Abstract
Background
Identification of risk factors for food allergy (FA) in infants is an active research area. An important reason is to identify optimal target infants for early introduction of specific food antigens. Although eczema has been used for this purpose, multivariable prediction scores have not been reported.
Objective
The aim of this research is to develop a multivariable prediction score for infants at high risk of FA.
Methods
We performed a cross-sectional analysis of a self-administered questionnaire for the parents of 18-month-old children at well-child visits between April 2016 and March 2017 (development dataset) and between April 2017 and March 2018 (validation dataset). We developed and validated the prediction score.
Results
The questionnaire collection rate was 18,549 of 20,198 (92%) in the development dataset and 18,620 of 19,977 (93%) in the validation dataset. Risk factors for FA were being born in August–December, first child, eczema, atopic dermatitis in father and mother, and FA in mother and sibling(s). For identifying infants with FA, the developed multivariable prediction score showed higher discrimination ability (area under the curve [AUC] = 0.75) than focusing on eczema (AUC = 0.70) in the validation dataset. The score was also useful for identifying infants with a history of anaphylaxis (AUC = 0.73) than focusing on eczema (AUC = 0.67) in the validation dataset.
Conclusion
The new prediction score enables more efficient identification of infants at high risk of FA, who may be the optimal target group for the early introduction of specific antigens.
Keywords: Infant, Food allergy, Anaphylaxis, Prediction model, Early introduction
INTRODUCTION
The prevalence of food allergy (FA) has been reported at 2% to 10% of children worldwide [1] and is increasing rapidly in certain countries [2,3,4]. FA influences several aspects of society [5,6], in addition to the health status of patients [7] and the quality of life of affected patients and their families [8].
As the recommendation of a delayed introduction of solid foods has shown undesirable results, 2 subsequent clinical studies [9,10] have led to the early introduction of solid foods being recommended to prevent the development of FA in infants, which includes peanuts in the USA [11] and hen's eggs in Japan [12]. This recommendation was aimed at infants with severe eczema and/or egg allergy [11] and atopic dermatitis (AD) [12], which followed the enrollment approached use in these 2 clinical studies [9,10].
In addition to eczema/AD, prior studies have identified earlier birth order [13], fall/winter season of birth [14], and positive family history of FA [15] as risk factors for FA. To date, however, a multivariable prediction model of FA has not been reported. In previous reports [16,17,18], we have found that multifactorial prediction is superior to use of a single factor. More efficient identification of children for which early introduction is recommended might be possible by using a multivariable prediction model.
The aim of the present study was to identify factors associated with FA in infants (age <2 years) and to develop a multivariable prediction model for infants at high risk of FA based on these large, population-based data with very high response rates. We hypothesized that the multivariable prediction model would perform better than predicting risk using eczema status alone.
MATERIALS AND METHODS
Dataset
The development and validation dataset were obtained from 18-month WCVs in Nagoya City, Japan (supplement material). Data collected between April 2016 and March 2017 were used for the development dataset, and data collected between April 2017 and March 2018 were used for the validation dataset.
The self-administered questionnaire for 18-month WCVs, which was made for this survey, included the following factors: birthday, sex, order of birth, history of eczema (with itch, with topical steroid use), history of wheezing, history of immediate reaction after ingesting foods or milk, clinician-diagnosed allergic disease (AD, bronchial asthma [BA], FA, anaphylaxis, allergic rhinitis [AR]/allergic conjunctivitis [AC], and hay fever), and family history of allergic disease (AD, BA, FA, AR/AC, and hay fever).
Regarding eczema, we asked parents “Has your child experienced eczema which repeated appearance and disappearance within 12 months?” and defined the infants as having eczema if their answers were “yes.” We also asked the parents who had experienced eczema “Did your child experienced itch with the eczema?” and “Did you used topical steroid for the eczema?”; we defined them “eczema with itch” and “eczema with topical steroid use” if their answers were “yes,” respectively. While regarding immediate reaction after ingesting foods or milk, we asked “Has your child experienced red flare or swelling of skin after ingestion foods or milk?” and defined the infants as having experience of immediate reaction if their answers were “yes.”
Definition of FA
The questionnaire includes questions regarding history of immediate reaction and diagnosis of FA by a medical doctor. As a result, the participants were divided into 4 groups: group 1, immediate reaction positive and diagnosis positive; group 2, immediate reaction negative and diagnosis positive; group 3, immediate reaction positive and diagnosis negative; and group 4, immediate reaction negative and diagnosis negative. Among these groups, group 1 was considered to have the highest likelihood of true FA. The participants in group 1 were considered to have FA in the primary analysis, with the other groups used in sensitivity analyses.
