Abstract
Background:
There have been conflicting results regarding clinical dexamethasone-sugammadex interactions in adults and pediatric patients under general anesthesia.
Methods:
This study used a systematic review with meta-analysis of randomized controlled trials and non-randomized studies based on the Cochrane Review Methods. A comprehensive literature search was conducted to identify clinical trials that investigated the effect of dexamethasone on sugammadex reversal of rocuronium-induced neuromuscular blockade in surgical patients undergoing general anesthesia.
Results:
Among the 314 patients in the 6 studies, 147 received intravenous dexamethasone (dexamethasone group), and 167 received intravenous saline or other antiemetics (control group). The primary outcome, the time to recovery after sugammadex administration (the time to recovery of the train-of-four ratio to 0.9 after sugammadex administration; s) was comparable between the 2 groups, the weighted mean difference (95% confidence interval [CI]) being –2.93 (–36.19, 30.33) (I2 = 94%). The time to extubation after sugammadex administration (s) and incidence of postoperative nausea and vomiting was not different between the 2 groups, the weighted mean difference (95% CI) being 23.31 (−2.26, 48.88) (I2 = 86%) and the pooled risk ratio (95% CI) being 0.25 (0.03, 2.11), respectively. The time to recovery after sugammadex administration might be different according to the study design or study region.
Conclusion:
This meta-analysis showed that use of dexamethasone in the perioperative period neither delayed nor facilitated the reversal of rocuronium-induced neuromuscular blockade with sugammadex in patients undergoing elective surgery with general anesthesia. However, given that the results showed high heterogeneity, further randomized controlled trials are needed to confirm these findings.
Keywords: adverse drug event, anesthesia recovery period, dexamethasone, general anesthesia, neuromuscular blockade, rocuronium, sugammadex, surgery, tracheal extubation
1. Introduction
Sugammadex reverses neuromuscular blockade (NMB) by binding with aminosteroidal nondepolarizing neuromuscular blocking agents (NMBAs) such as rocuronium.[1,2] Although sugammadex appears to be highly selective, it can interact with other drugs, especially corticosteroids, including dexamethasone.[3] Dexamethasone is one of the most widely used corticosteroids for treating many clinical conditions, such as laryngeal, cerebral, and surgical edema, as well as in combination with analgesics for multimodal analgesia, and for the prevention of postoperative nausea and vomiting (PONV).[4–7] It shares with rocuronium the same cyclopentanoperhydrophenanthrene structure and has very similar molecular dimensions. These characteristics could lead to a possible antagonistic interaction of dexamethasone with rocuronium to alter sugammadex binding.[6] Some experimental studies have demonstrated that dexamethasone inhibits in vitro neuromuscular reversal activity of sugammadex in innervated primary human muscle cells and concluded that the efficacy of sugammadex for reversal of rocuronium-induced NMB might be diminished by dexamethasone.[3,8]
In contrast, some clinical studies have reported conflicting results regarding in vivo clinical dexamethasone-sugammadex interactions in adults or pediatric patients undergoing surgeries under general anesthesia. Some clinical reports suggest that dexamethasone does not significantly affect NMB antagonism of sugammadex,[9–12] while some suggest that dexamethasone significantly extends or shortens sugammadex action times.[13,14] However, a meta-analysis of the topic has not yet been reported.[15]
Therefore, we performed a systematic review to determine the clinical relevance of the findings regarding the effect of dexamethasone on sugammadex reversal of rocuronium-induced NMB in surgical patients undergoing general anesthesia. We compared the time to recovery from NMB after sugammadex administration (primary endpoint) and the time to extubation after sugammadex administration, and incidence of PONV or other postoperative adverse events (secondary endpoints) between the dexamethasone group and the control group. This comparison was accomplished by performing a systematic review (SR) with meta-analysis of randomized controlled trials (RCTs) and non-randomized studies. We hypothesized that the times to recovery and extubation after sugammadex administration might be longer in the dexamethasone group than in the control group.
2. Methods
Since this SR and meta-analysis was performed using existing published literature and did not involve new human data, ethical approval was not required. We prospectively registered the protocol for this review with the UMIN clinical trials registry (unique trial number: UMIN000039035; registration number: R000044514; date of registration: December 30, 2019).
This SR and meta-analysis compared the recovery times and the time to extubation after sugammadex administration and PONV or other postoperative adverse events between the dexamethasone group and the control group undergoing administration of sugammadex for NMB reversal after surgery under general anesthesia. The primary outcome of this SR was the time to recovery (of the train-of-four [TOF] ratio to 0.9) after sugammadex administration. It was defined as the time from sugammadex administration (time = 0 s) to the recovery of the TOF ratio to 0.9. The secondary outcomes were the time to extubation after sugammadex administration and the incidence of PONV or other postoperative adverse events.
We conducted and reported this SR based on the Cochrane Collaboration methodology[16] and PRISMA statement.[17]
2.1. Data source & literature source
We searched PubMed, EMBASE, the Cochrane Library, the Web of Science, Scopus, Koreamed, and ClinicalTrials.gov, from inception to December 31, 2019, using medical subject headings (MeSH) and free-text terms without language restrictions.
The following keywords were searched using Medline: dexamethasone; corticosteroid; sugammadex; rocuronium; neuromuscular blockade; general anesthesia; surgery; tracheal extubation; adverse drug event; anesthesia recovery period. Search strategies were designed for each database (Supplementary Table 1). To identify unpublished or ongoing studies, we also searched the WHO International Clinical Trials Registry Platform and the Clinical Trial.gov website. After the original electronic search, we reviewed the bibliographies from identified studies.
2.2. Study selection
Potentially eligible studies were screened and selected by 2 independent reviewers (BG Lim and H Kim) using the pre-defined inclusion criteria. Two reviewers independently determined which of the identified studies were suitable for inclusion. Final selection was based on a screen of full texts. Discrepancies between reviewers were resolved by discussion.
Studies were included in our meta-analysis if they
-
1.
included patients undergoing administration of sugammadex for reversal of neuromuscular blockade after surgery under general anesthesia,
-
2.
administered intravenous (IV) dexamethasone as the intervention pretreatment (dexamethasone group) and placebo (IV saline) or other IV antiemetics as the control pretreatment (control group),
-
3.
reported the time to recovery after sugammadex administration in the dexamethasone and control group, and
-
4.
were RCTs or non-randomized studies.
