Abstract
Background:
Up to 80% of patients with pancreatic cancer experience abdominal and back pain. Although pharmacologic medications provide some relief, many report inadequate analgesia and adverse effects. Transcutaneous electrical nerve stimulation (TENS) is a non-invasive physical modality and had been widely applied for pain relieving, yet no study has investigated the effectiveness of TENS for pain in pancreatic cancer.
Methods:
Eligible patients were randomly assigned in a 1:1 ratio to TENS group or control group. The primary outcome was percentage change of numerous rating scale (NRS) after treatment. Secondary outcomes included percentage change of analgesic medication consumption and effect on constipation and poor appetite.
Results:
One hundred seventy-one patients were recruited (84 to control group and 87 to TENS group). NRS in TENS group has been largely decreased 77.9% right after treatment and 27.1% in 2 hours, before applying any analgesic medication, while that in control group was slightly downregulated right after treatment but gave a trend to increase at 1, 2, and 3 hours. When comparing both groups, pain was significantly well controlled without analgesic medication supplement in TENS group at 0 hour (difference in mean percent change in NRS = 50.0 [95% CI, 50–51.4], P < .01) and 3 hours (difference in mean percent change in NRS = 134.0 [95% CI, 130.0–142.7], P < .01) after treatment, and this analgesic effect last to 3 weeks after treatment cycle (difference in mean percent change in NRS = 22.5 [95% CI, 17.6–27.3], P < .01) without increase of analgesic medication consumption.
Conclusions:
TENS reduces pain without increase analgesic medication consumption in patients with pancreatic cancer pain. It provides an alternative therapy for pain in pancreatic cancer.
Clinical Trial Registration:
This study was registered at ClinicalTrials.gov, identifier NCT03331055.
Keywords: pain, pancreatic cancer, transcutaneous electrical nerve stimulation
1. Introduction
Pain in pancreatic cancer has come up to 80%, with 50% to 70% suffering from severe pain.[1] The experience of pain can either positively or negatively influence patient outcomes.[2] Conventionally, non-steroidal anti-inflammatory agents and/or opioid analgesics are used for alleviating pain according to the pain management strategy recommended by World Health Organization.[3] However, there are still many patients suffered from refractory pain, which presents a big challenge to the physicians. In addition, serious drug-related side effects bring more agony to many patients that can markedly reduce their quality of life. Celiac plexus neurolysis, in which the celiac plexus is chemically ablated, has been widely performed as an alternative treatment for alleviating cancer-associated pain, but would finally leads to an intractable pain.[4]
Transcutaneous electrical nerve stimulation (TENS) is a non-invasion and easy operated modality. It is applied by transcutaneous (over the skin) electrical stimulation and is primarily used for pain control in a wide range of acute and chronic pain conditions.[5–7] TENS units typically use adhesive electrodes applied to the skin surface to apply pulsed electrical stimulation that can be modified in terms of frequency (stimulation rate), intensity, and duration. It has been successfully applied in clinical treatment of various types of pain including: neuropathic pain,[8] bone pain,[9] postoperative pain,[10] etc. Although its analgesic mechanism is still unclear, electrical nerve stimulation results in consistent improvement of mechanical and thermal hyperalgesia[11] with reduction in the firing of spinal dorsal horn neurons,[12] increased inhibitory input to the pain pathways at the spinal cord level,[13] and decreases central excitability.[14]
We conducted prospective, randomized, and sham-controlled trial to evaluate the efficacy and safety TENS for cancer related pain in pancreatic cancer patients.
