Abstract
Background
Suspicion of beta-lactam (BL) hypersensitivity is often based on parental report. Evaluation is important as incorrect labelling has clinical consequence.
Objective
To describe the outcomes of drug provocation test (DPT) in children with suspected hypersensitivity.
Methods
A retrospective study of patients who completed BL DPT from 1 August 2016 to 31 December 2017 at a paediatric allergy centre in Singapore. Suspected hypersensitivity reactions were classified as immediate (onset ≤1 hour) or delayed (onset > 1 hour). Patients with immediate reactions underwent skin prick test (SPT) followed by DPT if SPT was negative. Patients with delayed reactions underwent DPT directly.
Results
We identified 120 children who reported 121 suspected hypersensitivity reactions. The median age at reaction was 2.0 years (interquartile range [IQR], 1.0–5.0 years) and the median age at DPT was 7.4 years (IQR, 4.2–11.1 years). The timing of suspected hypersensitivity reaction was immediate in 21% (25 of 121), delayed in 66% (80 of 121), and uncertain in 13% (16 of 121). Commonly implicated drugs were amoxicillin in 45% (54 of 121), amoxicillin-clavulanate in 37% (45 of 121), and cephalexin in 8% (10 of 121). Commonly reported symptoms were maculopapular rash 44% (53 of 121), urticaria 34% (41 of 121), and angioedema 22% (27 of 121). All SPTs (n = 26) were negative. There were 118 diagnostic DPTs to index drug and 3 DPTs to alternative drug. A negative challenge result was obtained in 93% (110 of 118) of diagnostic DPTs: 92% (96 of 104) and 100% (14 of 14) of DPTs to penicillin group and cephalosporins respectively. All challenge reactions were mild.
Conclusion
Our study supports the opinion that prior skin tests may not be necessary for children who report nonsevere reactions and directly performing diagnostic DPT is a safe approach in the evaluation of suspected childhood BL hypersensitivity.
Keywords: Antibiotic allergy, Child, Drug allergy, Drug hypersensitivity, Oral provocation test
INTRODUCTION
Drug hypersensitivity reaction is a common concern in children and beta-lactam (BL) antibiotics are commonly implicated [1,2]. In a review of the inpatient electronic medical records of 8,437 children in Singapore, adverse drug reactions were reported in 222 patients (2.6%), of which 45% were attributed to BL antibiotics [3]. In a questionnaire study of German children of median age 3.5 years, the lifetime prevalence of an adverse drug reaction was 7.5% and BL accounted for 79% of possible allergic reactions [4]. Similarly, in a questionnaire study of Singaporean school children age 7 to 16 years, the prevalence of a self-reported adverse drug reaction was 5% and 57% were related to BL antibiotics [5]. Phone interview of selected subjects revealed that although most patients visited a doctor upon suspected reaction, only 7% were referred to tertiary institutes for further investigation [5]. In the paediatric population, viral exanthems are often misinterpreted as drug hypersensitivity reactions [6]. Prior studies suggest that over 90% of children with suspected BL hypersensitivity do not react upon oral provocation, suggesting that true drug hypersensitivity reactions are uncommon or may wane with time [7,8].
It is important to confirm the diagnosis of BL hypersensitivity because reported antibiotic allergies are associated with increased use of broad-spectrum antibiotics, longer hospital stay, increased healthcare cost, and persistent parental fear of BL antibiotics [9,10]. Antibiotic allergy labelling is a public health issue and allergy testing is a recognised component of antimicrobial stewardship [11]. The diagnostic evaluation of suspected drug hypersensitivity helps to minimize unnecessary antibiotic avoidance and is ideally performed 1 to 6 months after complete recovery of the initial reaction [12]. Drug provocation test (DPT) is the gold standard in the evaluation of drug hypersensitivity. While BL skin tests prior to DPT are recommended by most guidelines [13,14], there is heterogeneity in clinical practice [15,16,17], particularly with regard to children [18].
In Singapore, there is limited literature on the evaluation of childhood BL hypersensitivity. A study of 111 children clinically diagnosed with drug eruption at a tertiary skin centre in Singapore showed that amoxicillin and ampicillin were the most commonly implicated drugs [19]. Drug hypersensitivity was evaluated based on history, patch test, penicillin specific immunoglobulin E antibodies and, in less than half of the cohort, by DPT. More recently, a study on DPT outcomes in Singaporean adults described 41 BL challenges, of which 3 were positive [20]. The aim of our study is to describe the outcomes of evaluation of children with suspected BL hypersensitivity in KK Women's and Children's Hospital, Singapore.