2.3. Data extraction
Two reviewers independently extracted data from each study using a pre-specified data extraction form. The following variables were extracted from the reviews:
-
1.
the mean and standard deviation of the times to recovery and extubation after sugammadex administration, and dichotomous data on the incidence of PONV or other postoperative adverse events in the intervention and control groups, and
-
2.
demographic, clinical, and treatment characteristics (e.g., the study population [pediatric or adult], the number of patients in the intervention and control groups).
Disagreements between reviewers were resolved in consultation with a third reviewer (YJ Won).
2.4. Assessment of methodological quality
The methodological quality of the included studies was evaluated by 2 blinded reviewers (H Kim and BG Lim). Quality assessment for the included RCTs was performed using the Cochrane Collaboration risk of bias tool, which provides for selection, performance, attrition, detection, and reporting bias through the assessment of the mentioned random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessments, incomplete outcome reporting, selective outcome reporting, other bias, and overall risk of bias.[18] For assessing the risk of bias in the included non-randomized studies, ROBINS-I (Risk Of Bias In Non-randomized Studies - of Interventions) was applied.[19] We evaluated the possible existence and direction of the bias and whether it is likely to have an impact on the effects of interventions.
2.5. Statistical analysis
The primary outcome of our review was the time to recovery (of the TOF ratio to 0.9) after sugammadex administration. It was defined as the time from sugammadex administration (time = 0 second) to the recovery of the TOF ratio to 0.9, as measured by the authors of the included studies. The secondary outcomes were the time to extubation after sugammadex administration and the incidence of PONV or other postoperative adverse events. The time to extubation after sugammadex administration was defined as the time from sugammadex administration (time = 0 second) to tracheal extubation. The incidence of PONV or other postoperative adverse events was defined as the number of patients complaining of or showing adverse events in the post-anesthesia care unit for 2 hour postoperatively.
Analysis of the times to recovery and extubation after sugammadex administration—understood here as continuous variables—was performed using the weighted mean difference (WMD) with a 95% confidence interval (CI). The incidence of PONV or other postoperative adverse events, defined in the study as a dichotomous variable, was analyzed using the risk ratio with a 95% CI.
We examined the heterogeneity between studies by scrutinizing the forest plots and quantifying the impact of heterogeneity using the I2 statistic. If heterogeneity between studies was identified (I2 statistic > 50% or any clinical heterogeneity), a random-effects model was conducted.
Initially, subgroup analyses were preplanned to compare different age groups (e.g., pediatric vs adult group), pretreated drugs in the control group (IV saline vs other IV antiemetics), NMB degrees at sugammadex administration (e.g., moderate vs deep NMB) or the doses of sugammadex (e.g., 2 mg kg−1 vs 4 mg kg−1) and dexamethasone (e.g., high vs others), the timing of IV dexamethasone pretreatment (during or after anesthesia induction vs 5–10 minutes before sugammadex injection) or the time intervals between dexamethasone and sugammadex administration (e.g., long vs short), and types of main anesthetics (sevoflurane vs desflurane). Also, we conducted sensitivity analysis according to the methodological quality (risk of bias) (e.g., low vs others), study design (e.g., RCT vs non-randomized studies), and the region in which the study was performed (e.g., Europe vs Africa). Publication bias (used for at least 10 studies) was not analyzed because of the small number of included studies. We used RevMan version 5.3 and STATA version 13.0 for these analyses.
3. Results
3.1. Identification of studies
Searches of the databases yielded 411 articles (Fig. 1). Of these, 400 publications were excluded, as parts of them were duplicated articles, or it was clear from the title and abstract that they did not fulfill the selection criteria. For the remaining 11 articles, we obtained full manuscripts, and following scrutiny of these, we identified 6 potentially relevant studies; 5 publications were excluded because they were either
Figure 1.
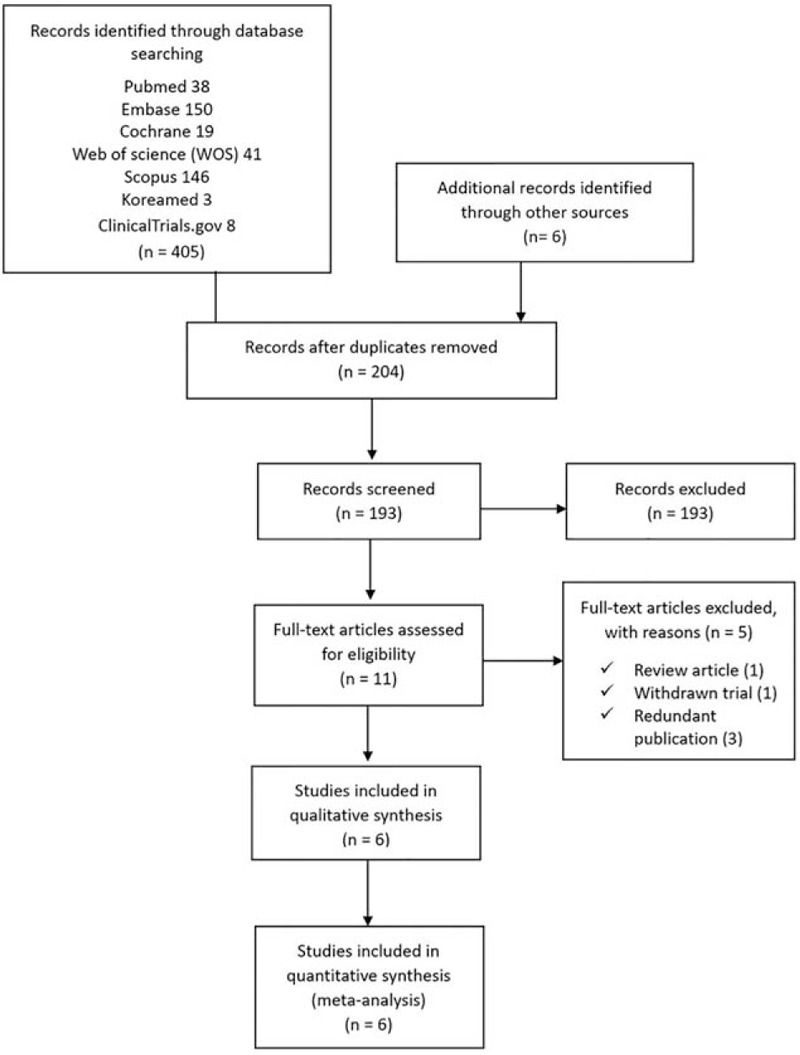
Flow diagram of included and excluded studies.
