Abstract
Community acquired-pneumonia (CAP) has varying causative pathogens and clinical characteristics. This study investigated the prevalence of Mycoplasma pneumoniae (M pneumoniae) and evaluated the clinical characteristics in infected hospitalized children by disease severity.
From throat swabs of hospitalized children (5 months to 14 years) with CAP collected between November 2017 and May 2018, M pneumoniae and other CAP pathogens were identified using polymerase chain reaction (PCR). Differences in clinical and laboratory test data were compared between severe and mild case groups.
Of 333 hospitalized children enrolled, 221/333 (66.4%) tested positive for M pneumoniae and 24/221 (10.9%) patients were (n = 9, aged <5 years vs n = 15, ≥5 years) single infection by PCR, however, only 170/333 (51.1%) patients were presented with M pneumoniae IgM-positive. M pneumoniae detection rate by PCR was higher than by immunoglobulin (IgM) serology. In 123/221 (55.7%) M pneumoniae infected patients, coinfection with bacterial pathogens (n = 61, <5 years vs n = 62, ≥5 years) occurred. Children (aged 3–8 years) had most M pneumoniae infection. Severe M pneumoniae pneumonia (MPP) in children occurred mostly in older age (7 [interquartile ranges {IQR}, 6–8] years; P < .0001), with longer cough days (14 [IQR, 10–19.5] days; P = .002) and hospitalization duration (9.5 [IQR, 7–12.3] days; P < .0001), lower lymphocyte ratio (24.1, [IQR, 20.0–31.1] %; P = .001), higher neutrophils ratio (66.0, [IQR, 60.2–70.3]%; P < .0001), and serum C-reactive protein (CRP) level (3.8, [IQR, 1.3–10.9] mg/L; P = .027).
M pneumoniae is the most commonly detected pathogen in CAP. High coinfection prevalence increases diagnosis difficulty by clinically nonspecific characteristics. M pneumoniae detection by PCR with IgM may improve precise and reliable diagnosis of community-acquired MPP.
Keywords: clinical characteristics, coinfection, community acquired-pneumonia, hospitalized children, Mycoplasma pneumoniae
1. Introduction
Community-acquired pneumonia (CAP), a leading infectious disease, is caused by multiple pathogens.[1] Bacteria usually cause “typical pneumonia,” while “atypical pathogens” including Mycoplasma pneumoniae (M pneumoniae), Chlamydophilia pneumoniae (C pneumoniae), and Legionella pneumophila (L pneumophila) cause “atypical pneumonia.” These 3 pathogens account for 21% to 28% of adult CAP worldwide.[2,3]
M pneumoniae is a major pathogen causing respiratory tract infection and atypical pneumonia at any age.[4]M pneumoniae infections remain one of the most common causes of CAP, but the prevalence of M pneumoniae is usually underestimated because in patients with pneumonia caused by M pneumoniae is usually asymptomatic. Hence, M pneumoniae pneumonia (MPP) is considered to be the “walking pneumonia.”[4] A prospective study of pathogens associated with pneumonia in adults (aged ≥19 years) in 7 cities (Beijing, Shanghai, Shenyang, Xi’an, Chengdu, Guangzhou, and Hangzhou) in China indicated M pneumoniae is the major causative agent of CAP with a prevalence rate of up to 20.7%.[5,6] Prevalence of M pneumoniae in hospitalized children with acute respiratory infection showed M pneumoniae to be the dominant pathogen with the highest detection rate (56.9%) among 10,435 specimens in Hubei, between May 2010 and April 2012.[7] Another study also indicated M pneumoniae as the most predominant pathogen with a positive rate of 40.78% among 1204 children aged 4 to 14 years, between August 2011 and August 2013 in Nanjing, China.[8]
MPP usually presents with fever, cough, diarrhea, and other non-specific symptoms,[9,10] however, MPP remains largely underdiagnosed. Lack of diagnostic test methods and other infections which either coexist or mimic M pneumoniae limit the understanding of the burden and epidemiology of hospitalized MPP. Case-control data indicate that 93.0% of cases and 74.1% of controls have >1 detected microorganisms in children <5 years with pneumonia.[11] Thus, determination of the etiological profile of MPP and the relationship with clinical features may provide information for improving the management and treatment of MPP.
This study aimed to determine the prevalence of M pneumoniae in hospitalized children with CAP at the No. 2 Clinical Teaching Hospital affiliated to Chengde Medical University and to evaluate differences in clinical characteristics of hospitalized children with MPP between severe and mild case groups.
2. Materials and methods
2.1. Study patients
A total of 333 hospitalized children between 5 months and 14 years who were admitted to the No.2 Clinical Teaching Hospital (affiliated to Chengde Medical University [Chengde, China]) with CAP were enrolled from November 2017 to May 2018.
