Abstract
Nowadays, herbal extracts are considered to be a potential source for developing new drugs that will overcome resistance to conventional chemotherapeutic agents. This study was aimed to explore the efficacy of several Egyptian plant extracts against Toxoplasma gondii infection in vitro for future development of a new, safe, and effective compound for T. gondii. Methanol extracts from Matricaria chamomilla (German chamomile), Laurus nobilis, Citrullus colocynthis, Cinnamum camphora, Boswellia scara, and Melissa officionalis plants and oil extracts (either essential or fixed oils) of some plants such as: lemon grass (Cymbopogon citratus), marjoram (Origanum majorana), watercress (Nasturtium officionale), wheat germ (Triticum aestivum), sesame (Sesamum indicum), rosemary (Salvia rosmarinus), citronella (Cymbopogon nardus), clove (Syzygum aromaticum), jojoba (Simmondsia chinesis), and basil (Ocimum basilicum) were investigated for their anti-Toxoplasma activities. The methanol extracts from C. colocynthis and L. nobilis and the oil extracts from lemon grass and marjoram were active against T. gondii with half maximal inhibitory concentrations (IC50) of 22.86 µg/ml, 31.35 µg/ml, 4.6 µg/ml, and 26.24 µg/ml, respectively. Their selectivity index (SI) values were <10. Interestingly, the methanol extract from M. chamomilla and oil from citronella had the lowest IC50 values for T. gondii (3.56 µg/ml and 2.54 µg/ml, respectively) and the highest SI values (130.33 and 15.02, respectively). In conclusion, methanol extract from M. chamomilla and oil from citronella might be potential sources of novel therapies for treating toxoplasmosis.
Keywords: inhibitory concentration, medicinal plants, oil extract, selectivity index, Toxoplasma gondii
Toxoplasmosis, a cosmopolitan zoonotic parasitic infection, is caused by an Apicomplexa intracellular protozoan, Toxoplasma gondii [38]. Members of the Felidae family are the definitive hosts of T. gondii. Humans can be infected by T. gondii through the ingestion of T. gondii cysts present in undercooked or raw meat of intermediate hosts such as cattle, sheep, goats, pigs, and chickens [26, 90]. It has been reported that up to one-third of the global population is affected by this disease with varying degrees of severity depending on immune status [88, 91]. Toxoplasmosis is usually asymptomatic in immune-competent people, causing chronic infection with parasite cyst formation in tissues. However, in hosts with a waned immune system as AIDS patients or chemotherapy-treated patients, it may lead to vigorous systemic disease and subsequently death [85]. Importantly, toxoplasmosis in pregnant women may lead to miscarriage or fetal abnormalities (hydrocephaly, calcification) or neurological illness in newborns [14].
Sulfadiazine and pyrimethamine are the two medications that are concomitantly used currently in the treatment of toxoplasmosis [63]. These drugs exert their action by blocking the Toxoplasma folate metabolism, which consequently inhibits the generation of the DNA and eventually blocks the replication of tachyzoites [19, 24]. However, there are many drawbacks that constrict the utilization of these current chemotherapeutic agents, including their restricted activity in eradicating T. gondii encysted bradyzoites [60, 84], which may cause serious undesirable effects such as suppression of the bone marrow, hematological reactions, embryopathies, gastrointestinal upset, and hypersensitivity [21, 31, 81]. Therefore, there is an urgent need for the development of novel, efficient, and safe compounds with less toxicity and short medicating courses.
For centuries, medicinal plant extracts have been utilized extensively by mankind in treating several diseases [39]. These herbal extracts possess a wide variety of bioactive compounds such as tannin, flavonoids, alkaloids, and phenolic substances, which may serve as potential alternatives to many synthetic drugs [23, 25]. Previously, several identified compounds for curing parasitic diseases such as malaria have been isolated from plants, for example, quinine and artemisinin [11]. In Egypt, the utilization of medicinal plants rose since Pharaonic times, and nowadays, many Egyptians still rely on medicinal plants for the treatment of several diseases [1, 55]. Considering the aforementioned drawbacks of the current anti-Toxoplasma remedies, it is imperative to search for novel and highly effective compounds for the treatment of toxoplasmosis with minimal side effects. Therefore, in an attempt to develop a new therapy from Egyptian herbal extracts for T. gondii, this study aimed to investigate the effect of methanol and oil extracts of some plants against T. gondii. The selected plants for this research are frequently used for treating many diseases in herbal therapy and are known to have diverse bioactive constituents. In addition, most of these plants have been previously reported to exhibit antiprotozoal and anthelmintic activity against organisms such as Matricaria chamomilla [37]; Laurus nobilis [10, 89]; Citrullus colocynthis [36]; Cinnamum camphor [76]; Boswellia scara [64]; Melissa officionalis [52]; Cymbopogon citratus [70]; Origanum majorana [46]; Salvia rosmarinus [7]; Syzygum aromaticum [50]; and Ocimum basilicum [20]. However, to the best of our knowledge, no literature on their potential anti-Toxoplasma activities is available.
MATERIALS AND METHODS
Parasites and cell cultures
T. gondii RH-GFP (a green fluorescent protein expressing- RH strain) was used in this study. African green monkey kidney (Vero) cells and human foreskin fibroblast (HFF) cells were used for parasite maintenance. Purification of the parasite was performed using a previously reported method [69].
Plant materials, oil extracts, and chemicals
Samples of M. chamomilla (German chamomile), L. nobilis (Bay laurel), C. colocynthis (Colocynth), C. camphora (Camphor tree), B. scara (Frankincens), and M. officionalis (Lemon balm) plants were procured from an herbal drug store in Mansoura, Egypt. Table 1 illustrates the name, family, utilized parts, and the traditional uses of plants that were employed in this experiment. Plant identification was carried out by Prof. Dr. Ibrahim Mashaly from Botany Department, Faculty of Science, Mansoura University, Egypt. Plant materials were dried and ground into min pieces. Afterwards, they were extracted thrice at ambient temperature using 70% methanol (48 hr/time). Crude extracts were obtained by evaporating the filtrates under vacuum in a rotary evaporator. The obtained extracts were mixed with dimethyl sulfoxide (DMSO) at 100 mg/ml and preserved at −30°C.