2. Patients and methods
2.1. Patients
The protocol was approved by relevant ethics committees and institutional academical review boards of Fuda Cancer Hospital (2017-TCM-01) and was registered at ClinicalTrials.gov (NCT03331055). All participants signed consent forms when recruited. Patients were recruited in 4 sites from March 2016 through March 2018. Key inclusion criteria were an age of 18 to 70 years, primary or metastatic pancreatic cancer or liver cancer with cancer related visceral pain, no neurolytic celiac plexus block was done in the past 1 month, with anticipatory survival of more than 3 months, and normal lung and heart function. Exclusion criteria included who could not tolerate of maintaining 30 min of side position without movement, who has been recruited in other clinical trial for pain relieving, who underwent radiotherapy or local radiactive seeds implantation in the past 1 month, who imaging diagnosed with encephalic tumor or metastasis, who with cardiac pacemaker or metal stand, who with risk in portal or other embolism, who with not well-controlled hypertension or diabetes.
2.2. Trial design
This randomized, single-blind, sham-controlled trial consisted of a screening visit, a 1-day pre-interventional analysis of numerous rating scale (NRS) and analgesic medication consumption baseline, a 1-week intervention duration, and a 4-weeks observation. On the basis of the screening visit and information collected in pre-interventional analysis process, patients were enrolled or were excluded if they were not eligible.
Eligible patients were randomly assigned in a 1:1 ratio of TENS group or control group. Randomization was performed by means of random number table, with stratification according to NRS of 3 to 6 and >6. Points on 1.5 cm away from middle line of T8 to T12 vertebra (belong to acupoints of B3, BL18, BL19, BL20, and BL21 in traditional Chinese meridian theory system), RN12, and pain point on abdomen. On back, acupoints at the same level were attached a pair of electrical poles, while acupoints on abdomen were stimulated with another pair of pole. TENS group was applied electrical stimulation in 2/100 Hz for 30 minutes. Tense was various and managed at the maximum comfortable critical points according to individual difference. Sham group was administered patches at the same acupoints which also attached electrical lines to electro-therapeutic apparatus but without electrical stimulation. Interventions were administered twice a day. Patients were unaware of the trial-group assignments.
2.3. Primary and secondary outcomes
The primary study outcome was pain relief for each patient before painkiller applied, at 0, 1, 2, and 3 hours after each intervention, and at 1, 2, 3, and 4 weeks after treatment cycle, quantified as percent change in NRS at 0, 1, 2, 3 hours and 1, 2, 3, 4 weeks, as compared with the patient's baseline NRS. Secondary outcomes were percentage change in morphine use (expressed as the change in morphine equivalent consumption compared with baseline).[15]
2.4. Complications
Complications were defined as any unplanned event considered related to TENS that required additional treatment after the procedure, and were recorded and classified in accordance with the Common Terminology Criteria of Adverse Events v4.0.[16]
2.5. Sample size calculation
Estimations based on trial of acupuncture for cervical cancer pain[17] of 64 patients with changing NRS from baseline of −4.2 in acupuncture group and −2.2 in control group (P < .01), indicated that a sample of 168 patients (equally divided into control and treatment groups) would suffice to achieve 90% statistical power for detecting a significant greater decrease of NRS in TENS group than in control group at a two-sided significance level of 0.05. For these calculations, patients undergoing TENS were assumed to themselves experience a decrease of 2.2 in pain scores (compared with their baseline pain scores) due to a placebo effect. Aggregate attrition rate at the 2-week mark (including both mortality and loss to follow-up) was assumed to be approximately 20%, therefore the total number of participants were 210.
2.6. Statistical analysis
Data were analyzed on an “intent to treat” basis conducted by using GraphPad Prism (San Diego, CA, version 6.0). Mean differences were expressed as coefficients in appropriate linear models and estimated using generalized estimating equations; this allowed the use of data from differing time points in the estimation procedure. CIs for these mean differences comparing outcomes between treatment groups were constructed using unpaired t test by assuming nonparametric test. Significant differences were indicated by P < .05 or P < .01.
3. Results
3.1. Patients recruitment
Between March 2016 through March 2018, a total of 254 patients were referred for this trial (Fig. 1). Eighty-three patients did not meet study entry criteria or refused to participate. One hundred seventy-one patients were randomly assigned, with 84 patients assigned to control group and 87 assigned to TENS group. Patients in both groups were comparable for all cogent variables (Table 1). All patients had locoregional disease. Patients who received TENS or sham TENS showed no evidence of early or late complications. Twenty-one patients were lost to follow-up (12 in the TENS group [mean baseline pain score 5.6] and 9 in the control group [baseline pain score 6.1]). All patients were included in the intention-to-treat analysis.
