Abstract
Background:
Controversy remains concerning the association of the all-cause mortality risk of hospitalized cardiovascular disease (CVD) patients with non-alcoholic fatty liver disease (NAFLD). This study investigated the risks of all-cause mortality among hospitalized CVD patients with NAFLD.
Methods:
We used related keywords to search for studies in 3 electronic databases: PubMed, EMBASE, and Cochrane Library. All eligible studies published up to April 2020 were reviewed. The findings of those studies reporting the mortality outcomes of hospitalized CVD patients with and without NAFLD were examined, and the various study results were pooled and analyzed using a random-effects model. A quality assessment using the Newcastle–Ottawa scale was performed on the studies selected for inclusion in a meta-analysis.
Results:
A total of 2135 studies were found, of which 3 were included in this meta-analysis. All studies were considered good quality. The mean age of the patients in the analysis was 73 years, and about half of them were men. The comorbidities reported were hypertension, diabetes mellitus, and dyslipidemia. The results showed that hospitalized CVD patients with NAFLD were at a significantly higher risk of all-cause mortality than non-NAFLD patients (adjusted hazard ratio of 2.08 [95% confidence interval, 1.56–2.59], P < .001). The included studies showed low heterogeneity (I2 = 0.0%, P = .473), and Begg and Egger tests revealed no apparent publication bias (P = .327 and P = .682, respectively).
Conclusions:
Hospitalized CVD patients with NAFLD were at a higher risk of all-cause mortality than those without NAFLD. More studies that further explore this association are needed.
Keywords: cardiovascular disease, hospitalization, meta-analysis, mortality, non-alcoholic fatty liver disease
1. Introduction
According to data from the World Health Organization, cardiovascular disease (CVD) is the most common cause of death worldwide. About one-third of mortality is associated with CVD. To reduce the number of deaths, risk factors that may predict the clinical end-points of CVD, including mortality, need to be identified. There is evidence that non-alcoholic fatty liver disease (NAFLD) is strongly associated with CVD.[1,2] A clear causal relationship and a pathophysiologic pathway between these 2 diseases still need to be determined as they both share many risk factors (such as obesity, diabetes, and dyslipidemia).[1,3] These 2 diseases also involve and affect multiple organ systems.[1] The prevalence of NAFLD has been estimated to be about 25.24%, which equates to approximately 1,900 million of the global population of 7,600 million.[4] Recent evidence has suggested that not only can NAFLD lead to liver-related complications, but it can also involve non-hepatic organs, leading to non-hepatic-related mortality.[1] NAFLD is considered to be the most common cause of chronic liver disease.[4,5] Surprisingly, the findings from a recent large population-based study in the United States showed that, next to cirrhosis, CVD is the most common underlying cause of death among NAFLD patients.[6] The mortality rate from CVD in NAFLD has been reported to be higher than those of cancers and hepatocellular carcinoma (HCC).[6] Even though patients with either NAFLD or CVD are known to have an associated higher risk of all-cause mortality than the general population,[7,8] the additional risk of mortality for more specific groups of patients—like CVD patients with NAFLD—remains unclear.[2,9] In 2016, 2 systematic reviews and meta-analyses showed inconsistent findings on whether NAFLD is associated with a higher all-cause mortality for CVD patients. This discrepancy should be resolved. There is a need for better practices that obviate the unfavorable outcome of premature death—a preventable event—for CVD patients with NAFLD, without unnecessarily raising the monetary and human resource burdens. The present work therefore sought to establish the all-cause mortality among hospitalized CVD patients with NAFLD, relative to that of patients without NAFLD. As the research focused on patients with a definite diagnosis of CVD, only studies previously undertaken on hospitalized CVD patients were included in the meta-analysis.
2. Methods
This article was performed and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[10] This study was registered with the trial registration number CRD42020185071 under the international prospective register of systematic reviews (PROSPERO: www.crd.york.ac.uk/PROSPERO).
2.1. Search strategy
We searched 3 international, validated, electronic medical databases—PubMed, EMBASE, and Cochrane Library—for relevant studies from inception till April 2020. The search terms drew upon the following relevant keywords: “non-alcoholic fatty liver disease,” “mortality,” “cardiovascular diseases,” “ischemic stroke,” and “hospital mortalities.” Also, the reference lists of relevant papers and previous review articles were searched for other additional relevant studies. The search was separately undertaken by 4 independent investigators (KT, PD, MW, and SKh). Duplicate studies were removed before record screening was conducted.
