To the Editor,
We read with great interest the letter by Prandoni et al. [1]about the hazard of fondaparinuxin noncritically ill patients with COVID-19. The authors showed a statistically significant increase of overall bleedings among patients on fondaparinux 2.5 mg daily compared to those on enoxaparin 40 mg daily. Moreover, they underlined the lack of real-world data about the use of fondaparinux for the thromboprophylaxis in COVID-19 patients, despite, according to current guidelines [2,3], it has the same clinical indication of enoxaparin for venous thromboembolism (VTE) prevention.
In a recentretrospective observational study [4] including 100 hospitalized COVID-19 patients, we did not find any statistically significant difference in bleeding events between patients on fondaparinux 2.5 mg daily compared to those enoxaparin 40 or 60 mg daily, despite the similar laboratory and clinical characteristics between the two groups.
The contrasting results about the prevalence of bleedings eventsbetween our studies might be driven bythecohort'snumerosity, the heterogeneity and not randomized dosage of anticoagulants, the differentlaboratory and clinical characteristics of the two study populations, which might justify the remarkably higher overall bleedings among COVID-19 patients treated with fondaparinux in the Prandoni's study cohort.
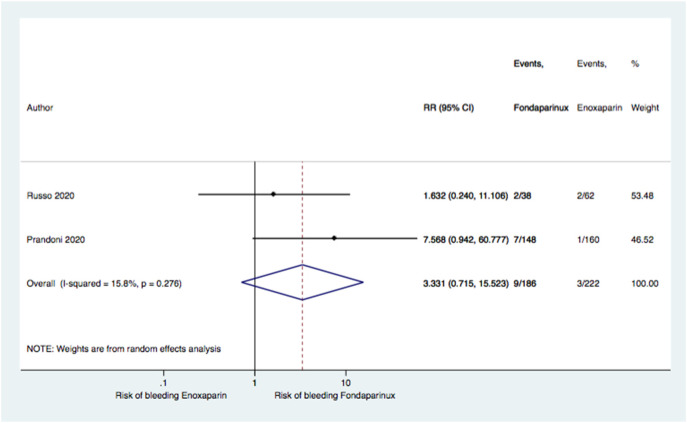
To clarify thereasons of differentbleeding hazardof fondaparinux among the two studies, the clinical and laboratory characteristics of Prandoni'sstudy population should be reported. We performed a pool-analysis of the two studies (Fig. 1 ) showingno significant difference in the bleeding risk ratio between patients on fondaparinux vs enoxaparin (RR 3.33, 95% CI 0.71–15.52; P = 0.276).
Fig. 1.
Forest plot of overall bleedings risk ratio between fondaparinux and enoxaparin.
Test for overall effect: Z-score: 1.53; P = 0.125
Test for heterogeneity: chi-squared: 1.19; P = 0.276; I2: 15.8%.
Solving the question about the bleeding risk of fondaparinux among COVID-19 patients is of pivotal importance, since no differences in the incidence of VTE events compared to enoxaparin has been shown and COVID-19 is considered acondition “per se”related at increased risk to both hemorrhagic and thrombotic events [5].
Moreover, since both studies included only non-critical COVID-19 patients, an extension of these clinical observationsshould be performed among those hospitalized in the intensive care unit (ICU), who usually show higher incidence of thrombotic complications [6].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Prandoni P., Cattelan A.M., Carrozzi L., Leone L., Filippi L., De Gaudenzi E., Villalta S., Pesavento R. FONDACOVIT Investigators [all in Italy]. The hazard of fondaparinux in non-critically ill patients with COVID-19: retrospective controlled study versus enoxaparin. Thromb Res. 2020 Sep 17;196:395–397. doi: 10.1016/j.thromres.2020.09.024. Epub ahead of print. PMID: 33007739; PMCID: PMC7497738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearon C., Akl E.A., Ornelas J., et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinides S.V., Meyer G., Becattini C., et al. The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur Respir J. 2019;54(3) doi: 10.1183/13993003.01647-2019. 2019.0000000000000893. PMID: 33027192. [DOI] [PubMed] [Google Scholar]
- 4.Russo V., Cardillo G., Viggiano G.V., Mangiacapra S., Cavalli A., Fontanella A., Agrusta F., Bellizzi A., Amitrano M., Iannuzzo M., Sacco C., Lodigiani C., Di Micco P. Fondaparinux use in patients with COVID-19: a preliminary multicenter real-world experience. J CardiovascPharmacol. 2020 Oct;76(4):369–371. doi: 10.1097/FJC. [DOI] [PubMed] [Google Scholar]
- 5.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., Goodarzi K., Bendapudi P.K., Bornikova L., Gupta S., Leaf D.E., Kuter D.J., Rosovsky R.P. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020 Jul 23;136(4):489–500. doi: 10.1182/blood.2020006520. PMID: 32492712; PMCID: PMC7378457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porfidia A., Valeriani E., Pola R., Porreca E., Rutjes A.W.S., Di Nisio M. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Aug 12;196:67–74. doi: 10.1016/j.thromres.2020.08.020. Epub ahead of print. PMID: 32853978; PMCID: PMC7420982. [DOI] [PMC free article] [PubMed] [Google Scholar]