-
1.
a review article,
-
2.
a withdrawn trial, or
-
3.
a redundant publication. Therefore, the total number of studies included in the review was 6 (Fig. 1).
3.2. Study characteristics and patient populations
The details of the selected 6 studies are summarized in Table 1. The included studies compared the times to recovery and extubation after sugammadex administration, or the incidence of postoperative adverse events including PONV between the dexamethasone control groups undergoing administration of sugammadex for NMB reversal after surgery under general anesthesia. In the 6 studies, a total of 314 patients including 147 patients who received IV dexamethasone (dexamethasone group) and 167 patients who received a placebo (IV saline) or other IV antiemetics (control group) were evaluated for the time to recovery after sugammadex administration.
Table 1.
Characteristics of the included studies.
| Study ID | Journal | Pt. age | Setting/Country | ASA class | Type of Surgery | Anesthetics (induction-maintenance) | Muscle relaxant | Interven--tion group | Control group | Timing of intervention (IV DEXA) | NMB degree at SUGA injection | Dose of SUGA | Time interval between DEXA and SUGA administra-tion∗ | Study design |
| Batistaki, 2019 | J Anaes-thesiol Clin Phar-macol. | 18–75 yr | University hospital/Greece | 1–3 | Elective laparo-scopic cholecyst-ectomy | Propofol-desflurane | Rocuro-nium | DEXA 5 mg | N/S | During anesthesia induction | Deep (TOF 0, PTC 1–2) | 4 mg kg−1 | Long | RCT |
| Buonanno, 2016 | Anesth Analg | 18–65 yr | University hospital/Italy | 1–2 | Various surgeries | Propofol-sevoflurane | Rocuro-nium | DEXA 8 mg | Ondans-etron 8 mg | 10 minutes after induction | Moderate (TOF 2) | 2 mg kg−1 | Long | Retro-spective case-control study |
| Gulec, 2016 | Anesth Analg | 3–8 yr | University hospital/Turkey | 1–2 | Elective adenoidec-tomy and/or tonsillectomy | Sevoflurane-sevoflurane | Rocuro-nium | DEXA 0.5 mg kg−1 | Saline | After anesthesia induction | Moderate (TOF 2) | 2 mg kg−1 | Long | RCT |
| Ozer, 2018 | Niger J Clin Pract | 18–60 yr | University hospital/Turkey | 1–4 | Elective direct laryngo-scopy/ biopsy | Thiopental-sevoflurane | Rocuro-nium | DEXA 8 mg | None | 10 minutes before SUGA injection | Moderate (TOF 1–2) | 2 mg kg−1 | Short | Prospective observa-tional study |
| Rezonja, 2016 | BMC Anes-thesiol | 18 yr or more | University hospital/ Slovenia | 1–3 | Elective abdominal or urological Surgery | Propofol or etomidate-sevoflurane | Rocuro-nium | DEXA 0.15 mg kg−1 | Granise-tron 1 mg | 5–10 minutes before SUGA injection | Moderate to deep (TOF 0–2) | 200 mg | Short | RCT |
| Saleh, 2017 | Egypt J Anaesth | 1–6 yr | University hospital/Egypt | 1–2 | Elective strabismus surgery | Propofol-sevoflurane | Rocuro-nium | DEXA 0.5 mg kg−1 | Metoclo-pramide 0.25 mg kg−1 | After anesthesia induction | Moderate (TOF 1) | 2 mg kg−1 | Long | RCT |
The included studies were RCTs (n = 4) or non-randomized studies (n = 2), and trial sizes ranged between 30 and 80 patients. One study was conducted in an African country,[14] and 5 studies were conducted in European countries.[9–13] The patients usually underwent surgeries that required anti-edema or antiemetic prophylaxis or deep NMB application such as laparoscopic cholecystectomy (n = 1), adenoidectomy or tonsillectomy (n = 1), direct laryngoscopy and biopsy (n = 1), abdominal or urological surgery (n = 1), strabismus surgery (n = 1), and others (n = 1). Two studies included pediatric patients aged 1 to 8 years.[11,14] Two studies included adult patients, except for elderly patients aged above 65 years,[10,13] and 2 studies included adult patients with elderly patients up to 75 years of age.[9,12] The selected studies generally excluded patients with American Society of Anesthesiology (ASA) physical status classification IV and those with a history of difficult intubation, current treatment with steroids or hormones, drugs interacting with neuromuscular blockers (e.g., magnesium and anticonvulsants), or allergy to NMBAs.
Only one study was performed with inhalational anesthetic (sevoflurane) induction,[11] and 5 studies were performed with intravenous anesthetic (propofol, thiopental, or etomidate) induction. In 5 studies, general anesthesia was maintained with sevoflurane, and in the other study, general anesthesia was maintained with desflurane.[9] All studies used rocuronium as an NMBA and performed the following neuromuscular monitoring method to assess the NMB state during surgery, at the end of surgery, and to accurately measure the recovery times from the NMB after sugammadex administration. Neuromuscular monitoring was initiated with an acceleromyograph (TOF-Watch S or SX; Organon Ireland Ltd., Dublin, Ireland), which measures the function of the adductor pollicis muscle after the induction of anesthesia. A transducer was attached over the thumb. Two electrodes were placed on cleaned skin corresponding to the ulnar nerve trajectory at the wrist. Stabilization and calibration were performed for the TOF-Watch according to the Good Clinical Research Practice guidelines in pharmacodynamic studies of NMBAs.[20] After calibration, TOF stimulations were applied repetitively every 15 second during the whole monitoring period. In 4 studies, the NMB degree at sugammadex administration was moderate (TOF count 1 or 2). Thus, the dose of sugammadex administered was 2 mg kg−1 in the studies,[10,11,13,14] and in 1 study, the NMB degree was deep (TOF count 0, post-tetanic count [PTC] 1–2), thus, the dose of sugammadex was 4 mg kg−1.[9] One study administered 200 mg of sugammadex in the range of TOF count 0–2 (median TOF count 0; deep NMB).[12]
Four studies included adult patients administered 5 mg (n = 1), 8 mg (n = 2), and 0.15 mg kg−1 (n = 1), respectively, and the other 2 studies, which included pediatric patients injected with 0.5 mg kg−1, a relatively high dose[11,14] as the dose of dexamethasone in the intervention group. Some studies used saline (n = 2) or no treatment (n = 1), while other studies used other antiemetics, including ondansetron, granisetron, or metoclopramide (n = 1, respectively) as the pretreatment in the control group. In 4 studies, the timing of intervention (IV dexamethasone) was during or after anesthesia induction. In other words, a time interval between dexamethasone and sugammadex was as long as the period from anesthesia induction until the end of surgery (above 30–60 minutes). In the other 2 studies, the timing of the intervention (IV dexamethasone) was 5–10 minutes before sugammadex injection, which means that a time interval between dexamethasone and sugammadex was as short as 10 minutes.[12,13]
All studies (n = 6) reported the time to recovery of the TOF ratio to 0.9 after sugammadex administration, the primary outcome for this review. Of the secondary outcomes, the time to extubation after sugammadex administration was reported in three studies (50%) and the incidence of PONV or other postoperative adverse events was reported in 2 studies (33%).