Background characteristics
For describing background characteristics, we compared the group with FA and without FA. High-risk birth months was defined as the month in which the proportion of FA is larger than the one in overall participants. Being the first child also was analyzed as a potential risk factor [13].
We also evaluate the concordance of eczema-related-factor (eczema, eczema with itch/topical steroid use, or clinician-diagnosed AD) separately. They were evaluated their discrimination ability for FA by using receiver operating characteristic (ROC) analysis; the factor with the largest AUC was accepted as the representative factor.
Development of the prediction model
Multivariable logistic regression analysis was used to identify factors associated with FA. In this analysis, we analyzed all factors considered to be present prior to the onset of FA. Family history of sibling(s) of the first child considered as negative in original analysis and later performed subgroup analysis. On the basis of the multivariable logistic regression analysis, we constructed a logistic regression model using the independent factors with a probability value p < 0.0026 (based on Bonferroni correction). A simple scoring model was then created by assigning the point scores of each variable in accordance to the adjusted β values calculated by the analysis [16,17,18].
The discriminative ability of these models was assessed using the area under the ROC curve (AUC) and compared with the eczema-related-factor (eczema, eczema with itch/topical steroid use, or clinician-diagnosed AD) with the highest AUC. We also evaluated net reclassification improvement (NRI) as an indicator of discrimination ability. The goodness-of-fit of the regression models was tested with the Hosmer–Lemeshow test, with p < 0.05 indicating a lack of deviation between the model and observed event rate.
Confirmation of model robustness: validation of model and sensitivity analysis
To confirm the robustness of the models, validation of the model was performed in the independent dataset, which was based on WCVs in Nagoya City between April 2017 and March 2018. The discriminatory ability was compared with the eczema-related-factor with the highest AUC. The goodness-of-fit of the model was also evaluated. Cutoff points for the prediction score were also evaluated for its sensitivity and specificity for FA and clinician-diagnosed anaphylaxis in the validation dataset.
We also performed some other sensitivity analyses including prediction for different definition of FA, prediction for FA of different antigen, subgroup analysis stratified by sibling status, and multivariable multilevel modeling with random intercept of resident area (16 wards in Nagoya city), which were considered to be associated with socioeconomic status.
Statistical analysis and ethical consideration
Mann–Whitney U test was used for continuous variables, whereas chi-square test was used for categorical data. All analyses were performed with the STATA (version 12.1 for Mac; STATA Inc., College Station, TX, USA) software program. A two-sided probability value p < 0.05 was considered to indicate statistical significance, but Bonferroni correction was accepted when multiple factors were analyzed.
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) in Aichi Children's Health and Medical Center, Obu, Japan (2018086) and Harvard University, Boston, USA (IRB number: 19-0435).
RESULTS
Participation in group WCVs and completion of the allergy survey
On the basis of the Certificate of Residence, a total of 20,198 children were qualified for the 18-month group WCVs in Nagoya City during the development dataset period (April 1, 2016–March 31, 2017). In this period, 19,313 questionnaires (95.6%) were submitted. Following data cleaning (e.g., removal of partially complete forms), the development dataset was based on 18,549 questionnaires (91.8%) (Fig. 1).
Fig. 1. Flowchart of data collection. WCVs, well-child visits.
In the same manner, 19,298 questionnaires were collected during the validation dataset period (April 1, 2017– March 31, 2018). Following data cleaning, the validation dataset was based on 18,620 questionnaires (93.2%) (Fig. 1).
Definition of FA
Among the 18,549 participants in the development dataset, 3,155 participants had experienced immediate allergic reactions and 2,528 parents answered that they have been diagnosed with FA by a clinician (Supplementary Table 1). Of the participants, 1,747 participants (9.4%) were allocated to group 1, 1,408 participants (7.6%) to group 2, 781 participants (4.2%) to group 3, and 14,613 participants (78.8%) to group 4. The proportion of each group in the validation dataset was similar (9.0%, 6.7%, 4.0%, 80.3%, respectively, Supplementary Table 2).
Background characteristics
The background characteristics of participants in development dataset are shown in Table 1. The high-risk birth months was defined as the months from August to December based on the distribution of the data (Supplementary Fig. 1). Eczema was defined as a factor among eczema-related factors (Supplementary Table 3).