2.2. Study selection
The titles and abstracts of all of the articles identified by the search process were screened, thereby enabling articles with unrelated objectives to be excluded. The full text of each of the remaining articles was subsequently read to identify which ones met the eligibility criteria. The authors independently assessed the studies; if there was any conflict, the disagreement was usually resolved through discussion. When a conflict could not be resolved, an expert was consulted to reach consensus.
2.3. Types of studies, and inclusion and exclusion criteria
All observational, prospective, and retrospective studies were included if they compared the risk of all-cause mortality of hospitalized CVD patients with NAFLD with that of CVD patients without NAFLD. However, the studies also needed to include hospitalized CVD patients without any restrictions on their gender or comorbid conditions. Studies that only had an abstract available were excluded, as were review articles, pamphlets, and academic articles. The following information was extracted from the included studies: their authors, publication year, country of research, study design, population characteristics, and outcomes of interest.
2.4. Quality and risk of bias assessment
The included studies underwent a risk-of-bias assessment, which was independently performed by 4 of the authors. Since the included studies were either a cohort or case-control design, the Newcastle–Ottawa Scale was used to appraise study quality, as recommended by Cochrane Collaboration. This scale uses a star system to assess the quality of a study in 3 domains: selection, comparability, and outcomes/exposure. Instances of disagreement between the investigators were solved by consensus and, if necessary, discussion with an expert.
2.5. Data synthesis
The interested outcome measure of this meta-analysis was the risk of all-cause mortality for hospitalized CVD patients with NAFLD relative to those without NAFLD. The presented outcomes of each study, including the adjusted odds ratio (aOR) and adjusted relative risk of each, were converted to hazard ratios (HRs) for final analysis. Thus, the outcomes of this study were presented as a pooled HR with a 95% confidence interval (CI). The conversions of the hazard ratios were made using the following equations[11]:
where “r” is the death rate from all causes of the reference group, which was the hospitalized CVD patients without NAFLD.
We analyzed the data using a random-effects model. The subgroup analyses were also done regarding to patients’ characteristics, including age (≥ 60 years vs < 60 years); smoking status; triglyceride levels (≥ 150 mg/dL vs < 150 mg/dL); comorbidities (chronic kidney disease and dyslipidemia); number of adjusted confounding; and study designs (cohort vs case-control study). The heterogeneity of the included studies was measured by I2 and Cochrane chi-squared (Q-test). If there were any indications of heterogeneity, the source of the heterogeneity was investigated with a L’Abbe plot. Publication bias was evaluated using a funnel plot, a Begg test, and an Egger test.
2.6. Ethics approval
The systematic review or meta-analysis is exempt from ethics approval because it collecting and synthesizing data from the previous studies. In addition, patient data is anonymized, and data are available in the public domain so that ethical permission is not needed. The authors followed applicable EQUATOR Network (https://www.equator-network.org) guidelines during the conduct of research project.
3. Results
A total of 2135 studies were identified through the database search. Based on their titles and abstracts, most were excluded due to duplication or irrelevance. The full text of 5 potentially relevant studies was reviewed, after which 2 were excluded because the information on outcomes was not relevant or the study objective was not consistent with that of the current investigation.[9,12] More specifically, the work by Wong et al did not clearly report whether the included patients were hospitalized,[9] while the investigation by Perera et al only reported the predicted risk of mortality, not the actual mortality.[12] Of the 3 studies that were finally included in the meta-analysis, 2 were cohort studies[13,14] and 1 was a case-control study.[15] The study review and selection process is illustrated in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram at Figure 1. Full details of the literature search are provided in supplemental digital content (see Table S1, Supplemental Digital Content 1, which illustrates the full details of search algorithms). The quality of each of the 3 studies was assessed and deemed to be good (Fig. 2) (see Table S2, Supplemental Digital Content 2 and Table S3, Supplemental Digital Content 3, which illustrate the risk of bias assessment of cohort and case-control studies included in the meta-analysis, respectively).
Figure 1.