2.2. Samples and clinical data collection
Throat swabs were collected from enrolled hospitalized children with CAP and stored in 2 mL germ free physiological saline. A total of 333 throat swabs were collected. The fresh samples were stored at –25 °C. Medical records of enrolled hospitalized children were reviewed and general clinical data including admission number, month of admission, sex, age, distribution of residence, use of antibiotics were also collected. Additional clinical data associated with disease severity such as: peak of fever, duration of fever, and duration of hospitalization were also collected. The laboratory data of white blood cell (WBC) count, neutrophils count, lymphocytes count, concentration of C-reactive protein (CRP), concentration of lactate dehydrogenase (LDH) were collected. The enrolled patients were divided into 2 groups: based on age (<5 years and ≥5 years), and disease severity (severe case group and mild case group), and the clinical data were compared. Written informed consent was obtained from the guardians of all study subjects and the study design was approved by Chengde Medical University (No.2017020).
2.3. Radiological assessment
Chest radiographs (chest x-ray or computed tomography scan) of the hospitalized children were done upon admission and the results were interpreted by 2 professional radiologists, and the final assessments were made by consensus of 2 pediatricians in accordance with the standardized definition of radiological pneumonia.[12]
2.4. Nucleotide extraction
DNA was extracted from 300 μL of throat swabs using QIAamp DNA mini kit (QIAGEN, Hilden, Germany). Viral DNA and RNA were extracted from 200 μL of throat swabs using Viral Nucleic Acid Extraction Kit II (Geneaid, New Taipei, China Taiwan). All processes were done according to the manufacturer's instructions. The nucleic acids were stored at –40 °C until further detection of bacterial or viral pathogens.
2.5. Detection of M pneumoniae by PCR
The 16S rDNA was amplified as the target gene of M pneumoniae by PCR amplification. The Mpn primer 1 (AAGGACCTGCAAGGGTTCGT) and Mpn primer 2 (CTCTAGCCATTACCTGCTAA) were as previously described.[13] The PCR amplification was conducted using 2×Easy-Load PCR Master Mix (Beyotime, Shanghai, China) in a final volume of 20 μL with 5 μL template DNA to generate 277 bp amplification fragment. The condition of PCR amplification was as follows: initial denaturation at 95 °C for 4 minutes; 40 cycles of denaturation 1 minute at 95 °C, annealing 1 minute at 60 °C, and extension 1.5 minutes at 72 °C, finally extension 10 minutes at 72 °C. The PCR products were screened using agarose gel electrophoresis with NA-Red staining (Beyotime, Shanghai, China) and the positive PCR products were sequenced by Tianyihuiyuan Co., Ltd. (Beijing, China).
2.6. Diagnostic for other respiratory pathogens
Bacterial pathogens including C pneumoniae,[14]L pneumophila,[15] group A Streptococcus (GAS),[16]Klebsiella pneumoniae (K pneumoniae),[17]Staphylococcus aureus (S aureus),[18]Pseudomonas aeruginosa (P aeruginosa),[19]Haemophilus influenzae (H influenzae)[20] were detected by PCR or real-time PCR. Additionally, viral pathogens including influenza A/B/C viruses[21]; parainfluenza virus (PIV) 1, 2, and 3[22]; adenovirus (AdV)[23]; human bocavirus (HBoV)[24]; human rhinovirus (HRV)[25]; human metapneumovirus (hMPV)[26]; respiratory syncytial virus (RSV)[21]; human coronavirus (HCoV)[27] were also detected by polymerase chain reaction or reverse transcription-polymerase chain reaction. The primers are described in the reference articles.
2.7. Immunoglobulin determination for M pneumoniae
The presences of M pneumoniae immunoglobulin (IgM) in peripheral blood of hospitalized children with CAP were tested by using the IgM-specific detection kit (VIRCELL, Granada, Spain). Nine pathogens (L pneumophila, M pneumoniae, Q-fever Rickettsia burneti, C pneumoniae, AdV, RSV, influenza A viruses [Flu A], influenza B viruses [Flu B], PIV 1-3) were tested for by indirect immunofluorescence assays (IFA). Tests and interpretation of results were in accordance with the manufacturer's instructions.
2.8. Case definitions and enrollment criteria
Patients having the following presentations were defined as having CAP, such as fever and body temperature >37.8 °C, cough, rales, or the presence of consolidation, infiltrate and pleural effusion supported by radiographic evidence. Hospitalized children with positive for M pneumoniae IgM or nucleic acid by PCR were defined as MPP. Patients who met one of the following criteria were defined as severe MPP, such as necrotizing pneumonia, dysfunction in respiratory tract or complication in other systems, complicated bronchiolitis obliterans, complicated systemic inflammatory response syndrome, less therapeutic response to single macrolides, persistent cough, and lung rale >1 week. Patients were excluded from this study if the age at hospitalization was ≤28 days, or ≥15 years; diagnosed with malignant tumor, or other pulmonary illness; or patients’ history was missing or incomplete.