Table 1. Background characteristics of participants in the development dataset.
| Characteristic | Total (n = 18,549) | With food allergy (n = 1,747) | Without food allergy (n = 16,802) | p value | |
|---|---|---|---|---|---|
| High-risk birth months (August–December) | 7,744 (41.7) | 912 (52.2) | 6,832 (40.7) | <0.001 | |
| Male sex | 9,480 (51.1) | 968 (55.4) | 8,512 (50.7) | <0.001 | |
| First child | 9,593 (51.7) | 984 (56.3) | 8,609 (51.2) | <0.001 | |
| Eczema | 7,229 (39.0) | 1,334 (76.4) | 5,895 (35.1) | <0.001 | |
| Family history of atopic dermatitis | |||||
| Father | 2,164 (11.7) | 337 (19.3) | 1,827 (10.9) | <0.001 | |
| Mother | 3,088 (16.6) | 437 (25.0) | 2,651 (15.8) | <0.001 | |
| Siblings* | 1,013/8,956 (11.3) | 122/763 (16.0) | 891/8,193 (10.9) | <0.001 | |
| Family history of food allergy | |||||
| Father | 824 (4.4) | 138 (7.9) | 686 (4.1) | <0.001 | |
| Mother | 1,006 (5.4) | 195 (11.2) | 811 (4.8) | <0.001 | |
| Siblings* | 1,254/8,956 (14.0) | 221/763 (29.0) | 1,033/8,193 (12.6) | <0.001 | |
| Family history of bronchial asthma | |||||
| Father | 1,374 (7.4) | 189 (10.8) | 1,185 (7.1) | <0.001 | |
| Mother | 1,395 (7.5) | 174 (10.0) | 1,221 (7.3) | <0.001 | |
| Siblings* | 741/8,956 (8.3) | 89/763 (11.7) | 652/8,193 (8.0) | 0.001 | |
| Family history of allergic rhinitis/conjunctivitis | |||||
| Father | 2,883 (15.5) | 353 (20.2) | 2,530 (15.1) | <0.001 | |
| Mother | 3,915 (21.1) | 422 (24.2) | 3,493 (20.8) | 0.001 | |
| Siblings* | 1,093/8,956 (12.2) | 108/763 (14.2) | 985/8,193 (12.0) | 0.14 | |
| Family history of hay fever | |||||
| Father | 6,553 (35.3) | 755 (43.2) | 5,798 (34.5) | <0.001 | |
| Mother | 6,614 (35.7) | 742 (42.5) | 5,872 (34.9) | <0.001 | |
| Siblings* | 1,205/8,956 (13.5) | 129/763 (16.9) | 1,076/8,193 (13.1) | 0.01 | |
Values are presented as number (%).
Each p value was calculated using chi-square test.
*The proportions were calculated based on the participants with at least one sibling.
Among 18,549 participants, eczema had been observed in 7,229 (39.0%) while in 1,334 of the 1,747 children (76.4%) with FA. The parents-reported causative antigens of participants' FA in development dataset (n = 1,749) are shown in Supplementary Fig. 2. Briefly, 84.8% for hen's egg, 36.6% for cow's milk, 14.8% for wheat, less than 5.0% for other antigens.
Development of the prediction model
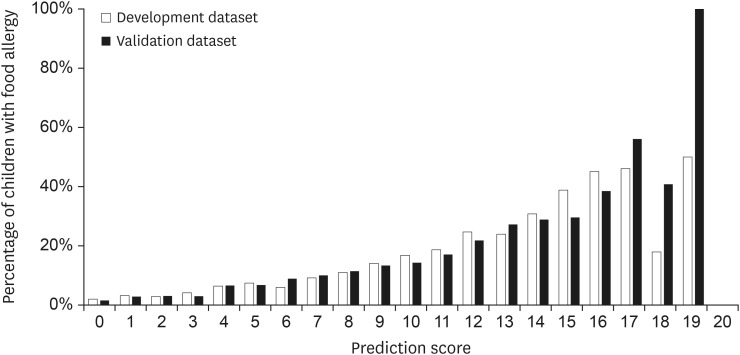
The results of the multivariable logistic regression analysis are shown in Table 2. High-risk birth months, first child, eczema, family history of AD of father and mother, and family history of FA in mother and sibling were identified as independent risk factors for FA (p < 0.0026). The prediction score consisted of 8 points for history of eczema (adjusted β = 1.65), 4 points for FA of siblings (adjusted β = 0.88), 3 points for FA of mother (adjusted β = 0.59), 2 points for high-risk birth months (August–December, adjusted β = 0.38), and 1 point each for the first child, AD of the father, and AD of the mother (adjusted β = 0.22, 0.29, and 0.22, respectively), resulting in a maximum of 20 points (theoretical maximum 19 points). The proportion of the children with FA increased in accordance with the increase of prediction score (Fig. 2). Only a few participants had a score of 18 (n = 11 and n = 17 in 2016 and 2017, respectively) and 19 (n = 2 both in 2016 and in 2017).