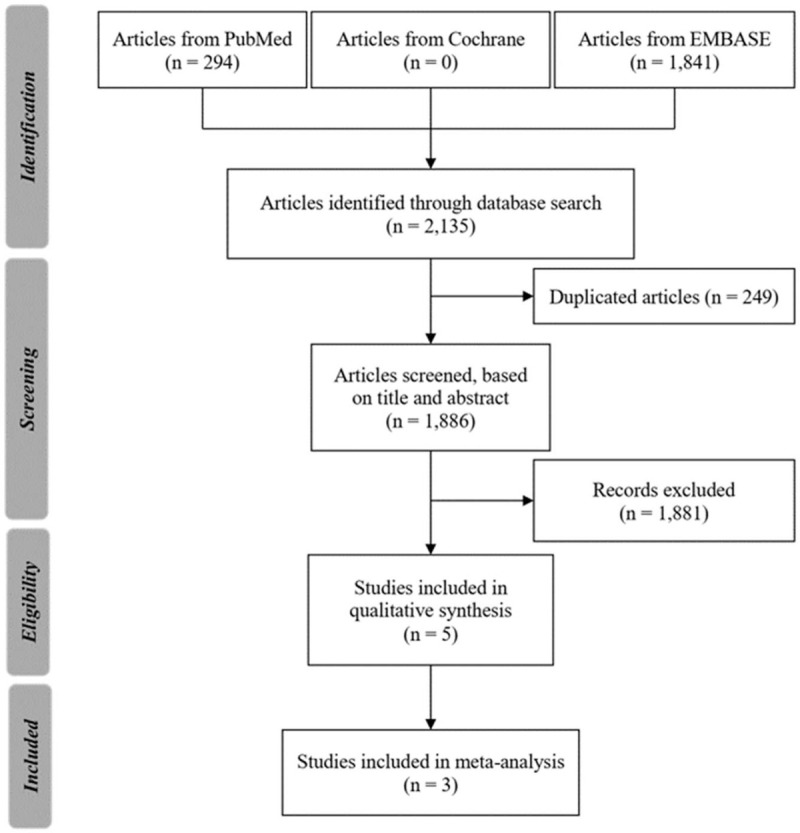
PRISMA flow diagram of the search strategy and study selection.
Figure 2.
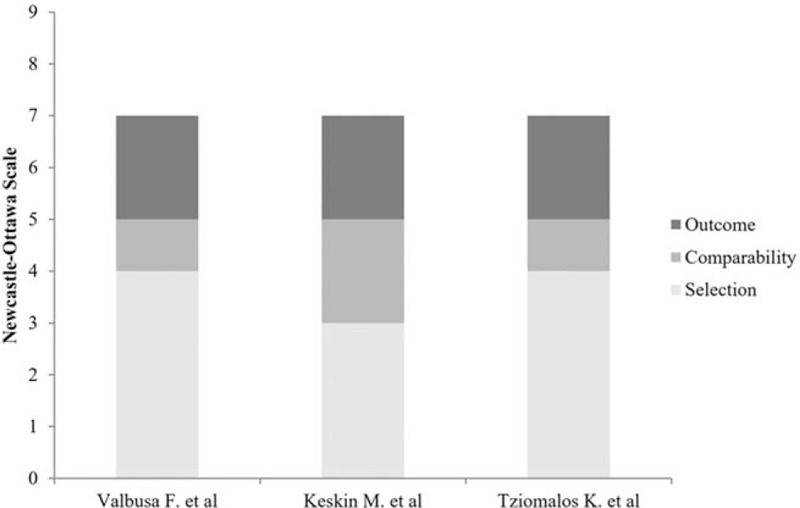
Summary of the quality of the included studies, using the Newcastle–Ottawa Scale.
3.1. Study characteristics
The 3 included studies were conducted in Europe (in Greece, Turkey, and Italy). The research by Keskin et al[15] enrolled patients hospitalized with myocardial infarction. Valbusa et al[14] investigated the outcomes of hospitalized, acute heart failure (HF) patients, and the work by Tziomalos et al[13] was undertaken on patients with acute ischemic stroke. The important characteristics and outcomes of interests from the included studies were aggregated, as summarized in Table 1. In all, 1,039 patients were included in the meta-analysis; of those, 36.19% were ultrasound-proven cases of NAFLD. The mean age of the patient cohort was 73 years, and about half were men (51.01%). The proportions of those with hypertension, atrial fibrillation, dyslipidemia, chronic renal failure, diabetes mellitus, and left ventricular ejection fraction were 62.23%, 47.62%, 46.11%, 35.47%, 32.85%, and 23.43%, respectively. About 1-quarter of the patients presented with a current smoking status, and a family history of CVD was found in 16.01% of the patients. The average body mass index of the included patients was 27.00 ± 4.81 kg/m2. The average hospital stay was 9.24 days.
Table 1.
