with the location of κ-Sμ bait used for LAM-HTGS and primers used for κ-CSR amplification by nested PCR (double arrows). (B) Mutation rates (mutations per kb) in recombined κ-Sμ/Sγ3 junctions are indicated over the bar graphs. κ-Sμ/Sγ3 junctions were obtained by nested-PCR from mCk+ cells from Peyer’s patches (n = 4 for each genotype). SHM was analyzed using CSReport. (C) κ-CSR junctions identified by LAM-HTGTS from in vitro activated B-cells. Map of junctions identified by LAM-HTGTS along the KI locus (left). Bar graphs (right) with percentages of ISD, cis κ-CSR internal to the Igκ locus and trans κ-CSR (Igκ-IgH) events obtained by LAM-HTGTS. Unique junctional sequences (each corresponding to multiple identical reads) were analyzed; the percentage of “Ig recombination events” (i.e. junctions of the bait with Ig sequences, either Igκ KI or IgH) is given. (D) κ-CSR junctions identified by LAM-HTGTS in vivo from Peyer’s patch B-cells. (E) Table indicating the number of junctional sequences analyzed, ratio of cis / trans events, ratio of ISD/CSR within the endogenous IgH locus, and ratio of cis/trans events.
To precisely analyze κ-CSR events in our models, we amplified CSR junctions using LAM-HTGTS [23]. By reference to a bait chosen upstream of a given DNA double-stranded break (DSB), this unbiased strategy detects junctions to “preys” located genome-wide. The κ-CSR events were captured by a 3’Jκ5 intron bait (κ-Sμ bait) chosen upstream of the knock-in donor κ-Sμ (Fig 5A). In order to identify and score the sequences joined to baits, non-redundant junctions were then analyzed using the algorithm published by Alt and colleagues [23].
Baits were found to be associated in cis or trans with sequences from either the Igκ KI (cis κ- CSR) or the endogenous IgH locus (trans κ-CSR). Analyzing the partners joined to the 3’Jκ5 intron bait (thereafter “κ-Sμ bait”) in both κ-CSR models, showed that the predominant type of recombination was local intra-Sμ deletion (ISD), whether the analysis concerned LPS-activated splenocytes or Peyer’s patches (Fig 5C and 5D). Alternatively, junctions to a distant κ-S region truly featuring cis κ-CSR also occurred, and were more frequent in the c3’RR-KIKS than in KIKS samples. This difference was dramatic when assessing ongoing cis κ-CSR in vitro by stimulating naive splenocytes, where Sμ-Sγ3 κ-CSR represented about 2.5% of all “Ig recombination events” in c3’RR-KIKS, while remaining undetectable in KIKS cells (Fig 5C and 5D).
LAM-HTGTS also confirmed that in vivo, Sμ-Sγ3 κ-CSR within the Igκ locus was more frequent in c3’RR-KIKS than in KIKS mice. Although less striking than in vitro, the difference was still significant (5.1% vs 4.1% of all “Ig recombination events”, p = 0.04) (Fig 5D).
Globally, the “ISD / κ-CSR” ratio (i.e. the proportion of local vs distant recombination) thus decreased in all conditions where c3’RR was included, and this effect was most significantly visible when activating naive cells in vitro and thus estimating de novo recombination (Fig 5E).
Altogether, these data were in agreement with flow cytometry analyses and also confirmed AID-dependence of the process since very few junctions to the bait were found in control c3’RR-KIKS / Aicda-/- mice and none of them featured CSR (Fig 5D). In addition to cis κ-CSR junctions internal to the Igκ locus (κ-Sμ to κ-Sγ3), some trans κ-CSR recombination events joined the κ-Sμ to acceptor S regions (Sγ3, Sγ1, Sγ2b, Sγ2a, Sε, Sα) from the endogenous IgH locus (Fig 5C and 5D). Trans IgH-CSR is known to abundantly occur between both IgH alleles and can account for up to 15% of all normal IgH CSR events [27, 28]. This figure appears even higher for κ-CSR, since more than one-third of κ-CSR junctions implicated an acceptor S region from the IgH locus (Fig 5C and 5D), corresponding to a trans κ-CSR rearrangement. This trans/cis κ-CSR ratio did not significantly differ in both κ-CSR models and the global stimulatory effect of the c3’RR on distant κ-CSR manifested equivalently for the prevalent cis junctions and the alternative trans pathway (Fig 5E).