M pneumoniae CAP was confirmed if the sample was positive for M pneumoniae PCR or IgM. With negative M pneumoniae PCR or IgM, the patient was considered to have CAP without M pneumoniae. With positive PCR detection of bacterial or viral pathogens, the patient was considered to have bacterial or viral CAP. Coinfection of M pneumoniae CAP was defined as the patient with ≥1 other bacterial or viral CAP-associated pathogen.
2.9. Statistical analysis
The database was generated in Microsoft Excel 2010 (Microsoft Corporation, CA). The categorical variables were presented as counts and percentages, and the continuous variables were described as median and interquartile ranges (IQR). The chi-squared test or Fisher exact test was used in comparisons of categorical variables. The Mann–Whitney U test was used to compare the difference of continuous variables. The significant difference was considered as the level of P < .05 with 2-tailed test. All statistical analyses were performed with the IBM SPSS Statistics 25.0 software package (IBM corp., Armonk, NY).
3. Results
3.1. Prevalence of M pneumoniae
Among the 333 hospitalized children with CAP, a total of 221/333 (66.4%) patients were positive for M pneumoniae genomic DNA by PCR detection, however, only 170/333 (51.1%) patients were presented with M pneumoniae IgM. The monthly distribution of M pneumoniae occurred throughout the study period from November 2017 to May 2018 and was peaked at December 2017 (Fig. 1A). A total of 115/221 (52.0%) male patients were infected with M pneumoniae detected by PCR, but there only 69/170 (40.6%) male patients were M pneumoniae IgM-positive. A total of 106/221 (48.0%) and 101/170 (59.4%) female patients were M pneumoniae PCR-positive and M pneumoniae IgM-positive, respectively (Fig. 1B). The residence distribution of patients showed that 186/221 (84.2%) and 145/170 (85.3%) patients infected with M pneumoniae detected by PCR and IgM were distributed in urban region of Chengde (Fig. 1C). Patients (age ranged 5 months to 13 years) were infected with M pneumoniae with most being 3 to 8 years of age, especially hospitalized children aged 3 to 4 years of age (Fig. 1D).
Figure 1.
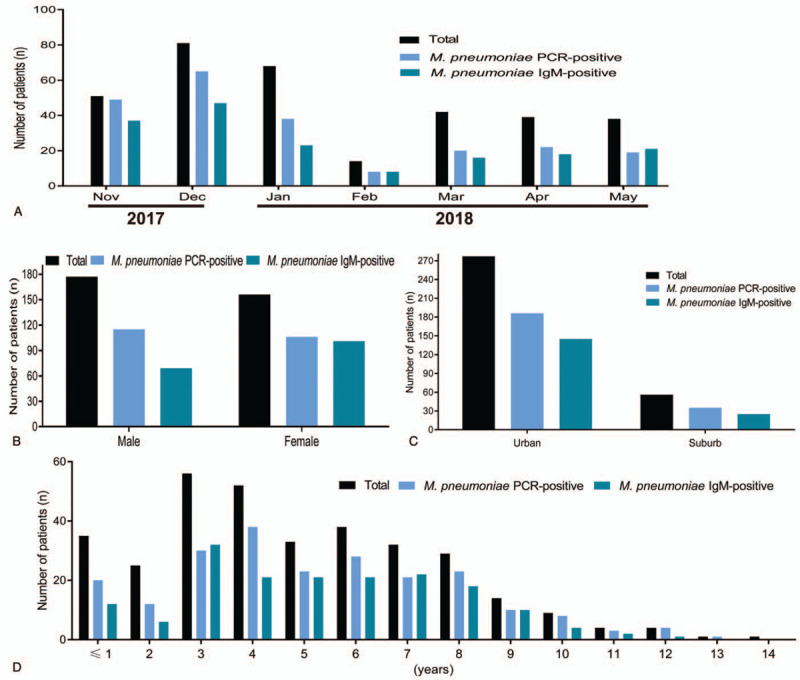
Prevalence of M pneumoniae in hospitalized children with CAP detected by PCR and IgM test. (A) Monthly distribution of M pneumoniae during 2017 to 2018; (B) gender distribution of M pneumoniae; (C) residence distribution of M pneumoniae; (D) age distribution of M pneumoniae. CAP = community acquired-pneumonia.
A total of 125/333 (37.5%) patients were positive with M pneumoniae both detection of PCR and IgM, 45/333 (13.5%) patients were positive for IgM of M pneumoniae but were PCR negative, however, 96/333 (28.8%) patients were positive for M pneumoniae with detection of PCR but were IgM negative, and 67/333 (20.1%) patients were both negative for M pneumoniae both detection of PCR and IgM (Table 1).
Table 1.