MATERIALS AND METHODS
We conducted a retrospective review and included all patients aged 18 years and below who underwent BL DPT for suspected BL hypersensitivity reaction at the paediatric allergy unit in KK Women's and Children's Hospital, Singapore from August 2016 to December 2017.
All patients were evaluated by an attending allergist. Suspected hypersensitivity reactions were defined based on clinical history as immediate if the onset of reaction was ≤1 hour and delayed if onset was >1 hour. Patients with immediate reactions underwent skin prick test (SPT). Patients with immediate reactions were offered DPT if skin tests were negative. Patients with delayed reactions were offered DPT directly. A DPT was considered diagnostic if the patient was challenged with the index drug which caused suspected hypersensitivity. In patients for whom diagnostic DPT was not possible, a DPT to an alternative BL was performed. The electronic case notes were reviewed and data were extracted onto standardized data collection forms. Statistical analysis was carried out using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA).
1. SPT and intradermal test
SPT was performed using the parenteral preparation of the index drug and a standard BL panel consisting of (1) histamine positive control, (2) diluent negative control, (3) benzylpenicilloyl octa-L-lysine, (PPL, Diater Laboratorios, Madrid, Spain) (4) sodium benzylpenilloate, (MD, Diater Laboratorios) (5) benzylpenicillin, (6) ampicillin, (7) cefazolin, and (8) ceftriaxone. Prior to September 2017, our unit used high concentration benzylpenicillin (333,333 U/mL), ampicillin (167 mg/mL), cefazolin (333 mg/mL), and ceftriaxone (333 mg/mL). Thereafter, we standardized the reagent concentrations to the recommendations of the European Network of Drug Allergy/European Academy of Allergy and Clinical Immunology (ENDA/EAACI) for benzylpenicillin (10,000 U/mL), ampicillin (20 mg/mL), cefazolin (2 mg/mL), and ceftriaxone (2 mg/mL) [21]. A positive result was defined as a mean wheal size of 3 mm or larger than the negative control.
Intradermal test (IDT) was performed using ENDA/EAACI [21] standardized concentration of drugs. Mean wheal size increase of 3 mm or larger than the initial bleb or persistence of wheal after 20 minutes with flare and itch were considered positive. Given the discomfort associated with IDT, children with mild reactions proceeded to diagnostic DPT after SPT.
2. Drug provocation test
The DPT consisted of a single therapeutic dose of BL antibiotic administered under physician supervision in an outpatient setting. For example, the single therapeutic dose of amoxicillin was 16.7 mg/kg (not exceeding adult dose 500 mg). If there was no initial reaction, the same dose was self-administered once daily for the next 4 days. A DPT was considered negative if the patient reported no reaction at the end of 5 days.
3. Ethical approval
The study is approved by the Institutional Review Board of KK Women's and Children's Hospital (reference number: 2015/3141). The parents of the study subjects had given their written informed consent.
RESULTS
1. Demographics and suspected hypersensitivity reaction
Over the 16-month study period, a total of 120 children with 121 suspected BL reactions were identified: 103 patients had reported suspected hypersensitivity reaction to a single penicillin, 16 patients to a single cephalosporin, and 1 patient to both penicillin and cephalosporin. Subject demographics are described in Table 1.
Table 1. Subject demographics (n = 120).
| Variable | Value | ||
|---|---|---|---|
| Age at DPT (yr) | 7.4 (4.2–11.1) | ||
| Male sex | 73 (61) | ||
| Race | |||
| Chinese | 86 (72) | ||
| Malay | 14 (12) | ||
| Indian | 8 (7) | ||
| Caucasian | 4 (3) | ||
| Others | 8 (7) | ||
| Family history | |||
| Paternal history | |||
| Atopy | 33 (28) | ||
| Drug hypersensitivity | 8 (7) | ||
| BL hypersensitivity | 4 (3) | ||
| Maternal history | |||
| Atopy | 38 (32) | ||
| Drug hypersensitivity | 17 (14) | ||
| BL hypersensitivity | 8 (7) | ||
| Sibling history | |||
| Atopy | 30 (25) | ||
| Personal history of atopy | |||
| Rhinitis | 72 (60) | ||
| Eczema | 32 (27) | ||
| Doctor-diagnosed asthma | 12 (10) | ||
| Food allergy | 7 (6) | ||
| Recurrent urticaria | 16 (13) | ||
| Chronic spontaneous urticaria | 6 (5) | ||
| Class of drug (other than BL) that patient reported suspected hypersensitivity | |||
| Nonsteroidal anti-inflammatory drug (NSAID) | 12 (10) | ||
| Macrolide antibiotic | 7 (6) | ||
Values are presented as median (interquartile range) or number (%).