Table 2. Results of multivariable logistic regression analysis and assigned score points for the prediction model.
| Variable | Adjusted β (95% CI) | Adjusted OR (95% CI) | p value | Score points | |
|---|---|---|---|---|---|
| High-risk birth months (August–December) | 0.38 (0.27–0.48) | 1.46 (1.31–1.62) | <0.001 | 2 | |
| Male sex | 0.16 (0.05–0.26) | 1.17 (1.05–1.30) | 0.003 | ||
| First child | 0.22 (0.10–0.34) | 1.24 (1.10–1.40) | <0.001 | 1 | |
| Eczema | 1.65 (1.54–1.77) | 5.22 (4.64–5.88) | <0.001 | 8 | |
| Family history of atopic dermatitis | |||||
| Father | 0.29 (0.15–0.43) | 1.33 (1.16–1.53) | <0.001 | 1 | |
| Mother | 0.22 (0.09–0.35) | 1.25 (1.10–1.42) | 0.001 | 1 | |
| Siblings | −0.17 (−0.40 to 0.06) | 0.84 (0.67–1.06) | 0.09 | ||
| Family history of food allergy | |||||
| Father | 0.27 (0.06–0.48) | 1.31 (1.07–1.61) | 0.04 | ||
| Mother | 0.59 (0.41–0.77) | 1.81 (1.50–2.17) | <0.001 | 3 | |
| Siblings | 0.88 (0.69–1.06) | 2.40 (1.99–2.90) | <0.001 | 4 | |
| Family history of bronchial asthma | |||||
| Father | 0.20 (0.02–0.37) | 1.22 (1.02–1.45) | 0.02 | ||
| Mother | −0.01 (−0.19 to 0.18) | 0.99 (0.83–1.20) | 0.69 | ||
| Siblings | 0.11 (−0.15 to 0.37) | 1.12 (0.86–1.45) | 0.45 | ||
| Family history of allergic rhinitis/conjunctivitis | |||||
| Father | 0.06 (−0.08 to 0.20) | 1.06 (0.93–1.22) | 0.38 | ||
| Mother | −0.17 (−0.3 to −0.04) | 0.85 (0.74–0.96) | 0.01 | ||
| Siblings | −0.11 (−0.36 to 0.14) | 0.89 (0.70–1.14) | 0.37 | ||
| Family history of hay fever | |||||
| Father | 0.17 (0.06–0.28) | 1.18 (1.06–1.32) | 0.003 | ||
| Mother | 0.16 (0.05–0.27) | 1.17 (1.05–1.31) | 0.005 | ||
| Siblings | −0.02 (−0.25 to 0.21) | 0.98 (0.78–1.24) | 0.88 | ||
OR, odds ratio; CI, confidence interval.
Fig. 2. Percentages of children with food allergy by prediction score. White bars indicate children with food allergy in the development dataset; black bars indicate those in the validation dataset.
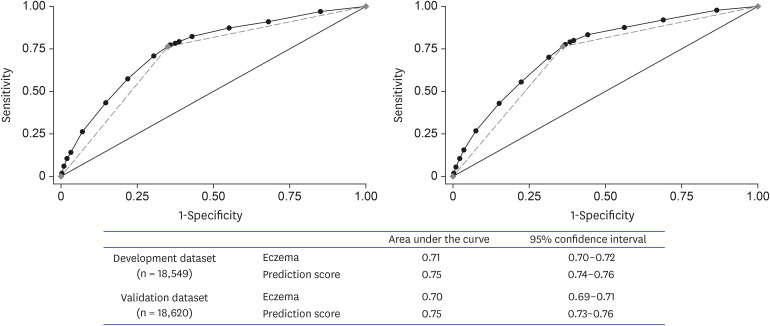
The Hosmer–Lemeshow statistic was not significant (p = 0.63 and p = 0.40 for the logistic model and the simple scoring model, respectively). The simple scoring model was superior in its discriminatory ability for predicting children with FA (AUC = 0.75) compared with prediction using repetitive eczema alone (AUC = 0.71; p < 0.001 for comparison; Fig. 3, left panel); NRI confirmed the results (p < 0.05).
Fig. 3. ROC analysis of prediction score and repetitive eczema as a prediction factor in development (left) and validation dataset (right). The black circles indicate the ROC curve of prediction score, and the gray diamond indicates the ROC curve of eczema alone. ROC, receiver operating characteristic.
Confirmation of the model robustness through validation of the model and sensitivity analysis
The distribution of questionnaire results regarding FA status in the validation dataset is shown in Supplementary Table 2. The background characteristics of the validation dataset are shown in Supplementary Table 4, which were similar results as development dataset (Supplementary Table 5). Multivariable multilevel modeling with random intercept of resident area showed similar results as original analysis (Supplementary Table 6).