Sensitivity comparison between PCR and serology in detection of M pneumoniae.
| Method | PCR positive | PCR negative | Total |
| Serology positive, n (%) | 125 (37.5) | 45 (13.5) | 170 (51.1) |
| Serology negative, n (%) | 96 (28.8) | 67 (20.1) | 163 (48.9) |
| Total | 221 (66.4) | 112 (33.6) | 333 (100) |
3.2. Epidemiology of M pneumoniae infection
The seasonal distribution of hospitalized children with MPP indicated that 160/221 (72.4%) patients were infected with M pneumoniae from November 2017 to February 2018; however, 61/221 (27.6%) patients were infected with M pneumoniae from March 2018 to May 2018 (Table 2). These results indicated that the prevalence rate of M pneumoniae in hospitalized children was higher in winter than spring. The patients were divided into 2 groups, 100/221 (45.2%) patients infected with M pneumoniae were <5 years, and 121/221 (54.8%) patients infected with M pneumoniae were ≥5 years (Table 3).
Table 2.
Seasonal distribution of M pneumoniae in hospitalized children with MPP.
| Oneset season | Total | Positive | Negative |
| Nov–Feb, n (%) | 214 | 160 (72.4) | 54 (48.2) |
| Mar–May, n (%) | 119 | 61 (27.6) | 58 (51.8) |
| Total | 333 | 221 | 112 |
Table 3.
Age distribution of M pneumoniae in hospitalized children with MPP.
| Age group | Total | Positive | Negative |
| <5 years, n (%) | 168 | 100 (45.2) | 68 (60.7) |
| ≥5 years, n (%) | 165 | 121 (54.8) | 44 (39.3) |
| Total | 333 | 221 | 112 |
3.3. Coinfections
A total of 24/221 (10.9%) patients were single infection among 221 patients positive for M pneumoniae infection (P = .419). Mixed infections (including C pneumoniae, viral and bacterial coinfections) were identified in 197/221 (89.1%) of studied cases. Bacterial and viral coinfection were found in 123/221 (55.7%) and 12/221 (5.4%) of cases, respectively. Viral/bacterial coinfection with M pneumoniae were identified in 59/221 (26.7%) cases (Table 4).
Table 4.
Age distribution of bacterial and viral pathogens coinfected with M pneumoniae.
| <5 yearsn = 100n (%) | ≥5 yearsn = 121n (%) | Totaln = 221n (%) | P | OR (95%CI) | |
| M pneumoniae | 9 (9.0) | 15 (12.4) | 24 (10.9) | .419 | 0.699 (0.292–1.673) |
| M pneumoniae + C pneumoniae | 0 (0) | 3 (2.5) | 3 (1.4) | .253 | – |
| M pneumoniae + bacteria∗ | 61 (61.0) | 62 (51.2) | 123 (55.7) | .146 | 1.488 (0.870–2.547) |
| M pneumoniae + virus¶ | 5 (5.0) | 7 (5.8) | 12 (5.4) | .798 | 0.857 (0.264–2.788) |
| M pneumoniae + bacteria + virus | 25 (25.0) | 34 (28.1) | 59 (26.7) | .604 | 0.853 (0.467–1.557) |
3.4. Clinical characteristics
Severe cases were older than mild cases (median, 7 [IQR, 6–8] years vs 4 [IQR, 3–7] years; P < .0001). The severe cases were present with higher peak of fever (39.6, [IQR, 39.1–40.0] °C; P < .0001); longer duration of fever (8, [IQR, 5–10] days; P < .0001), cough (15, [IQR, 12–21] days; P < .0001), and hospitalization (11, [IQR, 9–14] days; P < .0001) compared with mild cases. The severe cases with lower WBC count (7.4, [IQR, 6.4–8.8] × 109 cells/L; P = .003) and absolute lymphocyte count (23.8, [IQR, 18.1–29.7]%; P < .0001), but with higher absolute neutrophil count (66.0, [IQR, 60.7–70.6]%; P < .0001) and serum CRP level (3.1, [IQR, 1.3–12.0] mg/L; P < .0001) (Table 5).
Table 5.
Clinical characteristics of hospitalized children with CAP between severe and mild case groups.
