Abstract
Our study aims to summarize the clinical characteristics of patients with severe or critically ill coronavirus disease 2019 (COVID-19).
Five databases were electronically searched to collect studies describing clinical characteristics of severe or critically ill COVID-19 patients and published between January 1, 2020 and April 12, 2020. Three reviewers independently collected the literature, extracted the required data, and assessed the risk of publication bias of the included studies before including the studies in the meta-analysis.
A total of 40 studies involving 2459 patients with severe or critically ill COVID-19 patients were included. Meta-analysis showed that a greater proportion of severe or critically COVID-19 patients were male (62.3%), and the 2 main clinical symptoms were fever (87.4%) and cough (66.3%). Other common clinical symptoms included dyspnea (45.3%), chest tightness (37.4%), fatigue (36.6%), and expectoration (31.9%). Minor symptoms included myalgia (19.5%), dizziness (11.5%), headache (11.4%), diarrhea (11.2%), pharyngalgia (11.0%), nausea, and vomiting (5.9%). Most patients showed elevated levels of C-reactive protein (83.5%) and D-dimer (73.3%), lymphopenia (70.3%), and normal leukocyte counts (56.9%). Other findings included abnormal levels of liver function (39.8%), elevated procalcitonin (36.6%), leukocytosis (21.7%), thrombocytopenia (19.0%), and leucopenia (18.2%). Most patients showed acute respiratory distress syndrome (60.8%). Other complications included acute cardiac injury (37.1%), shock (32.0%), and acute kidney injury (22.0%).
The most common symptoms of severe or critically ill COVID-19 patients were fever and cough. Most patients showed lymphopenia, elevated levels of C-reactive protein and D-dimer. A large percentage of patients progress to ARDS, acute cardiac injury, acute kidney injury and shock were also common.
Keywords: clinical characteristics, coronavirus disease 2019, critically ill, meta-analysis, severe
1. Introduction
Since December 2019, many cases of novel coronavirus-infected pneumonia (NCIP) have been detected in Wuhan, China. In a short period, NCIP quickly spread to the whole world. On January 30, World Health Organization (WHO) declared NCIP to be an international public health emergency.[1] And on February 11, the disease was named coronavirus disease 2019 (COVID-19).[2] At present, the epidemic of COVID-19 has become a global outbreak. As of April 7, 2020, a total of 1,279,122 confirmed cases and 72,614 deaths have been recorded globally.[3] The cumulative number of confirmed cases in Spain, Italy, Germany and some other countries have exceeded 100,000,[3] and the number even exceeded 330,000 in the United States.
Although most COVID-19 patients had mild symptoms and got better after symptomatic support treatment, once they develop into a severe illness, many patients will quickly progress to acute respiratory distress syndrome (ARDS) or even multiple organ dysfunction syndrome (MODS) and increase the risk of death.[4] Since the fatality rate of severe or critically ill patients was over 50%,[5] the control of the number of patients with severe or critically ill COVID-19 has become one of the focal points and difficulties in epidemic prevention and control. Therefore, it is very important to master the clinical characteristics of severe or critically ill COVID-19 patients in order to help in judging the trend of severe disease as early as possible.
Various studies have been published on the clinical characteristics of patients with severe or critically ill COVID-19,[6–9] however, most of them were single-center studies with a small sample size, and the reported results were not completely consistent. Therefore, we carried out this meta-analysis to collect the latest literatures to systematically analyze the clinical characteristics of patients with severe or critically ill COVID-19, so as to provide references for further research and clinical decisions.
2. Materials and methods
2.1. Search strategy and study eligibility
This meta-analysis was conducted based on the guidelines of the Preferred Reporting Items for Meta-Analyses of Observational Studies in Epidemiology (MOOSE) Statement.[10]
PubMed, Embase, WanFang, Chinese Biomedical Literature Database and China National Knowledge Infrastructure databases were electronically searched to collect studies describing clinical characteristics of severe or critically ill COVID-19 patients and published between January 1, 2020 and April 12, 2020. We also manually searched all the references of the included studies in order to identify eligible studies. If duplicate studies describing the same population, only the most detailed or recent study was included. There was no language restriction during the literature search, but we only included the literatures published online. The terms we used, both separately and in combination, included: “Coronavirus” OR “SARS-CoV-2” OR “2019-nCoV” OR “COVID-19” AND “severe” OR “critical” OR “critically ill” OR “icu care” OR “death”.
2.2. Inclusion and exclusion criteria
The inclusion criteria included:
-
1.
Case-control studies, cohort studies and case series studies;
-
2.
The study population were the confirmed cases of severe or critically ill COVID-19 patients. Those received ICU care, mechanical ventilation or death also included.
-
3.
The outcomes were clinical symptoms, laboratory findings and complications.
The exclusion criteria included:
-
1.
Overlapping studies or duplicate studies describing the same population;
-
2.
Studies with a sample size less than 20.
All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
2.3. Data extraction and quality assessment
Three reviewers independently collected the literature, and extracted the required data. Disagreements were resolved by discussion or consultation with another researcher. We screened the titles and abstracts firstly to identify the eligible studies. After that, we performed a full-text review to extract the detailed data. If necessary, we would contact the authors in order to collect further information. The data we extracted included: the surname of the first author and the publication time of the included studies, sample size, study design, study population, age and outcomes; relevant information of bias risk assessment. All of the included studies were observational studies, therefore, 3 reviewers independently evaluated study quality based on the guidelines of the British National Institute for Clinical Excellence (NICE).[11] We conducted the evaluation based on 8 criteria, and studies with a score greater than 4 were considered high-quality studies (total score = 8).
2.4. Statistical analyses
The statistical analyses were performed by using STATA (version 12). Firstly, the original incidence rates r was converted by double arcsine to conform to the normal distribution, and then the meta-analysis of transformed rate tr was carried out. Finally, the pooled incidence rates R and its 95%CI are obtained by converting the results with the formula R = [sin (tr/2)]2. The heterogeneity among studies was analyzed by using a Chi-Squared test (P < . 10) and quantified by using the I2 statistic. If there was no statistical heterogeneity among the results, the fixed effect model was used for meta-analysis. If statistical heterogeneity existed among the results, sensitivity analysis was used to explore the source of heterogeneity, and the random effect model was used for meta-analysis after the exclusion of obvious clinical heterogeneity. According to the funnel plot and the Egger and Begg tests to judge whether there was publication bias. A two-tailed P < .05 was considered statistically significant.
3. Results
3.1. Literature screening and assessment
A total of 2538 articles were identified after initial retrieval from databases, 277 additional records were identified from the Chinese Medical Journal Network. After removing the duplicates and screening carefully based on the inclusion criteria and exclusion criteria, 40 unique studies[6–9,12–47] involving 2459 patients with severe COVID-19 were included in this meta-analysis (Fig. 1).