Table 1. Activities of methanolic extracts from some plants on Toxoplasma gondii at concentrations 50 µg/ml and 10 µg/ml.
| Plants | Plant part | Traditional uses | References | % inhibition of RH-GFP at 50 µg/ml |
% inhibition of RH-GFP at 10 µg/ml |
|---|---|---|---|---|---|
| Boswellia scara (Frankincens) | Resins | To cure bronchial and urinary infections | [42] | 18.28 ± 8.11 | 0.00 |
| Cinnamum camphora (Camphor tree) | Leaf | Used for treating for inflammatory diseases such as bronchitis, rheumatism, and sprains | [80] | 54.95 ± 18.97 | 34.73 ± 10.01 |
| Citrullus colocynthis (Colocynth) | Fruit | Used for treating respiratory diseases, diabetes, obesity, insecticide, purgative, anthelmintic, and mollusicide | [3, 28, 78] | 97.07 ± 8.7 | 65.73 ± 4.45 |
| Laurus nobilis (Bay laurel) | Leaf | Used for treating gastrointestinal diseases | [22] | 74.78 ± 12.01 | 0.00 |
| Matricaria chamomilla (German chamomile) | Flower | Used for skin irritations, wounds eczema, ulcers, bruises, burns, gout, neuralgia, sciatica, mastitis rheumatic pain, and hemorrhoids | [8, 79] | 83.36 ± 7.69 | 67.37 ± 14.52 |
| Melissa officionalis (Lemon balm) | Leaf | Used for treating CNS problems such as nervous agitation, sleep disorders, depression, and gastrointestinal diseases | [30, 34] | 17.96 ± 12.03 | 3.14 ± 4.29 |
% inhibition of a green fluorescent protein expressing-RH strain of T. gondii (RH-GFP) of 1 mg/ml Sulfadiazine: 67.93 ± 10.56. Values are the mean ± SD of triplicate samples, and data are a representative of three independent experiments.
Essential oils of some plants such as lemon grass (Cymbopogon citratus), marjoram (Origanum majorana), watercress (Nasturtium officionale), rosemary (Salvia rosmarinus), citronella (Cymbopogon nardus), clove (Syzygum aromaticum), basil (Ocimum basilicum), and fixed oils from other plants such as sesame (Sesamum indicum), wheat germ (Triticum aestivum), and jojoba (Simmondsia chinesis) were supplied by the National Research Center of Medicinal and Aromatic Plants, Qalyubia, Egypt. Sulfadiazine (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 1 N NaOH (stock solution 200 mg/ml) and was used.
In vitro Cytotoxic assay
The effect of the aforementioned plant extracts on the viability of HFF cells was investigated according to the method described by [47]. Briefly, HFF cells were grown in 96-well plates with 100 µl Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich) in each well (cell suspension, 1 × 105 cells/ml in DMEM supplemented with 10% FBS). After 48 hr of incubation at 37°C in a 5% CO2 atmosphere, 100 µl of the plant extracts dissolved in DMEM were added to the cells (final concentrations, 7.8–1,000 µg/ml). Wells with sulfadiazine or culture medium only served as the positive and negative controls, respectively. After 72 hr, cell counting kit-8 (CCK-8, Dojindo, Kumamoto, Japan) was added to determine cell viability. Their optical densities were detected at 450 nm using an MTP-120 microplate reader (Corona Electric, Hitachinaka, Japan). HFF cell growth suppression (%) was estimated using the following equation:
GraphPad Prism 5 software was used to determine the half maximal inhibitory concentration (IC50) values on HFF cells (GraphPad Software Inc., La Jolla, CA, USA).
Anti-Toxoplasma activity
HFF cells were grown at 100 µl/well in 96-well microtiter plates (cell suspension 1 × 105 cells/ml in DMEM supplemented with 10% FBS) and incubated at 37°C in a 5% CO2 atmosphere for 48 hr. Then, the cells were infected with T. gondii RH-GFP (5 × 104 tachyzoites/ well) for 4 hr, and the extracellular parasites were removed. Afterward, the infected cells were subjected to the herbal extracts prepared in DMEM. All plant extracts (methanol and oil extracts) were tested for their anti-Toxoplasma activity at 50 and 10 µg/ml. Then, the plant extracts showing percentage inhibition of RH-GFP growth at 50 µg/ml higher than that produced by sulfadiazine were investigated at concentrations (0.5–100 µg/ml) to determine their IC50 values against T. gondii. Sulfadiazine and media were added as positive and negative controls, respectively. After 3 days of incubation, a microplate reader (GloMax-Multi Detection System, Promega, Madison, WI, USA) was used to measure the fluorescence intensity of RH-GFP. The growth inhibition (%) of RH-GFP was determined as reported previously [47]. IC50 values of the herbal extract on T. gondii were determined with the aid of GraphPad Prism 5 software.
RESULTS
To investigate the anti-Toxoplasma effects of herbal extracts, we examined the fluorescence intensity of RH-GFP. Tables 1 and 2 show the activities of the aforementioned plant extracts against T. gondii at concentrations of 50 and 10 µg/ml. The methanol extracts obtained from M. chamomilla (German chamomile), C. colocynthis, and L. nobilis had high anti-Toxoplasma activities compared with 1 mg/ml sulfadiazine (67.93%). They inhibited RH-GFP growth at 50 µg/ml showing 83.36 ± 7.69, 97.07 ± 8.7, and 7478 ± 12.01% inhibition, respectively (Table 1). Moreover, among the oil extracts at 50 µg/ml, lemon grass, citronella, and marjoram showed high percentage inhibition of 93.20 ± 8.16, 87.69 ± 3.33, and 63.36 ± 6.66, respectively (Table 2). Figures 1 and 2
Fig. 2.