DPT, drug provocation test; BL, beta-lactam.
The median age at suspected reaction was 2.0 years (interquartile range [IQR], 1.0–5.0 years) and the median age at DPT was 7.4 years (IQR 4.2–11.1 years). The median time interval between suspected reaction and DPT was 2.6 years (IQR, 1.2–5.7 years).
The timing of suspected hypersensitivity reaction was immediate in 21% (25 of 121), delayed in 66% (80 of 121), and uncertain in 13% (16 of 121). The most commonly implicated drugs were amoxicillin in 45% (54 of 121), amoxicillin-clavulanate in 37% (45 of 121), and cephalexin in 8% (10 of 121). The most commonly reported symptoms were maculopapular rash in 44% (53 of 121), urticaria in 34% (41 of 121), and angioedema in 22% (27 of 121). The clinical characteristics of the suspected hypersensitivity reactions are described in Table 2.
Table 2. Description of suspected hypersensitivity reaction (n = 121).
| Variable | All reactions (n = 121) | Immediate (n = 25) | Delayed (n = 80) | Uncertain onset (n = 16) | ||
|---|---|---|---|---|---|---|
| Age at reaction (yr) | 2.0 (1.0–5.0) | 2.0 (1.0–5.0) | 3.0 (1.0–5.3) | 1.0 (1.0–2.5) | ||
| Beta-lactam antibiotic | ||||||
| Penicillin group | 104 (86) | 21 (84) | 69 (86) | 14 (88) | ||
| Amoxicillin | 54 (45) | 10 (40) | 38 (48) | 6 (38) | ||
| Ampicillin | 2 (2) | 2 (8) | - | - | ||
| Amoxicillin-clavulanate | 45 (37) | 9 (36) | 30 (38) | 6 (38) | ||
| Cloxacillin | 1 (1) | - | 1 (1) | - | ||
| Penicillin V | 2 (2) | - | - | 2 (13) | ||
| Cephalosporin group | 17 (14) | 4 (16) | 11 (14) | 2 (13) | ||
| Cephalexin | 10 (8) | 2 (8) | 7 (9) | 1 (11) | ||
| Cefuroxime | 2 (2) | 1 (4) | 1 (1) | - | ||
| Cefazolin | 1 (1) | - | 1 (1) | - | ||
| Cefaclor | 1 (1) | - | 1 (1) | - | ||
| Ceftibuten | 1 (1) | - | - | 1 (11) | ||
| Cefixime | 1 (1) | - | 1 (1) | - | ||
| Cefepime | 1 (1) | 1 (4) | - | - | ||
| Route | ||||||
| Oral | 113 (93) | 22 (88) | 75 (94) | 16 (100) | ||
| Intravenous | 8 (7) | 3 (12) | 5 (6) | - | ||
| Reaction with first course of BL | 83 (69) | 17 (68) | 58 (73) | 8 (50) | ||
| No. of doses to reaction | ||||||
| 1 Dose | 42 (35) | 16 (64) | 23 (29) | 3 (19) | ||
| 2–5 Doses | 19 (16) | 2 (8) | 15 (19) | 2 (13) | ||
| 6–10 Doses | 5 (4) | - | 5 (6) | - | ||
| >10 Doses | 3 (2) | - | 3 (4) | - | ||
| Clinical reaction | ||||||
| Angioedema | 27 (22) | 11 (44) | 13 (16) | 3 (19) | ||
| Urticaria | 41 (34) | 14 (56) | 24 (30) | 3 (19) | ||
| Maculopapular rash | 53 (44) | 7 (28) | 40 (50) | 6 (38) | ||
| Pustular rash | 2 (2) | - | 2 (3) | - | ||
| Nonspecific rash | 21 (17) | 5 (20) | 10 (13) | 6 (38) | ||
| Anaphylaxis* | 1 (1) | 1 (4) | - | - | ||
| Stevens-Johnson syndrome* | 1 (1) | - | 1 (1) | - | ||
Values are presented as median (interquartile range) or number (%).
BL, beta-lactam.
*Alternative etiologies to BL hypersensitivity were found more likely – further described in section Case Description.
2. SPTs and DPTs
A total of 26 SPTs were performed: 18 used high concentration reagents and 8 used ENDA/EAACI [21] concentrations. All patients had negative SPT results. One patient proceeded to IDT and is further described in the section Case Description.