3.3. The primary outcome
The time to recovery after sugammadex administration (the time to recovery of TOF ratio to 0.9 after sugammadex administration; s) was comparable between the dexamethasone and control groups, the WMD of which was −2.93 (95% CI: –36.19, 30.33; P = .86; I2 = 94%) (Fig. 2). This result showed high heterogeneity.
Figure 2.
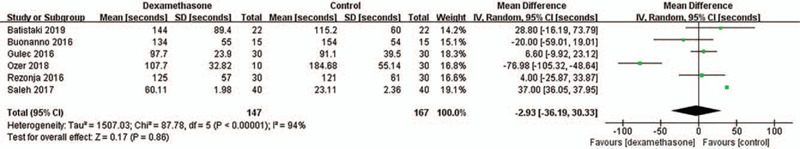
Time to recovery after sugammadex administration. SD = standard deviation, IV = inverse variance, CI = confidence interval.
3.4. The secondary outcomes
The time to extubation after sugammadex administration (s) was comparable between the 2 groups. The WMD was 23.31 (95% CI: −2.26, 48.88; P = .07; I2 = 86%) (Fig. 3). This result showed high heterogeneity. The incidence of PONV was not different between the 2 groups, and the pooled risk ratio was 0.25 (95% CI: 0.03, 2.11; P = .20) (Fig. 4). Heterogeneity in these results was not applicable because the incidence of PONV was reported in 2 studies, and the incidences in the 2 groups of one study were zero (%). The incidence of postoperative adverse events other than PONV was reported as zero (%) in the 2 studies. Thus, a meta-analysis for the outcome also was not applicable.
Figure 3.
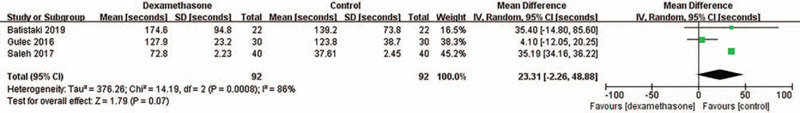
Time to extubation after sugammadex administration SD = standard deviation, IV = inverse variance, CI = confidence interval.
Figure 4.
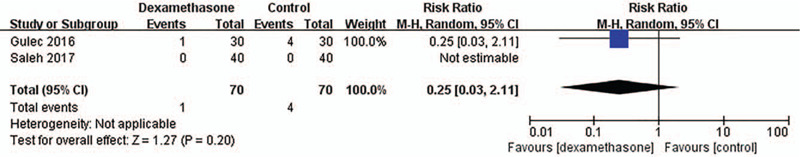
Incidence of postoperative nausea and vomiting (PONV). M-H = Mantel–Haenszel, CI = confidence interval.
3.5. Subgroup analysis
After we had performed the meta-analysis for all outcomes, we found that the number of the included studies only for the primary outcome (the time to recovery after sugammadex administration)—6 studies—were enough to identify each subgroup analysis. Also, after we conducted planned subgroup analyses for the primary outcome, we found that the subgroup analyses did not show a specific significance in the primary outcome (Table 2). Nevertheless, the subgroup analysis with different age groups showed that the time to recovery in the pediatric studies tended to be delayed in the dexamethasone group compared with the control group (WMD: 22.96, 95% CI: –6.74, 52.67; P = .13; I2 = 92%), although the time to recovery was not significantly different between the 2 groups regardless of whether the subjects were adults or children (Fig. 5). In addition, the subgroup analysis with different doses of dexamethasone showed the same results as those of the subgroup analysis with different age groups.
Table 2.
Summary of subgroup and sensitivity analysis for weighted mean difference (WMD) of primary outcome (time to recovery after sugammadex administration) among subgroups.
| Primary outcome or subgroups | N | participants | WMD (95% CI) | P value for heterogeneity | I2, % |
| The time to recovery after sugammadex administration | |||||
| All | 6 | 314 | –2.93 (–36.19, 30.33) | <.00001 | 94 |
| Subgroup or sensitivity analysis | |||||
| Age of population | |||||
| Adult | 4 | 174 | –17.53 (–64.54, 29.48) | <.00001 | 86 |
| Pediatric | 2 | 140 | 22.96 (–6.74, 52.67) | .0003 | 92 |
| Dose of dexamethasone in the intervention group | |||||
| Others (5 or 8 mg, 0.15 mg kg−1) | 4 | 174 | –17.53 (–64.54, 29.48) | <.00001 | 86 |
| High (0.5 mg kg−1) | 2 | 140 | 22.96 (–6.74, 52.67) | .0003 | 92 |
| Pretreated drugs in the control group | |||||
| IV saline (or none) | 3 | 144 | –14.96 (–75.82, 45.90) | <.00001 | 93 |
| Other IV antiemetics∗ | 3 | 170 | 10.94 (–24.46, 46.35) | .002 | 84 |
| Study design | |||||
| RCT | 4 | 244 | 19.86 (–2.29, 42.01) | .0005 | 83 |
| Non-randomized studies | 2 | 70 | –50.13 (–105.88, 5.62) | .02 | 81 |
| Study region | |||||
| Europe | 5 | 234 | –12.42 (–47.87, 23.02) | <.00001 | 86 |
| Africa | 1 | 80 | 37 (36.05, 37.95) | NA | NA |
| Risk of bias | |||||
| Low | 1 | 60 | 4 (–25.87, 33.87) | NA | NA |
| Others (unclear, high and moderate) | 5 | 254 | –4.4 (–42.89, 34.09) | <.00001 | 95 |
| NMB degrees at sugammadex administration | |||||
| Moderate | 4 | 210 | –11.5 (–55.74, 32.73) | <.00001 | 96 |
| Deep | 2 | 104 | 11.59 (–13.3, 36.48) | .37 | 0 |
| Time interval between DEXA and sugammadex administration# | |||||
| Long | 4 | 214 | 15.65 (–9.9, 41.2) | <.00001 | 86 |
| Short | 2 | 100 | –36.63 (–115.99, 42.72) | .0001 | 93 |
Figure 5.
