Abstract
This study aimed to elucidate the epidemiological status of hemoplasma infection and investigate the interaction between Theileria orientalis and hemoplasmas in Japanese Black breeding cows raised in the Kyushu and Okinawa regions. Blood samples were collected from 400 cows from 80 different farms in eight prefectures (five samples per farm and 10 farms per prefecture). Mycoplasma wenyonii (Mw), “Candidatus Mycoplasma haemobos” (CMh), and T. orientalis were examined by polymerase chain reaction (PCR) assay using whole blood samples. PCR results showed that 91.5% (366/400) of cows were positive for bovine hemoplasma: 40.3% were infected with Mw only, 9.5% with CMh only, and 41.8% with both species. T. orientalis was detected in 36% (144/400) of cows. The infection rate of T. orientalis was higher in the grazing group (P<0.001) than in the housed group, while the rate of CMh infection was higher (P<0.05) in the housed group than in the grazing group, suggesting that not only the tick but also other arthropod vectors may contribute to hemoplasma transmission. Although the cows with hemoplasma dual infection showed higher (P<0.05) white blood cell counts compared with hemoplasma-negative cows, there was no difference in hematologic parameters related to the anemia between the hemoplasma-positive and -negative animals. This may indicate that Japanese Black cattle could have resistance to the anemia caused by infection with hemoplasma.
Keywords: Candidatus Mycoplasma haemobos, Japanese Black breeding cow, Mycoplasma wenyonii, Theileria orientalis
Hemotropic mycoplasmas (hemoplasmas) are uncultivable and cell wall-less organisms that attach to erythrocytes in a variety of mammalian species, such as pigs, cattle, horses, cats, and dogs [1, 12]. Mycoplasma wenyonii (Mw) [14] and a provisional species “Candidatus Mycoplasma haemobos” (CMh) [8, 25] have been recognized as major hemoplasma species in cattle. The majority of Mw infections remain subclinical, but fever, anemia, and edema of the scrotum and hind limbs were reported in a bull infected with Mw [13]. In dairy heifers, Mw has been associated with swollen teats and distal portions of the hind limbs, prefemoral lymphadenopathy, transient fever, rough coat, decreased milk production, and subsequent weight loss and reproductive inefficiency [22]. On the other hand, CMh has recently been discovered in Japan using molecular methods such as polymerase chain reaction (PCR) and sequencing techniques [25]. The effect of CMh infection on the hematologic parameters might be stronger than that of Mw infection [26]. Thus, the clinical importance of hemoplasma infection in cattle is still controversial.
The epidemiology of bovine hemoplasma remains poorly understood, but possible transmission routes may include vectors, such as lice, flies, and mosquitoes [23]; hard ticks [21]; and blood-sucking flies [10]; or across the placenta [5, 10, 19].
Recently, grazing on mountain slopes, foot-hills, and abandoned agricultural land has been promoted for the purpose of labor-saving, low-cost production, and improved feed self-sufficiency rate in Japanese Black cattle [6], while the practice of pasturing cattle is associated with a high risk of contracting theileriosis [34] or hemoplasmosis [21]. Therefore, the preventive measures for these disease are of great concern in grazing feeding system. The both bovine hemoplasmas have been distributed worldwide, and the prevalence of their infections has been evaluated in the northern part of Japan, such as in Hokkaido [26, 28, 29], Iwate [20], and Miyagi [16] prefectures, but the study in the southern part of Japan is limited [3]. The beef breeding cow population in the Kyushu and Okinawa regions represents 896.9 thousand animals or 36% of the total beef breeding cow population in Japan. Almost all cattle previously examined were Holstein–Friesian dairy cows in Japan, and thus, little information is available on hemoplasma infection in Japanese Black cattle. Coinfections have been demonstrated with Anaplasma marginale, A. phagocytophilum, and Babesia and Theileria spp. [9]. Although the disease often is subclinical, coinfection with other hemotropic infectious agents has been suggested to increase the pathogenicity and clinical significance of bovine hemoplasmosis [11]. Conversely, Tagawa et al. [30] observed interference between T. orientalis and hemoplasmas in grazing Holstein cattle, and there is no report concerning Japanese Black cattle.
Accordingly, this study aimed to elucidate the epidemiological status of hemoplasma infection and investigate the interaction between T. orientalis and hemoplasmas in Japanese Black breeding cows raised in the Kyushu and Okinawa regions.
MATERIALS AND METHODS
Samples
The 10 farms per prefecture that raised >5 adult Japanese Black breeding cows were chosen randomly in eight prefectures of the Kyushu and Okinawa regions (Fig. 1). Blood samples anticoagulated with ethylenediaminetetraacetic acid dipotassium salt dihydrate (EDTA-2K) were collected from five clinically normal cows (age range, 1–16 years) per farm. Thus, 400 samples were collected in total between February 2018 and January 2019. The living condition (whether the cows experienced grazing or were housed in a barn year-round) was recorded by the veterinarians. Hematologic parameters, including white (WBC) and red (RBC) blood cell counts, hemoglobin (HGB) concentration, hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration, and platelet (PLT) count, were measured using an automated hematology analyzer (pocH-100iV; Sysmex Corp., Kobe, Japan; PCE-210N; Erma Inc., Tokyo, Japan; MEK-6550 and MEK-6458 Celltac α; Nihon Kohden Corp., Tokyo, Japan). Fifteen of 400 samples were analyzed only for HCT by the microhematocrit method. The remaining blood samples were stored at −40°C until use for PCR assay. All protocols were approved by the Animal Ethics Committee of the University of Miyazaki (Approval No. 2018-06-26-Z21).