In the validation dataset, the AUC was 0.75 for the prediction score, which was superior in its discriminatory ability compared with eczema alone (AUC = 0.70; p < 0.001 for comparison; Fig. 3, right panel), and the Hosmer–Lemeshow statistic was not significant (p = 0.14). These findings were confirmed by some sensitivity analyses (Supplementary Figs. 3, 4, 5). Moreover, the score has superior discrimination ability to identify the children with a history of anaphylaxis (AUC = 0.73 for the prediction score, 0.67 for repetitive eczema). This superiority of prediction model was confirmed by NRI (p < 0.05). Some cutoff of prediction score or solely eczema in validation dataset and its sensitivity, specificity, positive predictive value, and negative predictive value are shown in Table 3. When using a prediction score cutoff of ≥13, the specificity for both FA and anaphylaxis was >95%.
Table 3. Cutoff points and sensitivity/specificity of the prediction scores for food allergy and anaphylaxis in the validation dataset.
| Factor | Subjects | Food allergy | Anaphylaxis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||
| Prediction score | ||||||||||
| 1 | 16,312 | 97.7 | 13.4 | 10.0 | 98.3 | 97.0 | 12.4 | 0.40 | 99.9 | |
| 3 | 11,004 | 87.6 | 43.7 | 13.3 | 97.3 | 86.6 | 41.0 | 0.53 | 99.9 | |
| 8 | 7,439 | 76.7 | 63.7 | 17.3 | 96.5 | 73.1 | 60.2 | 0.66 | 99.8 | |
| 9 | 6,495 | 70.1 | 68.6 | 18.1 | 95.9 | 70.2 | 65.3 | 0.72 | 99.8 | |
| 10 | 4,723 | 55.6 | 77.6 | 19.7 | 94.7 | 58.2 | 74.8 | 0.83 | 99.8 | |
| 11 | 3,289 | 43.0 | 84.9 | 21.9 | 93.8 | 47.8 | 82.4 | 0.97 | 99.8 | |
| 12 | 1,711 | 26.7 | 92.5 | 26.1 | 92.7 | 32.8 | 90.9 | 1.29 | 99.7 | |
| 13 | 864 | 15.5 | 96.4 | 30.1 | 92.0 | 25.4 | 95.4 | 1.97 | 99.7 | |
| 14 | 557 | 10.5 | 97.8 | 31.6 | 91.7 | 20.9 | 97.1 | 2.51 | 99.7 | |
| 15 | 265 | 5.4 | 99.0 | 34.3 | 91.4 | 14.9 | 98.6 | 3.77 | 99.7 | |
| 16 | 84 | 2.2 | 99.7 | 44.0 | 91.2 | 7.5 | 99.6 | 5.95 | 99.7 | |
| Eczema | 7,400 | 76.5 | 63.9 | 17.3 | 96.5 | 73.1 | 60.4 | 0.66 | 99.8 | |
| Eczema with itch | 5,495 | 65.0 | 73.7 | 19.5 | 95.5 | 72.7 | 70.4 | 0.87 | 99.9 | |
Sensitivity/specificity of “eczema” and “eczema with itch” are also shown.
PPV, positive predictive value; NPV, negative predictive value.
DISCUSSION
In the present study, we developed a multivariable prediction model to identify infants at high risk of FA based on population-based data with a high response rate, and then validated the model. The prediction score performed as better predictor for high-risk infants of FA compared with eczema although it was not very accurate probably due to the small sample size in the highest and second highest scores. To the best of our knowledge, the present study is the first to report the usefulness of a multivariable model for predicting infants at high risk of FA.
Consistent with prior reports, the factors identified as being associated with FA by multivariable logistic regression analysis were: eczema [19], birth order [13], season of birth [14], and family history [15]. These findings are important in the sense of that the present study is based on a population-based survey. In addition, the magnitude of the association between each factor and the outcome was successfully identified in the present study.
Similar to a previous study [20], eczema was the factor that was most associated with FA. Judged from the discrimination ability, the factors “eczema with itch,” “eczema with topical steroid use,” and “clinician-diagnosed AD,” which may indicate more severe eczema, failed to show superior discrimination ability to “eczema.” However, judged from the significantly higher proportion of eczema with itch/topical steroid use or AD among participants with eczema and FA, these factors are considered to be high specificity but low sensitivity, therefore these questions are not suitable for primary screening. From the results obtained, “eczema” was considered the most suitable question for the general population to evaluate the risk of FA.