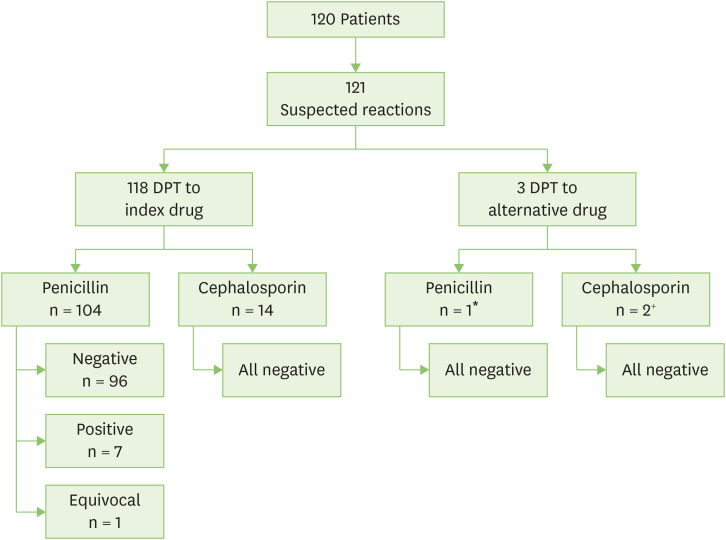
In 118 suspected hypersensitivity reactions, we performed diagnostic DPT to the index drug. This consisted of 104 challenges to the penicillin group of amoxicillin (n = 56, index drug amoxicillin [n = 54] and ampicillin [n = 2]), amoxicillin-clavulanate (n = 45), cloxacillin (n = 1), and penicillin V (n = 2), together with 14 diagnostic challenges to cephalosporin group of cephalexin (n = 11, index drug cephalexin [n = 10] and cefaclor [n = 1]), cefuroxime (n = 2), and ceftibuten (n = 1). In 3 cases, DPT to the index drug was not possible and DPT to an alternative drug was performed. Fig. 1 illustrates the outcomes of DPT to index and alternative drugs.
Fig. 1. Outcome of drug provocation test (DPT). *One patient had a suspected cefazolin DHR. As the patient's parent declined any evaluation of cephalosporin hypersensitivity, the patient underwent amoxicillin DPT. +One patient had a suspected cefixime DHR. As cefixime was unavailable in hospital formulary, the patient underwent ceftibuten DPT. One patient had cefepime hypersensitivity confirmed on IDT and underwent cefuroxime DPT. (Described in section Case Description).
A negative challenge result was obtained in 93% (110 of 118) of diagnostic DPTs. One DPT was considered to have an equivocal result. The index drug was well tolerated in 92% (96 of 104) of penicillin group drug challenges (95% [56 of 59] of penicillin only challenge; 89% [40 of 45] for amoxicillin-clavulanate challenges) and 100% (14 of 14) of cephalosporin challenges. An analysis of subject demographics and reported hypersensitivity reactions did not reveal significant differences in patients with positive and negative challenge result. Table 3 describes positive DPT reactions.
Table 3. Description of positive DPT reactions.
| Case | Sex | Index drug | SPT | Description of index reaction | Description of positive DPT reaction | Subsequent DPT result | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Type | Timing | Symptom | Age | Type | Timing | Symptom | |||||
| 1 | F | AX | - | 1 yr | Imm | 1st dose | R | 5 yr | Imm | 1 hr after first dose in clinic | UR | - |
| 2 | F | AX-CLV | Negative | Unk | Imm | Unk | AE, R | 15 yr | Imm | 20 min after home dose on day 2 to 5 | AE of eyes | - |
| 3 | F | AX | - | 10 yr | Del | 3rd dose | UR | 11 yr | Del | 8 hr after first dose | MPE | - |
| 4 | F | AX-CLV | - | 11 yr | Unk | Unk | UR | 12 yr | Del | A few hours after home dose on day 2 & 3 | MPE | - |
| 5 | M | AX-CLV | - | 11 yr | Del | 6th dose | MPE | 13 yr | Del | At least 4 hr after first dose | UR, AE of eyes and lip | Negative cephalexin DPT |
| 6 | M | AX-CLV | - | 2 yr | Unk | Unk | R | 3 yr | Del | 9 hr after first dose | UR, AE of eyes | - |
| 7 | M | AX-CLV | - | 5 yr | Del | Unk | MPE | 10 yr | Del | On day 6 | Lip ulcer | - |
DPT, drug provocation test; SPT, skin prick test; AX, amoxicillin; Imm, Immediate reaction; R, rash, nonspecific; UR, urticaria; AX-CLV, amoxicillin-clavulanate; Unk, unknown; AE, angioedema; Del, delayed reaction; MPE, maculopapular exanthem; Neg, Negative.
