Abstract
Introduction
Some patients with COVID-19 pneumonia present systemic disease involving multiple systems. There is limited information about the clinical characteristics and events leading to acute kidney injury (AKI). We described the factors associated with the development of AKI and explored the relation of AKI and mortality in Mexican population with severe COVID-19.
Methods
We retrospectively reviewed the medical records of individuals with severe pneumonia caused by SARS-CoV-2 hospitalized at the largest third-level reference institution for COVID-19 care in Mexico between March and April 2020. Demographic information, comorbidities, clinical and laboratory data, dates of invasive mechanical ventilation (IMV) and hospitalization, mechanical-ventilator settings and use of vasoactive drugs were recorded.
Results
Of 99 patients studied, 58 developed AKI (58.6%). The risk factors for AKI were older age (OR = 1.07, 95% CI = 1.01–1.13, p = 0.024); obesity (OR = 6.58, 95% CI = 1.8–24.05, p = 0.040); and the need for IMV (OR = 6.18, CI = 1.29–29.58, p = 0.023). The risk factors for mortality were obesity (OR = 5.57, 95% CI = 1.48–20.93, p = 0.011); requirement of vasoactive drugs on admission (OR = 5.35, 95% CI = 1.16–24.61, p = 0.031); and AKI (OR = 8.61, 95% CI = 2.24–33.1, p = 0.002). In-hospital mortality was significantly higher in patients with AKI stage 3 (79.3%) and AKI stage 2 (68.7%) compared with those with AKI stage 1 (25%; p = 0.004). Fifty-three patients underwent the furosemide stress test (FST) to predict progression to AKI stage 3. Of those, 12 progressed to AKI stage 3 (22%). The ROC curve for the FST had an AUC of 0.681 (p = 0.009); a sensitivity of 81.6% and a specificity of 54.5%.
Conclusions
AKI was common in our cohort of patients with severe pneumonia caused by SARS-CoV-2 infection. The risk factors for AKI were older age, obesity and the need for of IMV on admission. The risk factors for mortality were obesity, requirement of vasoactive drugs on admission and AKI. Mortality was more frequent in patients with AKI stages 2–3. The FST had an acceptable predictive capacity to identify patients progressing to AKI stage 3.
Introduction
In December 2019, a series of pneumonia cases of unknown cause emerged in Wuhan, Hubei Province, China, with clinical presentations resembling viral pneumonia [1]. The pneumonia spread quickly to other provinces of China and overseas. A novel coronavirus was identified by the Chinese Center for Disease Control and Prevention from the throat swab sample of a patient and was provisionally named 2019-nCoV by the World Health Organization (WHO) [2]. Based on phylogeny, taxonomy and established practice, the International Committee on Taxonomy of Viruses renamed the virus as Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [3]. WHO subsequently declared coronavirus disease 2019 (COVID-19) a public health emergency of international concern [4]. COVID-19 is primarily manifested as a respiratory tract infection, but emerging data indicate that it should be regarded as a systemic disease involving multiple systems, including cardiovascular, respiratory, gastrointestinal, neurological, hematopoietic, immune and renal [5–8]. Of note, after lung infection, the virus may enter the blood, accumulate in the kidney and cause damage to resident renal cells, with a significantly higher risk for in-hospital death [8]. Thus, understanding how the kidney is affected by SARS-CoV-2 is particularly relevant. The incidence of acute kidney injury (AKI) in hospitalized patients with COVID-19 varies across populations, but a large multicenter retrospective cohort study in New York reported AKI in 37% of hospitalized patients, and 35% of those died [9]. AKI initiation coincides with the development of Acute Respiratory Distress Syndrome (ARDS), and these alterations are typical of patients progressing to the most severe stage of illness involving extra-pulmonary systemic hyperinflammation [10].
The National Institute of Respiratory Diseases (INER) is the largest third-level national referral center for COVID-19 in Mexico City. Since early January 2020, this institution was gradually repurposed for the treatment of patients with COVID-19 exclusively. Since February 28, 2020, when the first Mexican patient was diagnosed with COVID-19, a high proportion of critically ill patients have been admitted at the INER. The aim of this study was to describe the factors associated with the development of AKI and explore the relation of AKI and mortality in the Mexican population with severe COVID-19.
Methods
Study population
The study was conducted at the INER, the largest third-level institution designated by the Mexican Government for COVID-19 care. All medical records of individuals with severe pneumonia caused by SARS-CoV-2 hospitalized at the INER between March and April 2020 were retrospectively reviewed. The Institutional Review Board (Comité de Ética en Investigación and Comité de Investigación del INER) approved the study and waived the requirement for informed consent due to the retrospective design of the study (Approval No. C39-20). Data were fully anonymized before being accessed. We included individuals with diagnosis of severe pneumonia caused by SARS-CoV-2, confirmed by real-time reverse transcription–polymerase chain reaction (rRT-PCR); 18 years of age or older; with no history of chronic kidney disease (CKD), as indicated by kidney ultrasound and direct interrogation of patients about CKD medical history; and ratio of partial arterial oxygen pressure/inspired oxygen fraction (PaO2/FiO2) <300 mm Hg on admission. Pregnant women were not included in the study. Patients with incomplete clinical records were excluded. Patients with SARS-CoV-2 severe pneumonia were defined as those with clinical data of respiratory distress, bilateral alveolar opacities in 2 or more lobes, a ratio of PaO2/FiO2 < 300 mm Hg and a positive result for SARS-CoV-2-rRT-PCR assay [11] in nasopharyngeal swab. Prone ventilation was used for the treatment of ARDS as a strategy to improve oxygenation when traditional modes of ventilation failed.
The primary outcome was the development of AKI. The secondary outcome was 30-day mortality in the group with AKI and the group without AKI. Recorded variables included demographic and anthropometric variables, symptoms, comorbidities, treatments, critical care variables, blood chemistry, blood count, initiation and termination dates of invasive mechanical ventilation (IMV), days in hospital, initial mechanical-ventilator settings, early use (in the first 24 hours after admission) of vasoactive drugs and outcomes.
Acute kidney injury
AKI staging was based on serum creatinine (sCr) levels. The urine output criterion was not used for diagnosis of AKI since nursing records were out of reach, in COVID-19 areas. The baseline sCr level was defined as the minimum inpatient value during the first 7 days of admission [12]. Diagnosis of AKI was based on the Kidney Disease Improving Global Outcomes (KDIGO) criteria [13]. AKI stage 1 corresponded to an increase in sCr by ≥ 0.3 mg/dL within 48 hours or increase in sCr 1.5 to 1.9 times baseline within the prior 7 days; AKI stage 2 corresponded to an increase in sCr of 2.0–2.9 times baseline; and AKI stage 3 corresponded to an increase in sCr of ≥3 times baseline or initiation of renal replacement therapy (RRT).
Furosemide stress test
Patients with AKI underwent the furosemide stress test (FST) for prediction of AKI severity. They were euvolemic before undertaking the furosemide challenge. The test was performed by administrating 1 mg/kg of furosemide i.v. or 1.5 mg/kg if the patient had received furosemide within the preceding 7 days, followed by observation of the urinary output in the first 2 hours. The result was considered positive if the patient urinated more than 200 ml per hour, in the following 2 hours after furosemide administration [14].