Fig. 1.
Location of sample collection in Kyushu and Okinawa region, southern part of Japan (modified from electronic map of Geospatial Information Authority of Japan).
Detection of M. wenyonii and “Candidatus Mycoplasma haemobos”
Direct PCR was conducted by the method of Tagawa et al. [27]. The primer pair F2 (5′-ACGAAAGTCTGATGGAGCAATA-3′) and R2 (5′-ACGCCCAATAAATCCGRATAAT-3′) amplify 16S rRNA genes of the hemoplasma. The expected amplicons of Mw and CMh were 193 and 170 base pairs (bp), respectively [25].
Detection of T. orientalis
DNA was extracted from 200 µl whole blood by MagLEAD 12gc (Precision System Science Co., Ltd., Chiba, Japan) according to the manufacturer’s instructions. PCR was conducted by the methods of Ota et al. [17]. A 776 bp DNA fragment from the major piroplasm surface protein (MPSP) gene of T. orientalis was amplified by PCR with the primers MPSP-F (5′-CTTTGCCTAGGATACTTCCT-3′) and MPSP-R (5′-ACGGCAAGTGGTGAGAACT-3′).
Sequence analysis of the 16S rRNA gene
Blood samples from cows that were singly infected with Mw or CMh and were born and raised in each prefecture were used for phylogenetic analysis. Genomic DNA was extracted from whole blood as described previously. To determine the longer 16S rRNA gene sequence (approximately 1,500 bp) of Mw and CMh, two PCRs were conducted by the method of Tagawa et al. [25] with some modifications using the following two primer sets: fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and R2, and F2 and Rp2 (5′-ACGGCTACCTTGTTACGACTT-3′). After confirming the specific amplification of each target gene by 2% agarose gel electrophoresis, the PCR products were cut from agarose gel and purified using a QIAquick Gel Extraction kit (Qiagen, Tokyo, Japan). The nucleotide sequences were determined directly from the PCR fragment in a PCR-based reaction using an ABI BigDye 3.1v (Applied Biosystems, Tokyo, Japan) and analyzed with a 3130 DNA sequencer (Applied Biosystems). ClustalW [32] was used to align the 16S rRNA gene sequence.
A phylogenetic tree was constructed using the neighbor joining method [18], and the topology of the tree was evaluated by 1,000 bootstrap trials with the sequence boot program in MEGA-X. The 16S rRNA gene sequence of MW (AY946266, AF016546, DQ641256, AY769937, FJ375309, MF377464, KX171205, EU367963, EU367964, MG948624, MG948626, and EF221880), M. ovis (AF338268), “Candidatus Mycoplasma haematoparvum” (AY383241), M. suis (U88565), M. haemomuris (U82963), M. haemocanis (AF407208), M. coccoides (AY171918), M. haemofelis (U88563), CMh (EF460765, EF616467, EU367965, MG948631, and MG948628), and M. pneumoniae (M29061) were obtained from the National Center for Biotechnology Information database.
Statistical analysis
All statistical analyses were conducted using SAS (Version 9.4, SAS Institute, Inc., Cary, NC, USA). The following variables were compared using the χ2 test: prefecture, living condition, age, and Theileria infection. The rates of Theileria infection between grazing and housed groups and between the hemoplasma-positive and -negative groups were also compared by the χ2 test. The Mann–Whitney U test was conducted to analyze hematologic findings. P<0.05 was considered statistically significant.
RESULTS
Hemoplasma was detected in 366 of the 400 (91.5%) blood samples: 161 (40.3%) were positive for Mw only and 38 (9.5%) for CMh only, whereas 167 (41.8%) showed dual infection. The remaining 34 cows were hemoplasma negative. T. orientalis was detected in 144 (36%) blood samples. Although no geographic difference in hemoplasma infection rate was noted among eight prefectures, significant differences were noted in the rates of Theileria infection (P<0.001) and animals that experienced grazing (P<0.001; Table 1). The prefectures with higher grazing rates tended to show higher Theileria infection rates except for Miyazaki prefecture.
Table 1. Numbers and percentages of cows infected with hemoplasma and Theileria and grazing in each prefecture.
| Organisms/ living condition | No. of PCR-positive and grazing cows (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fukuoka | Saga | Nagasaki | Kumamoto | Oita | Miyazaki | Kagoshima | Okinawa | P-value* | ||
| Hemoplasma | ||||||||||
| Only Mw | 16 (32) | 18 (36) | 22 (44) | 29 (58) | 16 (32) | 15 (30) | 22 (44) | 23 (46) | 0.07 | |
| Only CMh | 8 (16) | 5 (10) | 4 (8) | 2 (4) | 6 (12) | 4 (8) | 5 (10) | 4 (8) | 0.66 | |
| Mw + CMh | 20 (40) | 19 (38) | 17 (34) | 17 (34) | 26 (52) | 28 (56) | 20 (40) | 20 (40) | 0.23 | |
| Total hemoplasma | 44 (88) | 42 (84) | 43 (86) | 48 (96) | 48 (96) | 47 (94) | 47 (94) | 47 (94) | 0.18 | |
| Theileria | 4 (8) | 11 (22) | 30 (60) | 37 (74) | 36 (72) | 1 (2) | 22 (44) | 3 (6) | <0.001 | |
| Grazing | 0 (0) | 5 (10) | 45 (90) | 20 (40) | 31 (62) | 20 (40) | 17 (34) | 0 (0) | <0.001 | |
Mw, M. wenyonii; CMh, “Candidatus Mycoplasma haemobos”. *P-value: comparison of each infection rate and the rate of cattle that experienced grazing among eight prefectures by the χ2 test.