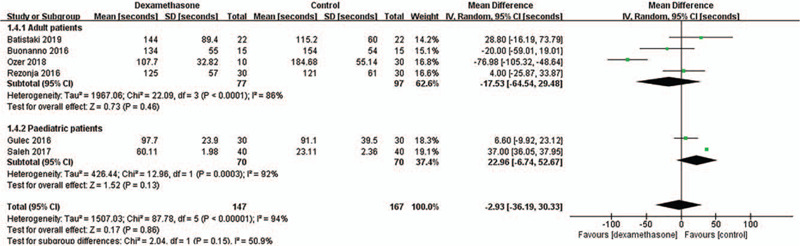
Subgroup analysis for the time to recovery after sugammadex administration according to age of study population. This figure shows the comparisons for the time to recovery within and between adult and pediatric subgroups. SD = standard deviation, IV = inverse variance, CI = confidence interval.
The tests for subgroup differences according to the pretreated drugs in the control group, NMB degrees at sugammadex administration, and the time interval between dexamethasone and sugammadex administration showed there were no significant differences in heterogeneity between the subgroups (P = .47, I2 = 0%; P = .37, I2 = 0%; P = .22, I2 = 33.8%, respectively).
3.6. Sensitivity analysis
We found that there were significant differences in heterogeneity according to the study design (e.g., RCT vs non-randomized studies) and the study region (e.g., Europe vs Africa) in the primary outcome of time to recovery. Although the time to recovery was not significantly different between the dexamethasone group and the control group regardless of the study design and study region (Table 2), the tests for subgroup differences according to the study design and study region showed significant differences in heterogeneity between the RCT and non-randomized studies subgroups (P = .02, I2 = 80.9%) (Fig. 6), and between the Europe and Africa subgroups (P = .006, I2 = 86.6%) (Fig. 7), respectively. These results indicate that the time to recovery after sugammadex administration may be different according to the study design or the study region.
Figure 6.
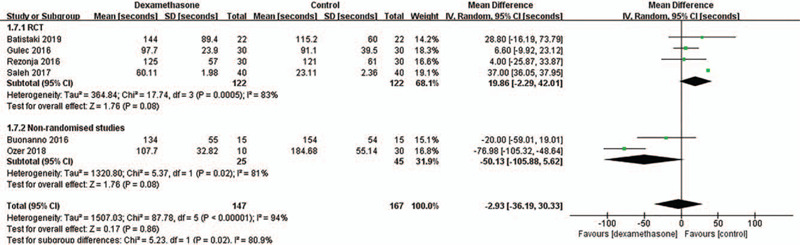
Sensitivity analysis for the time to recovery after sugammadex administration according to study design. This figure shows the comparisons for the time to recovery within and between RCT (randomized controlled trial) and non-randomized studies subgroups. SD = standard deviation, IV = inverse variance, CI = confidence interval.
Figure 7.
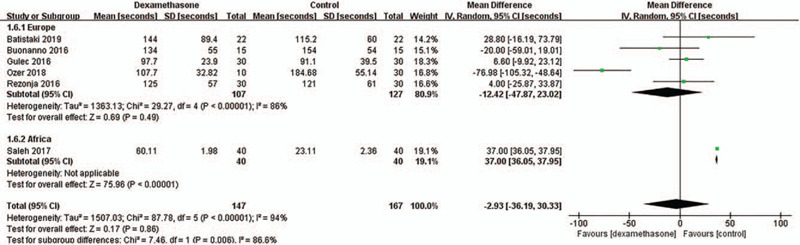
Sensitivity analysis for the time to recovery after sugammadex administration according to study region. This figure shows the comparisons for the time to recovery within and between Europe and Africa subgroups. SD = standard deviation, IV = inverse variance, CI = confidence interval.
The test for subgroup differences according to the risk of bias (e.g., low vs others) showed no significant difference in heterogeneity between the subgroups (P = .74, I2 = 0%).
3.7. Quality of the included studies (risk of bias in the included studies)
Figure 8.