Characteristics of the included observational studies assessing the risk of mortality in hospitalized cardiovascular disease patients associated with NAFLD.
| First author, publication year | Country | Study design | Number of subjects | Diagnostic method of NAFLD | Mean age of subjects (yr) | Cause of CVD hospitalization | Follow-up duration (mo) | Number of mortalities (cases) | All cause in-hospital mortality |
| Tziomalos K et al, 2013[13] | Greece | Prospective cohort study | 415 NAFLD = 32, non-NAFLD = 383 | US | 79 ± 7 | Acute ischemic stroke | N/A | NAFLD = 7% non-NAFLD = 8% | In-hospital mortality OR = 1.36 [95% CI: 0.25–4.85] |
| Keskin M et al, 2017[15] | Turkey | Retrospective case-control study | 360 NAFLD = 191, non-NAFLD = 169 | US | 59 ± 12 | STEMI | 31 ± 11 | Grade 0† = 8 Grade 1 = 7 Grade 2 = 8 Grade 3 = 12 | All-cause in-hospital mortality adjusted OR‡ of grade 1 = 1.5 [95% CI: 0.5–4.4] grade 2 = 2.0 [95% CI: 0.7–5.1] grade 3 = 4.0 [95% CI: 3.0–8.1] |
| Valbusa F et al, 2018[14] | Italy | Prospective cohort study | 264 NAFLD = 153, non-NAFLD = 111 | US and non-invasive fibrosis biomarker | 83 ± 9 | Acute heart failure | 23.2 | 140 deaths: 24 in-hospital and 116 post-discharge deaths NAFLD = 92 non-NAFLD = 48 | Adjusted HR§ = 1.82 [95% CI: 1.22–2.81], p < 0.005 |
3.2. All-cause mortality among hospitalized cardiovascular disease patients
The results showed that the hospitalized CVD patients with NAFLD were associated with a significantly higher risk of all-cause mortality than those without NAFLD (random-effect model, adjusted HR = 2.08 [95% CI: 1.56–2.59], I2 = 0.0%; Fig. 3). The results remained consistent in a subgroup analysis stratified by age (≥ 60 years vs < 60 years); smoking status (smoker vs non-smoker); triglyceride levels (≥ 150 mg/dl vs < 150 mg/dl); comorbidities (chronic kidney disease and dyslipidemia); number of adjusted confounding (≥ 5 factors vs < 5 factors) (see Table S4, Supplemental Digital Content 4, which describes the confounder adjustment of included studies; and study designs (cohort vs case-control study). The subgroup analysis confirmed that the risk of all-cause mortality for the hospitalized CVD patients with NAFLD was significantly higher than that for the patients without NAFLD. A forest plot of the subgroup-analysis results is presented at Figure 4.
Figure 3.
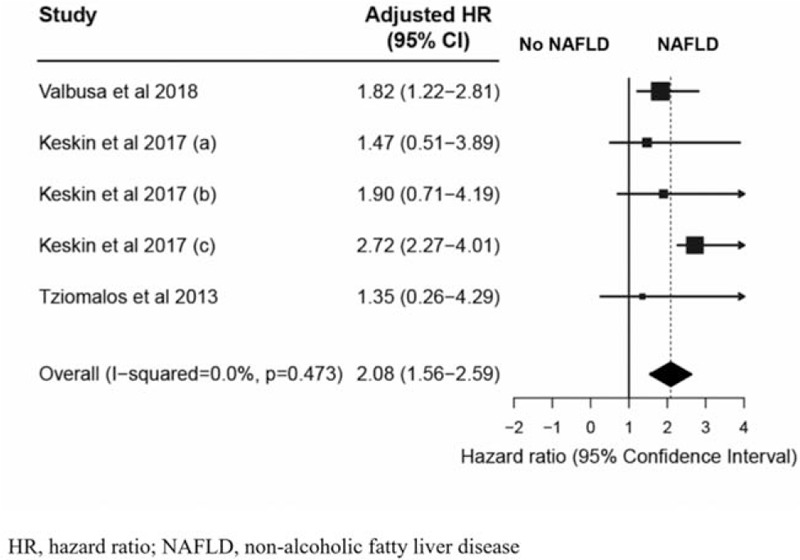
Forest plot of all-cause mortalities of hospitalized cardiovascular disease patients with and without NAFLD. Keskin (A), Grade 1 of NAFLD (mild fatty liver); Kessin (B), Grade 2 of NAFLD (moderate fatty liver); Keskin (C), Grade 3 of NAFLD (severe fatty liver).
Figure 4.