Statistical analysis
We performed descriptive statistics including means and standard deviations for normally distributed continuous variables, medians and interquartile ranges for non-parametric distributions, and proportions for categorical variables. Comparisons of the AKI vs. the non-AKI groups were made using Fisher´s exact test for categorical variables and U Mann-Whitey for continuous variables. Comparisons across AKI stages were made using Kruskal-Wallis rank sum test. Logistic regression analysis was used to identify the association between relevant covariates with AKI and mortality. No violations of the assumptions were detected. The multivariate models were built using a stepwise procedure, and variables were entered into the models when the alpha level of risk factor was <0.15 in the univariate analysis. All statistical tests were two-sided, and a P value <0.05 was considered statistically significant.
We also performed the Kaplan-Meier survival analyses for the time to death, comparing the group with AKI vs. the non-AKI group. Statistical analyses were performed using R version 3.6.3. Additionally, a Receiver Operating Characteristic (ROC) curve was constructed in order to define the optimal cut-off points of the variables for prediction to AKI stage 3. Sensitivity, specificity, and the area under the curve (AUC) were calculated with 95% confidence intervals and p values <0.05.
Results
Characteristics of study population
During the period between March 1st, 2020 and April 30, 2020, a total of 280 individuals were admitted at the INER due to suspected COVID-19. Of those, 12 died during the first 48 hours and 78 had a negative result for the SARS-CoV-2 rRT-PCR test. Therefore, we reviewed the clinical files of 190 individuals. Of those, 12 had pneumonia due to other causes; 19 were transferred to other hospitals due to local saturation; and 60 had incomplete clinical files. Thirty-six patients of the group with incomplete clinical files were not hospitalized because they had a ratio of PaO2/FiO2 > 300 mm Hg, with acceptable metabolic control (glycemic, kidney and hepatic functions). Therefore, they were trained on the use of supplemental oxygen, the recognition of worsening symptoms, and were monitored from home. The clinical files of the remaining 24 patients were unavailable due to the accelerated reconversion of our institution for exclusive treatment of patients with COVID-19. From the electronic records, we confirmed that five of those patients died and none of them developed AKI during the observation period at hospital. We thus included 99 individuals in the study (Fig 1).
Fig 1. Study diagram.
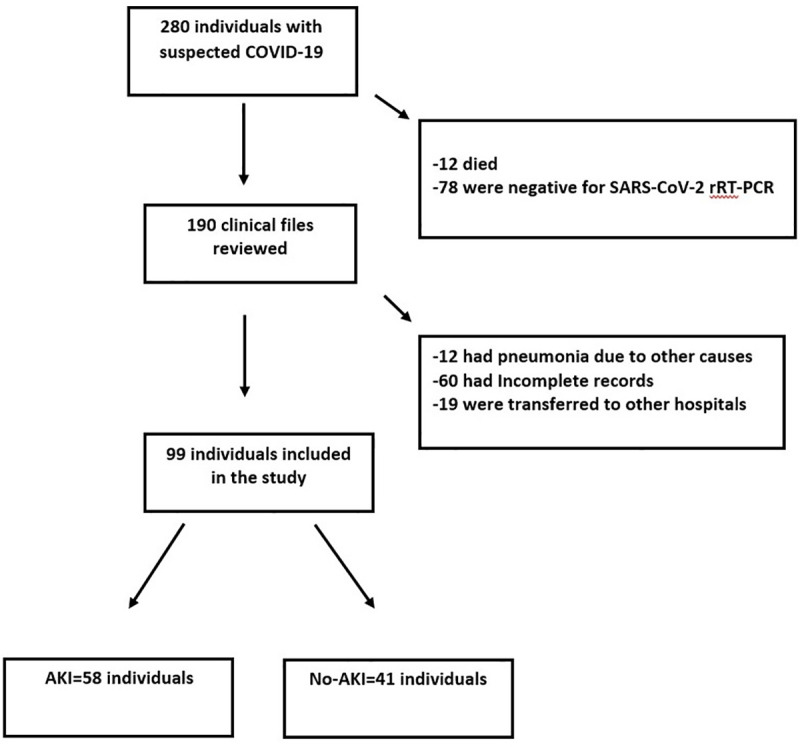
Numbers of individuals assessed for eligibility and individuals included in the study.
Of the 99 patients included, 74 were male (74.7%); the median age was 52.9 years (SD±13.27); 30 had hypertension (29.7%); 27 had diabetes (26.7%); and 56 had obesity (55.4%; Table 1).
Table 1. Baseline characteristics of the study population.
| Variables | Overall N = 99 | Non-AKI N = 41 | AKI N = 58 | AKI stage 1 N = 12 | AKI stage 2 N = 16 | AKI stage 3 N = 29 | p value Non-AKI vs. AKI | p value across AKI stages |
|---|---|---|---|---|---|---|---|---|
| Age, years‡ | 52.9 (13.2) | 48.4 (12.3) | 56.1(13.2) | 57.08 (11.9) | 57.25 (13.3) | 55.45 (14.1) | 0.01 | 0.67 |
| Male [n (%)] | 74 (74.7) | 30 (73) | 44 (73) | 9 (75) | 12 (75) | 22 (75.8) | 0.81 | 1.00 |
| Symptoms | ||||||||
| Diarrhea [n (%)] | 19 (18.8) | 8 (19) | 11 (18.9) | 2 (16) | 4 (25) | 5 (17) | 1.00 | 0.79 |
| Rhinorrhea [n (%)] | 36 (35.6) | 15 (36.5) | 21 (36.2) | 2 (16.6) | 3 (18.7) | 15 (51) | 1.00 | 0.02 |
| Anosmia [n (%)] | 4 (3.96) | 2 (4.8) | 3 (5.1) | 0 | 0 | 3 (10.3) | 1.00 | 0.22 |
| Cough [n (%)] | 4 (43.96) | 34 (82.9) | 54 (93.1) | 9 (75) | 16 (100) | 28 (96.5) | 0.19 | 0.02 |
| Dyspnea [n (%)] | 90 (89.1) | 33 (80.4) | 57 (98.2) | 11 (91.6) | 16 (100) | 29 (100) | 0.01 | 0.15 |