Regarding repair of κ-CSR, junctions analyzed after sequencing of nested-PCR products from mCκ+ cells from Peyer’s patches showed predominant blunt junctions, similar to classical CSR in wt mice. There was thus no apparent bias in the usage of either NHEJ or microhomology-mediated repair (Fig 6A). We consistently analyzed breakpoint distribution throughout the κ-S regions and found that breaks occurred as expected around AID hot spots. No difference in breakpoint dispersion in the κ-S region occurred between the two models (Fig 6B) suggesting that c3’RR does not impact the position break but rather the outcome of the breaks.
Fig 6. Structures and breakpoints distribution of κ-CSR junctions.
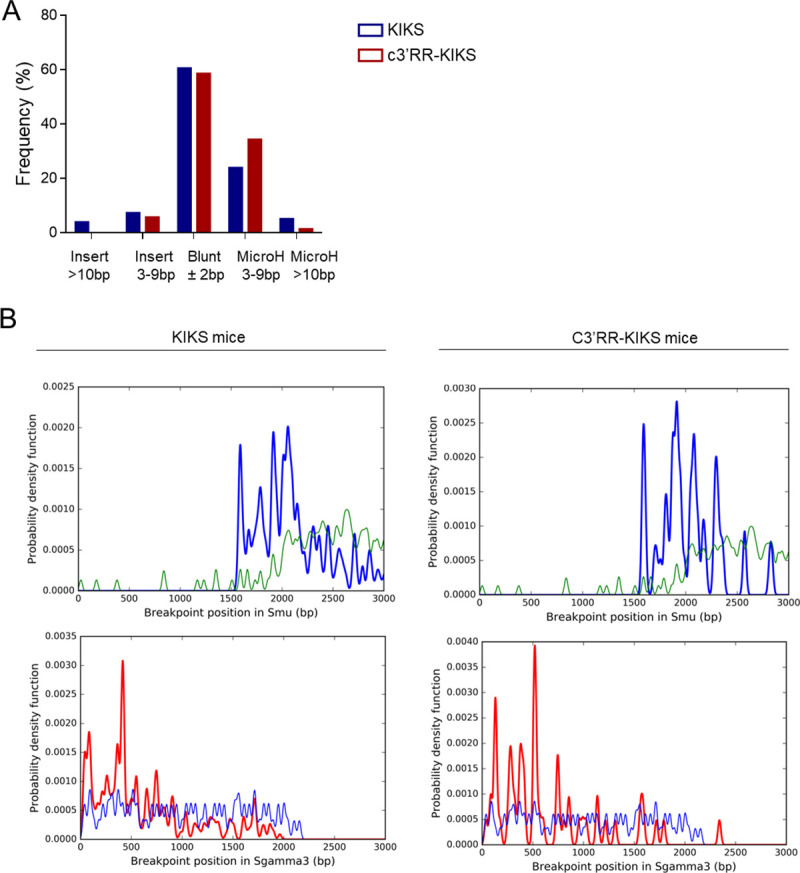
(A) Structures of κ-CSR junctions obtained by nested-PCR from mκ+ cells from Peyer’s patches and analyzed using CSReport. (B) Breakpoint distribution of κ-CSR junctions obtained by nested-PCR from mκ+ cells from Peyer’s patches and analyzed using CSReport. Thick and thin lines represented breakpoints and AID motifs respectively.
Globally considering κ-CSR (either in cis or trans), the present data indicate that integration of the c3’RR into the locus facilitated long distance synapses between AID-targeted DNA regions, rather than local deletions restricted to κ-Sμ. The c3’RR also tended to increase SHM of unrearranged κ-S regions (the level of which was higher in both models than observed in parallel for the IgH locus S regions) (Fig 4C). Strikingly, the c3’RR, however, facilitated the occurrence of junctions instead of less parallel local SHM. This change in the SHM/CSR ratio is in agreement with the observation made when comparing κ-CSR and parallel IgH-CSR, which can occur with minimal associated local SHM. In controls, we measured CSR at the endogenous IgH locus [23] and as expected, IgH-CSR was unaltered by the Igκ knock-in (with a majority of Sμ-Sα junctions in Peyer’s patches) (Fig 7A and 7B); the SHM load of these S-S junctions could thus be evaluated and was found to be 2 to 3 fold lower in the IgH locus (where the complete 3’RR likely optimized the CSR/SHM ratio) compared to the Igκ locus (Fig 7C and S5 Table).