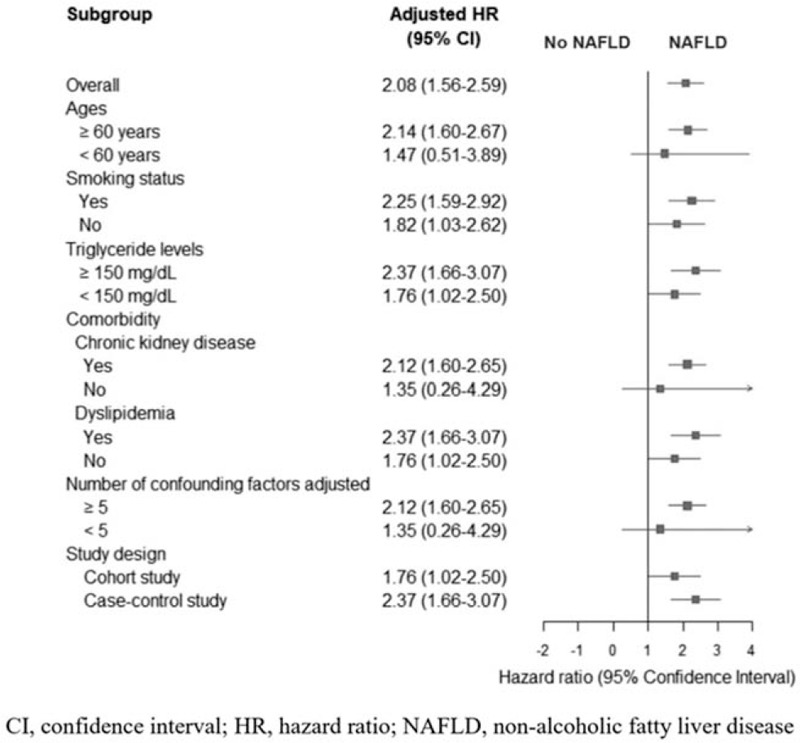
Forest plot of subgroup analysis.
3.3. Publication bias
The Egger (P = .682) and Begg (P = .327) tests revealed no apparent publication bias. However, the funnel plot was not supposed to perform since only 3 studies were included in the analysis.
4. Discussion
Since previously published meta-analyses have not provided a definite answer about the risk of mortality in hospitalized CVD patients with and without NAFLD, health system management may be adversely affected. More premature deaths may occur in these patients if an appropriate strategy of care has not been developed or, should NAFLD not actually increase the risk of mortality, financial and human resources would be wasted through the provision of nonessential care. These potential negative impacts are exacerbated by the high worldwide prevalence of both CVD and NAFLD.[4] Several studies have endeavored to establish whether NAFLD is associated with mortality in CVD patients; nevertheless, a definitive answer has not yet been provided. A meta-analysis by Targher et al, published in 2016, summarized the CVD outcomes of 34,043 adult patients with and without NAFLD. The analysis concluded that NAFLD was associated with an increased risk of fatal and non-fatal CVD events (random-effects model, odds ratio = 1.64 [95% CI: 1.26–2.13], I2 = 86%).[2] In contrast, a 2016 meta-analysis on 164,494 patients by Wu et al reported no association between NAFLD and overall mortality (HR = 1.14 [95% CI: 0.99–1.32], I2 = 65.4%), or between NAFLD and CVD mortality (HR = 1.10 [95% CI: 0.86–1.41], I2 = 64.9%).[16] Those researchers included all studies reporting CVD events and/or CVD mortalities among NAFLD patients, regardless of the diagnostic methods that had been employed. They also included a study with a large sample size in which NAFLD had been diagnosed by elevated liver enzymes, but without the recommended confirmatory imaging test.[17] Moreover, the studies published to date have defined NAFLD by various diagnostic methods, resulting in variations in their reported outcomes. The gold standard diagnostic method for NAFLD is a liver biopsy; unfortunately, this procedure is invasive and carries a high risk of potentially serious complications, such as severe pain, hemorrhage, and transient hypotension.[18] Also, CVD patients are typically prescribed an antiplatelet and/or anticoagulant agent, which theoretically could induce serious complications, including bleeding. Still, the number of evidence-based reports is limited, and the findings from those studies remain unclear.[18] In the current meta-analysis, all of the included studies reported diagnoses of NAFLD determined by ultrasonography; thus, a confounding bias from having a variety of diagnostic methods was eliminated. By comparison, many studies that diagnosed NAFLD by a liver biopsy have included patients with extremely elevated hepatic enzyme levels or obesity, which could confound the mortality outcomes. Moreover, the criteria used by studies to define CVD have varied greatly. In the present meta-analysis, only studies reporting the all-cause mortality of hospitalized CVD patients were included because we were concerned about inaccurate findings, such as a misdiagnosis of CVD, which may have led to an inconclusive finding.