| Odynophagia [n (%)] | 53 (52.4) | 26 (63.4) | 27 (46.5) | 4 (33.3) | 9 (56.2) | 13 (44.8) | 0.10 | 0.48 |
| Fever [n (%)] | 93 (92.07) | 38 (92.6) | 55 (94.8) | 11 (91.6) | 15 (93.7) | 28 (96.5) | 0.68 | 0.80 |
| Headache [n (%)] | 93 (92.0) | 31 (75.6) | 42 (72.4) | 9 (75) | 12 (75) | 20 (68.9) | 0.81 | 0.88 |
| Asthenia [n (%)] | 87 (86.1) | 34 (82.9) | 53 (91.3) | 11 (91.6) | 14 (87.5) | 27 (93.1) | 0.22 | 0.83 |
| Myalgia [n (%)] | 84 (83.1) | 36 (87.8) | 48 (82.7) | 10 (83.3) | 13 (81.2) | 24 (82.7) | 0.57 | 0.98 |
| Expectoration [n (%)] | 29 (28.7) | 10 (24.3) | 19 (32.7) | 4 (33.3) | 5 (31.2) | 10 (34.4) | 0.50 | 0.97 |
| Nausea [n (%)] | 15 (14.8) | 6 (14.6) | 9 (15.5) | 2 (16.6) | 1 (6.2) | 6 (20) | 1.00 | 0.50 |
| Comorbidities | ||||||||
| Hypertension [n (%)] | 30 (29.7) | 9 (21.9) | 21 (36.2) | 6 (50) | 4 (25) | 11 (37.9) | 0.18 | 0.39 |
| ACE2 i [n (%)] | 4 (3.9) | 1 (2.4) | 3 (5.1) | 0 | 1 (6.2) | 2 (6.8) | 0.63 | 0.65 |
| ARB [n (%)] | 15 (14.8) | 6 (14.6) | 9 (15.5) | 4 (33.3) | 1 (6.2) | 4 (13.7) | 1.00 | 0.17 |
| Obesity [n (%)] | 56 (55.4) | 10 (25.6) | 28 (50.9) | 3 (27.2) | 6 (37.5) | 18 (66.6) | 0.01 | 0.04 |
| Class 1 [n (%)] | 25 (62.5) | 7 (70) | 18 (60) | 3 (75) | 3 (50) | 11 (57.8) | 0.69 | 0.52 |
| Class 2 [n (%)] | 8 (20) | 2 (20) | 6 (20) | 1 (25) | 1 (16.6) | 4 (21) | ||
| Class 3 [n (%)] | 7 (17.5) | 1 (10) | 6 (20) | 0 | 2 (33.3) | 4 (21.0) | ||
| BMI, kg/m2 * | 29.7 (25.99–33.118) | 27.6 (25.1–30.1) | 31.1 (27.7–34.7) | 28.7 (26.3–31.8) | 29.7 (26.2–31.9 | 32.8 (29.3–37.5) | 0.01 | 0.05 |
| Diabetes [n (%)] | 27 (26.7) | 10 (19.6) | 17 (34.6) | 4 (36.3) | 3 (23.0) | 10 (40) | 0.11 | 0.58 |
| Dyslipidemia [n (%)] | 7 (6.9) | 2 (4.8) | 5 (8.6) | 3 (25) | 0 | 2 (6.8) | 0.69 | 0.06 |
| Heart Disease [n (%)] | 4 (3.9) | 1 (2.4) | 3 (5.1) | 1 (9.1) | 0 | 2 (8) | 0.35 | 0.56 |
| Pulmonary Disease [n (%)] | 5 (4.95) | 2 (3.9) | 3 (6) | 1 (9.09) | 0 | 2 (8) | 0.67 | 0.56 |
| Rheumatic Disease [n (%)] | 3 (2.9) | 1 (1.9) | 2 (4) | 2 (18.1) | 0 | 0 | 0.61 | 0.02 |
| Allergic [n (%)] | 10 (9.9) | 5 (9.8) | 5 (10.2) | 2 (18.1) | 0 | 3 (12) | 1.00 | 0.31 |
| Cancer [n (%)] | 3 (2.9) | 1 (1.9) | 2 (4) | 1 (9.09) | 0 | 1 (4) | 0.61 | 0.53 |
| Smoker [n (%)] | 3 (2.9) | 13 (25.4) | 7 (14.2) | 3 (27.2) | 0 | 4 (16) | 0.21 | 0.16 |
| Comorbidities [n (%)] | 2 (1–2) | 2 (1–3) | 2 (1–4) | 1 (1–1) | 2 (1–3) | 0.45 | 0.01 | |
| Treatment | ||||||||
| Drugs before admission [n (%)] | 67 (66.3) | 34 (66.6) | 34 (69.3) | 7 (63.6) | 8 (61.5) | 19 (76) | 0.83 | 0.59 |
| Quinines [n (%)] | 81 (80.1) | 43 (97.7) | 38 (95) | 10 (100) | 10 (90.9) | 18 (94.7) | 0.60 | 0.63 |
| Hydroxychloroquine [n (%)] | 43 (42.5) | 22 (43.1) | 16 (32) | 6 (66.6) | 5 (50) | 10 (55.5) | 0.62 | 0.90 |
| Chloroquine [n (%)] | 38 (37.6) | 21 (41.1) | 19 (38) | 3 (33.3) | 5 (50) | 8 (44.4) | ||
| Oseltamivir [n (%)] | 76 (75.24) | 37 (72) | 39 (79) | 10 (100) | 11 (100) | 18 (94.7) | 0.05 | 0.57 |
| Antibiotics [n (%)] | 79 (78.2) | 40 (78.4) | 39 (79.5) | 10 (25.6) | 10 (25.6) | 19 (48.7) | 0.38 | 0.42 |
| Ceftriaxone [n (%)] | 79 (78.2) | 38 (74.5) | 36 (73.4) | 10 (100) | 9 (90) | 17 (84.2) | ||
| Meropenem [n (%)] | 74 (73.2) | 1 (1.9) | 2 (4) | 0 | 1 (10) | 1 (5.2) | ||
| Amikacin [n (%)] | 3 (2.9) | 0 | 1 (2) | 0 | 0 | 1 (5.2) | ||
| Piperacillin/Tazobactam [n (%)] | 1 (0.99) | 1 (1.9) | 0 | 0 | 0 | 1 (5.2) | ||
| Enoxaparin [n (%)] | 81 (80.2) | 44 (86.2) | 37 (75.5) | 9 (90) | 10 (100) | 18 (94.7) | 0.21 | 0.60 |
AKI, acute kidney injury; ACE2 i, angiotensin converting enzyme inhibitor (enalapril, captopril); ARB, angiotensin II receptor blocker (losartan, telmisartan); BMI, body mass index; Class 1 obesity: BMI of 30 to < 35 kg/m2; Class 2 obesity: BMI of 35 to < 40 kg/m2; Class 3 obesity: BMI of 40 kg/m2 or higher.
*Data are expressed as medians (interquartile ranges).
‡Data are expressed as means (standard deviation).