3. Case descriptions
One case of Stevens-Johnson syndrome (SJS) was identified in this study population. This was a 5-year-old Chinese boy who presented with symptoms of fever and cough for 7 days and received oral cefuroxime on day 4 to 6 of illness. He experienced conjunctivitis on day 6 of illness. On day 7, the patient's chest radiograph showed left lung consolidation and he was admitted to the hospital. He received a dose of intravenous ceftriaxone and after 8 hours of observation, he experienced generalized rash with targetoid lesions and mucositis. Laboratory investigations confirmed mycoplasma pneumonia infection via particle agglutination antibody titre of 1:640. A diagnosis of SJS secondary to mycoplasma pneumonia was made. A DPT to exclude a cefuroxime hypersensitivity was performed and this was negative. Ceftriaxone drug challenge was not performed.
The only case of anaphylaxis was in a 10-year-old who presented with symptoms of acute angioedema, rhinorrhoea, breathlessness, and wheeze after simultaneous ingestion of amoxicillin-clavulanate (AX-CLV), ibuprofen, and chlorpheniramine. The patient had a background of Angelman syndrome, asthma, and allergic rhinitis. At age 11 years, he experienced facial angioedema and urticaria after paracetamol ingestion. Diagnostic evaluation revealed negative SPT to standard BL panel, negative AX-CLV DPT, negative paracetamol DPT, and positive ibuprofen DPT.
A 9-year-old boy, with a background of Fanconi's anaemia requiring bone marrow transplantation, was referred to the allergy service for the immediate reaction of angioedema and urticaria related to intravenous cefepime, administered for treatment of a central line infection. His SPT was negative to cefepime. Cefepime (2 mg/mL) IDT returned positive thus confirming immediate drug hypersensitivity. DPT to alternative cephalosporin of cefuroxime was negative.
DISCUSSION
This is the first report describing the diagnostic outcomes of suspected BL hypersensitivity in Singaporean children. In our study, 93% of diagnostic BL DPTs are negative. Results are concordant with adult data from Singapore [20] and large childhood studies from Europe [6,7], Canada [22], and Turkey [2]. Delayed-onset rashes are frequently observed in children treated with BL with subsequent labelling as drug hypersensitivity [23]. Vyles et al. [24] conducted a paediatric Emergency Department survey of 500 children with reported penicillin drug hypersensitivity and concluded that 76% had low-risk symptoms that were unlikely to be consistent with true allergy. We describe a similar trend in Singapore. In our study, suspicion of BL hypersensitivity occurred at a young median age of 2 years, with mild mucocutaneous involvement, and often upon the first encounter with BL. The baseline atopy background of our cohort seemed higher than the general population, with 60% of them having rhinitis, 18% having recurrent or chronic urticaria, likely due to the fact that this is a cohort derived from a tertiary allergy outpatient unit. Whilst a proportion of the cohort presented to the unit with a main concern of drug allergy, many of them were being followed up for other atopic conditions, noted with a label of drug allergy and opportunistically worked up after.
In 92% of our diagnostic penicillin challenges and 100% of cephalosporin challenges, the negative DPT allowed us to “de-label” the suspected antibiotic allergy. A recent systematic review published on the cost of self-reported penicillin allergy estimated a total inpatient cost savings of 1,145–4,254 United States dollar compared to a patient with no reported allergy [25]. Patients with self-reported penicillin allergies are more likely to receive fluoroquinolones, clindamycin, and vancomycin and are more likely to carry Clostridium difficile, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus [26]. Given that the majority of studied DPTs yielded a negative result, it is clear that all patients with suspected BL hypersensitivity should be referred for diagnostic evaluation.
In 2016, the ENDA/EAACI paediatric task force recommended a general algorithm for the evaluation of drug allergy in children [12]. In children with immediate reactions, the group recommended SPT and immediate reading of IDT prior to diagnostic provocation. The same group also published standards for non-irritant concentrations in skin tests [21]. Interestingly, none of our 18 children who underwent SPT with high concentration reagents experienced irritant effects or positive results.