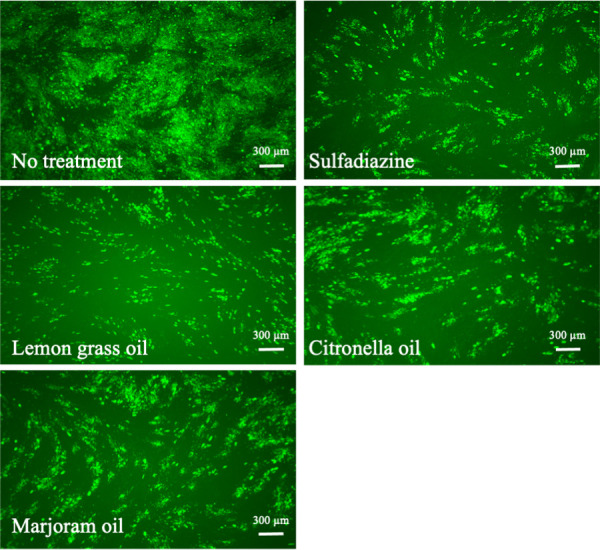
Anti-Toxoplasma activities of lemon grass, citronella, and marjoram oils: expressed as representative images of a green fluorescent protein expressing-RH strain of T. gondii-infected human foreskin fibroblast cells treated with sulfadiazine (1 mg/ml), or either lemon grass oil (10 µg/ml), citronella oil (10 µg/ml), or marjoram oil (50 µg/ml). Scale bar=300 µm.
show the anti-Toxoplasma activities of sulfadiazine (1 mg/ml), M. chamomilla (50 µg/ml), C. colocynthis (10 µg/ml), L. nobilis (50 µg/ml), lemon grass oil (10 µg/ml), citronella oil (10 µg/ml), and marjoram oil (50 µg/ml).
Table 2. Activities of oil extracts from some plants on Toxoplasma gondii at concentrations 50 µg/ml and 10 µg/ml.
| Oil extracts | Plant part | Traditional uses | References | % inhibition of RH-GFP at 50 µg/ml |
% inhibition of RH-GFP at 10 µg/ml |
|---|---|---|---|---|---|
| Basil | Leaf | For treatment of abdominal cramps, gastroenteritis, acne, wounds, and vitiligo | [33, 82] | 50.03 ± 4.46 | 22.53 ± 1.53 |
| Citronella | Aerial part | Treatment of intestinal parasites and digestive and menstrual disorders and also used as antipyretic | [44, 56] | 87.69 ± 3.33 | 48.60 ± 8.22 |
| Clove | Flower | For treating nausea, vomiting and flatulence, tuberculosis, cholera, malaria, candida, worms, viruses, different bacterial and protozoan infections | [13] | 59.51 ± 4.42 | 54.56 ± 4.54 |
| Jojoba | Seed | For treating sore throat, wounds, and warts | [59] | 16.29 ± 11.34 | 13.46 ± 6.56 |
| Lemon grass | Aerial part | Used for reducing cholesterol level, particularly in hypercholesterolemic patients | [40] | 93.20 ± 8.16 | 56.14 ± 2.26 |
| Marjoram | Leaf | For treating chest diseases, sore throat, cough, rheumatic pain, nervous disorders, insomnia | [15, 29] | 63.36 ± 6.66 | 6.10 ± 1.69 |
| Rosemary | Leaf | For treatment of bronchial asthma, abdominal colic, peptic ulcer, and cardiac diseases | [4, 77] | 24.05 ± 7.5 | 20.85 ± 7.57 |
| Sesame | Seeds | For treating urinary troubles and healing wound | [65] | 44.87 ± 0.82 | 1.74 ± 2.21 |
| Watercress | Leaf | For treatment of abdominal pain | [9, 72] | 53.62 ± 8.52 | 20.31 ± 9.12 |
| Wheat germ | Germ | Used for treating hypercholesteremia and diabetes | [16] | 43.51 ± 16.21 | 35.96 ± 1.57 |
Values are the mean ± SD of triplicate samples, and data are a representative of three independent experiments. RH-GFP, a green fluorescent protein expressing-RH strain of T. gondii.
Fig. 1.
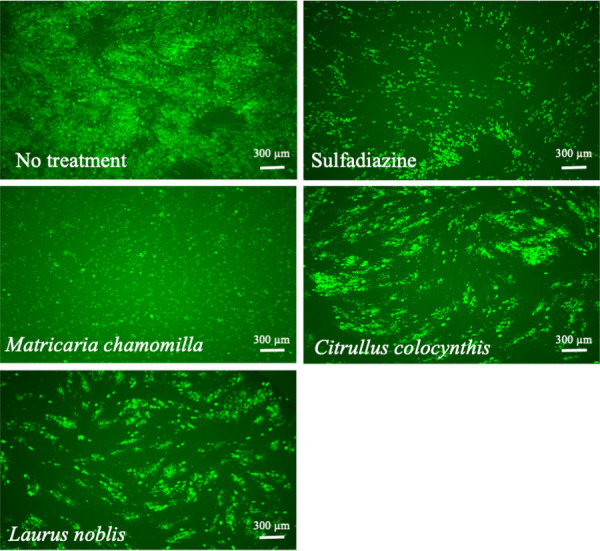
Anti-Toxoplasma activities of Matricaria chamomilla (German chamomile), Citrullus colocynthis, and Laurus nobilis: expressed as representative images of a green fluorescent protein expressing-RH strain of T. gondii-infected human foreskin fibroblast cells treated with sulfadiazine (1 mg/ml), or either M. chamomilla (German chamomile) (50 µg/ml), C. colocynthis (10 µg/ml), or L. nobilis (50 µg/ml). Scale bar=300 µm.
Table 3 illustrates, according to in vitro screening, that the IC50 values of plant extracts showed high anti-Toxoplasma activities. All examined plant extracts showed lower IC50 values than sulfadiazine (IC50=99.4 µg/ml). The methanol extracts of C. colocynthis and L. nobilis and the oil extracts of lemon grass and marjoram had high anti-Toxoplasma activities (IC50=22.86 µg/ml, 31.35 µg/ml, 4.6 µg/ml, and 26.24 µg/ml, respectively). However, their selectivity index values were relatively low, as the selectivity index (SI) values for T. gondii were 1.21, 5.5, 6.25, and 5.67, respectively. Interestingly, the methanol extract from M. chamomilla and oil extract from citronella had the lowest IC50 values (3.56 µg/ml and 2.54 µg/ml, respectively) and the highest SI values (130.33 and 15.02, respectively) for T. gondii.