The grazing and housed groups included 138 and 262 cows, respectively. The Theileria infection rate in the grazing group (68.1%; 94/138) was significantly higher (P<0.001) than that in the housed group (19.1%; 50/262). Concerning living condition, only CMh-positive rate was higher (P<0.05) in the housed group than that in the grazing group (Table 2). An association between hemoplasma infection rate and age was found with significant differences in only Mw-(P<0.001), Mw + CMh-(P<0.001), and the total hemoplasma-positive groups-(P<0.001). Cows 1 to 4 years old showed the lowest infection rate and the rate increased with increasing age in only Mw-positive group. In contrast, cows 1 to 4 and 5 to 7 years old showed the highest infection rates in Mw + CMh and the total hemoplasma-positive group, respectively, and the rates tended to decrease with increasing age. The only Mw infection rate was higher in the Theileria-positive cows (52.1%; P<0.001) than in the Theileria-negative animals (33.6%). Conversely, the dual infection rate in the Theileria-positive cows (33.3%; P<0.05) was lower than that in the Theileria-negative animals (46.5%).
Table 2. Sample prevalence of bovine hemoplasma infection according to living condition, age, and Theileria infection.
| Variable | Total number | PCR results (%) |
||||
|---|---|---|---|---|---|---|
| Only Mw positive | Only CMh positive | Mw + CMh positive | Total hemoplasma positive | |||
| Living condition | ||||||
| Grazing | 138 | 61 (44.2) | 7 (5.1) | 55 (39.9) | 123 (89.1) | |
| Housed | 262 | 100 (38.2) | 31 (11.8) | 112 (42.7) | 243 (92.7) | |
| P-value* | 0.24 | <0.05 | 0.58 | 0.22 | ||
| Age (years) | ||||||
| 1–4 | 124 | 32 (25.8) | 10 (8.1) | 77 (62.1) | 119 (96.0) | |
| 5–7 | 101 | 37 (36.6) | 13 (12.9) | 49 (48.5) | 99 (98.0) | |
| 8–10 | 96 | 47 (49.0) | 9 (9.4) | 26 (27.1) | 82 (85.4) | |
| ≥11 | 79 | 45 (57.0) | 6 (7.6) | 15 (19.0) | 66 (83.5) | |
| P-value† | <0.001 | 0.58 | <0.001 | <0.001 | ||
| Theileria infection | ||||||
| Positive | 144 | 75 (52.1) | 13 (9.0) | 48 (33.3) | 136 (94.4) | |
| Negative | 256 | 86 (33.6) | 25 (9.8) | 119 (46.5) | 230 (89.8) | |
| P-value‡ | <0.001 | 0.81 | <0.05 | 0.11 | ||
Mw, M. wenyonii; CMh, “Candidatus Mycoplasma haemobos”. *P-value: comparison of living condition in each hemoplasma group by the χ2 test. †P-value: comparison of age groups in each hemoplasma group by the χ2 test. ‡P-value: comparison of Theileria-positive and Theileria-negative in each hemoplasma group by the χ2 test.
Table 3 shows the Theileria infection rate in the hemoplasma-positive and -negative groups. The Theileria infection rate in only Mw-positive group was higher (46.6%; P<0.05) compared with hemoplasma-negative group (23.5%).
Table 3. Sample prevalence of Theileria infection according to hemoplasma infection.
| Variable | Total number | PCR results (%) |
P-value* | |
|---|---|---|---|---|
| Theileria positive | ||||
| Hemoplasma | ||||
| Only Mw | 161 | 75 (46.6) | <0.05 | |
| Only CMh | 38 | 13 (34.2) | 0.32 | |
| Mw + CMh | 167 | 48 (28.7) | 0.54 | |
| Total hemoplasma | 366 | 136 (37.2) | 0.11 | |
| Negative | 34 | 8 (23.5) | – | |
Mw, M. wenyonii; CMh, “Candidatus Mycoplasma haemobos”. *P-value: comparison of Theileria positive rate between the hemoplasma-positive and negative groups by the χ2 test.
The hematologic values in the hemoplasma-positive and hemoplasma-negative groups are compared in Table 4. The mean values of all parameters were within their reference ranges. Cows with hemoplasma dual infection had a higher (P<0.05) WBC count compared with hemoplasma-negative cows. In Theileria-infected and uninfected cows, there was no significant difference in hematologic values between the hemoplasma-positive and -negative groups. The data were shown in Table 5. Additionally, the comparison of hematologic values between the Theileria-positive and -negative groups was shown in Table 5. In Theileria-positive group, the WBC (P<0.01), HCT (P<0.05), MCV (P<0.01), and PLT (P<0.01) values of the only Mw-positive cows were higher than those in Theileria-negative group. The lower (P<0.05) RBC and HGB values, higher (P<0.01) WBC, and higher (P<0.05) PLT values were shown in only CMh-, Mw + CMh-positive, and hemoplasma-negative cows, respectively, in Theileria-positive group compared with Theileria-negative group.