Fig 7. c3’RR at KI locus does not affect CSR and SHM at IgH locus.
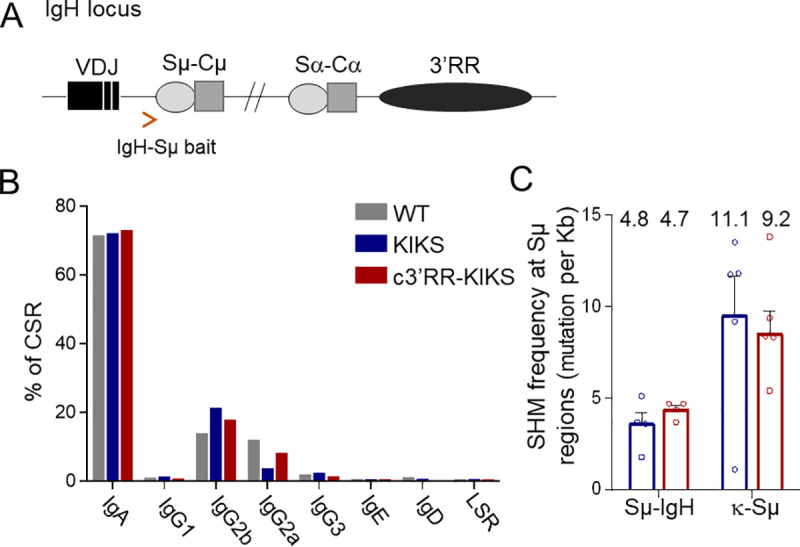
(A) IgH locus with the location of IgH-Sμ bait used for LAM-HTGS. () Percentage of switched IgH isotypes in Peyer’s patch B-cells from KIKS (n = 4), c3’RR-KIKS (n = 3) and wt (n = 3) mice obtained by LAM-HTGTS. (C) Mutation rate (mutation per kb) in IgH-Sμ (n = 4) and κ-Sμ(n = 5) after CSR recombination obtained by LAM-HTGTS from Peyer’s patches.
CSR and SHM are thoroughly intermingled processes in physiology and there are few situations where they appear uncoupled, as observed in patients with immune deficiencies and N-terminal mutations of AID, affecting SHM while preserving CSR [24, 29]. By contrast, normal IgH CSR usually associates with some local SHM focused on WRCY motifs even after short in vitro stimulation of B-cells. This minimal SHM level has long been considered as only supporting CSR in in vitro conditions [30]. AID targeting to S regions strongly relies on their structure, abundance of WRCY motifs, potential binding of these motifs by 14-3-3, G4-richness and ability to promote the formation of R-loops when transcribed [16, 31, 32]. This favors RNA Polymerase II stalling in association with Spt5 and recruits AID together with the exosome which locally degrades S region transcripts and exposes both strands of R-loops to cytidine deamination [33, 34]. The 3’RR promotes DNA looping within the IgH locus. Such loops facilitate transcription on one hand and specific interaction of the stalled polymerase II with Spt5, AID and other cofactors, and, on the other hand, ligation of paired S regions [16, 19]. By analogy in our κ-CSR model, it seems likely that the c3’RR can promote an Igκ locus conformation that is either more susceptible to single strand lesions and SHM or forms alternative loops that favor the occurrence of DSBs, terminates SHM and supports the synapsis of S regions prior to their ligation (as during classical CSR at the IgH locus).
As a conclusion, using a genetically engineered Igκ locus as a CSR substrate, we observed that inclusion of a surrogate 3’RR promoted CSR recombination in multiple aspects. It increased both the balance of CSR vs SHM on CSR-targeted S regions and the balance of recombination with distant partners vs local intra-Sμ deletion for the knocked-in donor Sμ region. The increased frequency of distant events primarily concerned cis-CSR within the modified Igκ locus, but also equally affected trans-CSR joining the Igκ / IgH loci.