Figure 1.
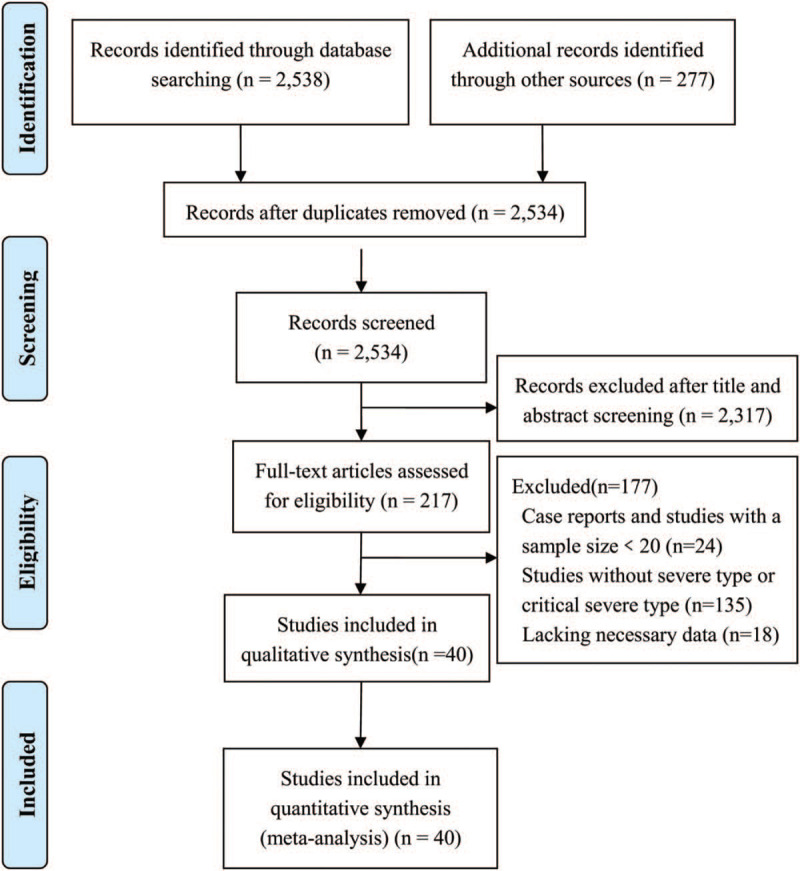
Flow chart of article screening.
3.2. Characteristics of included studies
A total of 40 retrospective studies[6–9,12–47] were included, including 25 in English and 15 in Chinese, which were published between February 7, 2020 and April 7, 2020. 38 of the studies were set in China, and the other 2 studies were set in America. All the included studies received quality scores of 6–8, and considered high-quality studies (Tab.1).
3.3. Meta-analysis results
3.3.1. Gender distribution
Relevant data regarding the clinical characteristics of 2459 COVID-19 patients were collected. Heterogeneity was significant across the included studies (I2 = 64.9%), therefore, we used a random-effects model in this meta-analysis. We found that 62.3% (95%CI 58.8–65.8) of the patients were male (Fig. 2).
Figure 2.
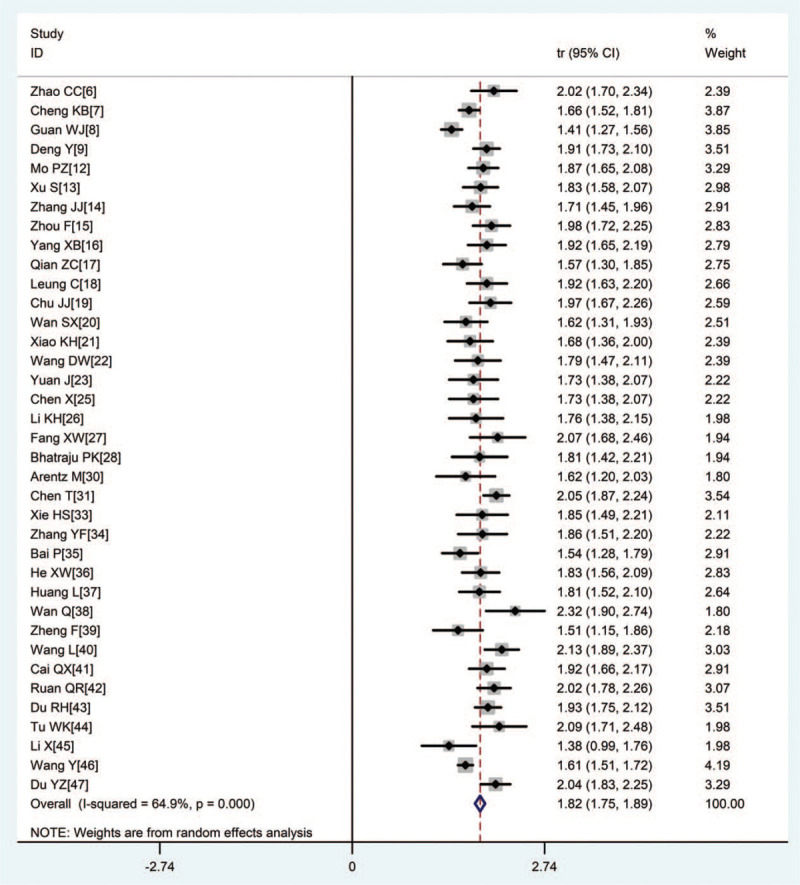
Forest plot of transformed proportion of male in severe or critically ill COVID-19 patients.
3.3.2. Clinical symptoms
Two main clinical symptoms prevalent among most patients were fever (87.4%) and cough (66.3%) (Figs. 3 and 4). Other common clinical symptoms included dyspnea (45.3%), chest tightness (37.4%), fatigue (36.6%), expectoration (31.9%), and myalgia (19.5%). Dizziness (11.5%), headache (11.4%), diarrhea (11.2%), pharyngalgia (11.0%), nausea, and vomiting (5.9%) occurred less frequently (Tab.2).
Figure 3.