Table 4. Comparison of hematologic values between hemoplasma-positive and negative groups.
| Parameter | PCR results |
|||
|---|---|---|---|---|
| Only Mw positive (n) | Only CMh positive (n) | Mw + CMh positive (n) | Negative (n) | |
| WBC (×102 µl) | 86.5 ± 35.0 (155) | 81.7 ± 25.9 (37) | 86.0 ± 34.2* (160) | 77.4 ± 38.3 (33) |
| RBC (×104 µl) | 718.2 ± 131.5 (155) | 745.6 ± 142.9 (37) | 735.6 ± 138.3 (160) | 723.6 ± 157.9 (33) |
| HGB (g/dl) | 11.9 ± 2.7 (155) | 11.9 ± 2.4 (37) | 11.9 ± 2.6 (160) | 12.2 ± 3.1 (33) |
| HCT (%) | 33.9 ± 7.1 (161) | 34.3 ± 6.3 (38) | 34.1 ± 6.8 (167) | 34.3 ± 8.4 (34) |
| MCV (fl) | 47.4 ± 4.6 (155) | 46.3 ± 4.5 (37) | 46.8 ± 4.9 (160) | 48.0 ± 6.2 (33) |
| MCH (pg) | 16.6 ± 1.8 (155) | 16.0 ± 1.7 (37) | 16.2 ± 1.9 (160) | 16.9 ± 1.7 (33) |
| MCHC (g/dl) | 35.2 ± 3.9 (155) | 34.7 ± 3.5 (37) | 34.9 ± 3.7 (160) | 35.6 ± 4.1 (33) |
| PLT (×104 µl) | 61.3 ± 105.7 (155) | 45.6 ± 83.5 (37) | 46.4 ± 71.2 (160) | 29.8 ± 15.0 (33) |
Mw, Mycoplasma wenyonii; CMh, “Candidatus Mycoplasma haemobos”; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet. Data are expressed as mean ± SD. *Significantly differs from the value in hemoplasma-negative group at P<0.05.
Table 5. Comparison of hematologic values between Theileria-positive and negative groups.
| Sample | Parameter | PCR results |
|||
|---|---|---|---|---|---|
| Only Mw positive (n) | Only CMh positive (n) | Mw + CMh positive (n) | Negative (n) | ||
| Theileria-positive | WBC (×102 µl) | 99.8 ± 38.5** (69) | 82.5 ± 19.9 (12) | 103.1 ± 40.1** (45) | 85.0 ± 40.8 (7) |
| RBC (×104 µl) | 715.1 ± 116.5 (69) | 677.8 ± 72.7* (12) | 736.6 ± 100.0 (45) | 699.9 ± 135.9 (7) | |
| HGB (g/dl) | 12.0 ± 2.4 (69) | 10.7 ± 1.6* (12) | 12.1 ± 2.1 (45) | 12.0 ± 1.9 (7) | |
| HCT (%) | 34.4 ± 6.1* (75) | 32.3 ± 3.8 (13) | 34.7 ± 5.6 (48) | 32.2 ± 6.6 (8) | |
| MCV (fl) | 48.6 ± 4.6** (69) | 47.4 ± 3.1 (12) | 47.6 ± 5.1 (45) | 48.3 ± 11.3 (7) | |
| MCH (pg) | 16.7 ± 1.7 (69) | 15.8 ± 1.5 (12) | 16.5 ± 2.0 (45) | 17.4 ± 2.6 (7) | |
| MCHC (g/dl) | 34.7 ± 3.9 (69) | 33.4 ± 3.5 (12) | 34.8 ± 3.8 (45) | 36.8 ± 3.8 (7) | |
| PLT (×104 µl) | 95.1 ± 142.3** (69) | 78.2 ± 143.3 (12) | 77.1 ± 116.6 (45) | 38.6 ± 9.0* (7) | |
| Theileria-negative | WBC (×102 µl) | 75.9 ± 27.8 (86) | 81.3 ± 28.7 (25) | 79.3 ± 29.1 (115) | 75.3 ± 38.2 (26) |
| RBC (×104 µl) | 720.7 ± 143.0 (86) | 778.1 ± 157.5 (25) | 735.2 ± 151.0 (115) | 730.0 ± 165.1 (26) | |
| HGB (g/dl) | 11.9 ± 2.9 (86) | 12.5 ± 2.5 (25) | 11.9 ± 2.8 (115) | 12.3 ± 3.4 (26) | |
| HCT (%) | 33.5 ± 7.9 (86) | 35.4 ± 7.2 (25) | 33.9 ± 7.3 (119) | 34.9 ± 8.9 (26) | |
| MCV (fl) | 46.4 ± 4.4 (86) | 45.8 ± 5.0 (25) | 46.4 ± 4.8 (115) | 47.9 ± 4.4 (26) | |
| MCH (pg) | 16.4 ± 1.9 (86) | 16.1 ± 1.9 (25) | 16.1 ± 1.8 (115) | 16.8 ± 1.4 (26) | |
| MCHC (g/dl) | 35.5 ± 3.9 (86) | 35.3 ± 3.4 (25) | 34.9 ± 3.7 (115) | 35.3 ± 4.2 (26) | |
| PLT (×104 µl) | 34.2 ± 48.9 (86) | 30.0 ± 15.8 (25) | 34.4 ± 36.1 (115) | 27.4 ± 15.5 (26) | |
Mw, Mycoplasma wenyonii; CMh, “Candidatus Mycoplasma haemobos”; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet. Data are expressed as mean ± SD. **, *Significantly differs from the value in Theileria-negative group at P<0.01 and P<0.05, respectively.