Altogether, this study shows that the IgH 3’RR can instrumentally promote the transformation of AID-initiated lesions into CSR breaks rather than SHM and promote junctions with distant CSR targets rather than local deletion.
This study thus directly confirms those roles of the 3’RR that have been previously postulated indirectly after analyzing 3’RR-deficient mice (which show anomalies of both IgH cis- and trans-CSR). While repair pathways of junctions did not vary between control loci or loci integrating the 3’RR, our data are consistent with the notion that the 3’RR promotes synapsis between distant CSR targets prior to recombination. This fits with a model where the 3’RR would participate in the assembly of a CSR factory, bringing both the loops formed in cis on a targeted locus, and potential other legitimate targets from other chromosomes (usually the other IgH allele but also here a knocked-in Igκ KIKS allele). This schema must thus accommodate not only the “loop model” [19] but also the data concurring to demonstrate the efficient interactions of S regions even when located on different chromosomes [27, 28, 35, 36].
That κ-CSR remains about 10 to 15-fold less frequent than IgH CSR in the c3’RR-KIKS locus is expected since the 3’ IgH super-enhancer stands as an extensive region with multiple functional modules in the IgH locus, which can hardly be replaced by a c3’RR cassette restricted to core enhancers. Notably, we previously demonstrated that the intervening sequences located in-between core enhancers architecturally contribute to the CSR-boosting effect exerted by the super-enhancer [13]. Dealing with chromosomal loops, the IgH locus context has by itself accumulated multiple elements favoring the occurrence of CSR loops, and the normal IgH 3’RR is notably followed by multiple CTCF binding elements which might ideally anchor the 3’RR within a CSR factory at the basis of a CSR loop [37]. Alt and coll thus recently proposed that CTCF and cohesin-bound elements dynamically support “loop extrusion” and enable the 3’RR to promote secondary loops for S region synapsis and cleavage, underlining the importance of the long range locus topology and chromosomal context [38]. In an attempt to identify the necessary constituents of a functional switching domain, our comparison of two artificial Ig loci with identical structures, AID accessibility, G4-density and location of S regions within introns, but only differing by inclusion of a c3’RR, confirms the instrumental contribution of the 3’RR enhancers to CSR competence beyond SHM.
Supporting information
The inserted cassette includes the core Sμ, followed by the hEiκ enhancer, constant hCκ exon from the human Igκ locus and then the core Sγ3 and c3’RR. A Vκ promoter and mutated leader exon provides transcription and splicing of Sγ3. The downstream floxed NeoR gene was removed by Cre-deletion to generate the germline c3’RR-KIKS locus. c3’RR-KIKS κ-CSR events join S regions, then excising hCk and yielding Ig with murine Ck.
(TIF)
(TIF)
A. Percentage of IgG1+ cells in spleens from NI or SRBC immunized mice (day 8) (KIKS, c3’RR-KIKS and c3’RR-KIKS / Aicda-/- mice). B. Representative dot plots of mκ and hκ staining in CD43 negative splenic B-cells. C Comparison of κ-CSR efficiency as evaluated by counting mCκ+ spleen B-cells (gated on B220+/IgM+ cells) by flow cytometry, in spleens from non-immunized (NI) or SRBC immunized (day 8) mice and from Peyers’ patches from KIKS, c3’RR-KIKS and c3’RR-KIKS / Aicda-/- mice. Percentages +/- SEM.
(TIF)
(XLSX)
B: Each individual experiment of κ-CSR efficiency as evaluated by counting mCκ+ in in vitro stimulated (LPS) B-cells (gated on B220+).
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We acknowledge the nucleic acid team and animal facility from BISCEm platforms of the Limoges University (France). We acknowledge Claire Carrion for cytometry analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files. Hight-throughput sequencing results have been deposited on ENA at https://www.ebi.ac.uk/ena/browser/home under the references PRJEB34781 and PRJEB34851.