| Severe cases(n = 40) | Mild cases(n = 293) | P | |
| Demographic and clinical presentation | |||
| Males, n (%) | 18 (45.0) | 159 (54.3) | .271 |
| Age (yrs), median (IQR) | 7 (6–8) | 4 (3–7) | <.0001 |
| Peak of fever (°C), median (IQR) | 39.6 (39.1–40.0) | 39.2 (38.8–39.7) | <.0001 |
| Duration of fever (days), median (IQR) | 8 (5–10) | 5 (3.5–8) | <.0001 |
| Cough (days), median (IQR) | 15 (12–21) | 11 (9–14) | <.0001 |
| Rales, n (%) | 18 (45.0) | 135 (46.1) | .898 |
| Clinical outcome | |||
| Duration of hospitalization (days), median (IQR) | 11 (9–14) | 6 (5–8) | <.0001 |
| Laboratory data | |||
| WBC (×109 cells/L), median (IQR) | 7.4 (6.4–8.8) | 7.7 (5.9–10.7) | .003 |
| Neutrophils (%), median (IQR) | 66.0 (60.7–70.6) | 58.2 (47.0–67.2) | <.0001 |
| Lymphocytes (%), median (IQR) | 23.8 (18.1–29.7) | 30.0 (21.8–40.9) | <.0001 |
| CRP (mg/L), median (IQR) | 3.1 (1.3–12.0) | 2.2 (0.6–8.7) | <.0001 |
| LDH (U/L), median (IQR) | 252 (226–312) | 263 (227–318) | .330 |
Severe MPP cases were older than the mild MPP cases (7 [IQR, 6–8] years vs 4 [IQR, 3–7] years; P < .0001). Duration of fever (7 [IQR, 4.8–10] days; P = .011), cough (14 [IQR, 10–19.5] days; P = .002), and duration of hospitalization (9.5 [IQR, 7–12.3] days; P < .0001) were longer in severe MPP cases. The severe MPP cases with lower ratio of lymphocytes (24.1 [IQR, 20.0–31.1] %; P = .001), but with higher ratio of neutrophils (66.0, [IQR, 60.2–70.3]%; P < .0001) and serum CRP level (3.8, [IQR, 1.3–10.9] mg/L; P = .027) (Table 6).
Table 6.
Clinical characteristics of hospitalized children with MPP between severe and mild case groups.
| Severe casesn = 33 | Mild casesn = 188 | P | |
| Demographic and clinical presentation | |||
| Males, n (%) | 16 (48.5) | 99 (52.7) | .658 |
| Age (years), median (IQR) | 7 (6–8) | 4 (3–7) | <.0001 |
| Peak of fever (°C), median (IQR) | 39.5 (39.0–40.0) | 39.2 (38.8–39.6) | .061 |
| Duration of fever (days), median (IQR) | 7 (4.8–10) | 5 (3–7) | .011 |
| Cough (days), median (IQR) | 14 (10–19.5) | 10 (8–13) | .002 |
| Rales, n (%) | 16 (48.5) | 99 (52.7) | .658 |
| Clinical outcome | |||
| Duration of hospitalization (days), median (IQR) | 9.5 (7–12.3) | 6 (5–7) | <.0001 |
| Laboratory data | |||
| WBC (×109cells/L), median (IQR) | 7.7 (6.5–9.0) | 7.3 (5.6–10.7) | .717 |
| Neutrophils (%), median (IQR) | 66.0 (60.2–70.3) | 57.4 (46.5–66.9) | <.0001 |
| Lymphocytes (%), median (IQR) | 24.1 (20.0–31.1) | 30.5 (23.1–41.1) | .001 |
| CRP (mg/L), median (IQR) | 3.8 (1.3–10.9) | 1.7 (0.5–6.8) | .027 |
| LDH (U/L), median (IQR) | 251 (219–306) | 261 (226–313) | .672 |
4. Discussion
In this study, we investigated the prevalence and clinical characteristics of hospitalized children infected with M pneumoniae. Our results showed that M pneumoniae was the dominant pathogen with different clinical characteristics of hospitalized children with MPP between severe and mild case groups, such as age, duration of fever, cough, duration of hospitalization, neutrophils, lymphocytes, and serum CRP level.
The etiology studies of acute respiratory tract infections (ARTI) in Hubei province showed that M pneumoniae were the leading causative agent in children with ARTI at Renmin Hospital of Wuhan University between May 2010 and April 2012,[7] and at Wuhan Children's Hospital between October 2010 and September 2012.[28] Children with ARTI in Guangzhou Women and Children's Medical center showed that M pneumoniae (33.15%) was also the top pathogen between 2011 and 2012.[29]M pneumoniae (40.78%) was the dominant pathogen in children with CAP at Zhongda Hospital in Nanjing between August 2011 and August 2013.[8]M pneumoniae (38.47%) was also the dominant pathogen at the affiliated Hospital of Southwest Medical University in Luzhou from July 2013 to December 2016.[30] In our study, 221 children were positive for M pneumoniae. Meanwhile, H influenzae and GAS were (209 children were positive for H influenzae and 125 children were positive for GAS) the 2 leading bacterial pathogens. hMPV and HBoV (80 cases were infected with hMPV, and 22 cases were infected with HBoV) were the 2 dominant viral pathogens.[31] Our study indicated that M pneumoniae is the dominant pathogen of hospitalized children with CAP in Chengde, China between 2017 and 2018. The key finding of this study is the increasing prevalence of M pneumoniae in hospitalized children with CAP.