Acute kidney injury
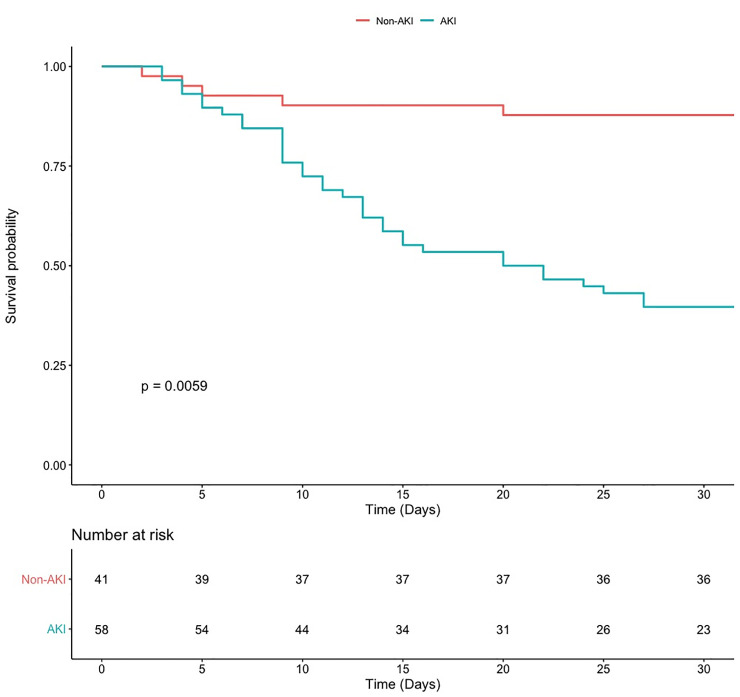
Fifty-eight patients developed AKI (the AKI group) and 41 individuals did not develop AKI (the non-AKI group, Table 1). Of those, 12 had AKI stage 1 (21.1%); 16 had AKI stage 2 (28.1%); and 29 had AKI stage 3 (50.9%). Forty-one patients of the AKI group (83.6%) required IMV, while 23 patients of the non-AKI group (45%) required IMV (p = 0.01). Twenty-six patients of the AKI group required prone mechanical ventilation (57.7%), compared with 2 patients (14%) in the non-AKI group (p = 0.01). On admission, 31 patients of the AKI group required vasoactive drugs (63.2%), compared with 18 patients (35.2%) of the non-AKI group (p = 0.01). Survival curves of both groups decreased with a similar rate on the first days in hospital, but since day 10 this decrease was steeper in the AKI group. By day 30, in-hospital mortality was significantly higher in the AKI group (38 patients in the AKI group (65.5%) vs. 6 patients (14.6%) in the non-AKI-group, p = 0.001; Fig 2).
Fig 2. Kaplan-Meier survival curves.
Time to death for the AKI group (blue line), and the non-AKI group (red line) during a follow-up period of 30 days. Time 0 corresponded to hospital admission.
In-hospital mortality was significantly higher in patients with AKI stage 3 (79.3%) and AKI stage 2 (68.7%) compared with those with AKI stage 1 (25%; p = 0.01). The body mass index (BMI) was significantly higher in the AKI group (31.1 kg/m2 vs. 27.6 kg/m2 in the non-AKI group; p = 0.01). Obesity was more frequent in the AKI group (28 patients, 50.9%) than in the non-AKI group (10 patients, 25.6%; p = 0.01). The median time to AKI development was 6.5 days (SD±8.33).
Fifty-three patients underwent the furosemide stress test. Of those, 39 had a positive result (73.5%) and 14 had a negative result (26.4%). Of the 53 patients undergoing the FST, 12 progressed to AKI stage 3 (22%). The ROC curve for the FST had an AUC of 0.681 (95% confidence interval (CI) = 0.514–0.847, p = 0.009), with a sensitivity of 81.6% and a specificity of 54.5% (Fig 3).
Fig 3. Receiver-operating characteristic (ROC) curve for the furosemide stress test (FST).
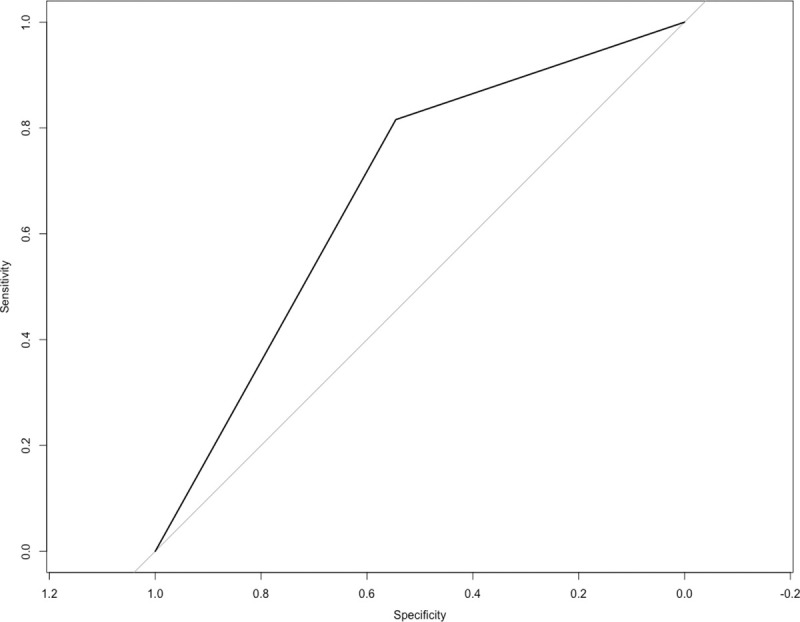
A ROC curve was constructed to evaluate FST performance in predicting AKI stage 3 development in 53 patients with COVID-19. AUC = 0.681 (95% CI = 0.514–0.847), p = 0.009, sensitivity = 81.6%, specificity = 54.5%.
A total of 11 patients (22.4%) required renal replacement therapy (RRT). Of those, 5 used continuous RRT, 3 used intermittent hemodialysis (IHD), and 3 used prolonged intermittent renal replacement therapies (PIRRT). The median time to RRT initiation after AKI initiation was 4.18 days (SD±3.8) and median time under RRT was 4.29 days (SD± 2.82). Five patients died after 30 days of follow-up, 2 of them had been discharged due to clinical improvement and 3 were hospitalized.
Inflammation markers and AKI
Some inflammation markers were higher in the AKI group, including C-reactive protein: 19.57 (±9.5) mg/dl in the AKI group vs. 15.03 (±9.3) mg/dl in the non-AKI group, p = 0.03; D-dimer: 1.5 μg/ml (interquartile range (IQR), 0.83–2.26) in the AKI group vs. 0.84 μg/ml (IQR, 0.6–1.29) in the non-AKI group, p = 0.01; procalcitonin: 0.25 ng/ml (IQR, 0.11–0.71) in the AKI group vs 0.18 ng/ml (IQR, 0.08–0.3) in the non-AKI group, p = 0.01; and troponin: 8 pg/ml (IQR, 4.25–38.6) in the AKI group vs. 3 pg/ml (IQR, 2–5.2) in the non-AKI group, p = 0.01. Lymphocytes were decreased in the AKI group: 0.7 10x3 mm3 (IQR, 0.5–1) compared to the non-AKI group: 0.9 10x3 mm3 (IQR, 0.8–1.33, p = 0.01; Table 2).