The present study examined 3, unique, observational studies that specifically focused on the all-cause mortality outcomes of hospitalized CVD patients with and without NAFLD. The meta-analysis encompassed 3 CVDs—acute ischemic stroke, myocardial infarction, and acute heart failure—and summarized the data of 1,039 patients (36.19% with NAFLD). We found that hospitalized CVD patients with NAFLD were significantly associated with a 2.08-times increased risk of in-hospital mortality, compared with those without NAFLD (adjusted HR = 2.08 [95% CI: 1.56–2.59], p < 0.001, I2 = 0.0%). This result is consistent with the meta-analysis conducted by Targher et al.[2] We also found that most of the characteristics of the hospitalized CVD patients with and without NAFLD were similar. Each of the included studies scored 7 out of a possible 9 stars on the Newcastle–Ottawa Scale.
The severity of NAFLD may also impact mortality outcomes. With our search strategy, we could identify only 1 published study that compared the risks of mortality of hospitalized CVD patients without NAFLD with those of patients with a different severity of NAFLD. This study, conducted by Keskin et al, had a small sample size.[15] The researchers reported higher observed mortality rates among the patients with more severe NAFLD. However, a statistically significant hazard ratio of mortality risk was found only when the mortality rates of grade-3 NAFLD patients and non-NAFLD patients were compared.
Given our inclusion and exclusion criteria, we excluded the study published by Wong et al in 2016 as it was unclear whether the patients had been admitted to a hospital. Still, this study reported that NAFLD was not significantly associated with an increased risk of mortality in coronary artery disease patients with coronary angiogram.[9] We also excluded the study by Perera et al because the research team reported the predicted risk of mortality, not the actual mortality.[12]
The results from our study may prove of value in the management of hospitalized CVD patients. The finding that there is a higher risk of all-cause mortality among patients with NAFLD than those without NAFLD should encourage health practitioners to give more attention to CVD patients with NAFLD. This would not only reduce in-hospital mortality, but would also lessen the economic burden on the health care system.
There are several strengths to our study. First, our findings extended the results of the meta-analyses conducted in 2016 by including 2 more recent studies.[14,15] The first of those studies, published in 2017 by Keskin et al, reported that the in-hospital mortality risks were higher for ST-elevation myocardial infarction patients with grade 3 NAFLD than for those without NAFLD.[15] The second study, published in 2018 by Valbusa et al, reported that NAFLD and its severity were independently associated with increased risks of in-hospital and post-discharge all-cause mortality in elderly patients admitted for acute HF. Nevertheless, the study by Tziomalos et al reported that the presence of NAFLD in patients admitted for acute ischemic stroke did not appear to be associated with more severe strokes, which may be due to the small number of patients with NAFLD. The second strength of the current work relates to the selection of studies. To provide a precise outcome for all-cause mortality in CVD patients, we only included studies with hospitalized CVD patients who had received a definite diagnosis of CVD. Thirdly, we analyzed only in-hospital mortality to increase the accuracy of the outcome analysis. The bias resulting from an increased lost-to-follow-up rate and data missing after discharge should also be reduced. Moreover, the effects of confounding factors (for instance, age, health status, and comorbidity) that vary over time should be decreased.
Nevertheless, this meta-analysis had a few limitations. For 1 thing, the study by Valbusa et al provided only the aOR for the combined in-hospital and post-discharge mortality. We were therefore unable to recalculate the aOR of the in-hospital mortality alone and pool it in our meta-analysis. However, we did calculate the OR from the number of in-hospital mortalities that the study had reported. This revealed a minimal clinical difference between the aOR of the combined in-hospital and post-discharge mortality rate and the OR of the only-in-hospital mortality. A second consideration is that we could not stratify the mortality outcomes by cause of death due to the limited information provided in the included studies. Moreover, because of those data limitations, we were unable to analyze the specific outcomes for each CVD subtype, nor the outcomes for the NAFLD subtypes and fibrosis stages. Furthermore, our study could not analyze mortality in cases of non-alcoholic steatohepatitis, a more severe subtype of NAFLD involving inflammation of the hepatocytes, because no related study was discovered with our search terms. However, we conducted the subgroup analysis among subgroups categorized by the risk factors of CVD, comorbidities, number of adjusted confounding factors, and study design. The summary effect estimates were consistent among most subgroups. Only 2 NAFLD groups did not show a significant HR for all-cause mortality: patients aged less than 60 years; and those without an established, chronic kidney disease. The third consideration is that the number of studies available for the analysis of the relationship between NAFLD and the mortality outcomes of hospitalized CVD patients may have been too few to provide definitive conclusions. However, the pooled effect showed the significant association between NAFLD and the mortality of hospitalized CVD patients. Moreover, all individual studies had provided the consistent trend to the conclusion. The fourth consideration is all those included studies were conducted only in Europe. Thus, the generalizability of our results should be concerned. Based on our findings, we suggest that patients with NAFLD should receive more intensive management and surveillance. Nonetheless, before a clinical practice strategy specifically for this group of patients can be endorsed, a larger number of high quality and reliable studies is needed to confirm the association between NAFLD and mortality in hospitalized CVD patients. Moreover, the answer to whether treating NAFLD would decrease the risk of mortality still needs to be determined.