Table 3. Half maximal inhibitory concentration (IC50) values and selectivity index values of methanol and oil herbal extracts.
| Herbal extracts | IC50 (µg/ml) on HFF cells | IC50 (µg/ml) on RH-GFP | Selectivity index (SI) |
|---|---|---|---|
| Citronella oil | 38.16 ± 8.16 | 2.54 ± 1.01 | 15.02 |
| Citrullus colocynthis (Colocynth) | 27.74 ± 0.74 | 22.86 ± 3.43 | 1.21 |
| Laurus nobilis (Bay laurel) | 173.50 ± 55.57 | 31.35 ± 17.01 | 5.53 |
| Lemon grass oil | 28.76 ± 2.67 | 4.57 ± 0.21 | 6.25 |
| Marjoram oil | 148.95 ± 61.87 | 26.24 ± 7.63 | 5.67 |
| Matricaria chamomilla (German chamomile) | 464 ± 9.05 | 3.56 ± 2.21 | 130.33 |
| Sulfadiazine | >1,000* | 99.40* | N.D. |
IC50 values were calculated based on three independent experiments. Data are expressed as mean ± SD. *Data from [47]. N.D.=not detected. HFF, human foreskin fibroblast; RH-GFP, a green fluorescent protein expressing-RH strain of T. gondii.
DISCUSSION
Over the last three decades, the high resistance of infectious organisms to chemotherapeutic agents has accounted for the failure of routine medication in the management of some infections [12]. Among the growing resistance problems, the current anti-Toxoplasma drugs have been reported to be less effective against parasites owing to genetic mutation [62]. Hence, there is an increasing demand for the development of new remedies for toxoplasmosis. Recently, great attention has been paid to the discovery of novel antiparasitic agents from medicinal plants. It has been reported that several herbal extracts were effective against T. gondii such as Eurycoma longifolia Jack [41, 43], Curcuma [5], Artemisia annua L. [27, 67], and Myristica fragrans Houtt [74]. The screening of herbal extracts to determine anti-Toxoplasma efficacy is considered a method for developing new drugs for toxoplasmosis. In the present study, the methanol extracts of six plants and oil extracts of ten plants were investigated in vitro, for the first time, for their antitoxoplasmal effects against T. gondii.
All the examined plant extracts revealed varying degrees of anti-Toxoplama activities at 50 µg/ml, ranging from 16.29 to 97.07%. Of all the extracts tested, the methanol extracts obtained from M. chamomilla (German chamomile), C. colocynthis, and L. nobilis and the essential oils of lemon grass, citronella, and marjoram exhibited higher growth inhibition than sulfadiazine. Importantly, M. chamomilla (German chamomile) methanol extract and citronella oil, with the lowest IC50 values for T. gondii RH-GFP (3.56 µg/ml and 2.54 µg/ml, respectively) and the highest SI values (130.33 and 15.02, respectively), were demonstrated to be the most potent. These SI values were considered as hallmarks for the selective activity of the therapy.
M. chamomilla (German chamomile) is a member of the Asteraceae family. In folk medicine, chamomile flowers have traditionally been used to alleviate upper respiratory and gastrointestinal tract illnesses and inflammatory conditions of the skin [73] and as a remedy for nightmares, insomnia, and hysteria. It has been reported to have anti-oxidant, antispasmodic, antidiarrheal, anti-inflammatory, anti-allergic, antimicrobial, and antidepressant features [2, 6, 17, 18, 58, 83]. Additionally, chamomile is known for its efficacy in the control and management of prostate, breast, and ovarian cancer [45]. Furthermore, Tunisian chamomile has been reported to have strong leishmanicidal activity [37]. M. chamomilla (German chamomile) possesses several compounds that are responsible for its biological activities [32, 35]. The major phenolic compounds found in chamomile flowers are flavonoids, quercetin, patuletin, apigenin, luteolin, glucosides, and coumarins (umbelliferone and herniarin) [54, 58]. The findings of our study indicate that the methanol extract of chamomile flowers is an effective anti-Toxoplasma agent. Hence, this effect on T. gondii growth may be attributed to any one or a combination of its bioactive ingredients.
Flavonoids have been shown to possess activity against Cryptosporidium parvum, Leishmania donovani, Entamoeba histolytica, and Plasmodium falciparum [48, 49, 57, 61]. Moreover, it has been revealed that apigenin was effective against Leishmania tropica amastigote [66]. In addition, previous reports have shown that coumarins exhibit activity against the promastigote form of Leishmania major and Trypanosma cruzi [53, 87]. Therefore, future studies on the anti-Toxoplasma activities of these compounds that were identified in M. chamomilla (German chamomile) flowers are needed.
Citronella grass (Cymbopogon nardus Rendle) belongs to the Poaceae family. Citronella oil is commonly used as an antipyretic, diuretic, and antispasmodic agent and for the treatment of intestinal parasites [44, 56]. It is widely used as an insect repellent, especially as a repellent against fleas, mosquitoes, and biting flies [68]. In addition, citronella oil is used in the manufacture of detergents, soaps, fragrances, and perfumes [71]. The major components detected in the citronella grass essential oil were monoterpenes, which include geraniol (28.62%), citronellal (23.62%), and citronellol (17.10%) [75]. The present study showed that citronella oil has acceptable activity against T. gondii RH in vitro, with an SI value >10. The anti-Toxoplasma activity of citronella oil is probably owed to its chemical constituents. Monoterpenes have been reported to exert antileishmanial activity [51, 86]. However, further studies are needed to explore the mechanism of action of citronella oil against T. gondii.
In conclusion, our results revealed that the methanol extract from M. chamomilla (German chamomile) and oil extract from citronella are effective against T. gondii in vitro and may serve as potential drugs for the treatment of toxoplasmosis. However, their efficacy in vivo needs to be investigated in a future study.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
Acknowledgments
This research was supported with a grant from the Research Program on Emerging and Re-emerging Infectious Diseases (20fk0108137h [Y.N.]). We would like to thank Dr. Gehad Abdelwahab Ali from the Department of Pharmacognosy, Faculty of Pharmacy, Mansoura University, Mansoura, Egypt for her help in preparing the methanol extracts from our studied medicinal plants. We also thank the staff members of the National Research Center of Medicinal and Aromatic plants, Qalyubia, Egypt for supplying us with the oils of the plants used in the present study. We appreciate Mr. Elsayed El-Alfy and Mr. Ahmed Abdou for their technical support in some experiments in this study. We thank Mrs. Rawia Abu El-Atta, a Master degree holder in English Literature, Al-Azhar University, Cairo for English editing.