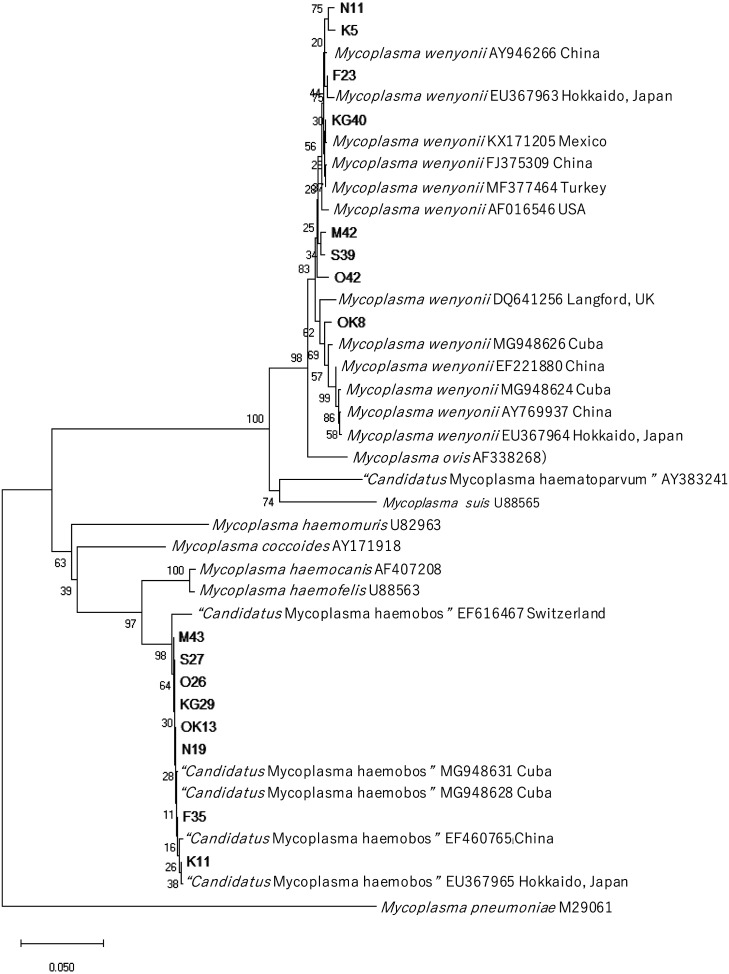
The results of phylogenetic tree analysis based on the nucleotide sequences of the 16S rRNA gene are shown in Fig. 2. The close evolutionary relationship between eight Mw isolates (N11, K5, F23, KG40, M42, S39, O42, OK8) and those from China, Japan (Hokkaido), Mexico, Turkey, the United States, the United Kingdom, and Cuba was confirmed. The sequences obtained from eight CMh isolates (M43, S27, O26, KG29, OK13, N19, F35, K11) were closely related to those from Switzerland, Cuba, China, and Japan (Hokkaido).
Fig. 2.
The results of phylogenetic tree analysis based on the nucleotide sequences of the 16S rRNA gene. F23, S39, N11, K5, O42, M42, KG40, and OK8 show Mw isolates. F35, S27, N19, K11, O26, M43, KG29, and OK13 show CMh isolates.
DISCUSSION
The total hemoplasma infection rate found in our study was 91.5% and was higher than rates reported in Hokkaido (22.3% [26], 64.7% [29], and 89.2% [28]) and Miyagi prefecture (71.6%) [16], which were located in the northern part of Japan. Fujihara et al. [3] reported a higher hemoplasma infection rate in the western part of Japan (Miyazaki, 93.8%) compared to Hokkaido and Miyagi prefecture and suggested that the geographic difference in morbidity might be due to the activity of arthropod vectors for hemoplasma transmission. Jersey cows showed a higher proportion of CMh-positive animals compared to Holstein cows [5]. Since blood samples were collected mainly from Holstein–Friesian cows in Hokkaido [26, 28, 29] and dairy and beef herds in Miyagi prefecture [16], the difference in cattle breeds may affect the prevalence of hemoplasma infection besides the geographic difference. Tagawa et al. [28] reported that 35.5% of cows were infected with MW only, 19.4% with CMh only, and 34.4% with both species. Similar findings were observed in our study. In contrast, Niethammer et al. [15] found that 56.59% of Simmental cows were positive for CMh, while 8.54% and 4.88% were positive for Mw or mixed infections, respectively. The difference in the prevalence of the single species may be due to the difference in climate, which could favor vector-borne transmission, breed susceptibility, housing system (barn vs. pasture), or the age of animals [15].
Lice, flies, and mosquitoes [23]; hard ticks [21]; and blood-sucking flies [10] have been considered as vectors of bovine hemoplasma, while the tick is the main vector of T. orientalis [33] and the infection rate increased after grazing [17]. In this study, although the cows that experienced grazing showed a higher Theileria infection rate compared to the housed animals, the CMh infection rate in the housed cows was higher than that in the grazing animals. This suggests that not only the tick but also other arthropod vectors may contribute to hemoplasma transmission.
Cows 1 to 4 years old had the lowest Mw infection rate and the rate increased with increasing age, whereas those showed the highest dual infection rate and the rate decreased as the age increased. Tagawa et al. [29] reported that cattle 1 to 3 years old had a higher prevalence of Mw and CMh infections compared to other age groups, and the detection of hemoplasma infections decreased progressively when cattle were 3 years of age or older. In this study, a similar tendency was found in co-infected cows. Most Japanese Black breeding cows experienced their first pregnancy and delivery from 1 to 3 years of age. Thus, immunosuppression during pregnancy and postpartum periods may be related to susceptibility to hemoplasma infection during these periods [2]. Additionally, reduced dual infection rate at >5 years of age may be attributed to maturation of the immunity and efficient removal of bacterial pathogens from the blood [29]. The reason for the reversed tendency observed in only Mw-infected animals was unclear.