Figure 1.
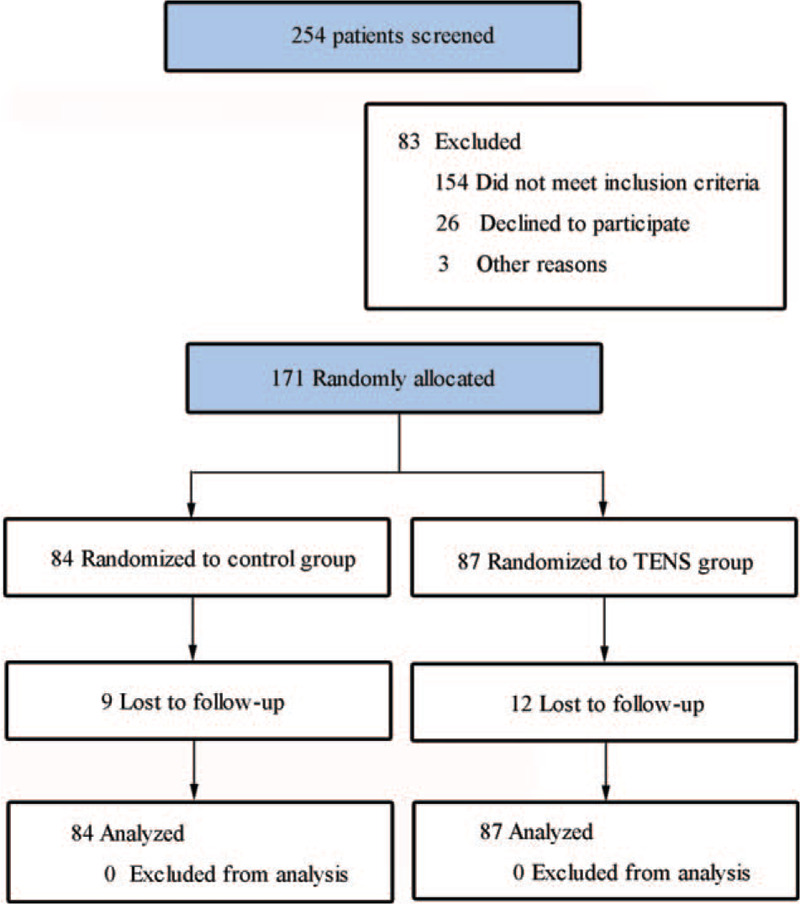
CONSORT diagram.
Table 1.
Baseline characteristics of all randomly assigned patients.