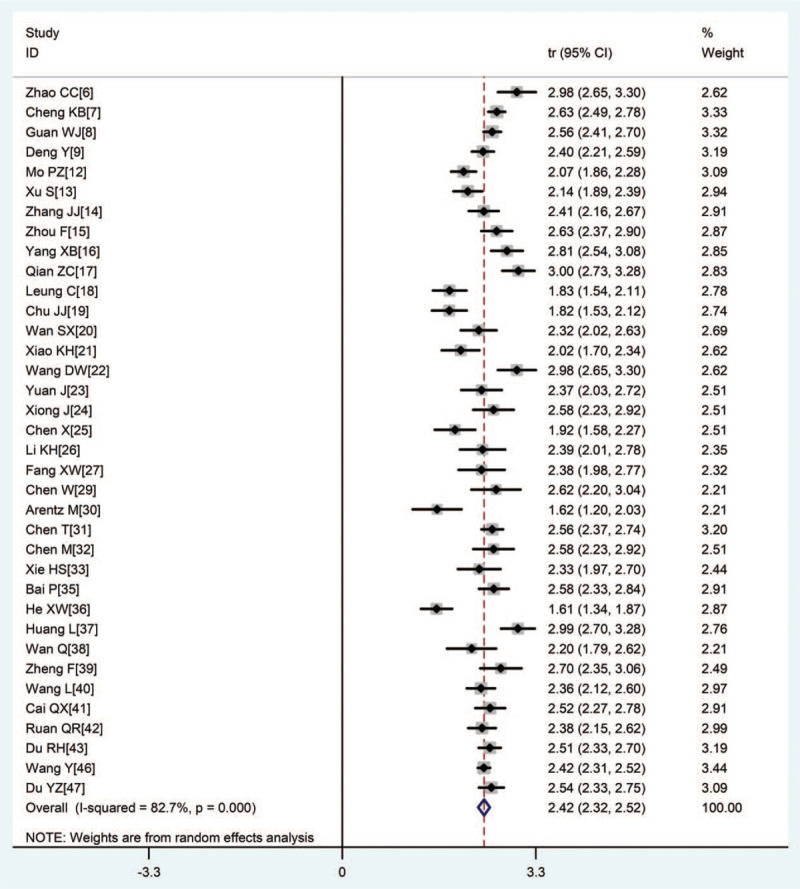
Forest plot of transformed incidence rate of fever in severe or critically ill COVID-19 patients.
Figure 4.
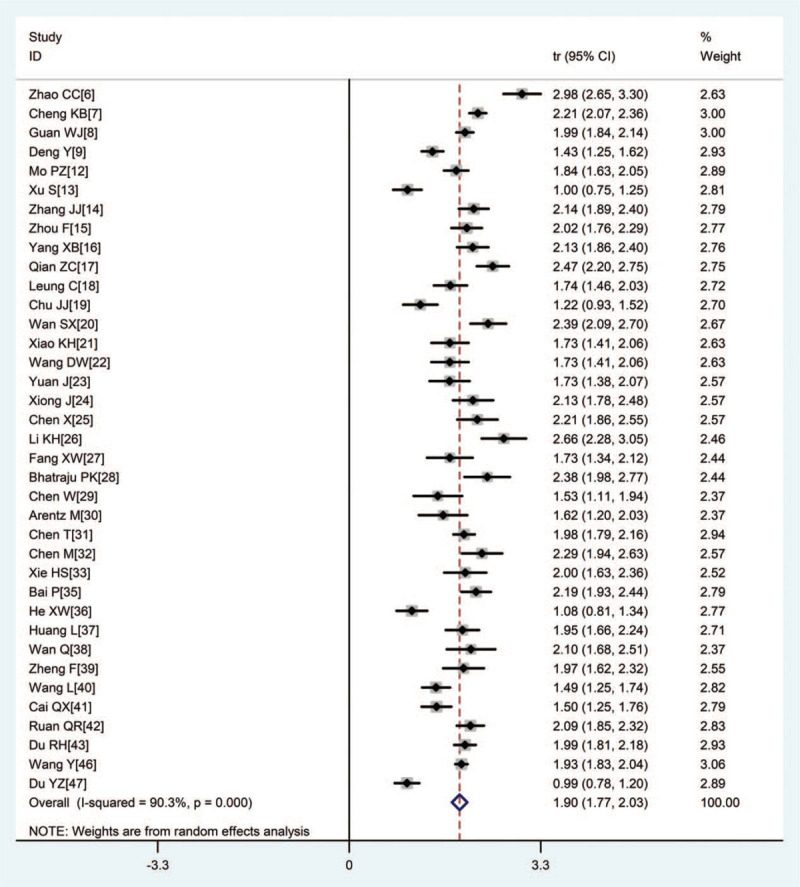
Forest plot of transformed incidence rate of cough in severe or critically ill COVID-19 patients.
3.3.3. Laboratory parameters
Most patients showed elevated C-reactive protein levels (83.5%), elevated D-dimer (73.3%), lymphopenia (70.3%), and normal leukocyte counts (56.9%). Other findings included abnormal levels of liver function (39.8%), elevated procalcitonin (36.6%), leukocytosis (21.7%), thrombocytopenia (19.0%), and leucopenia (18.2%) (Tab.3).
3.3.4. Complications
Most patients occurred acute respiratory distress syndrome (60.8%). Other complications included acute cardiac injury (37.1%), shock (32.0%), and acute kidney injury (22.0%) (Tab.3).
3.3.5. Sensitivity analysis
A sensitivity analysis was carried out by excluding each study one by one and reanalyzing the entire dataset. The pooled incidence rates did not change substantially, indicating that the results of this study were reliable and stable. Sensitivity analysis of the incidence rate of dyspnea in severe or critically ill COVID-19 patients were shown in Fig. 5.
Figure 5.
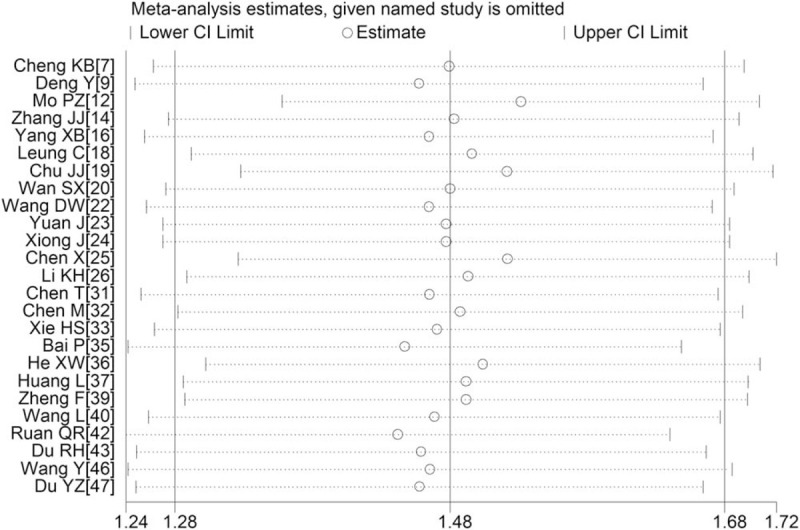
Sensitivity analysis of the incidence rate of dyspnea in severe or critically ill COVID-19 patients.