In the present study, the rate of cows co-infected with Mw and T. orientalis was higher than that in Theileria- or hemoplasma-negative animals, but the rate of cows co-infected with both hemoplasmas and T. orientalis was lower compared with Theileria-negative animals. Cattle infected with one of two pathogens tend to resist infection by the other pathogen, and this is called the “interference phenomenon” [30]. In splenectomized calves, suppression of A. marginale infection by Eperythrozoon teganodes [7] and depressed parasitemias in Babesia bovis and A. centrale infections by T. buffeli infection [24] have been reported. Gale et al. [4] demonstrated increased resistance to A. marginale infection in T. buffeli-infected cattle. Moreover, using PCR assays, Tagawa et al. [30] observed that single infection with T. orientalis or hemoplasma was more common than coinfection and the degree of anemia in co-infected animals was milder compared to those only infected with T. orientalis, suggesting interference between T. orientalis and bovine hemoplasma infections. Conversely, A. marginale reportedly was the major cause of the hemolytic anemia, but coinfection with other agents such as Theileria spp. or Mw exacerbated the disease in Swiss cattle [9]. Since different results between Mw- and co-infected cows according to Theileria infection were observed, further studies are needed to determine whether interference may exist between T. orientalis and hemoplasma infections.
Although WBC counts in the hemoplasma-positive and -negative groups were within the reference range, the cows with hemoplasma dual infection showed higher WBC counts compared with hemoplasma-negative cows. Higher WBC levels in Mw-infected cattle than in CMh-infected and hemoplasma-negative cattle have been reported [26]. Additionally, higher WBC values have been found in CMh-infected and dual infected Simmental cattle [15]. The increased WBC levels observed in our study may be due to a consequence of a presumably strong stimulation of immunity by coinfection and consequently higher numbers of leukocytes [15]. Furthermore, although the blood samples were collected from cattle without apparent clinical signs, lower RBC, HGB, and PCV levels and higher MCV levels than those in hemoplasma-negative cattle were found in CMh- [26], Mw-, CMh-, and co-infected cattle [29]. Lower MCV and MCH values and lower MCH values were observed in CMh- and Mw-infected cattle, respectively [15]. In this study, a hematologic difference was not found besides WBC values. This may indicate that Japanese Black cattle could have resistance to the anemia caused by infection with hemoplasma compared with Holstein or Simmental breeds. Interestingly, it was reported that the indigenous Japanese Black breed of cattle was more resistant to T. orientalis infection than the exotic Holstein [17, 31]. Concerning coinfection with T. orientalis, increased WBC counts were observed in Mw-infected and hemoplasma dual infected animals. This may indicate that coinfection with hemoplasma and Theileria induces a stronger inflammatory reaction than that in single infection. In the parameters related to the anemia, the constant alterations were not found in this study. The reasons for increased PLT counts observed in Mw-infected and hemoplasma-negative cows are unclear.
The pathogenic potential of bovine hemoplasmas remains difficult to interpret. The hemoplasma-positive cows found in our study were considered chronic carriers without apparent clinical signs. Decreased milk yield, abortion, and delayed estrus have been reported during the acute phase of hemoplasma infection in Holstein heifers [22]. Furthermore, lower milk yield and lower calf birth weight have been observed in Holstein dairy cows with chronic hemoplasma infection [28]. Since decreased reproductive efficiency is the main economic loss for breeding cows, further studies are required to elucidate the effect of subclinical hemoplasma infection on reproductive performance in Japanese Black cattle.
In conclusion, high prevalence rate (91.5%) in bovine hemoplasma infection was observed in Japanese Black breeding cows in the Kyushu and Okinawa regions. The cows that experienced grazing showed a higher Theileria infection rate compared to the housed animals, while the CMh infection rate in the housed cows was higher than that in the grazing animals. Since hematologic findings related to the anemia were not confirmed in hemoplasma-infected cattle, Japanese Black cattle may have resistance to the anemia due to hemoplasma infection. Although hemoplasma-infected cattle showed no apparent clinical signs, further studies are necessary to elucidate the effect of subclinical infection with bovine hemoplasma on reproductive performance.
POTENTIAL CONFLICTS OF INTEREST
The authors have nothing to disclose.
Acknowledgments
The authors would like to thank the members of veterinary clinics of Agricultural Mutual Aid Association in the Kyushu and Okinawa regions and Kurume Dairy Cattle Artificial Insemination Clinic, Fukuoka Prefecture Dairy Farming Cooperative for their assistance in collecting samples.