Mill et al. [27] performed direct DPT to a group of 818 children with suspected amoxicillin allergy, of whom almost 100 reported suspected immediate reactions. In the 17 children with proven immediate allergy upon oral provocation, only 1 patient had a positive SPT and IDT giving a low sensitivity of 6%, albeit with testing to a limited panel of benzylpenicillin and benzylpenicilloyl polylysine reagent. Hence selective amoxicillin allergy could have been missed if SPT and IDT were not performed with amoxicillin. In children with delayed reactions, investigative modalities include patch tests and delayed reading of IDT [14]. Atanaskovic-Markovic et al. [6] reported that when performing IDT in over 1,000 children with suspected delayed BL hypersensitivity, 5.5% had positive delayed IDT readings and thus avoided oral challenge. The IDT is painful and often not well tolerated in young children. In Asia, there may be limited resources to perform delayed IDT reading and patch testing given the requirement for trained personnel, additional clinic visits, and healthcare costs. The EAACI and British Society for Allergy and Clinical Immunology suggest performing oral provocation in children with mild delayed skin reactions without prior skin testing [12,13]. In 2015, Vezir et al. [28] proved that this approach was safe by conducting direct oral provocation in 119 children with mild delayed BL allergy. In the 4 patients (3%) who experienced drug reactions, they developed an urticarial rash that was not severe. In 2017, Moral and Caubet [29] wrote about the possibility of direct DPT in children with nonsevere immediate and delayed BL reactions and used the rostrum to call for large multicentric studies to provide strong evidence to change current skin tests guidelines. In our cohort, patients with delayed suspected reactions underwent direct DPT. We performed 26 SPTs for patients with immediate suspected reactions, and all SPTs returned negative. We performed IDT for the single patient with a suspected reaction to the parenteral drug of cefepime without an oral equivalent. In this patient, the positive IDT together with a clinical presentation consistent with an immediate hypersensitivity reaction confirmed the diagnosis and allowed the patient to avoid a high-risk DPT. The rest of our patients with negative SPT results proceeded to DPT, during which 1 patient experienced a mild reaction and 24 had negative DPT.
Similar to Vyles et al. [30], we performed single-dose oral challenge with a good safety profile. Patients with proven BL hypersensitivity had mild reactions of urticaria, angioedema, and macular exanthem within 2 days of oral challenge. Our study supports the opinion that prior skin tests may not be necessary for the evaluation of children who report nonsevere reactions and the direct oral challenge is a safe procedure. However, for patients with history of BL anaphylaxis, we would still recommend SPT and IDT to confirm the diagnosis, identify possible alternatives, followed by DPT to BL with negative skin test results, to confirm safe alternatives. The strength of our study is that this is the first report of challenge proven outcomes in the evaluation of suspected BL hypersensitivity in Singaporean children. This fills an important gap in the currently available local literature. A limitation of our study is the retrospective design. It would have been ideal to evaluate selective clavulanic hypersensitivity in our patients with proven amoxicillin-clavulanate reactions as well as determine cephalosporin tolerance in our patients with proven amoxicillin allergy. However, most parents declined a subsequent DPT.
In conclusion, given rising concerns of antibiotic resistance, it is important that unnecessary use of broad-spectrum antibiotics be avoided. The majority of children with suspected BL hypersensitivity do not react upon oral challenge. Skin tests may not be necessary for children who report nonsevere reactions and directly performing diagnostic DPT is a safe approach in the evaluation of suspected childhood BL hypersensitivity.
ACKNOWLEDGEMENTS
The authors would like to thank Nurse Lim Hwee Hoon, Nurse Ding Xiao Mei, and the SPT laboratory technicians for their contributions to patient care and clinical research.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Si Hui Goh, Wenyin Loh.
- Formal analysis: Si Hui Goh, Kok Wee Chong.
- Investigation: Si Hui Goh, Kok Wee Chong, Wen Chin Chiang, Anne Goh, Wenyin Loh.
- Methodology: Si Hui Goh, Wenyin Loh.
- Project administration: Wenyin Loh.
- Writing - original draft: Si Hui Goh, Kok Wee Chong.
- Writing - review & editing: Wen Chin Chiang, Anne Goh, Wenyin Loh.