| Characteristic | Control | TENS |
| No. of patients | 84 | 87 |
| Male sex | ||
| No. | 48 | 43 |
| % | 57.1 | 49.4 |
| Age, yr | ||
| Mean | 58.9 | 51.2 |
| SD | 9.9 | 10.7 |
| Pain history, wk | ||
| Mean | 9.4 | 8.7 |
| SD | 8.1 | 6.2 |
| Narcotic consumption, morphine-equivalent units | ||
| Mean | 44.8 | 39.7 |
| SD | 56.1 | 64.2 |
| Abdominal pain intensity, numeric rating scale | ||
| Mean | 5.4 | 5.8 |
| SD | 1.8 | 1.7 |
| Constipation | ||
| No. | 26 | 33 |
| % | 31.0 | 37.9 |
| Poor appetite | ||
| No. | 48 | 46 |
| % | 57.1% | 52.9% |
3.2. Primary outcome: percentage change in NRS
NRS in control group were tended to increase compared with baseline at 0 hour (mean percent change in NRS = −25.3 [95% CI, −26.7 to −24.0]), 1 hour (mean percent change in NRS = 26.2 [95% CI, 23.7–28.3]), 2 hours (mean percent change in NRS = 98.7 [95% CI, 95.1–102.3]) and 3 hours (mean percent change in NRS = 128.1 [95% CI, 124.6–131.7]) before taken analgesic medication after intervention, and was invariant at 1 week (mean percent change in NRS = −0.6 [95% CI, −1.5 to 0.3]), 2 weeks (mean percent change in NRS = 0.6 [95% CI, 0.6–1.9]), 3 weeks (mean percent change in NRS = 1.0 [95% CI, −0.5 to 2.4]), and 4 weeks (mean percent change in NRS = 1.1 [95% CI, −0.2 to 2.3]) after treatment cycle. In contrast, in TENS group, NRS were tended to decrease compared with baseline at 0 hour (mean percent change in NRS = −77.9 [95% CI, −79.0 to −76.9]), 1 hour (mean percent change in NRS = −37.9 [95% CI, −40.3 to −35.5]), 2 hours (mean percent change in NRS = −27.1 [95% CI, −29.0 to −25.1]), and 3 hours (mean percent change in NRS = −8.3 [95% CI, −10.8 to −5.7]) before taken analgesic medication after intervention, and was invariant at 1 week (mean percent change in NRS = −35.8 [95% CI, −38.8 to −32.8]), 2 weeks (mean percent change in NRS = −30.0 [95% CI, −34.2 to −25.9]), 3 weeks (mean percent change in NRS = −21.5 [95% CI, −26.2 to −16.8]) and 4 weeks (mean percent change in NRS = −0.8 [95% CI, −4.9 to 3.3]) after treatment cycle. When comparing both groups, pain was significantly well controlled without analgesic medication supplement in TENS group at 0 hour (difference in mean percent change in NRS = 50.0 [95% CI, 50–51.4], P < .01; Fig. 2A), 1 hour (difference in mean percent change in NRS = 63.0 [95% CI, 55.4–66.7], P < .01; Fig. 2A), 2 hours (difference in mean percent change in NRS = 116.7 [95% CI, 113.0–121.4], P < .01; Fig. 2A), and 3 hours (difference in mean percent change in NRS = 134.0 [95% CI, 130.0–142.7], P < .01; Fig. 2A) after intervention, and this analgesic effect last to 1 week (difference in mean percent change in NRS = 35.2 [95% CI, 32.1–38.3], P < .01; Fig. 2B), 2 weeks (difference in mean percent change in NRS = 30.6 [95% CI, 26.4–34.9], P < .01; Fig. 2B), and 3 weeks (difference in mean percent change in NRS = 22.5 [95% CI, 17.6–27.3], P < .01; Fig. 2B) after treatment cycle.
Figure 2.
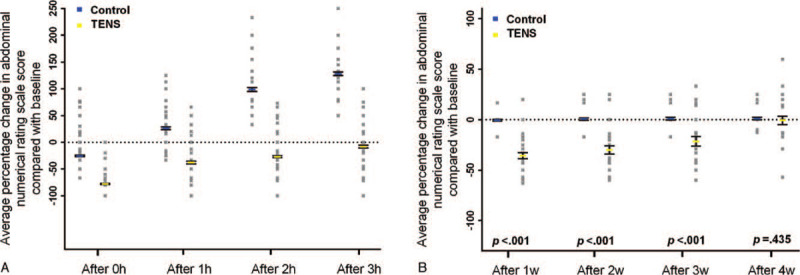
(A) Percent change of abdominal pain scores after each intervention. (B) Percent change of abdominal pain scores after treatment cycle.