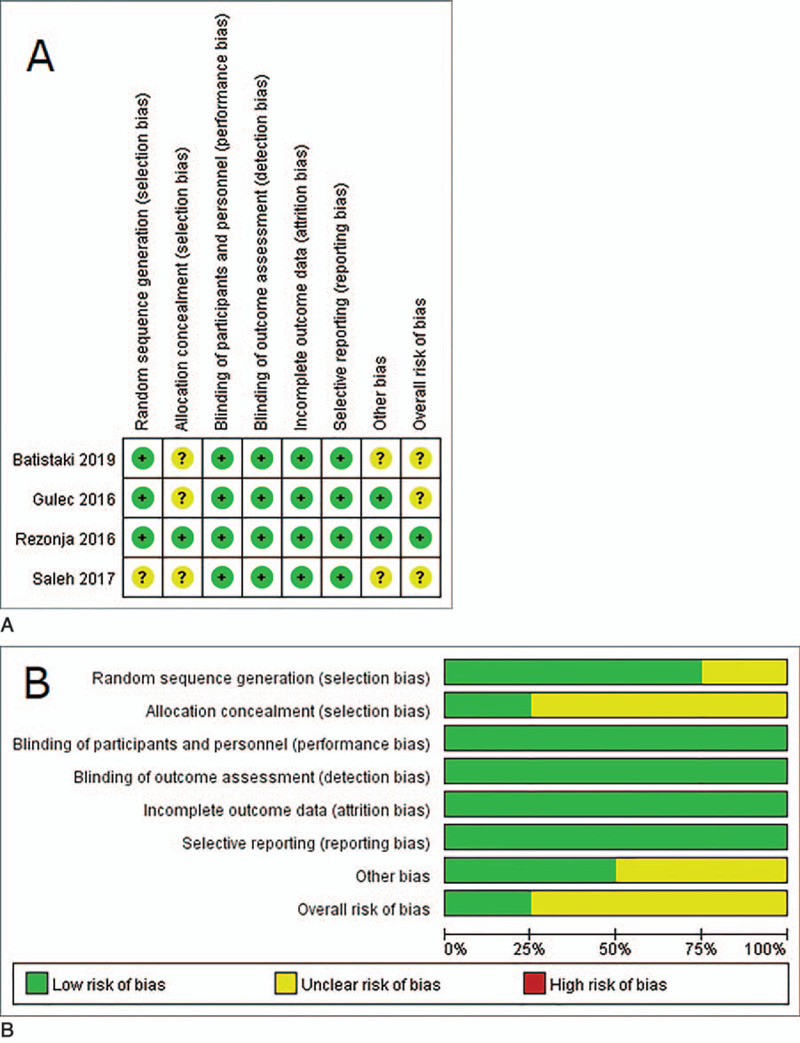
Quality assessment of the included studies. (A) Risk of bias summary of randomized controlled trials (RCTs): a review of authors judgments about each risk of bias item for each included RCT. Green circle: low risk of bias; yellow circle: unclear risk of bias; red circle: high risk of bias. (B) Risk of bias graph: review authors judgments about each risk of bias item presented as percentages across all included RCTs. Green color: low risk of bias; yellow color: unclear risk of bias; red color: high risk of bias.
Table 3.
Quality assessment of non-randomized studies. Summary of domain-level and overall risk of bias judgements using ROBINS-I∗.
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of intervention | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported results | Overall bias |
| Buonanno, 2016 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Ozer, 2018 | Moderate | Low | Low | Moderate | Low | Low | Low | Moderate |
3.7.1. Risk of bias in the 4 RCTs
All of the 4 included studies reported that the study was randomized, and three studies (75%) reported the method used for applying random sequence generation. Allocation concealment was adequately reported in only one study (Rezonja 2016). Given that all interventions were performed after anesthesia induction, participants were effectively blinded to the study intervention. All 4 studies were assessed as having a low risk of bias because each reported blinding of the outcome assessors, or it was not likely that assessment of the outcome was influenced by knowledge of the intervention received. All 4 studies that reported the completeness of outcome data for each primary outcome were assessed as having a low risk of bias. All 4 studies that had no selective or incomplete reporting of a specific outcome were assessed as having a low risk of bias. Two studies (Batistaki 2019, Saleh 2017) in which there were insufficient information related to the sample size calculation were assessed as having an unclear risk of bias. Based on the judgments regarding each risk of bias item, 1 study (Rezonja 2016) was assessed as having an overall low risk of bias, and the other 3 studies (Batistaki 2019, Gulec 2016, Saleh 2017) were assessed as having an overall unclear risk of bias.
3.7.2. Risk of bias in the 2 non-randomized studies
Of the 2 non-randomized studies, one was a prospective observational study and the other was a retrospective case-control study. They had an overall moderate risk of bias, as shown in Table 3.
4. Discussion
The purpose of this SR was to reveal the effects of dexamethasone on the reversal of NMB by sugammadex by comprehensively analyzing previous studies. Six relevant studies were identified using a comprehensive search strategy.
The main finding of this SR is that intraoperative dexamethasone administration does not significantly affect the sugammadex reversal of NMB. Sugammadex completely binds the aminosteroidal NMBAs in a 1: 1 ratio to remove them through the kidney, thus eliminating the effect of NMB.[1,2] Dexamethasone, with its steroid ring molecular structure, is similar to NMBAs and can bind to sugammadex, which may affect the reversal of NMB by sugammadex.[6] Therefore, several clinical studies have been conducted on whether dexamethasone affects sugammadexs reversal ability. In those studies, sugammadexs ability to reverse NMB was primarily assessed by the time to reach TOF 0.9, and the extubation time after sugammadex administration. All 6 studies included in this SR presented the time to recovery of the TOF ratio to 0.9 after sugammadex administration as the primary outcome, and it was concluded that intraoperative administration of dexamethasone does not affect the time to recovery of the TOF ratio to 0.9. Collectively, this finding objectively indicates that dexamethasone does not significantly affect NMB antagonism of sugammadex, unlike the hypothesis of our study.
However, the result of the primary outcome showed high heterogeneity (I2 = 94%), and thus subgroup and sensitivity analyses were performed to investigate the reason for the high heterogeneity.
First of all, we focused on the study design (RCTs vs non-randomized studies) of the included studies as a cause of the high heterogeneity. As a result of the sensitivity analysis, although the time to recovery was not significantly different between the dexamethasone and control groups regardless of the study design, the test for the subgroup differences according to the study design showed a significant difference in heterogeneity between the RCTs and non-randomized studies subgroups. In the case of RCTs, the time to recovery of the TOF ratio to 0.9 tended to be prolonged in the dexamethasone group, whereas in the non-randomized studies, it showed an opposite trend. Therefore, inclusion of more high-quality RCTs will make the results clearer.
Also, in terms of the study region (Europe vs Africa), the test for subgroup differences showed a significant difference in the heterogeneity of the time to recovery of the TOF ratio to 0.9 between the Europe and Africa subgroups, as only one African study showed the opposite trend for the results of other studies from Europe. Nevertheless, even if analyzing only the 5 European studies and omitting the 1 African study, there is no change in the overall result that dexamethasone does not affect the time to recovery of the TOF ratio to 0.9. Therefore, the impact of the African study indicating opposite results compared to the overall results may not be significant.