M pneumoniae is usually associated with infections in school-aged children and young adults,[32] however, the epidemiology and disease burden of hospitalized children with CAP caused by M pneumoniae is poorly understood. Currently, M pneumoniae is detected mainly by using the presence of IgM at admission or a ≥4-fold rise of IgG in serum.[10] Our results showed that 170 hospitalized children were positive for M pneumoniae IgM, however, 221 hospitalized children were M pneumoniae PCR-positive. M pneumoniae could be detected in asymptomatic children with a percentage of 21.2%,[33]M pneumoniae (0.6%) was also detected in asymptomatic control samples by PCR from hospitalized patients with radiographically confirmed pneumonia.[34] The studies on the prevalence of M pneumoniae also used the upper respiratory tract pathogens panel detection kit to test the presence of IgM against other pathogens, this method had confirmed that M pneumoniae was the dominant pathogen in children with respiratory tract infections in the cities of mainland China, such as Wuhan,[7,28] Nanjing,[8] and Luzhou.[30] We found the positive rate of M pneumoniae detected by PCR was higher than IgM. Thus, our research indicated that PCR should be the gold standard for diagnosis of M pneumoniae although its usage is not widespread in hospitals in mainland China yet. We also support the opinion proposed by previous report[34] that M pneumoniae is likely as the causative pathogen in hospitalized children with pneumonia when detected by PCR.
Our results showed that multiple pathogens (5 viruses and 4 bacteria) were coinfected with M pneumoniae among the hospitalized children with CAP. M pneumoniae coinfected with viral pathogens (RSV, AdV, PIV, and FluB)[7,10,35] or bacterial pathogen (S pneumoniae)[36] were also detected. At hence, we conclude that double and triple infections with M pneumoniae are common events.[11]
Previous study showed that children aged 2 to 5-year-old were more susceptible to M pneumoniae,[30] other study also confirmed that children >1-year-old were more prone to M pneumoniae, especially children aged 3 to 6-year-old.[7] Our results showed that children aged ≥5 years had higher detection rate of M pneumoniae than children <5 years, but children aged 3 to 8 years were the major target for M pneumoniae. The monthly distribution of M pneumoniae showed that October 2010 and October 2011 to December 2011 were the epidemic peak period,[32] however, another study showed that summer (June) and autumn (September) had high positive percentage of M pneumoniae.[8] One study showed the prevalence of M pneumoniae was relatively increased from November to January annually and peaked in winter (November 2013, December 2014 and 2015).[30] Our results showed that monthly distribution of M pneumoniae was observed throughout November 2017 and May 2018. Our result also confirmed that winter was high prevalence time of M pneumoniae and that it peaked in December 2017.
In this study, we compared the clinical features of MPP children between severe and mild case groups, we found the severe MPP cases had longer duration of hospitalization (9.5 days), higher neutrophils (66.0%), lower lymphocytes (24.1%), and higher serum CRP level (3.8 mg/L) compared with mild MPP cases. Clinical characteristics of children infected with M pneumoniae indicated that the children usually had more frequent cough, headache, and wheezing compared with non-M pneumoniae infected children, but the WBC, platelet, and CRP levels had no significant difference between the study groups.[37] Other study indicated M pneumoniae PCR-positive children usually presented with the following clinical presentation, such as fever, fatigue, chills, headache, sore throat, wheezing, runny nose, abnormal pain, chest pain. Examination findings included decreased breath sounds, rales, chest indrawing, rhonchi, and wheezing, however, laboratory test such as abnormal WBC count, abnormal platelet count, and hyponatremia had no significant difference between the study groups.[34] So, the clinicians could not distinguish M pneumoniae from viral or bacterial pneumonia just judging by clinical symptoms, signs, and the radiographic tests. We recommend using serology test combined with PCR assay for diagnosing hospitalized children with pneumonia, this will improve the quality of treatment and decrease the disease burden of patients.
This study was limited by the small number of samples (total 333 samples were collected from November 2017 to May 2018), which may have resulted in some loss in prevalence of M pneumoniae in hospitalized children throughout the year. Additionally, further studies such as using P1 gene or multilocus variable-number tandem repeat analysis (MLVA) to determine the genotypes of M pneumoniae, and determining antibiotic resistance especially macrolide-resistant M pneumoniae or minimum inhibitory concentrations of macrolides, are needed to conduct. Although this study had some above limitations, these limitations also point out our works’ focuses in the next step.
5. Conclusion
In conclusion, our study shows that M pneumoniae is the dominant pathogen of hospitalized children with CAP and winter is the peak prevalence time. We recommend that the use of PCR detection method may further improve etiological diagnosis of M pneumoniae where IgM detection is currently used.
Acknowledgments
The authors thank all enrolled hospitalized children and guardians in this study. Also, we thank all doctors and nurses who have participated in this study.
Author contributions
Conceptualization: Guang-Cheng Xie, Jian-Ying Liu, Luan-Ying Du.
Data curation: Meng Su, Qian Wang, Ling-Ling Wang, Dan Li.
Formal analysis: Meng Su, Qian Wang, Ling-Ling Wang, Dan Li, Chun-Yang Wang.
Investigation: Meng Su, Qian Wang, Ling-Ling Wang, Dan Li.