3.3. Secondary outcome: percentage change in morphine consumption
In the control group, morphine use (analgesic medication consumption in morphine-equivalent units) increased compared with baseline at 1 week (mean percent change in morphine consumption = 7.8 [95% CI, 5.2–10.5]), 2 weeks (mean percent change in morphine consumption = 8.1 [95% CI, 5.4–10.8]), 3 weeks (mean percent change in morphine consumption = 8.5 [95% CI, 6.0–11.1]), and 4 weeks (mean percent change in morphine consumption = 9.1 [95% CI, 6.5–11.6]). In TENS group, morphine use also increased at 1 week (mean percent change in morphine consumption = 5.6 [95% CI, 3.5–7.7]), 2 weeks (mean percent change in morphine consumption = 6.3 [95% CI, 4.1–8.4]), 3 weeks (mean percent change in morphine consumption = 6.7 [95% CI, 4.5–9.0]), and 4 weeks (mean percent change in morphine consumption = 6.7 [95% CI, 4.6–8.9]). However, the differences between control group and TENS group were not significant at 1 week (difference in mean percent change in morphine consumption = 2.2 [95% CI, −1.1 to 5.6], P < .20; Fig. 3), 2 weeks (difference in mean percent change in morphine consumption = 1.9 [95% CI, −1.6 to 5.3], P < .29; Fig. 3), 3 weeks (difference in mean percent change in morphine consumption = 1.8 [95% CI, −1.6 to 5.1], P < .30; Fig. 3), or 4 weeks (difference in mean percent change in morphine consumption = 2.3 [95% CI, −1.0 to 5.6], P < .17; Fig. 3).
Figure 3.
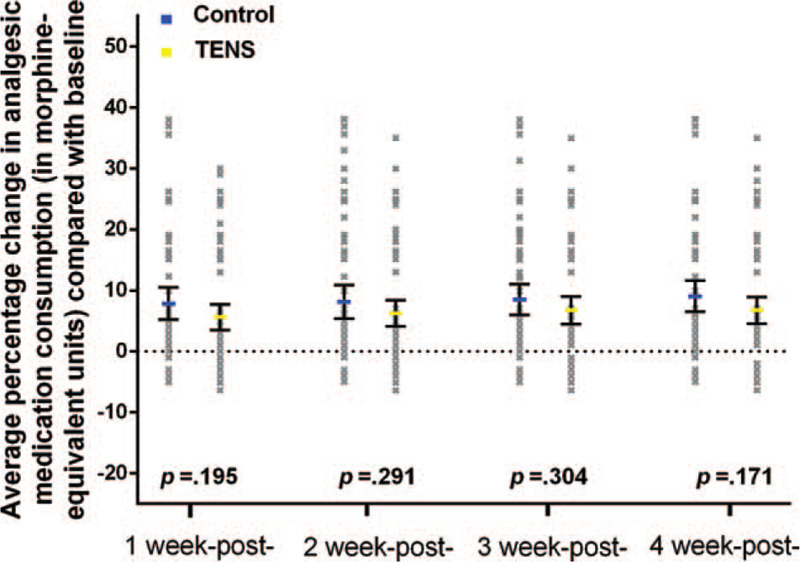
Percent change of morphine equivalent consumption.
No patient suffered constipation and poor appetite in control group was improved after treatment. Comparatively, in TENS group, all of 33 patients complained with constipation in TENS group told they had different degrees of improvements, and 42 out of 46 patients with poor appetite gain better appetite during and after treatment.
3.4. Safety
No side effect has occurred in any patient.
4. Discussion
TENS shows its benefits in reducing pancreatic cancer pain, which effect lasted till 3 weeks after treatment. More promising result is that TENS downregulated the NRS before analgesic medication had been applied within 2 hours after treatment procedure.
TENS has been widely studied and used for patients suffering cancer related pain with showing the potential to improve quality of life within specific types of cancer.[18] However, not all studies on TENS for cancer pain have the consistent results cause the sample size, study design, stimulation site, electric frequency, intensity, cycle frequency, method of administration, and outcome measures are various.[7,8] Hence in this study, we manage the methodological quality through methods of sample calculation by assuming result basing on a trial of acupuncture for cervical cancer pain, double-blind management, sham group design, etc. Besides these, core elements that would affect the results are mode of TENS, treatment frequency, and duration.