REFERENCES
- 1.AbouZid S. F., Mohamed A. A.2011. Survey on medicinal plants and spices used in Beni-Sueif, Upper Egypt. J. Ethnobiol. Ethnomed. 7: 18. doi: 10.1186/1746-4269-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Bahtiti N. H.2012. Chemical analysis and biological activity of Jordanian chamomile extracts. Adv. J. Food Sci. Technol. 4: 22–25. [Google Scholar]
- 3.AL-Qarawi A. A., Adam S. E.2003. Effect of combination of Capsicum frutescens and Citrullus colocynthis on growth, haematological and pathophysiological parameters of rats. Phytother. Res. 17: 92–95. doi: 10.1002/ptr.1094 [DOI] [PubMed] [Google Scholar]
- 4.al-Sereiti M. R., Abu-Amer K. M., Sen P.1999. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J. Exp. Biol. 37: 124–130. [PubMed] [Google Scholar]
- 5.Al-Zanbagi N. A.2009. In vivo effect of some home spices extracts on the toxoplasma gondii tachyzoites. J. Family Community Med. 16: 59–65. [PMC free article] [PubMed] [Google Scholar]
- 6.Amsterdam J. D., Shults J., Soeller I., Mao J. J., Rockwell K., Newberg A. B.2012. Chamomile (Matricaria recutita) may provide antidepressant activity in anxious, depressed humans: an exploratory study. Altern. Ther. Health Med. 18: 44–49. [PMC free article] [PubMed] [Google Scholar]
- 7.Anacarso I., Sabia C., de Niederhäusern S., Iseppi R., Condò C., Bondi M., Messi P.2019. In vitro evaluation of the amoebicidal activity of rosemary (Rosmarinus officinalis L.) and cloves (Syzygium aromaticum L. Merr. & Perry) essential oils against Acanthamoeba polyphaga trophozoites. Nat. Prod. Res. 33: 606–611. doi: 10.1080/14786419.2017.1399390 [DOI] [PubMed] [Google Scholar]
- 8.Awang-Dennis V. C.2006. The Herbs of Choice: The Therapeutic Use of Phytomedicinals, 3rd ed., CRC Press, Boca Raton. [Google Scholar]
- 9.Bahramikia S., Ardestani A., Yazdanparast R.2009. Protective effects of four Iranian, medicinal plants against free radical-mediated protein oxidation. Food Chem. 1159: 37–42. doi: 10.1016/j.foodchem.2008.11.054 [DOI] [Google Scholar]
- 10.Batiha G. E. S., Beshbishy A. M., Alkazmi L., Adeyemi O. S., Nadwa E., Rashwan E., El-Mleeh A., Igarashi I.2020. Gas chromatography-mass spectrometry analysis, phytochemical screening and antiprotozoal effects of the methanolic Viola tricolor and acetonic Laurus nobilis extracts. BMC Complement Med Ther 20: 87. doi: 10.1186/s12906-020-2848-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batista R., Silva A. J., Jr., de Oliveira A. B.2009. Plant-derived antimalarial agents: new leads and efficient phytomedicines. Part II. Non-alkaloidal natural products. Molecules 14: 3037–3072. doi: 10.3390/molecules14083037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertrand X., Hocquet D.2011. Antibiotic drug resistance: causes and solutions. Eur. J. Hosp. Pharm. Sci. Pract. 17: 58–59. [Google Scholar]
- 13.Bhowmik D., Kumar K. S., Yadav A., Srivastava S., Paswan S., Dutta A. S.2012. Recent trends in Indian traditional herbs Syzygium aromaticum and its health benefits. J. Pharmacogn. Phytochem. 1: 13–23. [Google Scholar]
- 14.Boothroyd J. C., Grigg M. E.2002. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr. Opin. Microbiol. 5: 438–442. doi: 10.1016/S1369-5274(02)00349-1 [DOI] [PubMed] [Google Scholar]
- 15.Bremness L.1994. The Complete Book of Herbs: A Practical Guide to Growing and Using Herbs, 5th ed., Studio Seattle Goodwill, Seattle. [Google Scholar]
- 16.Cara L., Borel P., Armand M., Senft M., Lafont H., Portugal H., Pauli A. M., Boulze D., Lacombe C., Lairon D.1991. Plasma lipid lowering effects of wheat germ in hypercholesterolemic subjects. Plant Foods Hum. Nutr. 41: 135–150. doi: 10.1007/BF02194082 [DOI] [PubMed] [Google Scholar]
- 17.Cemek M., Yilmaz E., Büyükokuroğlu M. E.2010. Protective effect of Matricaria chamomilla on ethanol-induced acute gastric mucosal injury in rats. Pharm. Biol. 48: 757–763. doi: 10.3109/13880200903296147 [DOI] [PubMed] [Google Scholar]
- 18.Chandrashekhar V. M., Halagali K. S., Nidavani R. B., Shalavadi M. H., Biradar B. S., Biswas D., Muchchandi I. S.2011. Anti-allergic activity of German chamomile (Matricaria recutita L.) in mast cell mediated allergy model. J. Ethnopharmacol. 137: 336–340. doi: 10.1016/j.jep.2011.05.029 [DOI] [PubMed] [Google Scholar]
- 19.Choi W. H., Lee I. A.2018. Evaluation of anti-Toxoplasma gondii effect of ursolic acid as a novel toxoplasmosis inhibitor. Pharmaceuticals (Basel) 11: 43. doi: 10.3390/ph11020043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Almeida I., Alviano D. S., Vieira D. P., Alves P. B., Blank A. F., Lopes A. H. C., Alviano C. S., Rosa M. S.2007. Antigiardial activity of Ocimum basilicum essential oil. Parasitol. Res. 101: 443–452. doi: 10.1007/s00436-007-0502-2 [DOI] [PubMed] [Google Scholar]
- 21.Değerli K., Kilimcioğlu A. A., Kurt O., Tamay A. T., Özbilgin A.2003. Efficacy of azithromycin in a murine toxoplasmosis model, employing a Toxoplasma gondii strain from Turkey. Acta Trop. 88: 45–50. doi: 10.1016/S0001-706X(03)00194-3 [DOI] [PubMed] [Google Scholar]
- 22.De Marino S., Borbone N., Zollo F., Ianaro A., Di Meglio P., Iorizzi M.2004. Megastigmane and phenolic components from Laurus nobilis L. leaves and their inhibitory effects on nitric oxide production. J. Agric. Food Chem. 52: 7525–7531. doi: 10.1021/jf048782t [DOI] [PubMed] [Google Scholar]