3.4. Evaluation of publication bias
The p values derived using the Egger and the Begg test for all outcomes showed no obvious publication bias (Tab.4). A funnel plot based on the incidence rate of fever showed p values of 0.022 in Egger test and 0.733 in Begg test (Fig. 6). These results indicated that there was no publication bias.
Figure 6.
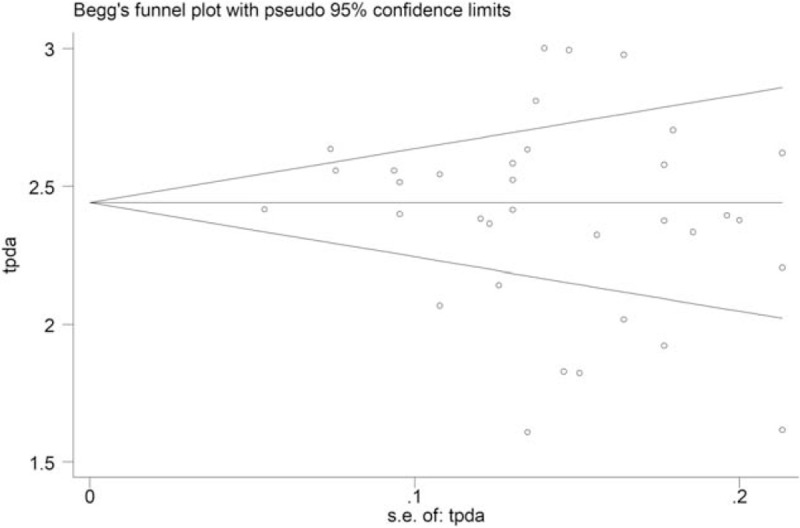
Funnel plot based on the incidence rate of fever to test the publication bias.
4. Discussion
There have been 3 epidemics of deadly coronavirus infections in human history, including severe acute respiratory syndrome (SARS) in 2002, Middle East respiratory syndrome (MERS) in 2012, and COVID-19 in 2019.[48] Although the fatality rate of COVID-19 was lower than that of SARS and MERS,[49–51] it had a stronger transmissibility.[52] With the rapid increase in the number of confirmed COVID-19 worldwide, the death toll has already surpassed that of SARS and MERS.[48] Since the fatality rate of severe or critically ill COVID-19 patients was high, it is of great significance to master the clinical characteristics of severe or critically ill patients so as to help to identify and diagnose severe cases at an early stage and reduce the number of deaths.
In this study, we analyzed the clinical characteristics of 2,459 patients with severe or critically ill COVID-19. The results showed that the 2 main clinical symptoms were fever (87.4%) and cough (66.3%), which was basically consistent with the results of Cao et al.[53] Compared to previous results,[53,54] our findings reveal the incidence rate of dyspnea (45.3%) was significant higher in severe or critically ill COVID-19 patients. This result highlights the importance of intense monitoring and evaluation of the disease of those presented with dyspnea.
Most severe or critically ill COVID-19 patients showed lymphopenia (70.3%) and normal leukocyte counts (56.9%), confirming the viral origin of the disease. Xu et al[55] pointed out in the pathologic anatomic report of COVID-19 patients that novel coronavirus induces lymphocyte clearance and inhibits immune function, which is a potential immunological mechanism for the occurrence and progression of COVID-19. As Du et al said in their study, a majority (82.6%) of decedents demonstrated a remarkable lymphopenia.[43] So dynamic monitoring of changes of lymphocyte count and immune indicators may be an important indicator for dynamic assessment of disease status and prediction of disease outcome.
However, there was a large proportion of patients also along with elevated levels of inflammatory markers such as C-reactive protein levels (83.5%) and procalcitonin (36.6%). A recent meta-analysis by Lippi et al,[56] showed that increased values of procalcitonin were associated with a nearly 5-fold higher risk of severe infection. Since the production and release into the circulation of procalcitonin from extrathyroidal sources is enormously amplified during bacterial infections,[56] suggesting that severe cases were more likely presented with bacterial infections, so serial procalcitonin measurement may play a role for predicting evolution towards a more severe form of the disease. We also found a substantial proportion of severe or critically ill COVID-19 patients showed elevated levels of D-dimer (73.3%). Another meta-analysis[57] indicated that D-dimer values were higher in severe COVID-19 patients than in those without severe disease (WMD: 2.97 mg/L;95% CI: 2.47–3.46 mg/L). Therefore, D-dimer measurement may be associated with evolution toward worse clinical picture in COVID-19 patients.
Grasselli et al retrospective analyzed 1591 consecutive patients requiring treatment in an intensive care unit (ICU) in Italy, showed that 99% of these patients needed respiratory support, including 88% who received mechanical ventilation and 11% who received noninvasive ventilation.[58] Also in our study, the incidence rate of ARDS in severe or critically ill cases was significantly increased compared to previous study.[60] In addition, other complications such as acute cardiac injury (37.1%), shock (32.0%), and acute kidney injury (22.0%) also common in severe or critically ill COVID-19 patients. So intense monitoring and evaluation of the functions of important organs in COVID-19 patients should be considered.
This meta-analysis included a large number of studies with large sample size, and all the included studies were of high quality. After the sensitivity analysis of each study was carried out, the results did not change substantially, indicating that the results of this study were reliable and stable. However, there were some limitations in our meta-analysis. Firstly, most of the included studies were single-center studies, so there may be admission deviation and selection deviation. Secondly, the sample size of included studies was small, the test efficiency may be insufficient. Thirdly, all the included studies in our meta-analysis were retrospective studies, it was hard to control the influence of confounding factors. Lastly, this meta-analysis indicated a significant heterogeneity among the studies which may affect the accuracy of the results of our meta-analysis.
5. Conclusion
In summary, the most common symptoms of severe or critically ill COVID-19 patients were fever and cough. Most patients showed lymphopenia, elevated levels of C-reactive protein and D-dimer. A large percentage of patients progress to ARDS, acute cardiac injury, acute kidney injury and shock were also common. Due to the limitation of quality and quantity of the included studies, the above conclusions need to be confirmed by more high-quality studies.
Table 1.