For electric frequency, even though some researches had gained negative results in pain relieving with TENS by using low electrical frequency or long treatment intervals (e.g., twice a week),[19–21] TENS has been used with varying success in analgesic treatment. Results from studies investigating the morphine-sparing effects of electrostimulation manifested that alternating-frequency (2/100 Hz), high-intensity (9–12 mA) stimulation of acupoints has >50% morphine-sparing effect in patients after lower abdominal gynecologic surgery.[22,23] Han and colleagues conducted a series of animal studies that have shown that low frequency TENS-induced anti-hyperalgesia (decreased sensitivity to pain) is mediated by enkephalin, b-endorphin, and endomorphin through d-opioid and m-opioid receptors, while high frequency TENS enhances dynorphin through k-opioid receptors.[24] But interestingly, it has been found that neither high nor low frequency alone could downregulate pain.[25] Similarly again, analgesic effect of alternating-frequency TENS was proved in this study with showing significant downregulation after intervention without taking painkiller, and maintaining a lower NRS after treatment cycle in 4 weeks without increase of analgesic medication consumption compared with control group.
Moreover, intensity is also close related to the “pain relieving duration.” It had been proved stronger intensity can help to reach a longer duration of staying at a higher pain threshold, and this dose–effect relationship would contribute to a better pain management results.[26] As it has been suggested the strongest comfortable intensity is normally work for pain in organs,[27] this study conduct strongest but comfortable intensity for each individual rather than implement a fix intensity, which might cause a vast various of sense to different person, might be an important factor help reaching an obvious decrease right after treatment process and maintain the effect of pain relieving for 2 hours without applying analgesic medication.
For cycle frequency, it seems that it has been underestimated by many studies. However, from the results of this study, a peak of pain-relieving effect by 77.9% of downregulation of NRS right after treatment procedure, decrease to 27.1% in 2 hours after treatment before analgesic medication have to be applied to. Hence, we assume that 2 hours should be a maximum of rest duration to maintain the pain-relieving effects by TENS only. However, implement the TENS treatment for every 2 hours for patients, which mean 6 times a day, could be with very low patient compliance.
One week of continuous treatment proved to prolong the analgesic effect. When finishing a week cycle of treatment, average level of NRS seems to be maintained at a lower level without increase of analgesic medication consumption, of a 35.2% downregulation of NRS at 1 week and 22.5% at 3 weeks. Despite other traditional standard therapy, such as intraoperative CPN, was proved to decrease 10% pain scores in pancreatic cancer patients at 1 month, TENS could benefit patients without invasion and too much additional economic burden.
In this initial research for intractable pancreatic cancer pain, we found that most patients’ pain was not well controlled due to various reasons, such as adverse function of analgesic medication of constipation and lost appetite. With the treatment of TENS, 100% of patients with constipation had different degrees of improvements, and 91% patients with poor appetite gain better appetite. Without increase of analgesic consumption, pain score was significantly reduced after TENS treatment cycle.
However, even during the treatment period, the pain was not disappeared or maintain at a low level for all day long. Average 3 hours of downregulation effect on NRS might call for a higher cycle frequency to achieve a more stable analgesic effect. Moreover, a higher cycle frequency may also reduce the use of painkiller. Therefore, further research on exploring more therapeutic characteristics of TENS and setting up an optimal application model in analgesia treatment is needed. For this, we have designed a portable and wearable acupoints electrical stimulation device (China patent: ZL201820740038.6), and will carry out further research in the future.
5. Conclusion
In final, we conclude TENS is safe and has significant effect on relieving pain in pancreatic cancer patients. In addition, it has many advantages such as non-invasive, seldom side effects, easy-operation, cheap, and with good patient compliance. Alternative frequency, maximum comfortable intensity, twice or more time treatment daily gave a model of TENS in pancreatic cancer pain treatment. TENS obviously may play a much more important role in dealing with cancer related pain in the future.