In the family history of allergic disease, FA of the mother and siblings are also associated with a high proportion of FA strongly. The association between maternal, but not paternal, FA and the delayed introduction of solid foods has been reported before [21]. The results of the present study suggest they were influenced by the delayed introduction of specific solid foods which are common as causative antigens of food allergies, such as hen's egg.
The association with season of birth has been reported previously [14]. The role of vitamin D deficiency has been suggested in previous reports [22,23] because the association between season of birth and sun exposure has also been reported [24] and sunlight is known to influence vitamin D status. Although no prospective study has shown the efficacy of supplementation of vitamin D for mothers for the prevention of FA [20,25], supplementation in infants is a possible avenue for future research.
Following confirmation of the efficacy of the early introduction of specific foods, the main question is how to apply these findings to practice in the real world [26]. Screening tests of FA for infants with eczema alone, which is recommended from academic community in USA [11], are not cost-effective from recent reports of economic perspective [27,28]. However, for the infants with higher risk of FA and anaphylaxis based on our prediction score (i.e., prediction score of ≥13), we should consider screening tests for some common antigens depending on the region. The cost-effectiveness of such screening tests requires evaluation given the more accurate prediction of our prediction score, which would be constructed with no effort except for providing a self-administered questionnaire.
The study has several potential limitations. Most importantly, we acknowledge some uncertainty about the FA case definition due to being based on a self-administered questionnaire. For that reason, we included a sensitivity analysis. The analysis compared children with the highest likelihood of true FA with those with the least likelihood, and the results were similar. This finding suggests the robustness of the prediction score.
It also was not possible to evaluate the severity or onset of eczema, although that is the USA recommendation in this context [11]. We agree that eczema severity is an important factor for identifying infants at high risk of peanut allergy. However, evaluation of eczema severity is difficult for the general population, as suggested by the results of the eczema-related-factors shows. Therefore, we suggest that the prediction model established here should be used as a primary screening tool for FA, and infants with a high prediction score then should be evaluated by an allergist/immunologist.
Although statistically better than solely eczema, the prediction score might be not substantially superior to the factor in terms of accuracy. However, the score could predict infants with a higher risk than eczema alone, which enables us to discuss and decide the optimal cutoff score in each society, considering the available human recourses and cost.
In conclusion, we used population-based data with a high response rate to develop a multivariable prediction model to identify infants at high risk of FA. The prediction model was validated using a separate dataset, other definitions of FA or other sensitivity analysis. Using the new prediction score, clinicians now can better identify infants at high-risk of FA, which should be useful for identifying the optimal target group of infants for early introduction or screening tests of specific foods. We encourage identification of target infants for early introduction or screening test depending on the society and based on the most accurate prediction possible.
ACKNOWLEDGEMENTS
This research was conducted with support from the Takemi Program in International Health, Harvard T.H. Chan School of Public Health. We thank Yasuto Kondo from Department of Pediatrics, Fujita Health University General Allergy Center, Nagoya, Japan for critical revise of the manuscript. We thank Nakanishi Rieko from Allergies Support Network, Nagoya, Japan for providing interpretation of data form the view point of the patients.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Shiro Sugiura.
- Formal analysis: Shiro Sugiura.
- Investigation: Shiro Sugiura, Yoshimichi Hiramitsu, Masaki Futamura, Naomi Kamioka, Chikae Yamaguchi, Harue Umemura, Komei Ito.
- Methodology: Shiro Sugiura, Carlos A Camargo Jr.
- Project administration: Shiro Sugiura, Yoshimichi Hiramitsu, Masaki Futamura, Naomi Kamioka, Chikae Yamaguchi, Harue Umemura, Komei Ito.
- Writing - original draft: Shiro Sugiura.
- Writing - review & editing: Carlos A Camargo Jr.
SUPPLEMENTARY MATERIALS
Definition of the food allergy in the development dataset
Definition of food allergy based on the self-administered survey in the validation dataset
The discrimination ability of each eczema-related-factors
Background characteristics of participants in the validation dataset
Comparison of Background characteristics of participants in the development and validation dataset
Results of multivariable logistic regression analysis in original analysis, sensitivity analyses 1 and 2
The association between proportion of participants with food allergies and their birth month.
Causative antigens of the participants with food allergy (n = 1,749) in development dataset (n = 18,549).
Receiver operating characteristic curves of prediction score and repetitive eczema as predictive factors in sensitivity analyses 1 and 2.
Receiver operating characteristic curves of prediction score and repetitive eczema as predictive factors in sensitivity analyses 3 and 4.
Receiver operating characteristic curves of prediction score and repetitive eczema as predictive factors in sensitivity analyses 5 and 6.