Funding Statement
M. C. was supported by Agence nationale de la recherche grant No. 16-CE15-0019-01 and Fondation pour la recherche sur le cancer grant No. PGA1 RF20180207070. F. B. was supported by ALURAD. B.H. was supported by a FEDER European grant. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Conticello SG, Thomas CJF, Petersen-Mahrt SK, Neuberger MS. Evolution of the AID/APOBEC family of polynucleotide (deoxy)cytidine deaminases. Mol Biol Evol. 2005;22: 367–377. 10.1093/molbev/msi026 [DOI] [PubMed] [Google Scholar]
- 2.Boehm T, McCurley N, Sutoh Y, Schorpp M, Kasahara M, Cooper MD. VLR-based adaptive immunity. Annu Rev Immunol. 2012;30: 203–220. 10.1146/annurev-immunol-020711-075038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol. 2012;12: 517–531. 10.1038/nri3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cogné M, Lansford R, Bottaro A, Zhang J, Gorman J, Young F, et al. A class switch control region at the 3’ end of the immunoglobulin heavy chain locus. Cell. 1994;77: 737–747. 10.1016/0092-8674(94)90057-4 [DOI] [PubMed] [Google Scholar]
- 5.Pinaud E, Khamlichi AA, Le Morvan C, Drouet M, Nalesso V, Le Bert M, et al. Localization of the 3′ IgH Locus Elements that Effect Long-Distance Regulation of Class Switch Recombination. Immunity. 2001;15: 187–199. 10.1016/s1074-7613(01)00181-9 [DOI] [PubMed] [Google Scholar]
- 6.Vincent-Fabert C, Fiancette R, Pinaud E, Truffinet V, Cogné N, Cogné M, et al. Genomic deletion of the whole IgH 3’ regulatory region (hs3a, hs1,2, hs3b, and hs4) dramatically affects class switch recombination and Ig secretion to all isotypes. Blood. 2010;116: 1895–1898. 10.1182/blood-2010-01-264689 [DOI] [PubMed] [Google Scholar]
- 7.Péron S, Laffleur B, Denis-Lagache N, Cook-Moreau J, Tinguely A, Delpy L, et al. AID-driven deletion causes immunoglobulin heavy chain locus suicide recombination in B cells. Science. 2012;336: 931–934. 10.1126/science.1218692 [DOI] [PubMed] [Google Scholar]
- 8.Chaudhuri J, Alt FW. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol. 2004;4: 541–552. 10.1038/nri1395 [DOI] [PubMed] [Google Scholar]
- 9.Saintamand A, Vincent-Fabert C, Garot A, Rouaud P, Oruc Z, Magnone V, et al. Deciphering the importance of the palindromic architecture of the immunoglobulin heavy-chain 3’ regulatory region. Nat Commun. 2016;7: 10730 10.1038/ncomms10730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honjo T, Kinoshita K, Muramatsu M. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu Rev Immunol. 2002;20: 165–196. 10.1146/annurev.immunol.20.090501.112049 [DOI] [PubMed] [Google Scholar]
- 11.Pefanis E, Wang J, Rothschild G, Lim J, Kazadi D, Sun J, et al. RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell. 2015;161: 774–789. 10.1016/j.cell.2015.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauveau C, Cogné M. Palindromic structure of the IgH 3’locus control region. Nat Genet. 1996;14: 15–16. 10.1038/ng0996-15 [DOI] [PubMed] [Google Scholar]
- 13.Le Noir S, Boyer F, Lecardeur S, Brousse M, Oruc Z, Cook-Moreau J, et al. Functional anatomy of the immunoglobulin heavy chain 3΄ super-enhancer needs not only core enhancer elements but also their unique DNA context. Nucleic Acids Res. 2017;45: 5829–5837. 10.1093/nar/gkx203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinaud E, Marquet M, Fiancette R, Péron S, Vincent-Fabert C, Denizot Y, et al. The IgH locus 3’ regulatory region: pulling the strings from behind. Adv Immunol. 2011;110: 27–70. 10.1016/B978-0-12-387663-8.00002-8 [DOI] [PubMed] [Google Scholar]