5. Conclusion
In conclusion, hospitalized CVD patients with NAFLD were associated with a significantly greater risk of all-cause mortality than non-NAFLD patients. Clinicians should be aware of this finding and develop protocols designed to prevent premature deaths among this group of patients. More studies that further explore this association are needed.
Acknowledgments
We thank the University of Phayao for its financial support of this research project. The authors are also grateful to Miss Pinyapat Ariyakunaphan, research assistant, for coordinating the project and Mr. David Park for language editing.
Author contributions
Conceptualization: Surasak Saokaew, Sukrit Kanchanasurakit, Kanitta Thawichai, Prommanee Duangprom, Monnapha Wannasri, Sirintip Khankham, Chayanis Kositamongkol, Nathorn Chaiyakunapruk, Pochamana Phisalprapa.
Data curation: Surasak Saokaew, Sukrit Kanchanasurakit, Kanitta Thawichai, Prommanee Duangprom, Monnapha Wannasri, Sirintip Khankham.
Formal analysis: Surasak Saokaew, Sukrit Kanchanasurakit, Kanitta Thawichai, Prommanee Duangprom, Monnapha Wannasri, Sirintip Khankham.
Funding acquisition: Surasak Saokaew.
Investigation: Surasak Saokaew, Sukrit Kanchanasurakit, Kanitta Thawichai, Prommanee Duangprom, Monnapha Wannasri, Sirintip Khankham, Chayanis Kositamongkol, Pochamana Phisalprapa.
Methodology: Surasak Saokaew, Sukrit Kanchanasurakit, Kanitta Thawichai, Prommanee Duangprom, Monnapha Wannasri, Sirintip Khankham, Chayanis Kositamongkol, Pochamana Phisalprapa.
Project administration: Surasak Saokaew, Pochamana Phisalprapa.
Resources: Surasak Saokaew.
Software: Surasak Saokaew.
Supervision: Surasak Saokaew, Chayanis Kositamongkol, Nathorn Chaiyakunapruk, Pochamana Phisalprapa.
Validation: Surasak Saokaew, Sukrit Kanchanasurakit, Chayanis Kositamongkol, Nathorn Chaiyakunapruk, Pochamana Phisalprapa.
Visualization: Surasak Saokaew, Chayanis Kositamongkol, Nathorn Chaiyakunapruk, Pochamana Phisalprapa.
Writing – original draft: Sukrit Kanchanasurakit, Kanitta Thawichai, Prommanee Duangprom, Monnapha Wannasri, Sirintip Khankham, Chayanis Kositamongkol.
Writing – review & editing: Surasak Saokaew, Sukrit Kanchanasurakit, Chayanis Kositamongkol, Nathorn Chaiyakunapruk, Pochamana Phisalprapa.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: aOR = adjusted odds ratio, CI = confidence interval, CVD = cardiovascular disease, HF = heart failure, HR = hazard ratio, NAFLD = non-alcoholic fatty liver disease, US = ultrasonography.
How to cite this article: Saokaew S, Kanchanasurakit S, Thawichai K, Duangprom P, Wannasri M, Khankham S, Kositamongkol C, Chaiyakunapruk N, Phisalprapa P. Association of non-alcoholic fatty liver disease and all-cause mortality in hospitalized cardiovascular disease patients: a systematic review and meta-analysis. Medicine. 2021;100:5(e24557).
This research project was supported by The University of Phayao (grant number FF64-UoE003).