Table 2. Baseline laboratory values of the study population.
| Variables | Overall N = 99 | Non-AKI N = 41 | AKI N = 58 | AKI stage 1 N = 12 | AKI stage 2 N = 16 | AKI stage 3 N = 29 | p value Non-AKI vs. AKI | p value across AKI stages |
|---|---|---|---|---|---|---|---|---|
| Critical Care Variables | ||||||||
| IMV [n (%)] | 65 (64.3) | 23 (45) | 41 (83.6) | 8 (72.7) | 12 (92.3) | 21 (84) | 0.01 | 0.44 |
| Prone MV [n (%)] | 28 (47.4) | 2 (14) | 26 (57.7) | 6 (75) | 11 (84) | 9 (39) | 0.01 | 0.01 |
| Vasoactive Drugs [n (%)] | 49 (48.5) | 18 (35.2) | 31(63.2) | 6 (75) | 8 (72.7) | 17 (80.9) | 0.01 | 0.85 |
| Laboratory studies | ||||||||
| Creatinine, mg/dL* | 0.98 (0.84–1.51) | 0.87 (0.74–0.99) | 1.23 (0.9–1.72) | 1.02 (0.91–1.56) | 1.13 (0.92–1.5) | 1.51 (0.89–1.9) | 0.01 | 0.55 |
| Nitrogen urea mg/dL* | 18.11 (13.9–26.2) | 14.2 (10.9–20.24) | 23 (16–36.5) | 22.1 (17.0–26.6) | 19.05 (15.5–23.8) | 28.7 (16.1–37.03) | 0.01 | 0.26 |
| Creatinine mg/dL, day 7* | 1.04 (0.71–1.58) | 0.72 (0.62–0.91) | 1.4 (1–1.92) | 1.19 (0.77–1.47) | 1.32 (0.93–1.84) | 1.68 (1.12–2.57) | 0.01 | 0.06 |
| Creatinine, mg/dL on AKI* | 1.62 (1.37–2.06) | NA | 1.62 (1.37–2.06) | 1.31 (1.1–1.48) | 1.52 (1.35–1.79) | 1.93 (1.62–2.99) | NA | 0.01 |
| Maximum Creatinine, mg/dL* | 2.5 (1.71–5.36) | NA | 2.5 (1.71–5.36) | 1.4 (1.18–1.59) | 1.87 (1.56–2.3) | 5.5 (2.99–6.63) | NA | 0.01 |
| CPK, U/I* | 98.8 (60–193.3) | 89.4 (50.4–268.62 | 108.8 (69.1–177.1) | 139.5 (103.0–311.7) | 107.7 (64.7–168.4) | 89 (71.2–174.9) | 0.55 | 0.38 |
| Glucose, mg/dL* | 111.6 (98.2–153) | 101.3 (90.5–111.9) | 124.7 (108.8–184.2) | 127.9 (119.2–237.6) | 112.15 (102–129.4) | 136.5 (110.2–194.7) | 0.01 | 0.07 |
| Uric acid, mg/dL* | 5.17 (4.07–7.58) | 4.52 (3.6–5.9) | 5.7 (4.6–7.3) | 6.3 (5.06–6.9) | 4.9 (3.5–6.7) | 6.27 (4.73–7.58) | 0.01 | 0.26 |
| PaO2/FiO2, mm/Hg* | 194.05 (123.14–238.81) | 211.67 (153–248) | 181.19 (121–228) | 212.5 (146–213) | 194 (146–224) | 172.8 (108–224) | 0.12 | 0.76 |
| Lactate dehydrogenase, UI/L* | 373.1 (235–512.3) | 346.45 (291.3–492.4) | 378 (208–519.7) | 369.9 (263.5–643.3) | 398.2 (296–453.1) | 390.8 (214.6–535.4) | 0.56 | 0.97 |
| C-reactive protein, mg/dL‡ | 17.72 (9.65) | 15.03 (9.3) | 19.57 (9.5) | 21.5 (9.7) | 20.9 (6.6) | 16.8(8.7) | 0.03 | 0.09 |
| D-dimer, μg/ml* | 1.1 (0.7–1.78) | 0.84 (0.6–1.29) | 1.5 (0.83–2.26) | 1.09 (0.79–1.8) | 1.43 (0.88–2.34) | 1.75 (0.97–2.32) | 0.01 | 0.32 |
| Procalcitonin, ng/ml* | 0.2 (0.09–0.58) | 0.18 (0.08–0.3) | 0.25 (0.11–0.71) | 0.22 (0.14–0.67) | 0.26 (0.19–0.46) | 0.24 (0.1–1.2) | 0.01 | 0.85 |
| Troponin, pg/ml* | 4.8 (2.5–12.9) | 3 (2–5.2) | 8 (4.25–38.6) | 8.1 (2.1–55.1) | 12 (7.55–14.8) | 6.05 (4.35–51.2) | 0.01 | 0.82 |
| Albumin, g/dL‡ | 3.44 (0.5) | 3.63 (0.5) | 3.3 (0.46) | 3.56 (0.3) | 3.12 (0.38) | 3.28 (0.52) | 0.01 | 0.02 |
| Hemoglobin, g/dL* | 15.3 (14.4–16.3) | 15.2 (14.6–15.8) | 15.3 (14.4–16.6) | 16.1 (15.1–16.7) | 14.7 (13.3–16.03) | 15.4 (14.8–16.2) | 0.48 | 0.23 |
| Leucocytes, 10x3 mm3* | 8.9 (6.6–11.5) | 8.05 (6.5–11) | 9 (6.7–11.5) | 9.45 (6.3–11.5) | 7.3 (6.38–9.2) | 9.5 (7.25–12.5) | 0.34 | 0.21 |
| Lymphocytes, 10x3 mm3* | 0.8 (0.6–1.2) | 0.9 (0.8–1.33) | 0.7 (0.5–1) | 0.7 (0.48–0.72) | 0.7 (0.5–1.02) | 0.85 (0.5–1.2) | 0.01 | 0.194 |
| Platelets 10x3mm3* | 202 (171–266) | 217.5 (178.5–266.75) | 201 (162–257) | 196.5 (158.25–251.75) | 186 (165.5–267.2) | 201.5 (165–261.25) | 0.34 | 0.85 |
| Platelets/Lymphocytes Index* | 246.25 (173.64–340) | 233.75 (162–264) | 300 (202.2–424) | 317.4 (267.7–460) | 308.1 (234.5–419.9) | 282.7 (176.6–383.4) | 0.01 | 0.45 |
| Platelets/Neutrophils Index* | 29.13 (20.49–41.19) | 34.9 (25.08–44.91) | 27.66 (19–36.5) | 27.1 (19.3–33.04) | 28.8(25.4–39.5) | 26.1 (18.8–36.5) | 0.02 | 0.56 |
| Outcomes | ||||||||
| Mortality (%)* | 44 (44.4) | 6 (14.6) | 38 (65.5) | 3 (25) | 11 (68.7) | 23 (79.3) | 0.01 | 0.01 |
| Days in hospital* | 12 (8.5–23.5) | 9 (8–16) | 14 (9–30) | 14.5 (9.75–32.25) | 13.5 (9–24.75) | 15(9–33) | 0.02 | 0.91 |
| Days to death* | 12 (8.5–23.5) | 7 (4.25–17.25) | 12.5 (9–20) | 9 (8–18) | 13 (9–15) | 12 (8–21) | 0.01 | 0.96 |
| Days to AKI* | 2 (1–9) | NA | 2 (1–9) | 3 (1–9) | 4 (1–10.75) | 2 (1–8) | NA | 0.77 |
| Days on IMV* | 1 (0–1) | 1 (0–1) | 1 (0–1) | 1 (0–1) | 1(0–1) | 1(0–1) | 0.96 | 0.80 |
AKI, acute kidney injury; CPK, creatine phosphokinase; PaO2/FiO2 arterial partial pressure of oxygen/fraction inspired oxygen; IMV, invasive mechanical ventilation; Prone MV, prone mechanical ventilation; vasoactive drugs (norepinephrine and vasopressin on admission). Admission laboratory test results were used unless otherwise specified.