- 23.Djeussi D. E., Noumedem J. A., Seukep J. A., Fankam A. G., Voukeng I. K., Tankeo S. B., Nkuete A. H. L., Kuete V.2013. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement. Altern. Med. 13: 164. doi: 10.1186/1472-6882-13-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunay I. R., Gajurel K., Dhakal R., Liesenfeld O., Montoya J. G.2018. Treatment of Toxoplasmosis: historical perspective, animal models, and current clinical practice. Clin. Microbiol. Rev. 31: e00057–e17. doi: 10.1128/CMR.00057-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duraipandiyan V., Ayyanar M., Ignacimuthu S.2006. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement. Altern. Med. 6: 35. doi: 10.1186/1472-6882-6-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsheikha H. M.2008. Congenital toxoplasmosis: priorities for further health promotion action. Public Health 122: 335–353. doi: 10.1016/j.puhe.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 27.El Zawawy L. A.2008. Effect of artesunate on Toxoplasma gondii: in vitro and in vivo studies. J. Egypt. Soc. Parasitol. 38: 185–201. [PubMed] [Google Scholar]
- 28.Ezzat-Ali Esmail O.2012. A Possible protective effect of Citrullus colocynthis melon against diabetes mellitus type-2 associated with non-alcoholic fatty liver syndrome in rats. J. Am. Sci. 8: 1054–1061. [Google Scholar]
- 29.Faleiro L., Miguel G., Gomes S., Costa L., Venâncio F., Teixeira A., Figueiredo A. C., Barroso J. G., Pedro L. G.2005. Antibacterial and antioxidant activities of essential oils isolated from Thymbra capitata L. (Cav.) and Origanum vulgare L. J. Agric. Food Chem. 53: 8162–8168. doi: 10.1021/jf0510079 [DOI] [PubMed] [Google Scholar]
- 30.Farzaei M. H., Rahimi R., Abdolladi Z., Abbasabahi Z.2013. An evidence-based review on medicinal plants used for the treatment of peptic ulcer in traditional iranian medicine. Int. J. Pharmacol. 9: 108–124. doi: 10.3923/ijp.2013.108.124 [DOI] [Google Scholar]
- 31.Furtado J. M., Smith J. R., Belfort R., Jr., Gattey D., Winthrop K. L.2011. Toxoplasmosis: a global threat. J. Glob. Infect. Dis. 3: 281–284. doi: 10.4103/0974-777X.83536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardiner P.2007. Complementary, holistic, and integrative medicine: chamomile. Pediatr. Rev. 28: e16–e18. doi: 10.1542/pir.28-4-e16 [DOI] [PubMed] [Google Scholar]
- 33.Ghazanfar S. A.1994. Handbook of Arabian Medicinal Plants,1st ed., CRC press, Boca Raton. [Google Scholar]
- 34.Guginski G., Luiz A. P., Silva M. D., Massaro M., Martins D. F., Chaves J., Mattos R. W., Silveira D., Ferreira V. M., Calixto J. B., Santos A. R.2009. Mechanisms involved in the antinociception caused by ethanolic extract obtained from the leaves of Melissa officinalis (lemon balm) in mice. Pharmacol. Biochem. Behav. 93: 10–16. doi: 10.1016/j.pbb.2009.03.014 [DOI] [PubMed] [Google Scholar]
- 35.Guimarães R., Barros L., Dueñas M., Calhelha R. C., Carvalho A. M., Santos-Buelga C., Queiroz M. J., Ferreira I. C.2013. Infusion and decoction of wild German chamomile: bioactivity and characterization of organic acids and phenolic compounds. Food Chem. 136: 947–954. doi: 10.1016/j.foodchem.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 36.Haddad M. H. F., Mahbodfar H., Zamani Z., Ramazani A.2017. Antimalarial evaluation of selected medicinal plant extracts used in Iranian traditional medicine. Iran. J. Basic Med. Sci. 20: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajaji S., Sifaoui I., López-Arencibia A., Reyes-Batlle M., Jiménez I. A., Bazzocchi I. L., Valladares B., Akkari H., Lorenzo-Morales J., Piñero J. E.2018. Leishmanicidal activity of α-bisabolol from Tunisian chamomile essential oil. Parasitol. Res. 117: 2855–2867. doi: 10.1007/s00436-018-5975-7 [DOI] [PubMed] [Google Scholar]
- 38.Innes E. A.2010. A brief history and overview of Toxoplasma gondii. Zoonoses Public Health 57: 1–7. doi: 10.1111/j.1863-2378.2009.01276.x [DOI] [PubMed] [Google Scholar]
- 39.Jones F. A.1996. Herbs—useful plants. Their role in history and today. Eur. J. Gastroenterol. Hepatol. 8: 1227–1231. doi: 10.1097/00042737-199612000-00018 [DOI] [PubMed] [Google Scholar]
- 40.Jones P. J., Demonty I., Chan Y. M., Herzog Y., Pelled D.2007. Fish-oil esters of plant sterols differ from vegetable-oil sterol esters in triglycerides lowering, carotenoid bioavailability and impact on plasminogen activator inhibitor-1 (PAI-1) concentrations in hypercholesterolemic subjects. Lipids Health Dis. 6: 28. doi: 10.1186/1476-511X-6-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kavitha N., Noordin R., Chan K. L., Sasidharan S.2012. In vitro anti-Toxoplasma gondii activity of root extract/fractions of Eurycoma longifolia Jack. BMC Complement. Altern. Med. 12: 91–98. doi: 10.1186/1472-6882-12-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan A. J.2012. Medicinal properties of frankincense. Int. J. Nutr. Pharmacol. Neurol. Dis. 2: 79. doi: 10.4103/2231-0738.95925 [DOI] [Google Scholar]
- 43.Khanam Z., Wen C. S., Bhat I. U. H.2015. Phytochemical screening and antimicrobial activity of root and stem extracts of wild Eurycoma longifolia Jack (Tongkat Ali). J. King Saud Univ. Sci. 27: 23–30. doi: 10.1016/j.jksus.2014.04.006 [DOI] [Google Scholar]
- 44.Konwar B. K., Gohain A. K.1999. Nutritive value of spent citronella grass (Cymbopogon nardus) in cattle. Ind. J. An. Nutr. 16: 324–325. [Google Scholar]