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study had no direct interaction with patients as we acquired data from previously published articles. Informed consent was not directly obtained for this study. The ethical permission is not needed. The authors followed applicable EQUATOR Network (https://www.equator-network.org) guidelines during the conduct of research project.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests: Surasak Saokaew (SS), Sukrit Kanchanasurakit (SK), Kanitta Thawichai (KT), Prommanee Duangprom (PD), Monnapha Wannasri (MW), Sirintip Khankham (SKh), Chayanis Kositamongkol (CK), Nathorn Chaiyakunapruk (NC), and Pochamana Phisalprapa (PP) declare that there are no potential conflicts of interest with respect to the research, organization, or publication of this work.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
CVD = cardiovascular disease, HR = hazard ratio, N/A = not available, OR = odds ratio, STEMI = ST-elevation myocardial infarction, US = ultrasonography.
The patients were classified into 4 groups according to the severity of the NAFLD diagnosis using ultrasonography. Grade 0 referred to no fatty liver, Grade 1 referred to mild fatty liver, Grade 2 referred to moderate fatty liver, and Grade 3 referred to severe fatty liver.
Adjusted for demographics (age, sex, body mass index, waist circumference); first measurement of SBP and HR; first measurement during hospitalization of the following laboratory values: admission estimated glomerular filtration rate (eGFR) calculated by CKD-EPI, blood urea nitrogen, hematocrit, white blood cell, neutrophil, lymphocyte and platelet count; creatine kinase-MB, C-reactive protein, troponin I, SYNTAX score and comorbidities (diabetes, chronic kidney disease, hypertension) and medications.
Adjusted for age, sex, past history of HF, diabetes, coronary heart disease, chronic kidney disease (defined as glomerular filtration rate estimated by the 4-variable modification of diet in renal disease (eGFRMDRD) <60 ml/min/1.73 m2), chronic obstructive pulmonary disease, presence of pacemakers, implantable cardiac defibrillators, hospital ward, body weight, systolic blood pressure, LV-ejection fraction, use of angiotensin converting enzyme inhibitors/angiotensin receptor blockers, daily furosemide dosages, and plasma albumin, NT pro-brain natriuretic peptide (NT-proBNP), and gamma-glutamyl transferase (GGT) concentration.
References
- [1].Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341–50. [DOI] [PubMed] [Google Scholar]
- [2].Targher G, Byrne CD, Lonardo A, et al. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol 2016;65:589–600. [DOI] [PubMed] [Google Scholar]
- [3].Morningstar JE, Syn WK, Litwin SE. The emerging epidemic of nonalcoholic fatty liver disease and cardiovascular risk: true, true, and related? Dig Dis Sci 2020;65:1885–7. [DOI] [PubMed] [Google Scholar]
- [4].Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- [5].Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- [6].Paik JM, Henry L, De Avila L, et al. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun 2019;3:1459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu Y, Zhong GC, Tan HY, et al. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: a meta-analysis. Sci Rep 2019;9:11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006;113:791–8. [DOI] [PubMed] [Google Scholar]
- [9].Wong VW, Wong GL, Yeung JC, et al. Long-term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: a prospective cohort study. Hepatology 2016;63:754–63. [DOI] [PubMed] [Google Scholar]
- [10].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shor E, Roelfs D, Vang ZM. The “Hispanic mortality paradox” revisited: Meta-analysis and meta-regression of life-course differentials in Latin American and Caribbean immigrants’ mortality. Soc Sci Med (1982) 2017;186:20–33. [DOI] [PubMed] [Google Scholar]
- [12].Perera N, Indrakumar J, Abeysinghe WV, et al. Non alcoholic fatty liver disease increases the mortality from acute coronary syndrome: an observational study from Sri Lanka. BMC Cardiovasc Disord 2016;16:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tziomalos K, Giampatzis V, Bouziana SD, et al. Association between nonalcoholic fatty liver disease and acute ischemic stroke severity and outcome. World J Hepatol 2013;5:621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Valbusa F, Agnoletti D, Scala L, et al. Non-alcoholic fatty liver disease and increased risk of all-cause mortality in elderly patients admitted for acute heart failure. Int J Cardiol 2018;265:162–8. [DOI] [PubMed] [Google Scholar]
- [15].Keskin M, Hayiroglu MI, Uzun AO, et al. Effect of nonalcoholic fatty liver disease on in-hospital and long-term outcomes in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2017;120:1720–6. [DOI] [PubMed] [Google Scholar]
- [16].Wu S, Wu F, Ding Y, et al. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: a systematic review and meta-analysis. Sci Rep 2016;6:33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]
- [18].Khalifa A, Rockey DC. The utility of liver biopsy in 2020. Curr Opin Gastroenterol 2020;36:184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