References
- 1.Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn JK, Fiocchi A, Ebisawa M, Sampson HA, Beyer K, Lee BW. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013;6:21. doi: 10.1186/1939-4551-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy J, Matthews S, Bateman B, Dean T, Arshad SH. Rising prevalence of allergy to peanut in children: Data from 2 sequential cohorts. J Allergy Clin Immunol. 2002;110:784–789. doi: 10.1067/mai.2002.128802. [DOI] [PubMed] [Google Scholar]
- 3.Venter C, Hasan Arshad S, Grundy J, Pereira B, Bernie Clayton C, Voigt K, Higgins B, Dean T. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010;65:103–108. doi: 10.1111/j.1398-9995.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 4.Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatr. 2013;167:1026–1031. doi: 10.1001/jamapediatrics.2013.2376. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki K, Sugiura S, Matsui T, Nakagawa T, Nakata J, Kando N, Ito K. A workshop with practical training for anaphylaxis management improves the self-efficacy of school personnel. Allergol Int. 2015;64:156–160. doi: 10.1016/j.alit.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Umasunthar T, Leonardi-Bee J, Hodes M, Turner PJ, Gore C, Habibi P, Warner JO, Boyle RJ. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2013;43:1333–1341. doi: 10.1111/cea.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flokstra-de Blok BM, Dubois AE, Vlieg-Boerstra BJ, Oude Elberink JN, Raat H, DunnGalvin A, Hourihane JO, Duiverman EJ. Health-related quality of life of food allergic patients: comparison with the general population and other diseases. Allergy. 2010;65:238–244. doi: 10.1111/j.1398-9995.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 9.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, Turcanu V, Sever ML, Gomez Lorenzo M, Plaut M, Lack G LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natsume O, Kabashima S, Nakazato J, Yamamoto-Hanada K, Narita M, Kondo M, Saito M, Kishino A, Takimoto T, Inoue E, Tang J, Kido H, Wong GW, Matsumoto K, Saito H, Ohya Y PETIT Study Team. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:276–286. doi: 10.1016/S0140-6736(16)31418-0. [DOI] [PubMed] [Google Scholar]
- 11.Togias A, Cooper SF, Acebal ML, Assa'ad A, Baker JR, Jr, Beck LA, Block J, Byrd-Bredbenner C, Chan ES, Eichenfield LF, Fleischer DM, Fuchs GJ, 3rd, Furuta GT, Greenhawt MJ, Gupta RS, Habich M, Jones SM, Keaton K, Muraro A, Plaut M, Rosenwasser LJ, Rotrosen D, Sampson HA, Schneider LC, Sicherer SH, Sidbury R, Spergel J, Stukus DR, Venter C, Boyce JA. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139:29–44. doi: 10.1016/j.jaci.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuie T, Ohya Y, Ebisawa M, Itoh K, Aihara Y, Itoh S, et al. Proposal on the prevention of hen's egg allergy. Jpn J Pediatr Allergy Clin Immunol. 2017;31:i–x. (Japanese) [Google Scholar]
- 13.Kikkawa T, Yorifuji T, Fujii Y, Yashiro M, Okada A, Ikeda M, Doi H, Tsukahara H. Birth order and paediatric allergic disease: a nationwide longitudinal survey. Clin Exp Allergy. 2018;48:577–585. doi: 10.1111/cea.13100. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Matsui T, Sato A, Sasaki K, Nakata J, Nakagawa T, Sugiura S, Kando N, Nishiyama T, Kojima S, Ito K. The relationship between the season of birth and early-onset food allergies in children. Pediatr Allergy Immunol. 2015;26:607–613. doi: 10.1111/pai.12440. [DOI] [PubMed] [Google Scholar]
- 15.Nwaru BI, Hickstein L, Panesar SS, Muraro A, Werfel T, Cardona V, Dubois AE, Halken S, Hoffmann-Sommergruber K, Poulsen LK, Roberts G, Van Ree R, Vlieg-Boerstra BJ, Sheikh A EAACI Food Allergy and Anaphylaxis Guidelines Group. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014;69:62–75. doi: 10.1111/all.12305. [DOI] [PubMed] [Google Scholar]
- 16.Sugiura S, Matsui T, Nakagawa T, Sasaki K, Nakata J, Kando N, Ito K. Development of a prediction model of severe reaction in boiled egg challenges. Allergol Int. 2016;65:293–299. doi: 10.1016/j.alit.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Sugiura S, Sasaki K, Matsui T, Nakagawa T, Kando N, Ito K. Development of a prediction model for a severe reaction in cow's milk challenges. Allergol Int. 2017;66:493–494. doi: 10.1016/j.alit.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Sugiura S, Matsui T, Furuta T, Sasaki K, Kando N, Ito K. Development of a prediction model for severe wheat allergy. Pediatr Allergy Immunol. 2018;29:93–96. doi: 10.1111/pai.12806. [DOI] [PubMed] [Google Scholar]