- 15.Saintamand A, Rouaud P, Saad F, Rios G, Cogné M, Denizot Y. Elucidation of IgH 3’ region regulatory role during class switch recombination via germline deletion. Nat Commun. 2015;6: 7084 10.1038/ncomms8084 [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Wuerffel R, Feldman S, Khamlichi AA, Kenter AL. S region sequence, RNA polymerase II, and histone modifications create chromatin accessibility during class switch recombination. J Exp Med. 2009;206: 1817–1830. 10.1084/jem.20081678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li G, White CA, Lam T, Pone EJ, Tran DC, Hayama KL, et al. Combinatorial H3K9acS10ph Histone Modification in IgH Locus S Regions Targets 14-3-3 Adaptors and AID to Specify Antibody Class-Switch DNA Recombination. Cell Rep. 2013;5: 702–714. 10.1016/j.celrep.2013.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeevan-Raj BP, Robert I, Heyer V, Page A, Wang JH, Cammas F, et al. Epigenetic tethering of AID to the donor switch region during immunoglobulin class switch recombination. J Exp Med. 2011;208: 1649–1660. 10.1084/jem.20110118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wuerffel R, Wang L, Grigera F, Manis J, Selsing E, Perlot T, et al. S-S Synapsis during Class Switch Recombination Is Promoted by Distantly Located Transcriptional Elements and Activation-Induced Deaminase. Immunity. 2007;27: 711–722. 10.1016/j.immuni.2007.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonaud A, Lechouane F, Le Noir S, Monestier O, Cogné M, Sirac C. Efficient AID targeting of switch regions is not sufficient for optimal class switch recombination. Nat Commun. 2015;6: 7613 10.1038/ncomms8613 [DOI] [PubMed] [Google Scholar]
- 21.Boyer F, Boutouil H, Dalloul I, Dalloul Z, Cook-Moreau J, Aldigier J-C, et al. CSReport: A New Computational Tool Designed for Automatic Analysis of Class Switch Recombination Junctions Sequenced by High-Throughput Sequencing. J Immunol Baltim Md 1950. 2017;198: 4148–4155. 10.4049/jimmunol.1601924 [DOI] [PubMed] [Google Scholar]
- 22.Martin OA, Garot A, Le Noir S, Aldigier J-C, Cogné M, Pinaud E, et al. Detecting Rare AID-Induced Mutations in B-Lineage Oncogenes from High-Throughput Sequencing Data Using the Detection of Minor Variants by Error Correction Method. J Immunol Baltim Md 1950. 2018;201: 950–956. 10.4049/jimmunol.1800203 [DOI] [PubMed] [Google Scholar]
- 23.Hu J, Meyers RM, Dong J, Panchakshari RA, Alt FW, Frock RL. Detecting DNA double-stranded breaks in mammalian genomes by linear amplification-mediated high-throughput genome-wide translocation sequencing. Nat Protoc. 2016;11: 853–871. 10.1038/nprot.2016.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong J, Panchakshari RA, Zhang T, Zhang Y, Hu J, Volpi SA, et al. Orientation-specific joining of AID-initiated DNA breaks promotes antibody class switching. Nature. 2015;525: 134–139. 10.1038/nature14970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frock RL, Hu J, Meyers RM, Ho Y-J, Kii E, Alt FW. Genome-wide detection of DNA double-stranded breaks induced by engineered nucleases. Nat Biotechnol. 2015;33: 179–186. 10.1038/nbt.3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gel B, Serra E. karyoploteR: an R/Bioconductor package to plot customizable genomes displaying arbitrary data. Bioinforma Oxf Engl. 2017;33: 3088–3090. 10.1093/bioinformatics/btx346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laffleur B, Bardet SM, Garot A, Brousse M, Baylet A, Cogné M. Immunoglobulin genes undergo legitimate repair in human B cells not only after cis- but also frequent trans-class switch recombination. Genes Immun. 2014;15: 341–346. 10.1038/gene.2014.25 [DOI] [PubMed] [Google Scholar]
- 28.Reynaud S, Delpy L, Fleury L, Dougier H-L, Sirac C, Cogné M. Interallelic class switch recombination contributes significantly to class switching in mouse B cells. J Immunol Baltim Md 1950. 2005;174: 6176–6183. 10.4049/jimmunol.174.10.6176 [DOI] [PubMed] [Google Scholar]