*Data are expressed as medians (interquartile ranges).
‡Data are expressed as means (standard deviation).
On admission, 82.8% had a positive result for SARS-CoV-2-rRT-PCR, but all patients had a positive result when the test was performed for the third time. Sixty-five patients (64.3%) required IMV. The median positive end-expiratory pressure (PEEP) level was 11.5 cm H2O (SD ±2.4); and 28 required prone ventilation due to refractory hypoxemia. Forty-nine patients (48.5%) required vasoactive drugs on admission; and overall mortality was 44.4%. All patients had ground glass opacities; 85.2% had crazy paving pattern; 94.3% had consolidation; 5.6% had pleural effusion; 79.5% had bronchiectasis; 54.5% had atelectasis; 59% had peripheral distribution; and 39.7% had central and peripheral distribution.
Risk factors for acute kidney injury
The univariate analysis indicated that patients with AKI were older (unadjusted odds ratio (OR) = 1.05, 95% CI = 1.01–1.08, p = 0.007); had a higher BMI (OR = 1.10, CI = 1.02–1.18, p = 0.012); a higher frequency of obesity (OR = 2.59, 95% CI = 1.11–6.04, p = 0.028); a higher requirement of IMV (OR = 6.78, 95% CI = 2.69–17.04, p = 0.001); a higher requirement of vasoactive drugs (OR = 4.26, CI = 1.08–10.06, p = 0.001); a higher ratio of platelet/lymphocyte (OR = 1.0037, CI = 1.0005–1.0068, p = 0.021); a lower count of lymphocytes (OR = 0.23, CI = 0.08–0.67, p = 0.007); a higher level of C-reactive protein (OR = 1.05, CI = 1.01–1.1, p = 0.028); and a lower level of albumin (OR = 0.26, CI = 0.11–0.64, p = 0.003). After adjusting for possible confounding variables, the multivariate analysis indicated that the risk factors for AKI were older age (OR = 1.07, 95% CI = 1.01–1.13, p = 0.024); obesity (OR = 6.58, 95% CI = 1.8–24.05, p = 0.040); and requirement of IMV (OR = 6.18, CI = 1.29–29.58, p = 0.023, Table 3).
Table 3. Risk factors for acute kidney injury.
| Variables | Unadjusted OR (95% CI) | p value | Adjusted OR (95% CI)* | p value |
|---|---|---|---|---|
| Age, years | 1.05 (1.01–1.08) | 0.007 | 1.07 (1.01–1.13) | 0.024 |
| Male | 1.15 (0.46–2.88) | 0.762 | 0.88 (0.24–3.21) | 0.849 |
| BMI | 1.10 (1.02–1.18) | 0.012 | - | - |
| Obesity (BMI ≥ 30 kg/m2) | 2.59 (1.11–6.04) | 0.028 | 6.58 (1.8–24.05) | 0.040 |
| Hypertension | 2.02 (0.81–5.03) | 0.132 | 0.25 (0.05–1.15) | 0.750 |
| Diabetes | 2.56 (0.96–6.79) | 0.060 | 1.91 (0.05–7.3) | 0.342 |
| PaO2/FIO2, mmHg | 0.99 (0.99–1.00) | 0.302 | - | - |
| IMV | 6.78 (2.69–17.04) | 0.001 | 6.18 (1.29–29.58) | 0.023 |
| Vasoactive drugs | 4.26 (1.08–10.06) | 0.001 | 1.54 (0.37–6.43) | 0.551 |
| Platelet/Lymphocyte ratio | 1.0037 (1.0005–1.0068) | 0.021 | 1.0033 (0.9983–1.0084) | 0.198 |
| Lymphocytes 10x3 mm3 | 0.23 (0.08–0.67) | 0.007 | 0.69 (0.12–3.83) | 0.670 |
| Procalcitonin, ng/ml | 1.1 (0.93–1.32) | 0.259 | - | - |
| D-dimer, μg/ml | 1.29 (0.95–1.75) | 0.102 | 1.23 (0.89–1.71) | 0.202 |
| Troponin, pg/ml | 1.00 (0.99–1.001) | 0.984 | - | - |
| C-reactive protein, mg/dL | 1.05 (1.01–1.1) | 0.028 | 0.9984 (0.9366–1.0642) | 0.961 |
| Albumin g/dL | 0.26 (0.11–0.64) | 0.003 | 1.24 (0.29–5.28) | 0.769 |
OR, odds ratio; CI, confidence interval; BMI, body mass Index; IMV, invasive mechanical ventilation; PaO2/FiO2, arterial partial pressure of oxygen/fraction inspired oxygen ratio; AKI, acute kidney injury; vasoactive drugs (norepinephrine and vasopressin on admission).
Admission laboratory test results were used.
* Variables were entered into the model when the alpha level of risk factor was less than 0.15. Age and gender were added into the model regardless of alpha level.
Risk factors for mortality
The univariate analysis indicated that deceased patients had a higher BMI (OR = 1.14, 95% CI = 1.06–1.23, p = 0.001); a higher frequency of obesity (OR = 3.6, 95% CI = 1.56–8.32, p = 0.003); a higher requirement of IMV (OR = 7.47, IC = 2.71–20.57, p = 0.001) and of vasoactive drugs (OR = 7.18, IC = 2.95–17.46, p = 0.001); a higher level of C-reactive protein (OR = 1.06, CI = 1.02–1.11, p = 0.008); a lower level of albumin (OR = 0.25, IC = 0.1–0.62, p = 0.003); and a higher frequency of AKI (OR = 12.96; IC = 4.63–36.28, p = 0.001). After adjusting for possible confounding variables, the multivariate analysis indicated that the risk factors for mortality were obesity (OR = 5.57, 95% CI = 1.48–20.93, p = 0.011); requirement of vasoactive drugs on admission (OR = 5.35, 95% CI = 1.16–24.61, p = 0.031); and AKI (OR = 8.61, 95% CI = 2.24–33.1, p = 0.002, Table 4).