Acknowledgments
We acknowledge the contributions of staff at the Fuda Cancer Hospital, Jinan University, Provincial Tongde Hospital of Zhejiang, Third Affiliated Hospital of Zhejiang University of Traditional Chinese Medicine, and First Affiliated Hospital, Guangzhou University of Chinese Medicine. We also thank of the Provincial Tongde Hospital of Zhejiang for his careful technical review of this manuscript.
Author contributions
Conceptualization: Lihua He, Lizhu Lin.
Data curation: Lihua He, Xueliang Wang.
Formal analysis: Keping Tan.
Funding acquisition: Lihua He, Hui Yi, Xueliang Wang.
Investigation: Hui Yi, Keping Tan, Jiangsong Zhang, Jietao Lin.
Methodology: Lihua He, Keping Tan, Xianming Lin.
Project administration: Lihua He, Lizhu Lin.
Resources: Lihua He, Keping Tan, Xianming Lin, Lizhu Lin.
Supervision: Xianming Lin.
Validation: Xianming Lin, Lizhu Lin.
Visualization: Lihua He, Keping Tan.
Writing – original draft: Lihua He, Keping Tan.
Writing – review & editing: Xianming Lin, Lizhu Lin.
Footnotes
Abbreviations: NRS = numerous rating scale, TENS = transcutaneous electrical nerve stimulation.
How to cite this article: He L, Tan K, Lin X, Yi H, Wang X, Zhang J, Lin J, Lin L. Multicenter, randomized, double-blind, controlled trial of transcutaneous electrical nerve stimulation for pancreatic cancer related pain. Medicine. 2021;100:5(e23748).
This study is supported by Guangdong provincial administration of traditional Chinese medicine (20162040) and National Clinical Key Specialty (ZX001232).
The authors alone are responsible for the views expressed in this paper. These views do not necessarily represent the decisions, policies, or views of the United States Centers for Disease Control and Prevention.
Written informed consent from patient involved in this study was received prior to study inclusion. All details that might disclose the identity of the subjects under study were omitted or anonymized.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
SD = standard deviation; TENS = transcutaneous electrical nerve stimulation.
References
- [1].Arcidiacono PG, Calori G, Carrara S, et al. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev 2011;2011:CD007519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Beck SL, Towsley GL, Berry PH, et al. Core aspects of satisfaction with pain management: cancer patients’ perspectives. J Pain Symptom Manage 2010;39:100–15. [DOI] [PubMed] [Google Scholar]
- [3].Turk DC, Dworkin RH, Burke LB, et al. Developing patient-reported outcome measures for pain clinical trials: IMMPACT recommendations. Pain 2006;125:208–15. [DOI] [PubMed] [Google Scholar]
- [4].Nagels W, Pease N, Bekkering G, et al. Celiac plexus neurolysis for abdominal cancer pain: a systematic review. Pain Med 2013;14:1140–63. [DOI] [PubMed] [Google Scholar]
- [5].Jeffrey L, Amitabh G. The use of transcutaneous electrical nerve stimulation (TENS) in a major cancer center for the treatment of severe cancer-related pain and associated disability. Pain Med 2015;16:1204–10. [DOI] [PubMed] [Google Scholar]
- [6].Lison JF, Amer-Cuenca JJ, Piquer-Marti S, et al. Transcutaneous nerve stimulation for pain relief during office hysteroscopy: a randomized controlled trial. Obstet Gynecol 2017;129:363–70. [DOI] [PubMed] [Google Scholar]
- [7].Johnson MI, Paley CA, Howe TE, et al. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database Syst Rev 2015;(6):CD006142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gibson W, Wand BM, O’Connell NE. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database Syst Rev 2017;9:CD011976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bennett MI, Johnson MI, Brown SR, et al. Feasibility study of transcutaneous electrical nerve stimulation (TENS) for cancer bone pain. J Pain 2010;11:351–9. [DOI] [PubMed] [Google Scholar]