REFERENCES
- 1.Dieckmann S. M., Winkler M., Groebel K., Dieckmann M. P., Hofmann-Lehmann R., Hoelzle K., Wittenbrink M. M., Hoelzle L. E.2010. Haemotrophic Mycoplasma infection in horses. Vet. Microbiol. 145: 351–353. doi: 10.1016/j.vetmic.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 2.Evermann J. F.2015. Immunosuppression during pregnancy and postpartum periods. pp. 1576–1577. In: Large Animal Internal Medicine, 5th ed. (Smith, B. P. ed.), Elsevier, St. Louis. [Google Scholar]
- 3.Fujihara Y., Sasaoka F., Suzuki J., Watanabe Y., Fujihara M., Ooshita K., Ano H., Harasawa R.2011. Prevalence of hemoplasma infection among cattle in the western part of Japan. J. Vet. Med. Sci. 73: 1653–1655. doi: 10.1292/jvms.11-0269 [DOI] [PubMed] [Google Scholar]
- 4.Gale K. R., Leatch G., Dimmock C. M., Gartside M. G.1997. Increased resistance to Anaplasma marginale infection in cattle chronically infected with Theileria buffeli (syn. T. orientalis). Vet. Parasitol. 69: 187–196. doi: 10.1016/S0304-4017(96)01125-9 [DOI] [PubMed] [Google Scholar]
- 5.Girotto-Soares A., Soares J. F., Bogado A. L. G., de Macedo C. A. B., Sandeski L. M., Garcia J. L., Vidotto O.2016. ‘Candidatus Mycoplasma haemobos’: Transplacental transmission in dairy cows (Bos taurus). Vet. Microbiol. 195: 22–24. doi: 10.1016/j.vetmic.2016.08.020 [DOI] [PubMed] [Google Scholar]
- 6.Gotoh T., Nishimura T., Kuchida K., Mannen H.2018. The Japanese Wagyu beef industry: current situation and future prospects - A review. Asian-Australas. J. Anim. Sci. 31: 933–950. doi: 10.5713/ajas.18.0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadani A., Guglielmone A. A., Anziani O. S., Tarablas H., Mangold A., Bermudez A., Castor J. C., Gonzalez de Rios L.1982. A case of apparent suppression of Anaplasma marginale infection by eperythrozoonosis (Eperythrozoon teganodes). Vet. Parasitol. 9: 267–272. doi: 10.1016/0304-4017(82)90071-1 [DOI] [PubMed] [Google Scholar]
- 8.Hoelzle K., Winkler M., Kramer M. M., Wittenbrink M. M., Dieckmann S. M., Hoelzle L. E.2011. Detection of Candidatus Mycoplasma haemobos in cattle with anaemia. Vet. J. 187: 408–410. doi: 10.1016/j.tvjl.2010.01.016 [DOI] [PubMed] [Google Scholar]
- 9.Hofmann-Lehmann R., Meli M. L., Dreher U. M., Gönczi E., Deplazes P., Braun U., Engels M., Schüpbach J., Jörger K., Thoma R., Griot C., Stärk K. D. C., Willi B., Schmidt J., Kocan K. M., Lutz H.2004. Concurrent infections with vector-borne pathogens associated with fatal hemolytic anemia in a cattle herd in Switzerland. J. Clin. Microbiol. 42: 3775–3780. doi: 10.1128/JCM.42.8.3775-3780.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornok S., Micsutka A., Meli M. L., Lutz H., Hofmann-Lehmann R.2011. Molecular investigation of transplacental and vector-borne transmission of bovine haemoplasmas. Vet. Microbiol. 152: 411–414. doi: 10.1016/j.vetmic.2011.04.031 [DOI] [PubMed] [Google Scholar]
- 11.Meli M. L., Willi B., Dreher U. M., Cattori V., Knubben-Schweizer G., Nuss K., Braun U., Lutz H., Hofmann-Lehmann R.2010. Identification, molecular characterization, and occurrence of two bovine hemoplasma species in Swiss cattle and development of real-time TaqMan quantitative PCR assays for diagnosis of bovine hemoplasma infections. J. Clin. Microbiol. 48: 3563–3568. doi: 10.1128/JCM.02224-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messick J. B.2004. Hemotrophic mycoplasmas (hemoplasmas): a review and new insights into pathogenic potential. Vet. Clin. Pathol. 33: 2–13. doi: 10.1111/j.1939-165X.2004.tb00342.x [DOI] [PubMed] [Google Scholar]
- 13.Montes A. J., Wolfe D. F., Welles E. G., Tyler J. W., Tepe E.1994. Infertility associated with Eperythrozoon wenyonii infection in a bull. J. Am. Vet. Med. Assoc. 204: 261–263. [PubMed] [Google Scholar]
- 14.Neimark H., Kocan K. M.1997. The cell wall-less rickettsia Eperythrozoon wenyonii is a Mycoplasma. FEMS Microbiol. Lett. 156: 287–291. doi: 10.1111/j.1574-6968.1997.tb12742.x [DOI] [PubMed] [Google Scholar]
- 15.Niethammer F. M., Ade J., Hoelzle L. E., Schade B.2018. Hemotrophic mycoplasma in Simmental cattle in Bavaria: prevalence, blood parameters, and transplacental transmission of ‘Candidatus Mycoplasma haemobos’ and Mycoplasma wenyonii. Acta Vet. Scand. 60: 74. doi: 10.1186/s13028-018-0428-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishizawa I., Sato M., Fujihara M., Sato S., Harasawa R.2010. Differential detection of hemotropic Mycoplasma species in cattle by melting curve analysis of PCR products. J. Vet. Med. Sci. 72: 77–79. doi: 10.1292/jvms.09-0338 [DOI] [PubMed] [Google Scholar]