- 45.Kozak M., Sobczak P., Żukiewicz-Sobczak W.2016. Health properties of selected herbal plants. Health Probl. Civiliz. 2: 64–70. doi: 10.5114/hpc.2016.59635 [DOI] [Google Scholar]
- 46.Kozłowska M., Laudy A. E., Starościak B. J., Napiórkowski A., Chomicz L., Kazimierczuk Z.2010. Antimicrobial and antiprotozoal effect of sweet marjoram (Origanum majorana L.). Acta Sci. Pol. Hortorum Cultus 9: 133–141. [Google Scholar]
- 47.Leesombun A., Boonmasawai S., Nishikawa Y.2019. Ethanol extracts from Thai plants have anti-plasmodium and anti-toxoplasma activities in vitro. Acta Parasitol. 64: 257–261. doi: 10.2478/s11686-019-00036-w [DOI] [PubMed] [Google Scholar]
- 48.Lehane A. M., Saliba K. J.2008. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Res. Notes 1: 26. doi: 10.1186/1756-0500-1-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewin G., Cojean S., Gupta S., Verma A., Puri S. K., Loiseau P. M.2011. In vitro antileishmanial properties of new flavonoids against Leishmania donovani. Biomed. Prev. Nutr. 13: 168–171. doi: 10.1016/j.bionut.2011.06.012 [DOI] [Google Scholar]
- 50.Machado M., Dinis A. M., Salgueiro L., Custódio J. B., Cavaleiro C., Sousa M. C.2011. Anti-Giardia activity of Syzygium aromaticum essential oil and eugenol: effects on growth, viability, adherence and ultrastructure. Exp. Parasitol. 127: 732–739. doi: 10.1016/j.exppara.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 51.Machado M., Pires P., Dinis A. M., Santos-Rosa M., Alves V., Salgueiro L., Cavaleiro C., Sousa M. C.2012. Monoterpenic aldehydes as potential anti-Leishmania agents: activity of Cymbopogon citratus and citral on L. infantum, L. tropica and L. major. Exp. Parasitol. 130: 223–231. doi: 10.1016/j.exppara.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 52.Malatyali E., Tepe B., Degerli S., Berk S.2012. In vitro amoebicidal activities of Satureja cuneifolia and Melissa officinalis on Acanthamoeba castellanii cysts and trophozoites. Parasitol. Res. 110: 2175–2180. doi: 10.1007/s00436-011-2744-2 [DOI] [PubMed] [Google Scholar]
- 53.Mandlik V., Patil S., Bopanna R., Basu S., Singh S.2016. Biological activity of coumarin derivatives as anti-leishmanial agents. PLoS One 11: e0164585. doi: 10.1371/journal.pone.0164585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann C., Staba E. J.1986. The chemistry, pharmacology, and commercial formulations of chamomile. J. Herbs Spices Med. Plants 1: 235–280. [Google Scholar]
- 55.Manniche L.1999. An Ancient Egyptian Herbal, First University of Texas Press, Austin. [Google Scholar]
- 56.Manzoor I., Khuda M., Rahman M., Yusuf M., Chowdhrury J. U.1984. Essential oils of Cymbopogonspecies of Bangladesh. J. Bangladesh Acad. Sci. 8: 77–80. [Google Scholar]
- 57.Martínez-Castillo M., Pacheco-Yepez J., Flores-Huerta N., Guzmán-Téllez P., Jarillo-Luna R. A., Cárdenas-Jaramillo L. M., Campos-Rodríguez R., Shibayama M.2018. Flavonoids as a natural treatment against Entamoeba histolytica. Front. Cell. Infect. Microbiol. 8: 209. doi: 10.3389/fcimb.2018.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKay D. L., Blumberg J. B.2006. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L.). Phytother. Res. 20: 519–530. doi: 10.1002/ptr.1900 [DOI] [PubMed] [Google Scholar]
- 59.McKeon T. A.2016. Emerging industrial oil crops. p. 275–341. In: Industrial Oil Crops, AOCS Press, Urbana. [Google Scholar]
- 60.McLeod R., Van Tubbergen C., Montoya J. G., Petersen E.2014. Chapter 4. Human Toxoplasma Infection, 2nd ed., Elsevier, London. [Google Scholar]
- 61.Mead J., McNair N.2006. Antiparasitic activity of flavonoids and isoflavones against Cryptosporidium parvum and Encephalitozoon intestinalis. FEMS Microbiol. Lett. 259: 153–157. doi: 10.1111/j.1574-6968.2006.00263.x [DOI] [PubMed] [Google Scholar]
- 62.Montazeri M., Mehrzadi S., Sharif M., Sarvi S., Tanzifi A., Aghayan S. A., Daryani A.2018. Drug resistance in Toxoplasma gondii. Front. Microbiol. 9: 2587. doi: 10.3389/fmicb.2018.02587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montoya J. G., Liesenfeld O.2004. Toxoplasmosis. Lancet 363: 1965–1976. doi: 10.1016/S0140-6736(04)16412-X [DOI] [PubMed] [Google Scholar]
- 64.Monzote L., Herrera I., Satyal P., Setzer W. N.2019. In-vitro evaluation of 52 commercially-available essential oils against Leishmania amazonensis. Molecules 24: 1248. doi: 10.3390/molecules24071248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mukta N., Neeta P. M.2017. A review on sesame-an ethno medicinally significant oil crop. In. J. Life. Sci. Pharm. Res. 7: L58–L63. [Google Scholar]
- 66.Naddaf N., Haddad S.2020. Apigenin effect against Leishmania tropica amastigotes in vitro. J. Parasit. Dis. 44: 574–578. doi: 10.1007/s12639-020-01230-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagamune K., Beatty W. L., Sibley L. D.2007. Artemisinin induces calcium-dependent protein secretion in the protozoan parasite Toxoplasma gondii. Eukaryot. Cell 6: 2147–2156. doi: 10.1128/EC.00262-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nerio L. S., Olivero-Verbel J., Stashenko E.2010. Repellent activity of essential oils: a review. Bioresour. Technol. 101: 372–378. doi: 10.1016/j.biortech.2009.07.048 [DOI] [PubMed] [Google Scholar]
- 69.Nishikawa Y., Xuenan X., Makala L., Vielemeyer O., Joiner K. A., Nagasawa H.2003. Characterisation of Toxoplasma gondii engineered to express mouse interferon-gamma. Int. J. Parasitol. 33: 1525–1535. doi: 10.1016/S0020-7519(03)00204-2 [DOI] [PubMed] [Google Scholar]