In the sensitivity analysis regarding the risk of bias (low vs others), the test for the subgroup differences showed there was no significant difference in the heterogeneity of the time to recovery of the TOF ratio to 0.9 between the low subgroup and other (risk of bias) subgroup, which could be caused by a comparable risk of bias among all included studies. In this SR, an assessment of the risk of bias to evaluate the quality of RCTs or non-randomized studies was performed with different tools that depend on the study design. The Cochrane Collaboration's risk of bias tool was applied to the RCTs, whereas ROBINS-I was applied to the non-randomized studies. There was no high (or serious) risk for each domain of bias in all included studies. The overall risk of bias of one RCT was low and unclear in the other 3, and the 2 non-randomized studies had a moderate overall risk of bias. Herein, the reason why the risk of bias was not high is that the study outcomes such as the time to recovery, extubation time and incidence of PONV could be objectively evaluated with measurement of time or by presence or absence and thus, the risk in some domains of bias was decreased and homogenized. Therefore, a comparable risk of bias among all included studies could lead to no significant difference in heterogeneity between the low subgroup and other (risk of bias) subgroup.
Two of the 6 studies included in this SR were performed in pediatric patients. In addition, the 2 studies used a higher dose (0.5 mg kg−1) of dexamethasone in the intervention group than those of the other 4 studies. The subgroup analysis regarding the age of the study population or the dose of dexamethasone showed that contrary to adults with use of lower doses of dexamethasone, the time to recovery of the TOF ratio to 0.9 in the pediatric studies with use of a higher dose of dexamethasone had shifted to the right, which indicates that dexamethasone may prolong the time to recovery of the TOF ratio to 0.9 after sugammadex administration. However, this shift is not statistically significant. The 2 studies performed in pediatric patients with use of a higher dose of dexamethasone were RCTs, and, interestingly, the results of the 2 studies that the use of dexamethasone tended to prolong time to recovery of the TOF ratio to 0.9 are consistent with the results of the sensitivity analysis of the subgroup with RCT designs.
In the subgroup analysis regarding the pretreatment drugs in the control group, the test for subgroup differences showed there was no significant difference in the heterogeneity of the time to recovery of the TOF ratio to 0.9 between the saline and other antiemetics subgroups, which indicates that like saline, other antiemetics including ondansetron, granisetron, or metoclopramide do not affect the NMB reversal action of sugammadex.
It is known that a single dose of dexamethasone attenuated rocuronium-induced NMB by 15% to 20% if administered 2 to 3 hour before induction of anesthesia.[21] The mechanism is not clear, but an animal experiment showed that dexamethasone treatment led to desensitization of the rat diaphragm to rocuronium, as demonstrated by a shift of the rocuronium concentration–twitch tension curves to the right, and indicated that dexamethasone treatment could induce alterations in muscle function and susceptibility to rocuronium.[22] Conversely, the use of dexamethasone during surgery has little effect on the action of NMBAs, such as rocuronium.[21] For this reason, dexamethasone was administered during anesthesia or surgery in most of the clinical studies investigating whether dexamethasone affects NMB or its reversal.[9–14,21] Meanwhile, if dexamethasone combined with sugammadex prolongs the time to recovery of the TOF ratio to 0.9, the more likely it is to be extended when given simultaneously, considering the blood concentration of dexamethasone. We defined “short” as a time interval between dexamethasone and sugammadex administration of less than 10 minutes and “long” as the time interval greater than 30 to 60 minutes (from anesthesia induction until the end of surgery) (Table 1). We predicted that the shorter the interval between drug administration, the higher the effect of dexamethasone on the reversal of NMB by sugammadex. However, the subgroup analysis regarding the time interval between dexamethasone and sugammadex administration, contrary to expectations, showed that NMB reversal in the dexamethasone group tended to be faster in the case of the “short” interval subgroup, even though there was no statistically significant difference between the dexamethasone group and the control group. This finding may be due to the statistically significant shortening of the time to recovery of the TOF ratio to 0.9 in the dexamethasone group in one (Ozer 2018) of the 2 studies included in the “short” interval subgroup. Given that the study was a prospective observational study,[13] more RCTs are needed to verify the effect of the time interval between dexamethasone and sugammadex administration on the times to recovery of the TOF ratio to 0.9.
The subgroup analysis regarding the degree of NMB (moderate vs deep NMB) at the time of sugammadex administration showed there was no significant difference in the time to recovery of the TOF ratio to 0.9 between the dexamethasone group and the control groups in the 2 (moderate and deep NMB) subgroups, and no significant difference in heterogeneity between the subgroups, which could be caused by the required dose of sugammadex, as determined by the level of NMB at the time of reversal (the deeper the level of NMB, the higher the dose of sugammadex required for NMB reversal; e.g., sugammadex 2 mg kg−1 for moderate NMB reversal and 4 mg kg−1 for deep NMB reversal).[2]
Secondary outcomes were the time to extubation after sugammadex administration and the incidence of PONV. Three RCTs examined the time to extubation, which tended to be longer in the dexamethasone group than in the control group but showed no statistically significant difference between the 2 groups. It is noteworthy that the results for extubation time reported in the 3 RCTs are similar to the results for the time to recovery of the TOF ratio to 0.9 reported in the 4 RCTs (Fig. 6). Given the consistent trend of the results, more RCTs are needed.
The incidence of PONV was investigated in 2 pediatric RCTs, indicating that dexamethasone did not affect the incidence of PONV. Unexpectedly, the reason why the dexamethasone group did not show antiemetic effects superior to the control group was that the incidence of PONV itself was very low in the studies, and the incidence appeared to be zero in the 2 groups as metoclopramide, an antiemetic agent, was used instead of saline in the control group in 1 study (Saleh 2017). It was also challenging to determine the effect of sugammadex on the antiemetic properties of dexamethasone for the same reason.
This SR may be limited by high heterogeneity in the results of the outcomes, which could have been caused by the differences in the study design and region of the included studies. Another limitation is that we could not assess publication bias because the number of the included studies was small (less than 10 studies).
In conclusion, this SR revealed that the time to recovery of the TOF ratio to 0.9 and extubation after sugammadex administration and the incidence of PONV were comparable between the dexamethasone and control groups, and suggested that the use of dexamethasone in the perioperative period neither delayed nor facilitated the reversal of rocuronium-induced NMB with sugammadex in patients undergoing elective surgery with general anesthesia. However, given that these results showed high heterogeneity and there were significant differences in heterogeneity according to the study design (RCT vs. non-randomized studies) and study region (Europe vs. Africa) in the primary outcome of the time to recovery of TOF ratio to 0.9, further studies, especially RCTs are needed to confirm these findings.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Author contributions
Conceptualization: Byung Gun Lim, Heezoo Kim.