- 17.Ota N., Mizuno D., Kuboki N., Igarashi I., Nakamura Y., Yamashina H., Hanzaike T., Fujii K., Onoe S., Hata H., Kondo S., Matsui S., Koga M., Matsumoto K., Inokuma H., Yokoyama N.2009. Epidemiological survey of Theileria orientalis infection in grazing cattle in the eastern part of Hokkaido, Japan. J. Vet. Med. Sci. 71: 937–944. doi: 10.1292/jvms.71.937 [DOI] [PubMed] [Google Scholar]
- 18.Saitou N., Nei M.1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425. [DOI] [PubMed] [Google Scholar]
- 19.Sasaoka F., Suzuki J., Hirata T., Ichijo T., Furuhama K., Harasawa R., Satoh H.2015. Vertical transmission of Mycoplasma wenyonii in cattle, supported by analysis of the ribonuclease P RNA gene - Short communication. Acta Vet. Hung. 63: 271–274. doi: 10.1556/004.2015.025 [DOI] [PubMed] [Google Scholar]
- 20.Sasaoka F., Suzuki J., Watanabe Y., Fujihara M., Nagai K., Hirata T., Harasawa R.2013. Two genotypes among ‘Candidatus Mycoplasma haemobos’ strains based on the 16S-23S rRNA intergenic spacer sequences. J. Vet. Med. Sci. 75: 361–364. doi: 10.1292/jvms.12-0349 [DOI] [PubMed] [Google Scholar]
- 21.Shi H., Duan L., Liu F., Hu Y., Shi Z., Chen X., Yang H., Yan B., Yao L.2019. Rhipicephalus (Boophilus) microplus ticks as reservoir and vector of ‘Candidatus Mycoplasma haemobos’ in China. Vet. Parasitol. 274: 108929. doi: 10.1016/j.vetpar.2019.108929 [DOI] [PubMed] [Google Scholar]
- 22.Smith J. A., Thrall M. A., Smith J. L., Salman M. D., Ching S. V., Collins J. K.1990. Eperythrozoon wenyonii infection in dairy cattle. J. Am. Vet. Med. Assoc. 196: 1244–1250. [PubMed] [Google Scholar]
- 23.Song Q., Wang L., Fang R., Khan M. K., Zhou Y., Zhao J.2013. Detection of Mycoplasma wenyonii in cattle and transmission vectors by the loop-mediated isothermal amplification (LAMP) assay. Trop. Anim. Health Prod. 45: 247–250. doi: 10.1007/s11250-012-0197-y [DOI] [PubMed] [Google Scholar]
- 24.Stewart N. P., de Vos A. J., Standfast N. F.1990. Concurrent infection with Theileria buffeli caused depression of parasitaemia in Babesia bovis and Anaplasma centrale infections in splenectomised calves but not in B bigemina infections. Res. Vet. Sci. 49: 346–348. doi: 10.1016/0034-5288(90)90071-B [DOI] [PubMed] [Google Scholar]
- 25.Tagawa M., Matsumoto K., Inokuma H.2008. Molecular detection of Mycoplasma wenyonii and ‘Candidatus Mycoplasma haemobos’ in cattle in Hokkaido, Japan. Vet. Microbiol. 132: 177–180. doi: 10.1016/j.vetmic.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 26.Tagawa M., Matsumoto K., Yokoyama N., Inokuma H.2010. Comparison of the effect of two hemoplasma species on hematological parameters in cattle. J. Vet. Med. Sci. 72: 113–115. doi: 10.1292/jvms.09-0304 [DOI] [PubMed] [Google Scholar]
- 27.Tagawa M., Takeuchi T., Fujisawa T., Konno Y., Yamamoto S., Matsumoto K., Yokoyama N., Inokuma H.2012. A clinical case of severe anemia in a sheep coinfected with Mycoplasma ovis and ‘Candidatus Mycoplasma haemovis’ in Hokkaido, Japan. J. Vet. Med. Sci. 74: 99–102. doi: 10.1292/jvms.11-0296 [DOI] [PubMed] [Google Scholar]
- 28.Tagawa M., Yamakawa K., Aoki T., Matsumoto K., Ishii M., Inokuma H.2013. Effect of chronic hemoplasma infection on cattle productivity. J. Vet. Med. Sci. 75: 1271–1275. doi: 10.1292/jvms.13-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tagawa M., Ybanez A. P., Matsumoto K., Yokoyama N., Inokuma H.2012. Prevalence and risk factor analysis of bovine hemoplasma infection by direct PCR in Eastern Hokkaido, Japan. J. Vet. Med. Sci. 74: 1171–1176. doi: 10.1292/jvms.12-0118 [DOI] [PubMed] [Google Scholar]
- 30.Tagawa M., Ybañez A. P., Matsumoto K., Yokoyama N., Inokuma H.2013. Interference between Theileria orientalis and hemotropic Mycoplasma spp. (hemoplasmas) in grazing cattle. Vet. Parasitol. 195: 165–168. doi: 10.1016/j.vetpar.2012.12.041 [DOI] [PubMed] [Google Scholar]
- 31.Terada Y., Ishida M., Yamanaka H.1995. Resistibility to Theileria sergenti infection in Holstein and Japanese Black cattle. J. Vet. Med. Sci. 57: 1003–1006. doi: 10.1292/jvms.57.1003 [DOI] [PubMed] [Google Scholar]
- 32.Thompson J. D., Gibson T. J., Higgins D. G.2002. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinformatics Chapter 2, Unit 2.3. [DOI] [PubMed] [Google Scholar]
- 33.Watts J. G., Playford M. C., Hickey K. L.2016. Theileria orientalis: a review. N. Z. Vet. J. 64: 3–9. doi: 10.1080/00480169.2015.1064792 [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama N., Ueno A., Mizuno D., Kuboki N., Khukhuu A., Igarashi I., Miyahara T., Shiraishi T., Kudo R., Oshiro M., Zakimi S., Sugimoto C., Matsumoto K., Inokuma H.2011. Genotypic diversity of Theileria orientalis detected from cattle grazing in Kumamoto and Okinawa prefectures of Japan. J. Vet. Med. Sci. 73: 305–312. doi: 10.1292/jvms.10-0263 [DOI] [PubMed] [Google Scholar]