Table 4. Risk factors for mortality.
| Variables | Unadjusted OR (95% CI) | p value | Adjusted OR (95% CI)* | p value |
|---|---|---|---|---|
| Age, years | 1.02 (0.99–1.06) | 0.119 | 1.009 (0.9457–1.0593) | 0.975 |
| Male | 1.42 (0.06–3.57) | 0.455 | ||
| BMI | 1.14 (1.06–1.23) | 0.001 | ||
| Obesity (BMI ≥ 30 kg/m2) | 3.6 (1.56–8.32) | 0.003 | 5.57 (1.48–20.93) | 0.011 |
| Hypertension | 1.8 (0.76–4.29) | 0.182 | ||
| Diabetes | 1.65 (0.68–4.03) | 0.269 | ||
| Comorbidities | ||||
| PaO2/FIO2, mmHg | 0.9963 (0.9912–1.0014) | 0.152 | ||
| IMV | 7.47 (2.71–20.57) | 0.001 | 1.4 (0.22–8.77) | 0.720 |
| Vasoactive drugs | 7.18 (2.95–17.46) | 0.001 | 5.35 (1.16–24.61) | 0.031 |
| Platelet/Lymphocyte ratio | 1.0013 (0.999–1.0036) | 0.274 | ||
| Lymphocytes 10x3 mm3 | 0.54 (0.2–1.44) | 0.217 | ||
| Procalcitonin, ng/ml | 1.0068 (0.9749–1.0397) | 0.680 | ||
| D-dimer, μg/ml | 1.17 (0.97–1.41) | 0.100 | 1.04 (0.84–1.29) | 0.723 |
| Troponin, pg/ml | 1.01 (1–1.02) | 0.118 | 1.0062 (0.9962–1.0163) | 0.228 |
| C-reactive protein, mg/dL | 1.06 (1.02–1.11) | 0.008 | 1.02 (0.95–1.08) | 0.653 |
| Albumin g/dl | 0.25 (0.1–0.62) | 0.003 | 0.83 (0.2–3.51) | 0.802 |
| AKI | 12.96 (4.63–36.28) | 0.001 | 8.61 (2.24–33.1) | 0.002 |
OR, odds ratio; CI, confidence interval; BMI, body mass Index; IMV, invasive mechanical ventilation; PaO2/FiO2, arterial partial pressure of oxygen/fraction inspired oxygen ratio; AKI, acute kidney injury; vasoactive drugs (norepinephrine and vasopressin on admission).
Admission laboratory test results were used.
* Variables were entered into the model when the alpha level of risk factor was less than 0.15. Age and gender were added into the model regardless of alpha level.
Discussion
Multiple organ involvement including the liver, gastrointestinal tract and kidney have been reported in patients with COVID-19 [8]. Since information about causes leading to severe kidney disease in these patients is still limited, here we determined the factors associated with the development of AKI and explored the relation between AKI and mortality in Mexican population with severe COVID-19.
In our cohort of patients with severe COVID-19, the risk factors for AKI were older age, obesity and requirement of IMV on admission. The risk factors for mortality were obesity, requirement of vasoactive drugs on admission and AKI. Moreover, in-hospital mortality was particularly elevated in patients with AKI stages 2 and 3.
In contrast with initial studies reporting low incidences of AKI between 5–7% in hospitalized patients with COVID-19 in China [8, 15, 16], the incidence of AKI in our cohort was 58.6%, and half of those had severe AKI (stage 3). Differences between studies might be partially explained by the fact that our institution is a national referral center for respiratory diseases, where mostly patients with COVID-19 severe disease are being admitted. Our study population was similar to that studied in another Mexican, third-level, national referral center for patients with severe COVID-19, reporting an AKI incidence of 60.7% [17]; and to the cohort studied in a New York City medical center, where up to 78% of the patients developed AKI and most of them required IMV [18].
As previously reported, we found that older age was a risk factor for AKI in hospitalized patients with COVID-19 [9]. Obesity was a risk factor for AKI and mortality in our cohort, and it has been reported as a common comorbidity in hospitalized patients with COVID-19 [19, 20], and as a risk factor for hospital admission and need for critical care [21]. This is particularly relevant for countries with high obesity rates, such as Mexico. In the adult Mexican population, the combined prevalence of overweight and obesity is approximately 71% [22]. It has been suggested that chronic inflammation in obesity is apparent, with an increased level of interleukin-6, adipokines and pro-inflammatory cytokines (e.g., TNF-alpha, interferon), inducing a chronic low-grade inflammatory state and impairing immune response [23, 24]. A possible mechanism related to COVID-19 severity in obese persons is speculated to occur through a functional restrictive capacity of the obese lung. It would also be interesting to understand whether the obese patients had higher PEEP or driving pressure that could account for the higher risk for AKI. Unfortunately, PEEP data retrieval was incomplete in our retrospective review of medical records, so we were unable to perform this analysis. The need for mechanical ventilation on admission was also a risk factor for AKI in our cohort. It is well known that the main causes of AKI are hypoxia, ischemia and nephrotoxicity. The kidney is particularly susceptible to ischemia and toxins, resulting in vasoconstriction, endothelial damage, and activation of inflammatory processes [25]. In hospitalized patients with severe COVID-19, the important relationship between AKI and respiratory failure was previously reported [9].
The requirement of vasoactive drugs on admission was a risk factor for mortality in our cohort. This is not surprising if we consider that vasoactive drugs are used in the most critically ill patients with septic shock and evidence of renal dysfunction [26]. In this context, early start of vasopressor support is aimed at having a more rapid restoration of blood flow in combination with lower fluid accumulation, allowing early restitution of tissue perfusion while avoiding fluid overload-mediated harm [27].
We found that elevated serum creatinine on admission was more common in patients with AKI, which was previously reported in a cohort in China [8]. This means that patients with kidney involvement on admission were more likely to develop AKI. Lymphopenia was more common in the group with AKI, and low lymphocyte counts have been associated with severe COVID-19 and longer hospital stay [28]. COVID-19-associated lymphopenia might derive from retention of lymphocytes in the lung. Also, lymphocytes express the angiotensin-converting enzyme 2 (ACE2) receptor on their surface [29]. Thus, SARS-CoV-2 infection may directly induce lysis of these cells. In addition, elevated levels of pro-inflammatory cytokines, may promote lymphocyte apoptosis.
D-dimer elevation on admission was more common in patients with AKI. This molecule is a product of cross-linked fibrin degradation and is a sensitive marker of thrombosis and coagulation activation [30]. Elevated D-dimer level has been consistently reported in patients with COVID-19 [31, 32], and its gradual increase during disease course is particularly associated with disease worsening [33]. Elevated C-reactive protein on admission was more common in patients with AKI. Higher C-reactive protein has been linked to unfavorable aspects of COVID-19 disease, such as ARDS development [34], higher troponin-T levels and myocardial injury [35], and death [36].
Troponin levels on admission were higher in the group with AKI. High-sensitivity cardiac troponin T (hs-cTnT) and cardiac troponin I (cTnI) have been associated with AKI and are useful plasma biomarkers of cardiac injury [37]. In patients with severe COVID-19, elevated troponin level might indicate cardiovascular stress resulting from direct SARS-CoV-2 infection of the heart [38], hemodynamic changes, or underlying cardiac injury and dysfunction [39].
The original study describing the furosemide stress test for prediction of AKI outcome reported an AUC of 0.87 in the first two hours, with a sensitivity of 87.1% and specificity of 84.1% [14]. That study was performed in a heterogeneous population of patients exposed to different nephrotoxic factors (nonsteroidal anti-inflammatory drugs, aminoglycosides, amphotericin, contrast, post-cardiac surgery and sepsis). We found lower values of AUC (0.681), sensitivity (81.6%) and specificity (54.5%). Differences between studies may be partially explained by the fact that we only included patients with sepsis related with SARS-CoV-2 infection. Despite these considerations, we deem that the furosemide stress test is an easy, non-invasive and accessible technique which may contribute to predict the severity of AKI in patients with SARS-CoV-2 infection. Larger, prospective validations of the furosemide stress test in patients with severe COVID-19 are required because improving risk prediction in those with early AKI would be of high value for patient care and clinical decision making.