Basic characteristics of included studies.
| Study | Publication date | Sample size (n) | Study design | Study population | Age∗ (year) | Follow up | Outcomes reported | Quality score |
| Zhao CC[6] | Mar 24 | 36 | retrospective, multi-centre | Severe and critical ill COVID-19 patients in the First Affiliated Hospital of Bengbu Medical College and Fuyang Second People's Hospital | 55.9 ± 15.4 | Jan 24 to Feb 17 | ①② | 7 |
| Cheng KB[7] | Mar 12 | 181 | retrospective, single-centre | Severe COVID-19 patients in Wuhan Jin Yin-tan hospital | 54 (46-64) | Dec 2019 to Feb 6, 2020 | ①② | 6 |
| Guan WJ[8] | Feb 28 | 173 | retrospective, multi-centre | Severe COVID-19 patients in 552 hospitals in 30 provinces, autonomous regions, and municipalities in China | 52 (40–65) | Dec 2019 to Jan 29, 2020 | ①②③ | 7 |
| Deng Y[9] | Feb 20 | 109 | retrospective, multi-centre | Deceased COVID-19 patients in two tertiary hospitals in Wuhan | 69 (62-74) | Jan 1 to Feb 21 | ①③ | 7 |
| Mo PZ[12] | Mar 16 | 85 | retrospective, single-centre | Refractory COVID-19 patients in Zhongnan Hospital of Wuhan University | 61 (51-70) | Jan 1 to Feb 5 | ① | 6 |
| Xu S[13] | Mar 16 | 62 | retrospective, single-centre | Critical ill COVID-19 patients in Zhongnan Hospital of Wuhan University | 62.9 ± 15.3 | Jan 8 to Feb 14 | ①②③ | 7 |
| Zhang JJ[14] | Feb 19 | 58 | retrospective, single-centre | Severe COVID-19 patients in No.7 hospital of Wuhan | 64 (25-87) | Jan 16 to Feb 3 | ①② | 6 |
| Zhou F[15] | Mar 28 | 54 | retrospective, multi-centre | Non-survivor with COVID-19 in Wuhan Jinyintan Hospital and Wuhan Pulmonary Hospital | 69.0 (63–76) | Dec 2019 to Jan 31, 2020 | ①②③ | 6 |
| Yang XB[16] | Feb 24 | 52 | retrospective, single-centre | Critical ill COVID-19 patients in Wuhan Jin Yin-tan hospital | 59.7 ± 13.3 | Dec 2019 to Jan 26, 2020 | ①③ | 6 |
| Qian ZC[17] | Mar 17 | 50 | retrospective, single-centre | Severe and critical ill COVID-19 patients in Renmin Hospital of Wuhan University | 57. 6 | Jan to Feb | ①② | 7 |
| Leung C[18] | Mar 16 | 46 | retrospective, multi-centre | Deceased COVID-19 patients in Hospitals in Hubei,Chongqing, Henan,Heilongjiang, Sichuan | 70.6 (52.0-80.5) | Dec 2019 to Feb 2, 2020 | ① | 6 |
| Chu JJ[19] | Mar 29 | 43 | retrospective, single-centre | Severe COVID-19 patients in Tongji Hospital | 38 (26-66) | Jan 7 to Feb 11 | ① | 6 |
| Wan SX[20] | Mar 21 | 40 | retrospective single-centre | Severe cases with COVID-19 in Chongqing University Three Gorges Hospital | 56 (52-73) | Jan 23 to Feb 8 | ①②③ | 6 |
| Xiao KH[21] | Feb 27 | 36 | retrospective, single-centre | Severe and critical ill COVID-19 patients in Chongqing University Three Gorges Hospital | NA | Jan 23 to Feb 8 | ①② | 6 |
| Wang DW[22] | Feb 07 | 36 | retrospective, single-centre | COVID-19 patients admitted and transferred to the ICU in Zhongnan Hospital of Wuhan University | 66 (57-78) | Jan 1 to Jan 28 | ①③ | 8 |
| Yuan J[23] | Mar 06 | 31 | retrospective, single-centre | Severe and critical ill COVID-19 patients in Chongqing public health medical treatment center | 56.4 ± 12.4 | Jan 24 to Feb 23 | ①② | 6 |
| Xiong J[24] | Mar 03 | 31 | retrospective, single-centre | Severe and critical ill COVID-19 patients in Renmin Hospital of Wuhan University | NA | Jan 17 to Feb 20 | ① | 6 |
| Chen X[25] | Mar 13 | 31 | retrospective, single-centre | Severe and critical ill COVID-19 patients in Chongqing University Three Gorges Hospital | 53 (45-68) | Jan to Feb | ① | 7 |
| Li KH[26] | Feb 29 | 25 | retrospective, single-centre | Severe and critical ill COVID-19 patients in the Second Affiliated Hospital of Chongqing Medical University | 53.7 ± 12.3 | Jan to Feb | ①② | 6 |
| Fang XW[27] | Feb 25 | 24 | retrospective, single-centre | Severe and critical ill COVID-19 patients in Anhui Provincial Hospital | 56.7 ± 14.4 | Jan 22 to Feb 18 | ① | 7 |
| Bhatraju PK[28] | Mar 30 | 24 | retrospective, multi-centre | Critical ill COVID-19 patients in nine Seattle-area hospitals | 64 ± 18 | Dec 2019 to Mar 23,2020 | ①② | 6 |
| Chen W[29] | Mar 17 | 21 | retrospective, single-centre | Severe and critical ill COVID-19 patients in Jingzhou first people's hospital | NA | Dec 2019 to Feb 21,2020 | ①② | 6 |
| Arentz M[30] | Mar 19 | 21 | retrospective, single-centre | Critical ill COVID-19 patients in Evergreen Hospital | 70 (43-92) | Feb 20 to Mar 5 | ①③ | 6 |
| Chen T[31] | Mar 26 | 113 | retrospective, single-centre | Deceased COVID-19 patients in Tongji Hospital | NA | Dec 2019 to Feb 28,2020 | ①②③ | 6 |
| Chen M[32] | Feb 27 | 31 | retrospective, single-centre | Severe, critical ill and Deceased COVID-19 patients in Hubei No. 3 People's Hospital of Jianghan University | NA | Jan 24 to Feb 8 | ①③ | 6 |
| Xie HS[33] | Apr 2 | 28 | retrospective, single-centre | Severe COVID-19 patients in Wuhan Jin Yin-tan hospital | 62.5 (50.5–67.8) | Feb 2 to Feb 23 | ① | 6 |