References
- 1.Rebelo Gomes E, Fonseca J, Araujo L, Demoly P. Drug allergy claims in children: from self-reporting to confirmed diagnosis. Clin Exp Allergy. 2008;38:191–198. doi: 10.1111/j.1365-2222.2007.02870.x. [DOI] [PubMed] [Google Scholar]
- 2.Erkoçoğlu M, Kaya A, Civelek E, Ozcan C, Cakır B, Akan A, Toyran M, Ginis T, Kocabas CN. Prevalence of confirmed immediate type drug hypersensitivity reactions among school children. Pediatr Allergy Immunol. 2013;24:160–167. doi: 10.1111/pai.12047. [DOI] [PubMed] [Google Scholar]
- 3.Kidon MI, See Y. Adverse drug reactions in Singaporean children. Singapore Med J. 2004;45:574–577. [PubMed] [Google Scholar]
- 4.Lange L, Koningsbruggen SV, Rietschel E. Questionnaire-based survey of lifetime-prevalence and character of allergic drug reactions in German children. Pediatr Allergy Immunol. 2008;19:634–638. doi: 10.1111/j.1399-3038.2007.00702.x. [DOI] [PubMed] [Google Scholar]
- 5.Tan VAK, Gerez IFA, Van Bever HP. Prevalence of drug allergy in Singaporean children. Singapore Med J. 2009;50:1158–1161. [PubMed] [Google Scholar]
- 6.Atanaskovic-Markovic M, Gaeta F, Medjo B, Gavrovic-Jankulovic M, Cirkovic Velickovic T, Tmusic V, Romano A. Non-immediate hypersensitivity reactions to beta-lactam antibiotics in children - our 10-year experience in allergy work-up. Pediatr Allergy Immunol. 2016;27:533–538. doi: 10.1111/pai.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zambonino MA, Corzo JL, Muñoz C, Requena G, Ariza A, Mayorga C, Urda A, Blanca M, Torres MJ. Diagnostic evaluation of hypersensitivity reactions to beta-lactam antibiotics in a large population of children. Pediatr Allergy Immunol. 2014;25:80–87. doi: 10.1111/pai.12155. [DOI] [PubMed] [Google Scholar]
- 8.Tonson la Tour A, Michelet M, Eigenmann PA, Caubet JC. Natural History of benign nonimmediate allergy to beta-lactams in children: a prospective study in retreated patients after a positive and a negative provocation test. J Allergy Clin Immunol Pract. 2018;6:1321–1326. doi: 10.1016/j.jaip.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Norton AE, Konvinse K, Phillips EJ, Broyles AD. Antibiotic allergy in pediatrics. Pediatrics. 2018;141:e20172497. doi: 10.1542/peds.2017-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picard M, Paradis L, Nguyen M, Bégin P, Paradis J, Des Roches A. Outpatient penicillin use after negative skin testing and drug challenge in a pediatric population. Allergy Asthma Proc. 2012;33:160–164. doi: 10.2500/aap.2012.33.3510. [DOI] [PubMed] [Google Scholar]
- 11.Trubiano J, Phillips E. Antimicrobial stewardship's new weapon? A review of antibiotic allergy and pathways to ‘de-labeling’. Curr Opin Infect Dis. 2013;26:526–537. doi: 10.1097/QCO.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes ER, Brockow K, Kuyucu S, Saretta F, Mori F, Blanca-Lopez N, Ott H, Atanaskovic-Markovic M, Kidon M, Caubet JC, Terreehorst I ENDA/EAACI Drug Allergy Interest Group. Drug hypersensitivity in children: report from the pediatric task force of the EAACI Drug Allergy Interest Group. Allergy. 2016;71:149–161. doi: 10.1111/all.12774. [DOI] [PubMed] [Google Scholar]
- 13.Mirakian R, Leech SC, Krishna MT, Richter AG, Huber PAJ, Farooque S, Khan N, Pirmohamed M, Clark AT, Nasser SM Standards of Care Committee of the British Society for Allergy and Clinical Immunology. Management of allergy to penicillins and other beta-lactams. Clin Exp Allergy. 2015;45:300–327. doi: 10.1111/cea.12468. [DOI] [PubMed] [Google Scholar]
- 14.Romano A, Atanaskovic-Markovic M, Barbaud A, Bircher AJ, Brockow K, Caubet JC, Celik G, Cernadas J, Chiriac AM, Demoly P, Garvey LH, Mayorga C, Nakonechna A, Whitaker P, Torres MJ. Towards a more precise diagnosis of hypersensitivity to beta-lactams - an EAACI position paper. Allergy Eur J Allergy Clin Immunol. 2020;75:1300–1315. doi: 10.1111/all.14122. [DOI] [PubMed] [Google Scholar]