The main limitation of our study was its retrospective design. Also, the number of patients included in the study was low. Another study limitation was that patients with incomplete clinical files or those who were transferred to other hospitals due to local saturation were not included in the study, and this may represent a selection bias.
Considering that standardized definitions of AKI are based on sCr and urine output [40], then inaccessibility to nursing records restricted to COVID-19 areas represents an important study limitation because urine output was not used for diagnosis of AKI, and sCr was not adjusted for fluid-balance. The lack of pre-hospital baseline sCr measurements was also a study limitation because baseline sCr values were an estimation. Since we could not assess the baseline renal status, we could not explore whether CKD itself is a risk factor for AKI in the context of COVID-19. One additional study limitation is that we retrieved information during hospitalization, but a longer observation period would have provided additional information regarding the clinical outcome and the impact of AKI in the population studied. That is, we were not able to report the proportion of individuals developing CKD in the group with AKI. Finally, our study was conducted at a national referral center for respiratory diseases receiving disproportionately more patients with severe COVID-19, and this represents a potential source of referral bias. Nevertheless, this particular cohort was suitable for identifying the risk factors for AKI in Mexican individuals with severe COVID-19.
Conclusions
AKI was common in our cohort of patients with severe pneumonia caused by SARS-CoV-2 infection. The risk factors for AKI were older age, obesity and requirement of IMV on admission. The risk factors for mortality were obesity, requirement of vasoactive drugs on admission and AKI. Mortality was more frequent in patients with AKI stages 2–3. The FST had an acceptable predictive capacity to identify patients progressing to AKI stage 3. Still, larger, prospective validations of the FST in patients with severe COVID-19 are required.
Supporting information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.World Health Organization. Novel coronavirus–China. Jan 12, 2020. Available at: https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ Accessed June 20, 2020.
- 2.World Health Organization. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. Jan 11, 2020. Available at: https://apps.who.int/iris/handle/10665/330893. Accessed June 20, 2020.
- 3.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. 10.1038/s41564-020-0695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Coronavirus disease (COVID-19) outbreak. https://www.who.int. Accessed June 20, 2020.
- 5.Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020. Available at: 10.1016/j.jacc.2020.03.031. Accessed June 27, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:428–430. 10.1016/S2468-1253(20)30057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall R, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. 10.1016/j.kint.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqu HK, Mehra MR. COVID-19 Illness in Native and Immunosuppressed States: A Clinical Therapeutic Staging Proposal. Journal of Heart and Lung Transplantation. 2020;39:405–407. 10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Published January 28, 2020. Available at: https://www.who.int/publicationsdetail/clinical-managementof-severe-acute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected. Accessed June 20, 2020.
- 12.Moore PK, Hsu RK, Liu KD. Management of Acute Kidney Injury: Core Curriculum 2018. Am J Kidney Dis. 2018; 72:136–48. 10.1053/j.ajkd.2017.11.021 [DOI] [PubMed] [Google Scholar]
- 13.KDIGO Clinical Practice Guideline for Acute Kidney Injury. Published March 2012. Available at: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf. Accessed: June 30, 2020.
- 14.Chawla LS, Davison DL, Brasha-Mitchell E, Koyner JL, Arthur JM, Shaw AD, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17:R207 10.1186/cc13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramirez-Sandoval JC, Gaytan-Arocha JE, Xolapa-Chávez P, Mejia-Vilet JM, Arvizu-Hernandez M, et al. Prolonged intermittent renal replacement therapy for acute kidney injury in COVID-19 patients with acute respiratory distress syndrome. Blood Purif. 2020;1–9. 10.1159/000510996 [DOI] [PubMed] [Google Scholar]
- 18.Argenziano MG, Bruce SL, Slater CL, Tiao JL, Baldwin MR, Chang RG, et al. Characterization and clinical course of 1000 Patients with COVID-19 in New York: retrospective case series. BMJ. 2020;369:m1996 10.1136/bmj.m1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;April 9;ciaa415 10.1093/cid/ciaa415 Epub ahead of print. 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Secretaría de Salud. Encuesta Nacional de Salud y Nutrición de Medio Camino 2016 (ENSANUT MC 2016). Secretaría de Salud e Instituto Nacional de Salud Pública. Available at: https://ensanut.insp.mx/encuestas/ensanut2016/index.php Accessed: June 25, 2020.
- 23.De Heredia FP, Gomez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332–338. 10.1017/S0029665112000092 [DOI] [PubMed] [Google Scholar]
- 24.Hegde V, Dhurandhar NV. Microbes and obesity-interrelationship between infection, adipose tissue and the immune system. Clin Microbiol Infect. 2013;19:314–320. 10.1111/1469-0691.12157 [DOI] [PubMed] [Google Scholar]
- 25.Seller-Pérez G, Más-Font S, Pérez-Calvo C, Villa-Díaz P, Celaya-López M, Herrera-Gutiérrez ME. Acute kidney injury: Renal disease in the ICU. Medicina intensiva. 2016;40:374–382. 10.1016/j.medin.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 26.Bellomo R, Wan L, May C. Vasoactive drugs and acute kidney injury. Critical care medicine. 2008; 36:S179–S186. 10.1097/CCM.0b013e318169167f [DOI] [PubMed] [Google Scholar]
- 27.Opina-Tascón GA, Hernández G, Álvarez I, Calderón-Tapia LE, Manzano-Núñez R, Sánchez-Ortíz AI, et al. Effects of very early start of norepinephrine in patients with septic shock: a propensity score-based analysis. 2020;24:52 10.1186/s13054-020-2756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu R, Ling Y, Zhang YH, Wei LY, Chen X, Li XM, et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020; 10.1002/jmv.25767 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Long Y, Xiao H, Yang J, Toulon P, Zhang Z. Use of D-dimer in oral anticoagulation therapy Int J Lab Hematol. 2018; 10.1111/ijlh.12864 10.1111/ijlh.12864. [DOI] [PubMed] [Google Scholar]
- 31.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95:834–47. 10.1002/ajh.25829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;e200950 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng Y, Liu W, Liu K, Fang YY, Shang J, Zhou L, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020; 133:1262–67. 10.1097/CM9.0000000000000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan Park K, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovascular Res. 2017;1708–1718. 10.1093/cvr/cvx183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bose R, McCarthy JR. Direct SARS-CoV-2 infection of the heart potentiates the cardiovascular sequelae of COVID-19. Drug discovery today 2020, 25:1559–1560. 10.1016/j.drudis.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh CR, Puthumana J, Shlipak MG, Koyner JL, Thiessen-Philbrook H, McArthur E, et al. Relationship of Kidney Injury Biomarkers with Long-Term Cardiovascular Outcomes after Cardiac Surgery. J Am Soc Nephrol. 2017;12:3699–3707. 10.1681/ASN.2017010055 10.1681/ASN.2017010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Rosa S, Samoni S, Ronco C. Creatinine-based definitions: from baseline creatinine to serum creatinine adjustment in intensive care. Critical Care. 2016; 20:69 10.1186/s13054-016-1218-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.