- 19.Du Toit G, Roberts G, Sayre PH, Plaut M, Bahnson HT, Mitchell H, Radulovic S, Chan S, Fox A, Turcanu V, Lack G Learning Early About Peanut Allergy (LEAP) Study Team. Identifying infants at high risk of peanut allergy: the Learning Early About Peanut Allergy (LEAP) screening study. J Allergy Clin Immunol. 2013;131:135–43.e1-12. doi: 10.1016/j.jaci.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Norizoe C, Akiyama N, Segawa T, Tachimoto H, Mezawa H, Ida H, Urashima M. Increased food allergy and vitamin D: randomized, double-blind, placebo-controlled trial. Pediatr Int. 2014;56:6–12. doi: 10.1111/ped.12207. [DOI] [PubMed] [Google Scholar]
- 21.McKean M, Caughey AB, Leong RE, Wong A, Cabana MD. The timing of infant food introduction in families with a history of atopy. Clin Pediatr (Phila) 2015;54:745–751. doi: 10.1177/0009922815584927. [DOI] [PubMed] [Google Scholar]
- 22.Allen KJ, Koplin JJ, Ponsonby AL, Gurrin LC, Wake M, Vuillermin P, Martin P, Matheson M, Lowe A, Robinson M, Tey D, Osborne NJ, Dang T, Tina Tan HT, Thiele L, Anderson D, Czech H, Sanjeevan J, Zurzolo G, Dwyer T, Tang ML, Hill D, Dharmage SC. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. J Allergy Clin Immunol. 2013;131:1109–1116. 1116.e1–1106. doi: 10.1016/j.jaci.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Matsui T, Yamashita H, Saneyasu KI, Tanaka H, Ito K, Inagaki N. Vitamin D deficiency exacerbates sensitization and allergic diarrhea in a murine food allergy model. Allergol Int. 2018;67:289–291. doi: 10.1016/j.alit.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Matsui T, Tanaka K, Nakagawa T, Sasaki K, Nakata J, Sugiura S, Kando N, Ito K. Sun exposure inversely related to food sensitization during infancy. Pediatr Allergy Immunol. 2015;26:628–633. doi: 10.1111/pai.12445. [DOI] [PubMed] [Google Scholar]
- 25.Tuokkola J, Luukkainen P, Kaila M, Takkinen HM, Niinistö S, Veijola R, Virta LJ, Knip M, Simell O, Ilonen J, Virtanen SM. Maternal dietary folate, folic acid and vitamin D intakes during pregnancy and lactation and the risk of cows' milk allergy in the offspring. Br J Nutr. 2016;116:710–718. doi: 10.1017/S0007114516002464. [DOI] [PubMed] [Google Scholar]
- 26.Koplin JJ, Peters RL, Dharmage SC, Gurrin L, Tang MLK, Ponsonby AL, Matheson M, Togias A, Lack G, Allen KJ HealthNuts study investigators. Understanding the feasibility and implications of implementing early peanut introduction for prevention of peanut allergy. J Allergy Clin Immunol. 2016;138:1131–41.e2. doi: 10.1016/j.jaci.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Shaker M, Stukus D, Chan ES, Fleischer DM, Spergel JM, Greenhawt M. “To screen or not to screen”: comparing the health and economic benefits of early peanut introduction strategies in five countries. Allergy. 2018;73:1707–1714. doi: 10.1111/all.13446. [DOI] [PubMed] [Google Scholar]
- 28.Shaker M, Verma K, Greenhawt M. The health and economic outcomes of early egg introduction strategies. Allergy. 2018;73:2214–2223. doi: 10.1111/all.13565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definition of the food allergy in the development dataset
Definition of food allergy based on the self-administered survey in the validation dataset
The discrimination ability of each eczema-related-factors
Background characteristics of participants in the validation dataset
Comparison of Background characteristics of participants in the development and validation dataset
Results of multivariable logistic regression analysis in original analysis, sensitivity analyses 1 and 2
The association between proportion of participants with food allergies and their birth month.
Causative antigens of the participants with food allergy (n = 1,749) in development dataset (n = 18,549).
Receiver operating characteristic curves of prediction score and repetitive eczema as predictive factors in sensitivity analyses 1 and 2.
Receiver operating characteristic curves of prediction score and repetitive eczema as predictive factors in sensitivity analyses 3 and 4.
Receiver operating characteristic curves of prediction score and repetitive eczema as predictive factors in sensitivity analyses 5 and 6.