| Zhang YF[34] | Apr 2 | 31 | retrospective, single-centre | Severe COVID-19 patients in Zhongnan Hospital of Wuhan University | 64.58 ± 13.26 | Jan 18 to Feb 22 | ② | 6 |
| Bai P[35] | Mar 7 | 58 | retrospective, single-centre | Severe and critical ill COVID-19 patients in Huazhong University of Science and Technology | 62.12 ± 12.95 | Jan 29 to Feb 26 | ①② | 6 |
| He XW[36] | Mar 15 | 54 | retrospective, single-centre | Severe and critical ill COVID-19 patients in Tongji Hospital | 68.0 (59.8–74.3) | Feb 3 to Feb 24 | ①③ | 7 |
| Huang L[37] | Feb 11 | 45 | retrospective, single-centre | Severe and critical ill COVID-19 patients in Tongji Hospital | NA | Jan | ①② | 6 |
| Wan Q[38] | Feb 24 | 21 | retrospective, single-centre | Severe and critical ill COVID-19 patients in Chongqing Public Health Medical Center | 57.7 ± 12.8 | Jan 26 to Feb 5 | ① | 6 |
| Zheng F[39] | NA | 30 | retrospective, single-centre | Severe COVID-19 patients in the North Hospital of Changsha first Hospital | 57 (46.5–66) | Jan 17 to Feb 7 | ①② | 6 |
| Wang L[40] | Apr 6 | 30 | retrospective, single-centre | Deceased COVID-19 patients in Renmin Hospital of Wuhan University | 76 (70–83) | Jan 1 to Feb 6 | ①③ | 6 |
| Cai QX[41] | Apr 1 | 65 | retrospective, single-centre | Severe COVID-19 patients in the Third People's Hospital of Shenzhen | 62.5 (56–66) | Jan 11 to Mar 6 | ①②③ | 6 |
| Ruan QR[42] | Mar 30 | 58 | retrospective, single-centre | Deceased COVID-19 patients in Wuhan | 67 (15–81) | NA | ①③ | NA |
| Du RH[43] | Apr 2 | 68 | retrospective, multi-centre | Deceased COVID-19 patients in three hospitals in Wuhan | 70.7 ± 10.9 | Dec 2019 to Feb 24,2020 | ①② | 7 |
| Tu WK[44] | Apr 6 | 109 | retrospective, single-centre | Fatal cases of COVID-19 in Zhongnan Hospital of Wuhan University | 70 (64–80) | Jan 3 to Feb 24 | ②③ | 6 |
| Li X[45] | Apr 7 | 25 | retrospective, single-centre | Death cases with COVID-19 in Renmin Hospital of Wuhan University | 73 | Jan 14 to Feb 13 | ② | 6 |
| Wang Y[46] | Apr 6 | 25 | retrospective, single-centre | Intensive care patients with COVID-19 in Tongji hospital | 64 (52–72) | Jan 25 to Feb 25 | ①③ | 6 |
| Du YZ[47] | Apr 3 | 344 | retrospective, multi-centre | Fatal cases of COVID-19 in two hospitals in Wuhan | 65.8 ± 14.2 | Jan 9 to Feb 15 | ①③ | 7 |
Table 2.
Meta analysis of clinical symptoms in patients with severe or critically ill COVID-19.
| Heterogeneity | Meta analysis | ||||||
| Symptoms | No. studies | No. patients | P | I2 | Model | R (95%CI) | P |
| Fever | 36 | 2354 | <.001 | 82.7% | Random | 0.874 (0.839,0.906) | <.001 |
| Cough | 37 | 2378 | <.001 | 90.3% | Random | 0.663 (0.599,0.723) | <.001 |
| Dyspnea | 25 | 1798 | <.001 | 94.5% | Random | 0.453 (0.354,0.554) | <.001 |
| Chest tightness | 20 | 1098 | <.001 | 91.1% | Random | 0.374 (0.280,0.474) | <.001 |
| Fatigue | 29 | 2107 | <.001 | 89.1% | Random | 0.366 (0.303,0.431) | <.001 |
| Expectoration | 24 | 1793 | <.001 | 81.9% | Random | 0.319 (0.267,0.373) | <.001 |
| Myalgia | 26 | 1413 | <.001 | 83.7% | Random | 0.195 (0.145,0.249) | <.001 |
| Dizziness | 9 | 473 | <.001 | 86.7% | Random | 0.115 (0.048,0.208) | <.001 |
| Headache | 19 | 1265 | <.001 | 76.7% | Random | 0.114 (0.079,0.154) | <.001 |
| Pharyngalgia | 19 | 1139 | <.001 | 88.0% | Random | 0.110 (0.062,0.169) | <.001 |
| Diarrhea | 26 | 1962 | <.001 | 85.0% | Random | 0.112 (0.077,0.152) | <.001 |
| Nausea and vomiting | 12 | 857 | .523 | 0.0% | Fixed | 0.059 (0.045,0.076) | <.001 |
Table 3.
Meta analysis of laboratory parameters and complications in patients with severe or critically ill COVID-19.
| Heterogeneity | Meta analysis | ||||||
| Laboratory indicators | No. studies | No. patients | P | I2 | Model | R (95%CI) | P |
| Laboratory findings | |||||||
| Leukocytosis | 20 | 1252 | <.001 | 90.6% | Random | 0.217 (0.146,0.297) | <.001 |
| Normal leukocytes | 16 | 1092 | <.001 | 88.0% | Random | 0.569 (0.481,0.655) | <.001 |
| Leukopenia | 16 | 1092 | <.001 | 90.6% | Random | 0.182 (0.112,0.264) | <.001 |
| Lymphopenia | 20 | 1245 | <.001 | 94.7% | Random | 0.703 (0.585,0.808) | <.001 |
| Elevated C-reactive protein | 17 | 1028 | <.001 | 93.1% | Random | 0.835 (0.738,0.913) | <.001 |
| Elevated procalcitonin | 13 | 888 | <.001 | 97.3% | Random | 0.366 (0.188,0.565) | <.001 |
| Elevated D-dimer | 9 | 622 | <.001 | 95.7% | Random | 0.733 (0.552,0.881) | <.001 |
| Abnormal liver function | 14 | 826 | <.001 | 83.5% | Random | 0.398 (0.316,0.484) | <.001 |
| Thrombocytopenia | 8 | 693 | <.001 | 93.8% | Random | 0.190 (0.085,0.325) | <.001 |
| Complications | |||||||
| Acute respiratory distress syndrome | 14 | 1226 | <.001 | 97.8% | Random | 0.608 (0.412,0.787) | <.001 |
| Acute kidney injury | 15 | 1217 | <.001 | 90.8% | Random | 0.220 (0.145,0.305) | <.001 |
| Acute cardiac injury | 15 | 1118 | <.001 | 91.2% | Random | 0.371 (0.274,0.474) | <.001 |
| Shock | 11 | 899 | <.001 | 97.1% | Random | 0.320 (0.164,0.501) | <.001 |
Table 4.