- 70.Okere S. O., Sangodele J. O., Ogunwole E., Adams M. D., Shafe M. O.2014. Antiplasmodial activity of aqueous leaf extract of Cymbopogon citratus against Plasmodium falciparum infected rats. Am. J. Biomed. Life Sci 2: 60–64. doi: 10.11648/j.ajbls.20140203.12 [DOI] [Google Scholar]
- 71.Oladeji O. S., Adelowo F. E., Ayodele D. T., Odelade K. A.2019. Phytochemistry and pharmacological activities of Cymbopogon citratus: a review. Sci. Am. 6: e00137. [Google Scholar]
- 72.Ozen T.2009. Investigation of antioxidant properties of Nasturtium officinale (watercress) leaf extracts. Acta Pol. Pharm. 66: 187–193. [PubMed] [Google Scholar]
- 73.Petronilho S., Maraschin M., Coimbra M. A., Rocha S. M.2012. In vitro and in vivo studies of natural products: achallenge for their valuation. The case study of chamomile (Matricaria recutita L.). Ind. Crops Prod. 40: 1–12. doi: 10.1016/j.indcrop.2012.02.041 [DOI] [Google Scholar]
- 74.Pillai S., Mahmud R., Lee W. C., Perumal S.2012. Anti-parasitic activity of Myristica Fragrans Houtt. Essential oil against Toxoplasma Gondii parasite. APCBEE Procedia 2: 92–96. doi: 10.1016/j.apcbee.2012.06.017 [DOI] [Google Scholar]
- 75.Pinheiro P. F., Queiroz V. T. D., Rondelli V. M., Costa A. V., Marcelino T. D. P., Pratissoli D.2013. Insecticidal activity of citronella grass essential oil on Frankliniella schultzei and Myzus persicae. Cienc. Agrotec. 37: 138–144. doi: 10.1590/S1413-70542013000200004 [DOI] [Google Scholar]
- 76.Rabiul H., Subhasish M., Parag G.2011. Investigation of in vitro anthelmintic activity of Cinnamomum Camphor leaves. Int. J. Drug Dev. & Res 3: 295–300. [Google Scholar]
- 77.Rampart M., Beetens J. R., Bult H., Herman A. G., Parnham M. J., Winkelmann J.1986. Complement-dependent stimulation of prostacyclin biosynthesis: inhibition by rosmarinic acid. Biochem. Pharmacol. 35: 1397–1400. doi: 10.1016/0006-2952(86)90289-3 [DOI] [PubMed] [Google Scholar]
- 78.Rizvi T. S., Shahina F.2014. Nematicidal activity of Citrullus colocynthis extracts against root-knot nematodes. Pak. J. Nematol. 32: 101–112. [Google Scholar]
- 79.Rombi M.1993. One Hundered Medicinal Plants. pp. 63–65. New Italian Institute of Graphic Arts, Bergamo (in Italian). [Google Scholar]
- 80.Salman A. S., Farghaly A. A., Donya S. M., Shata F.2012. Protective effect of Cinnamomum camphora leaves extract against atrazine induced genotoxicity and biochemical effect on mice. J. Am. Sci. 81: 190–196. [Google Scholar]
- 81.Schmidt D. R., Hogh B., Andersen O., Hansen S. H., Dalhoff K., Petersen E.2006. Treatment of infants with congenital toxoplasmosis: tolerability and plasma concentrations of sulfadiazine and pyrimethamine. Eur. J. Pediatr. 165: 19–25. doi: 10.1007/s00431-005-1665-4 [DOI] [PubMed] [Google Scholar]
- 82.Schopen A.1983.Traditional Remedies in Yemen. pp. 66–68. Steiner, Wiesbaden (in German). [Google Scholar]
- 83.Sebai H., Jabri M. A., Souli A., Hosni K., Rtibi K., Tebourbi O., El-Benna J., Sakly M.2015. Chemical composition, antioxidant properties and hepatoprotective effects of chamomile (Matricaria recutita L.) decoction extract against alcohol-induced oxidative stress in rat. Gen. Physiol. Biophys. 34: 263–275. doi: 10.4149/gpb_2014039 [DOI] [PubMed] [Google Scholar]
- 84.Serranti D., Buonsenso D., Valentini P.2011. Congenital toxoplasmosis treatment. Eur. Rev. Med. Pharmacol. Sci. 15: 193–198. [PubMed] [Google Scholar]
- 85.Shen G., Wang X., Sun H., Gao Y.2016. Seroprevalence of Toxoplasma gondii infection among HIV/AIDS patients in Eastern China. Korean J. Parasitol. 54: 93–96. doi: 10.3347/kjp.2016.54.1.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Silva A. R. S. T., Scher R., Santos F. V., Ferreira S. R., Cavalcanti S. C. H., Correa C. B., Bueno L. L., Alves R. J., Souza D. P., Fujiwara R. T., Dolabella S. S.2017. Leishmanicidal activity and structure-activity relationships of essential oil constituents. Molecules 22: 815. doi: 10.3390/molecules22050815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soares F. G. N., Göethel G., Kagami L. P., das Neves G. M., Sauer E., Birriel E., Varela J., Gonçalves I. L., Von Poser G., González M., Kawano D. F., Paula F. R., de Melo E. B., Garcia S. C., Cerecetto H., Eifler-Lima V. L.2019. Novel coumarins active against Trypanosoma cruzi and toxicity assessment using the animal model Caenorhabditis elegans. BMC Pharmacol. Toxicol. 20 Suppl 1: 76. doi: 10.1186/s40360-019-0357-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tenter A. M., Heckeroth A. R., Weiss L. M.2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30: 1217–1258. doi: 10.1016/S0020-7519(00)00124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Uchiyama N., Matsunaga K., Kiuchi F., Honda G., Tsubouchi A., Nakajima-Shimada J., Aoki T.2002. Trypanocidal terpenoids from Laurus nobilis L. Chem. Pharm. Bull. (Tokyo) 50: 1514–1516. doi: 10.1248/cpb.50.1514 [DOI] [PubMed] [Google Scholar]
- 90.Weiss L. M., Dubey J. P.2009. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 39: 895–901. doi: 10.1016/j.ijpara.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan C., Liang L. J., Zheng K. Y., Zhu X. Q.2016. Impact of environmental factors on the emergence, transmission and distribution of Toxoplasma gondii. Parasit. Vectors 9: 137. doi: 10.1186/s13071-016-1432-6 [DOI] [PMC free article] [PubMed] [Google Scholar]