Methodology: Meng Su, Qian Wang, Ling-Ling Wang, Dan Li, Jiang-Li Wang, Qing Zhang.
Project administration: Guang-Cheng Xie, Chun-Yang Wang.
Supervision: Guang-Cheng Xie, Luan-Ying Du.
Validation: Jiang-Li Wang, Qing Zhang.
Writing – original draft: Meng Su, Qian Wang.
Writing – review & editing: Guang-Cheng Xie, Jian-Ying Liu, Chun-Yang Wang.
Footnotes
Abbreviations: AdV = adenovirus, ARTI = acute respiratory tract infection, CAMP = community-acquired mycoplasma pneumonia, CAP = community acquired-pneumonia, CRP = C-reactive protein, Flu A = influenza A virus, Flu B = influenza B virus, GAS = group A Streptococcus, HBoV = human bocavirus, HCoV = human coronavirus, hMPV = human metapneumovirus, HRV = human rhinovirus, IFA = indirect immunofluorescence assay, IQR = interquartile range, LDH = lactate dehydrogenase, MPP = M pneumoniae pneumonia, PIV = parainfluenza virus, RSV = respiratory syncytial virus, WBC = white blood cell.
How to cite this article: Su M, Wang Q, Li D, Wang LL, Wang CY, Wang JL, Zhang Q, Du LY, Liu JY, Xie GC. Prevalence and clinical characteristics of hospitalized children with community-acquired Mycoplasma pneumoniae pneumonia during 2017/2018, Chengde, China. Medicine. 2021;100:5(e23786).
MS and QW have contributed equally to the article.
This work was supported by Youth Science Fund Project of National Natural Science Foundation of China (81702008, 81702010), Youth Science Fund Project of Natural Science Foundation of Hebei Province (H2018406024), Foundation for High-level Talents of Chengde Medical University (201702), Program of Shanxi Respiratory Project Center (No. 2017GCKF04).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
MPP = M pneumoniae pneumonia.
MPP = M pneumoniae pneumonia.
Bacteria including H influenzae, GAS, K pneumoniae, S aureus.
Virus including RSV, hMPV, HBoV, HCoV, AdV.
LDH = lactate dehydrogenase.
CRP = C-reactive protein; LDH = lactate dehydrogenase.
References
- [1].Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430–40. [DOI] [PubMed] [Google Scholar]
- [2].Arnold FW, Summersgill JT, Lajoie AS, et al. A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am J Respir Crit Care Med 2007;175:1086–93. [DOI] [PubMed] [Google Scholar]
- [3].Bartlett JG. Is activity against “atypical” pathogens necessary in the treatment protocols for community-acquired pneumonia? Issues with combination therapy. Clin Infect Dis 2008;47: suppl: S232–6. [DOI] [PubMed] [Google Scholar]
- [4].Bajantri B, Venkatram S, Diaz-Fuentes G. Mycoplasma pneumoniae: a potentially severe infection. J Clin Med Res 2018;10:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu Y, Chen M, Zhao T, et al. Causative agent distribution and antibiotic therapy assessment among adult patients with community acquired pneumonia in Chinese urban population. BMC Infect Dis 2009;9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Song JH, Thamlikitkul V, Hsueh PR. Clinical and economic burden of community-acquired pneumonia amongst adults in the Asia-Pacific region. Int J Antimicrob Agents 2011;38:108–17. [DOI] [PubMed] [Google Scholar]
- [7].Wu Z, Li Y, Gu J, et al. Detection of viruses and atypical bacteria associated with acute respiratory infection of children in Hubei, China. Respirology 2014;19:218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen K, Jia R, Li L, et al. The aetiology of community associated pneumonia in children in Nanjing, China and aetiological patterns associated with age and season. BMC Public Health 2015;15:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother 2010;16:162–9. [DOI] [PubMed] [Google Scholar]
- [10].Sun H, Chen Z, Yan Y, et al. Epidemiology and clinical profiles of Mycoplasma pneumoniae infection in hospitalized infants younger than one year. Respir Med 2015;109:751–7. [DOI] [PubMed] [Google Scholar]
- [11].Benet T, Sanchez PV, Messaoudi M, et al. Microorganisms associated with pneumonia in children < 5 years of age in developing and emerging countries: The GABRIEL Pneumonia Multicenter, Prospective, Case-Control Study. Clin Infect Dis 2017;65:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kumar S, Garg IB, Sethi GR. Mycoplasma pneumoniae in community-acquired lower respiratory tract infections. Indian J Pediatr 2018;85:415–9. [DOI] [PubMed] [Google Scholar]
- [14].Maraha B, Berg H, Kerver M, et al. Is the perceived association between Chlamydia pneumoniae and vascular diseases biased by methodology? J Clin Microbiol 2004;42:3937–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rafiee M, Jahangiri-Rad M, Hajjaran H, et al. Detection and identification of Legionella species in hospital water supplies through Polymerase Chain Reaction (16S rRNA). J Environ Health Sci Eng 2014;12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang HB, Song YY, You YH, et al. Molecular epidemiological analysis of group A Streptococci isolated from children in Chaoyang District of Beijing, 2011: emm types, virulence factor genes and erythromycin resistant genes. Biomed Environ Sci 2013;26:782–4. [DOI] [PubMed] [Google Scholar]