Evaluation of publication bias using the Egger's and the Begg's test.
| Characteristic | p (Egger's) | p (Begg's) | Characteristic | p (Egger's) | p (Begg's) |
| Proportion of male | 0.658 | 0.588 | Leukocytosis | 0.989 | 0.535 |
| Fever | 0.434 | 0.421 | Normal leukocytes | 0.614 | 0.230 |
| Cough | 0.857 | 0.619 | Leukopenia | 0.657 | 0.051 |
| Dyspnea | 0.107 | 0.049 | Lymphopenia | 0.455 | 0.448 |
| Chest tightness | 0.688 | 0.845 | Elevated C-reactive protein | 0.271 | 0.734 |
| Fatigue | 0.293 | 0.638 | Elevated procalcitonin | 0.817 | 0.059 |
| Expectoration | 0.146 | 0.157 | Elevated D-dimer | 0.883 | 0.296 |
| Myalgia | 0.291 | 0.580 | Abnormal liver function | 0.454 | 0.851 |
| Dizziness | 0.854 | 0.529 | Thrombocytopenia | 0.224 | 0.296 |
| Headache | 0.189 | 0.150 | ARDS | 0.487 | 0.801 |
| Pharyngalgia | 0.276 | 0.063 | Acute kidney injury | 0.521 | 0.458 |
| Diarrhea | 0.134 | 0.741 | Acute cardiac injury | 0.253 | 0.448 |
| Nausea and vomiting | 0.877 | 0.582 | Shock | 0.491 | 1.000 |
Author contributions
Data curation: Hongyuan Li, Pan Ji, Bocheng Li, Jielong Pang.
Methodology: Zhimei Zhong, Hongyuan Li, Jieyun Zhu.
Software: Pan Ji, Bocheng Li, Jielong Pang.
Supervision: Jianfeng Zhang, Xiangdong Liang.
Writing – original draft: Zhimei Zhong, Hongyuan Li.
Writing – review & editing: Jianfeng Zhang, Xiangdong Liang.
Footnotes
Abbreviations: ARDS = acute respiratory distress syndrome, CI = confidence interval, COVID-19 = coronavirus disease 2019, ICU = intensive care unit, MERS = middle east respiratory syndrome, MODS = multiple organ dysfunction syndrome, MOOSE = Meta-Analyses of Observational Studies in Epidemiology, NCIP = novel coronavirus-infected pneumonia, NICE = National Institute for Clinical Excellence, R = rate, SARS = severe acute respiratory syndrome, tr = transformed rate, WHO = World Health Organization, WMD = weighted mean difference.
How to cite this article: Zhong Z, Li H, Zhu J, Ji P, Li B, Pang J, Zhang J, Liang X. Clinical characteristics of 2,459 severe or critically ill COVID-19 patients: a meta-analysis. Medicine. 2021;100:5(e23781).
This study was supported by grants from the National Natural Science Foundation of China (81960343); the Emergency Science and Technology Brainstorm Project for the Prevention and Control of COVID-19, which is part of the Guangxi Key Research and Development Plan (GuikeAB20058002).
The authors have declared that no competing interest exists.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Reported variously as range or mean ± SD or median, and interquartile range (IQR) values.
① Symptoms; ② Laboratory findings; ③ Complications; NA = not available.
CI = confidence interval, R = rate.
CI = confidence interval, R = rate.
ARDS = acute respiratory distress syndrome.
References
- [1].WHO. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). (2020-03-02)[2020-04-15]. https://www.who.int/newsroom/detail/30-01-2020-statement-on-thesecondmeeting-of-the-international-healthregulations-(2005)-emergency-committeeregarding-the-outbreak-of-novel-coronavirus-(2019-ncov). [Google Scholar]
- [2].WHO. Naming the coronavirus disease (COVID-19) and the virus that causes it.(2020-02-11)[2020-04-15]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. [Google Scholar]
- [3].WHO. Coronavirus disease 2019 (COVID-19)SituationReport-78).(2020-04-07)[2020-04-15]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200407-sitrep-78-covid-19.pdf?sfvrsn=bc43e1b_2. [Google Scholar]
- [4].National Health Commission Office. The guideline for treatment plan for severe andcritical cases of novel coronavirus pneumonia (Trial edition 2).(2020-04-01)[2020-04-15]. http://www.nhc.gov.cn/yzygj/s7653p/202004/c083f2b0e7eb4036a59be419374ea89a/files/0f4be6a0f4f0419cae3ab6b6efd7cead.pdf. [Google Scholar]
- [5].Li SX, Shan Y. Latest research advances on novel coronavirus pneumonia. Journal of Shandong University(Health Sciences).(2020-03-08)[2020-04-15]. http://kns.cnki.net/kcms/detail/37.1390.r.20200306.0947.004.html. [Google Scholar]
- [6].Zhao CC, XU H, LI SH, et al. Comparison of CT imaging and clinical featuresbetween common and severe/critical type of COVID-19 patients. Int J Med Radiol [Google Scholar]
- [7].Cheng KB, Wei M, Shen H, et al. Clinical characteristics of 463 patients with common and severe type coronavirus disease 2019. Shanghai Med J [Google Scholar]
- [8].Guan W, Ni Z, Yu H, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases ofcoronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (2020-02-20)[2020-04-15]. DOI:10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [11].NICE. Appendix 4 Quality of case series form. [Internet][2020-04-15]. Available: https://www.nice.org.uk/guidance/cg3/documents/appendix-4-quality-of-case-series-form2. [Google Scholar]
- [12].Mo PZ, Xing YY, Xiao Y, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis (2020-03-16)[2020-04-15]. doi:10.1093/cid/ciaa270. [Google Scholar]
- [13].Xu S, Hu HT, Hu YG, et al. Clinical features of 62 cases of Coronavirus Disease 2019 complicated with acute renal injury. Medical Journal of Wuhan University. (2020-03-16) [2020-04-15]. http://kns.cnki.net/kcms/detail/42.1677.r.20200315.1403.001.html. [Google Scholar]
- [14].Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected bySARS-CoV-2 in Wuhan, China. Allergy. (2020-02-19) [2020-04-15]. DOI: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- [15].Zhou F, Yu T, Du RH, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang XB, Yu Y, Xu JQ, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Qian ZC, Song XY, Li SS, et al. Epidemiological and clinical characteristics analysisof severe and critical corona virus disease 2019. Med J Wuhan Univers [Google Scholar]
- [18].Leung C. Clinical features of deaths in the novel coronavirus epidemic in China. Rev Med Virol. (2020-03-16) [2020-04-15] . 10.1002/rmv.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chu JJ, Yang N, Wei YQ. Clinical Characteristics of 54 medical staff with COVID-19: a retrospective study in a single center in Wuhan, China. XXXX Check for updates. (2020-03-29) [2020-04-15] DOI:10.1002/jmv.25793.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wan SX, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol (2020-03-21) [2020-04-15] DOI:10.1002/jmv.257831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xiao KH, Shui LL, Pang XH, et al. The clinical features of the 143 patients with COVID-19 in North-East of Chongqing. J Third Military Med Univers [Google Scholar]