Data curation: Byung Gun Lim, Young Ju Won.
Formal analysis: Byung Gun Lim, Heezoo Kim.
Methodology: Byung Gun Lim, Young Ju Won, Heezoo Kim.
Software: Byung Gun Lim, Young Ju Won, Heezoo Kim.
Supervision: Heezoo Kim.
Validation: Byung Gun Lim, Young Ju Won.
Writing – original draft: Byung Gun Lim.
Writing – review & editing: Byung Gun Lim, Young Ju Won, Heezoo Kim.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, IV = intravenous, MeSH = medical subject headings, NMB = neuromuscular blockade, NMBAs = neuromuscular blocking agents, PONV = postoperative nausea and vomiting, PTC = post-tetanic count, RCTs = randomized controlled trials, ROBINS-I = Risk of Bias in Non-randomized Studies - of Interventions, SR = systematic review, TOF = train-of-four, WMD = weighted mean difference.
How to cite this article: Lim BG, Won YJ, Kim H. The effect of dexamethasone on sugammadex reversal of rocuronium-induced neuromuscular blockade in surgical patients undergoing general anesthesia: a systematic review and meta-analysis. Medicine. 2021;100:5(e23992).
This work was supported by a Korea University Grant (K2005171).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
Long, an interval from anesthesia induction until the end of surgery (above 30–60 minutes); Short: an interval within 10 minutes.
Pt = patient, ASA class = American Society of Anesthesiologists physical status classification, IV = intravenous, DEXA = dexamethasone, NMB = neuromuscular blockade, SUGA = sugammadex, TOF = train-of-four count, PTC = post-tetanic count, RCT = randomized controlled trial.
Indicates ondansetron, granisetron, or metoclopramide.
Long, an interval from anesthesia induction until the end of surgery (above 30–60 minutes); Short: an interval within 10 minutes.
95% CI = 95% confident interval, DEXA = dexamethasone, N = the number of studies, NA = not applicable, NMB = neuromuscular blockade, RCT = randomized controlled trial, WMD = weighted mean difference.
ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 2016; 355: i4919.
References
- [1].Gijsenbergh F, Ramael S, Houwing N, et al. First human exposure of Org 25969, a novel agent to reverse the action of rocuronium bromide. Anesthesiology 2005;103:695–703. [DOI] [PubMed] [Google Scholar]
- [2].Kovac AL. Sugammadex: the first selective binding reversal agent for neuromuscular block. J Clin Anesth 2009;21:444–53. [DOI] [PubMed] [Google Scholar]
- [3].Rezonja K, Sostaric M, Vidmar G, et al. Dexamethasone produces dose-dependent inhibition of sugammadex reversal in in vitro innervated primary human muscle cells. Anesth Analg 2014;118:755–63. [DOI] [PubMed] [Google Scholar]
- [4].Lee CH, Peng MJ, Wu CL. Dexamethasone to prevent postextubation airway obstruction in adults: a prospective, randomized, double-blind, placebo-controlled study. Crit Care 2007;11:R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee MJ, Lee KC, Kim HY, et al. Comparison of ramosetron plus dexamethasone with ramosetron alone on postoperative nausea, vomiting, shivering and pain after thyroid surgery. Korean J Pain 2015;28:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg 2000;90:186–94. [DOI] [PubMed] [Google Scholar]
- [7].De Oliveira GS, Jr, Almeida MD, Benzon HT, et al. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 2011;115:575–88. [DOI] [PubMed] [Google Scholar]
- [8].Rezonja K, Lorenzon P, Mars T. Opposing effects of dexamethasone, agrin and sugammadex on functional innervation and constitutive secretion of IL-6 in in vitro innervated primary human muscle cells. Neurosci Lett 2013;549:186–90. [DOI] [PubMed] [Google Scholar]
- [9].Batistaki C, Pandazi A, Kyttari A, et al. Is there an interaction between dexamethasone and sugammadex in real clinical conditions? A randomized controlled trial in patients undergoing laparoscopic cholecystectomy. J Anaesthesiol Clin Pharmacol 2019;35:215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Buonanno P, Laiola A, Palumbo C, et al. Dexamethasone does not inhibit sugammadex reversal after rocuronium-induced neuromuscular block. Anesth Analg 2016;122:1826–30. [DOI] [PubMed] [Google Scholar]
- [11].Gulec E, Biricik E, Turktan M, et al. The effect of intravenous dexamethasone on sugammadex reversal time in children undergoing adenotonsillectomy. Anesth Analg 2016;122:1147–52. [DOI] [PubMed] [Google Scholar]
- [12].Rezonja K, Mars T, Jerin A, et al. Dexamethasone does not diminish sugammadex reversal of neuromuscular block - clinical study in surgical patients undergoing general anesthesia. BMC Anesthesiol 2016;16:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ozer AB, Bolat E, Erhan OL, et al. Sugammadex improves neuromuscular function in patients receiving perioperative steroids. Niger J Clin Pract 2018;21:139–42. [DOI] [PubMed] [Google Scholar]
- [14].Saleh RS, Moustafa MA. Recovery from rocuronium with sugammadex in children premedicated with dexamethasone for prevention of postoperative nausea and vomiting. Egyptian J Anaesth 2017;33:1–4. [Google Scholar]
- [15].Held CR, Sullivan MD. Effects of dexamethasone on sugammadex reversal times of rocuronium: a systematic review protocol. JBI Database Syst Rev Implement Rep 2017;15:1543–51. [DOI] [PubMed] [Google Scholar]
- [16].Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: https://training.cochrane.org/handbook/archive/v5.1/. [Google Scholar]
- [17].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fuchs-Buder T, Claudius C, Skovgaard LT, et al. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anaesthesiol Scand 2007;51:789–808. [DOI] [PubMed] [Google Scholar]
- [21].Soltesz S, Fraisl P, Noe KG, et al. Dexamethasone decreases the duration of rocuronium-induced neuromuscular block: a randomised controlled study. Eur J Anaesthesiol 2014;31:417–22. [DOI] [PubMed] [Google Scholar]
- [22].Chen D, Yang MR, Huang LN, et al. Dexamethasoneinduced hyposensitivity to rocuronium in rat diaphragm associated with musclefiber transformation. Mol Med Rep 2014;9:527–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