- [17].Kiratisin P, Apisarnthanarak A, Laesripa C, et al. Molecular characterization and epidemiology of extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates causing health care-associated infection in Thailand, where the CTX-M family is endemic. Antimicrob Agents Chemother 2008;52:2818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brakstad OG, Aasbakk K, Maeland JA. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol 1992;30:1654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Valadbeigi H, Tabatabaei RR, Malek A, et al. Genomic diversity and virulence genes among clinical isolates of Pseudomonas aeruginosa. Clin Lab 2014;60:363–7. [DOI] [PubMed] [Google Scholar]
- [20].Wang Y, Guo G, Wang H, et al. Comparative study of bacteriological culture and real-time fluorescence quantitative PCR (RT-PCR) and multiplex PCR-based reverse line blot (mPCR/RLB) hybridization assay in the diagnosis of bacterial neonatal meningitis. BMC Pediatr 2014;14:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Coiras MT, Perez-Brena P, Garcia ML, et al. Simultaneous detection of influenza A, B, and C viruses, respiratory syncytial virus, and adenoviruses in clinical samples by multiplex reverse transcription nested-PCR assay. J Med Virol 2003;69:132–44. [DOI] [PubMed] [Google Scholar]
- [22].Coiras MT, Aguilar JC, Garcia ML, et al. Simultaneous detection of fourteen respiratory viruses in clinical specimens by two multiplex reverse transcription nested-PCR assays. J Med Virol 2004;72:484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Allard A, Girones R, Juto P, et al. Polymerase chain reaction for detection of adenoviruses in stool samples. J Clin Microbiol 1990;28:2659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chung JY, Han TH, Kim CK, et al. Bocavirus infection in hospitalized children, South Korea. Emerg Infect Dis 2006;12:1254–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jin Y, Yuan XH, Xie ZP, et al. Prevalence and clinical characterization of a newly identified human rhinovirus C species in children with acute respiratory tract infections. J Clin Microbiol 2009;47:2895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Peiris JS, Tang WH, Chan KH, et al. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis 2003;9:628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Woo PC, Lau SK, Chu CM, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol 2005;79:884–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu J, Ai H, Xiong Y, et al. Prevalence and correlation of infectious agents in hospitalized children with acute respiratory tract infections in Central China. PLoS One 2015;10:e119170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lin ZF, Zhao MQ, Guo M, et al. The seroprevalence of some pathogen specific IgM in children with acute respiratory tract infections in Guangzhou Region, 2011-2012. Clin Lab 2015;61:917–24. [DOI] [PubMed] [Google Scholar]
- [30].Chen A, Song L, Chen Z, et al. Immunoglobulin M profile of viral and atypical pathogens among children with community acquired lower respiratory tract infections in Luzhou, China. BMC Pediatr 2019;19:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang Q, Su M, Li D, et al. Molecular prevalence and clinical characteristics of human metapneumovirus and human bocavirus in 333 hospitalized children with community-acquired pneumonia. Chinese J Exp Clin Virol 2019;33:261–6. [Google Scholar]
- [32].Sondergaard MJ, Friis MB, Hansen DS, et al. Clinical manifestations in infants and children with Mycoplasma pneumoniae infection. PLoS One 2018;13:e195288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Spuesens EB, Fraaij PL, Visser EG, et al. Carriage of Mycoplasma pneumoniae in the upper respiratory tract of symptomatic and asymptomatic children: an observational study. PLoS Med 2013;10:e1001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kutty PK, Jain S, Taylor TH, et al. Mycoplasma pneumoniae among children hospitalized with community-acquired pneumonia. Clin Infect Dis 2019;68:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Del VJ, Silva-Caso W, Cornejo-Tapia A, et al. Molecular etiological profile of atypical bacterial pathogens, viruses and coinfections among infants and children with community acquired pneumonia admitted to a national hospital in Lima, Peru. BMC Res Notes 2017;10:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen CJ, Lin PY, Tsai MH, et al. Etiology of community-acquired pneumonia in hospitalized children in northern Taiwan. Pediatr Infect Dis J 2012;31:e196–201. [DOI] [PubMed] [Google Scholar]
- [37].Medjo B, Atanaskovic-Markovic M, Radic S, et al. Mycoplasma pneumoniae as a causative agent of community-acquired pneumonia in children: clinical features and laboratory diagnosis. Ital J Pediatr 2014;40:104. [DOI] [PMC free article] [PubMed] [Google Scholar]