- [22].Wang DW, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumoniain Wuhan, China. JAMA (2020-02-07) [2020-04-15] DOI:10.1001/jama.2020.1585.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yuan J, Sun YY, Zuo YJ, et al. Clinical Characteristics of 223 Cases of COVID - 19 in Chongqing. J Southwest Univers (Natural Science Edition) 2020;42:1–7. [Google Scholar]
- [24].Xiong J, Jiang WL, Zhou Q, et al. Clinical characteristics, treatment, and prognosis in 89 cases of COVID-2019. Med J Wuhan Univers [Google Scholar]
- [25].Chen X, Tong J, Xiang JH. Retrospective study on the epidemiological characteristics of 139 patients with novel coronavirus pneumonia on the effects of Severity. Chongqing Med [Google Scholar]
- [26].Li KH, Wu J, Wu FQ, et al. The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia. Investigative Radiol (2020-02-29) [2020-04-15]. DOI:10.1097/RLI.0000000000000672.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fang XW, Mei Q, Yang TJ, et al. Clinical features and treatment of 79 cases of pneumonia infected by COVID-19JT. Chin Pharmacological Bull [Google Scholar]
- [28].Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in Critically Ill Patients inthe Seattle Region - Case Series. N Engl J Med. (2020-03-30) [2020-04-15]. DOI: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen W, Xu L, Zhang Q, et al. Clinical Characteristics of 91 Novel Coronavirus Pneumonia Patients In Jingmen First People's Hospital. Inner Mongolia Med Univers [Google Scholar]
- [30].Arentz M, Yim E, Klaff L, et al. Characteristicsand Outcomes of 21 Critically Ill PatientsWith COVID-19 in Washington State. JAMA (2020-03-19) [2020-04-15] doi:10.1001/jama.2020.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen T, Wu D, Chen HL, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. JAMA (2020-03-26) [2020-04-15] DOI: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen M, An W, Xia F, et al. Retrospective Analysis of COVID-19 Patients with Different Clinical Subtypes. Herald Med [Google Scholar]
- [33].Xie HS, Zhao JM, Lian NF, et al. Clinical characteristics of Non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. XXX Check for updates. (2020-04-02) [2020-04-15] DOI:10.1111/LIV.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang YF, Liang Z, Lan L, et al. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single center in Wuhan city, China. XXXX Check for updates. (2020-04-02) [2020-04-15] DOI:10.1111/LIV.14455. [DOI] [PubMed] [Google Scholar]
- [35].Bai P, He W, Zhang XC, et al. Analysis of clinical features of 58 patients with severe or critical 2019 novel coronavirus pneumonia. Chin J Emerg Med [Google Scholar]
- [36].He XW, Lai JS, Cheng J, et al. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Chin J Cardiol [DOI] [PubMed] [Google Scholar]
- [37].Huang L, Han R, Yu PX. A correlation study of CT and clinical features ofdifferent clinical types of 2019 novel coronavirus pneumonia. Chin J Radiological Med [Google Scholar]
- [38].Wan Q, Shi AQi, He T, et al. Analysis of clinical features of 153 patients with novel coronavirus pneumonia in Chongqing. Chin J Clin Infect Dis (2020-02-24) [2020-04-15] http://rs.yiigle.com/yufabiao/1182652.htm. DOI: 10.3760/cma.j.cn115673-20200212-00030. [Google Scholar]
- [39].Zheng F, Tang W, Li H, et al. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci 2020;24:3404–10. DOI: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- [40].Wang L, He W, Yu X, et al. Coronavirus Disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cai QX, Huang DL, Yu H, et al. COVID-19 in a Designated Infectious Diseases Hospital Outside Hubei Province, China. XXXX Check for updates. (2020-04-02)[2020-04-15]. DOI: 10.1111/ALL.14309. [DOI] [PubMed] [Google Scholar]
- [42].Ruan Q, Yang K, Wang W, et al. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med.(2020-04-06)[2020-04-15]. DOI:10.1007/s00134-020-06028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Du RH, Liu LM, Yin W, et al. Hospitalization and Critical Care of 109 Decedents with COVID-19 Pneumonia in Wuhan, China. Ann Am Thorac Soc.(2020-04-07)[2020-04-15]. doi:10.1513/AnnalsATS.202003-225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tu WJ, Cao J, Yu L, et al. Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Med.(2020-04-06)[2020-04-15]. 10.1007/s00134-020-06023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li X, Wang L, Yan S, et al. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China, International Journal of Infectious Diseases.(2020-04-03)[2020-04-15]. 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang Y, Lu X, Chen H, et al. Clinical Course and Outcomes of 344 Intensive Care Patients with COVID-19. Am. J. Respir. Crit. Care Med .(2020-04-08)[2020-04-15]. DOI:10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Du Y, Tu L, Zhu P, et al. Clinical Features of 85 Fatal Cases of COVID-19 fromWuhan: A Retrospective Observational Study. Am. J. Respir. Crit. Care Med.(2020-04-03)[2020-04-15]. DOI:10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yi M, Hu C, Xu GG, et al. Epidemiological patterns of coronavirus diseases. Chinese Journal of Multiple Organ Diseases in the Elderly 2020;19:187–9. DOI:10.11915/j.issn.1671-5403.2020.03.042. [Google Scholar]
- [49].Special expert group for control of the epidemic of novel coronavirus pneumonia ofthe Chinese preventive medicine association. An update on the epidemiological charac teristics of novel coronavirus pneumoniaCOVID-19. Zhonghua liu xing bing xue za zhi 2020;41:139–44.32057211 [Google Scholar]
- [50].Hui DSC, Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am 2019;33:869–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Azhar EI, Hui DSC, Memish ZA, et al. The Middle East Respiratory Syndrome (MERS). Infect Dis Clin North Am 2019;33:891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020;25(3): DOI: 10.2807/1560-7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cao YH, Liu XL, Xiong LJ, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Med Virol (2020-04-03) [2020-04-15] DOI: 10.1002/jmv.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Li LQ, Huang T, Wang YQ, et al. 2019 novel coronavirus patients’ clinical characteristics, discharge rate and fatality rate of meta-analysis. J Med Virol 2020;DOI:10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta 2020;505:190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lippi G. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost 2020;DOI:10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Grasselli G, Zangrillo A, Zanella A, et al. Baseline Characteristics and Outcomes of 1591Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;DOI:10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. DOI: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]