- [10].Rakel BA, Zimmerman MB, Geasland K, et al. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: a randomized, blinded, placebo-controlled trial. Pain 2014;155:2599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Inoue T, Takenoshita M, Shibata M, et al. Long-lasting effect of transcutaneous electrical nerve stimulation on the thermal hyperalgesia in the rat model of peripheral neuropathy. J Neurol Sci 2003;211:43–7. [DOI] [PubMed] [Google Scholar]
- [12].Leem JW, Park ES, Paik KS. Electrophysiological evidence for the antinociceptive effect of transcutaneous electrical stimulation on mechanically evoked responsiveness of dorsal horn neurons in neuropathic rats. Neurosci Lett 1995;192:197–200. [DOI] [PubMed] [Google Scholar]
- [13].Maeda Y, Lisi TL, Vance CG, et al. Release of GABA and activation of GABA (A) in the spinal cord mediates the effects of TENS in rats. Brain Res 2007;1136:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lisi TL, Sluka KA. A new electrochemical HPLC method for analysis of enkephalins and endomorphins. J Neurosci Methods 2006;150:74–9. [DOI] [PubMed] [Google Scholar]
- [15].Network NCC. NCCN clinical practice guidelines in oncology: adult cancer pain. Opioid Principles, Prescribing, Titration, Maintenance, And Safety 2018;7. [Google Scholar]
- [16].Chen AP, Setser A, Anadkat MJ, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol 2012;67:1025–39. [DOI] [PubMed] [Google Scholar]
- [17].Meng FF, Feng YH. A pilot study of acupuncture at pain acupoints for cervical cancer pain. Medicine 2018;97:e13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hurlow A, Bennett MI, Robb KA, et al. Transcutaneous electric nerve stimulation (TENS) for cancer pain in adults. Cochrane Database Syst Rev 2012;(6):CD006276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Robb K, Oxberry SG, Bennett MI, et al. A cochrane systematic review of transcutaneous electrical nerve stimulation for cancer pain. J Pain Symptom Manage 2009;37:746–53. [DOI] [PubMed] [Google Scholar]
- [20].Zheng YC, Yuan TT, Liu T. Is acupuncture a placebo therapy? Complement Ther Med 2014;22:724–30. [DOI] [PubMed] [Google Scholar]
- [21].Choi TY, Lee MS, Kim TH, et al. Acupuncture for the treatment of cancer pain: a systematic review of randomised clinical trials. Support Care Cancer 2012;20:1147–58. [DOI] [PubMed] [Google Scholar]
- [22].Hamza MA, White PF, Ahmed HE, et al. Effect of the frequency of transcutaneous electrical nerve stimulation on the postoperative opioid analgesic requirement and recovery profile. Anesthesiology 1999;91:1232–8. [DOI] [PubMed] [Google Scholar]
- [23].Chen L, Tang J, White PF, et al. The effect of location of transcutaneous electrical nerve stimulation on postoperative opioid analgesic requirement: acupoint versus nonacupoint stimulation. Anesth Analg 1998;87:1129–34. [PubMed] [Google Scholar]
- [24].Han JS, Chen XH, Sun SL, et al. Effect of low- and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain 1991;47:295–8. [DOI] [PubMed] [Google Scholar]
- [25].Bergeron-Vezina K, Corriveau H, Martel M, et al. High- and low-frequency transcutaneous electrical nerve stimulation does not reduce experimental pain in elderly individuals. Pain 2015;156:2093–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kuai L, Chen H, Zhang TT, et al. Study on dose–effect relationship of electroacupuncture with different current intensities alleviating tibial cancer pain and inhibition of expression of spinal GFAP in rats. Chin Acupunct Moxibustion 2012;32:331–7. [PubMed] [Google Scholar]
- [27].Zhao TY, Xi Q, Guo Y. Current research status and analysis of electroacupuncture parameters for post-operative pain. Shanghai J Acupunct Moxibustion 2015;5:464–7. [Google Scholar]