- 15.Thong BYH, Mirakian R, Castells M, Pichler W, Romano A, Bonadonna P, Diana D, Kowalski M, Yanez A, Lleonart R, Sanchez-Borges M, Demoly P. A world allergy organization international survey on diagnostic procedures and therapies in drug allergy/hypersensitivity. World Allergy Organ J. 2011;4:257–270. doi: 10.1097/WOX.0b013e31823dc02c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richter AG, Nasser SM, Krishna MT. A UK national survey of investigations for beta-lactam hypersensitivity - heterogeneity in practice and a need for national guidelines - on behalf of British Society for Allergy and Clinical Immunology (BSACI) Clin Exp Allergy. 2013;43:941–949. doi: 10.1111/cea.12134. [DOI] [PubMed] [Google Scholar]
- 17.Torres MJ, Celik GE, Whitaker P, Atanaskovic-Markovic M, Barbaud A, Bircher A, Blanca M, Brockow K, Caubet JC, Cernadas JR, Chiriac A, Demoly P, Garvey LH, Merk HF, Mosbech H, Nakonechna A, Romano A. A EAACI drug allergy interest group survey on how European allergy specialists deal with β-lactam allergy. Eur J Allergy Clin Immunol. 2019;74:1052–1062. doi: 10.1111/all.13721. [DOI] [PubMed] [Google Scholar]
- 18.Foong RXM, Logan K, Perkin MR, du Toit G. Lack of uniformity in the investigation and management of suspected β-lactam allergy in children. Pediatr Allergy Immunol. 2016;27:527–532. doi: 10.1111/pai.12557. [DOI] [PubMed] [Google Scholar]
- 19.Khoo BP, Giam YC. Drug eruptions in children: a review of 111 cases seen in a tertiary skin referral centre. Singapore Med J. 2000;41:525–529. [PubMed] [Google Scholar]
- 20.Thalayasingam M, Davies LJ, Llanora GV, Gerez IF, Van Bever HP, Shek LP. Clinical characteristics and outcomes of patients undergoing drug provocation tests (DPTs) Ann Acad Med Singapore. 2013;42:184–189. [PubMed] [Google Scholar]
- 21.Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, Bircher A, Blanca M, Bonadonna B, Campi P, Castro E, Cernadas JR, Chiriac AM, Demoly P, Grosber M, Gooi J, Lombardo C, Mertes PM, Mosbech H, Nasser S, Pagani M, Ring J, Romano A, Scherer K, Schnyder B, Testi S, Torres M, Trautmann A, Terreehorst I ENDA/EAACI Drug Allergy Interest Group. Skin test concentrations for systemically administered drugs -- an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013;68:702–712. doi: 10.1111/all.12142. [DOI] [PubMed] [Google Scholar]
- 22.Abrams EM, Wakeman A, Gerstner TV, Warrington RJ, Singer AG. Prevalence of beta-lactam allergy: a retrospective chart review of drug allergy assessment in a predominantly pediatric population. Allergy Asthma Clin Immunol. 2016;12:59. doi: 10.1186/s13223-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caubet JC, Kaiser L, Lemaître B, Fellay B, Gervaix A, Eigenmann PA. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218–222. doi: 10.1016/j.jaci.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vyles D, Chiu A, Simpson P, Nimmer M, Adams J, Brousseau DC. Parent-reported penicillin allergy symptoms in the Pediatric Emergency Department. Acad Pediatr. 2017;17:251–255. doi: 10.1016/j.acap.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Mattingly TJ, Fulton A, Lumish RA, Williams AMC, Yoon S, Yuen M, Heil EL. The cost of self-reported penicillin allergy: a systematic review. J Allergy Clin Immunol Pract. 2018;6:1649–54.e4. doi: 10.1016/j.jaip.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 26.Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133:790–796. doi: 10.1016/j.jaci.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Mill C, Primeau MN, Medoff E, Lejtenyi C, O'Keefe A, Netchiporouk E, Dery A, Ben-Shoshan M. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016;170:e160033. doi: 10.1001/jamapediatrics.2016.0033. [DOI] [PubMed] [Google Scholar]
- 28.Vezir E, Dibek Misirlioglu E, Civelek E, Capanoglu M, Guvenir H, Ginis T, Toyran M, Kocabas CN. Direct oral provocation tests in non-immediate mild cutaneous reactions related to beta-lactam antibiotics. Pediatr Allergy Immunol. 2016;27:50–54. doi: 10.1111/pai.12493. [DOI] [PubMed] [Google Scholar]
- 29.Moral L, Caubet JC. Oral challenge without skin tests in children with non-severe beta-lactam hypersensitivity: Time to change the paradigm? Pediatr Allergy Immunol. 2017;28:724–727. doi: 10.1111/pai.12800. [DOI] [PubMed] [Google Scholar]
- 30.Vyles D, Adams J, Chiu A, Simpson P, Nimmer M, Brousseau DC. Allergy testing in children with low-risk penicillin allergy symptoms. Pediatrics. 2017;140:e20170471. doi: 10.1542/peds.2017-0471. [DOI] [PubMed] [Google Scholar]