- 29.Shinkura R, Ito S, Begum NA, Nagaoka H, Muramatsu M, Kinoshita K, et al. Separate domains of AID are required for somatic hypermutation and class-switch recombination. Nat Immunol. 2004;5: 707–712. 10.1038/ni1086 [DOI] [PubMed] [Google Scholar]
- 30.Yeap L-S, Hwang JK, Du Z, Meyers RM, Meng F-L, Jakubauskaitė A, et al. Sequence-Intrinsic Mechanisms that Target AID Mutational Outcomes on Antibody Genes. Cell. 2015;163: 1124–1137. 10.1016/j.cell.2015.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu K, Chedin F, Hsieh C-L, Wilson TE, Lieber MR. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat Immunol. 2003;4: 442–451. 10.1038/ni919 [DOI] [PubMed] [Google Scholar]
- 32.Xu Z, Fulop Z, Wu G, Pone EJ, Zhang J, Mai T, et al. 14-3-3 adaptor proteins recruit AID to 5′-AGCT-3′–rich switch regions for class switch recombination. Nat Struct Mol Biol. 2010;17: 1124–1135. 10.1038/nsmb.1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandra V, Bortnick A, Murre C. AID targeting: old mysteries and new challenges. Trends Immunol. 2015;36: 527–535. 10.1016/j.it.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basu U, Meng F-L, Keim C, Grinstein V, Pefanis E, Eccleston J, et al. The RNA Exosome Targets the AID Cytidine Deaminase to Both Strands of Transcribed Duplex DNA Substrates. Cell. 2011;144: 353–363. 10.1016/j.cell.2011.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kingzette M, Spieker-Polet H, Yam PC, Zhai SK, Knight KL. Trans-chromosomal recombination within the Ig heavy chain switch region in B lymphocytes. Proc Natl Acad Sci U S A. 1998;95: 11840–11845. 10.1073/pnas.95.20.11840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Noir S, Laffleur B, Carrion C, Garot A, Lecardeur S, Pinaud E, et al. The IgH locus 3’ cis-regulatory super-enhancer co-opts AID for allelic transvection. Oncotarget. 2017;8: 12929–12940. 10.18632/oncotarget.14585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garrett FE, Emelyanov AV, Sepulveda MA, Flanagan P, Volpi S, Li F, et al. Chromatin architecture near a potential 3’ end of the igh locus involves modular regulation of histone modifications during B-Cell development and in vivo occupancy at CTCF sites. Mol Cell Biol. 2005;25: 1511–1525. 10.1128/MCB.25.4.1511-1525.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Zhang Y, Ba Z, Kyritsis N, Casellas R, Alt FW. Fundamental roles of chromatin loop extrusion in antibody class switching. Nature. 2019. 10.1038/s41586-019-1723-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The inserted cassette includes the core Sμ, followed by the hEiκ enhancer, constant hCκ exon from the human Igκ locus and then the core Sγ3 and c3’RR. A Vκ promoter and mutated leader exon provides transcription and splicing of Sγ3. The downstream floxed NeoR gene was removed by Cre-deletion to generate the germline c3’RR-KIKS locus. c3’RR-KIKS κ-CSR events join S regions, then excising hCk and yielding Ig with murine Ck.
(TIF)
(TIF)
A. Percentage of IgG1+ cells in spleens from NI or SRBC immunized mice (day 8) (KIKS, c3’RR-KIKS and c3’RR-KIKS / Aicda-/- mice). B. Representative dot plots of mκ and hκ staining in CD43 negative splenic B-cells. C Comparison of κ-CSR efficiency as evaluated by counting mCκ+ spleen B-cells (gated on B220+/IgM+ cells) by flow cytometry, in spleens from non-immunized (NI) or SRBC immunized (day 8) mice and from Peyers’ patches from KIKS, c3’RR-KIKS and c3’RR-KIKS / Aicda-/- mice. Percentages +/- SEM.
(TIF)
(XLSX)
B: Each individual experiment of κ-CSR efficiency as evaluated by counting mCκ+ in in vitro stimulated (LPS) B-cells (gated on B220+).
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Hight-throughput sequencing results have been deposited on ENA at https://www.ebi.ac.uk/ena/browser/home under the references PRJEB34781 and PRJEB34851.


