Abstract
Background
The aim of this study was to evaluate sonographic features that may aid in risk stratification and to propose a focused cardiac and lung ultrasound (LUS) algorithm in patients with coronavirus disease 2019.
Methods
Two hundred consecutive hospitalized patients with coronavirus disease 2019 underwent comprehensive clinical and echocardiographic examination, as well as LUS, irrespective of clinical indication, within 24 hours of admission as part of a prospective predefined protocol. Assessment included calculation of the modified early warning score (MEWS), left ventricular systolic and diastolic function, hemodynamic and right ventricular assessment, and a calculated LUS score. Outcome analysis was performed to identify echocardiographic and LUS predictors of mortality or the composite event of mortality or need for invasive mechanical ventilation and to assess their adjunctive value on top of clinical parameters and MEWS.
Results
A simplified echocardiographic risk score composed of left ventricular ejection fraction < 50% combined with tricuspid annular plane systolic excursion < 18 mm was associated with mortality (P = .0002) and with the composite event (P = .0001). Stepwise analyses evaluating echocardiographic and LUS parameters on top of existing clinical risk scores showed that addition of tricuspid annular plane systolic excursion and stroke volume index improved prediction of mortality when added to clinical variables but not when added to MEWS. Once echocardiography was added, and patients were recategorized as high risk only if having both high-risk MEWS and high-risk cardiac features, specificity increased from 63% to 87%, positive predictive value from 28% to 48%, and accuracy from 66% to 85%. Although LUS was not associated with incremental risk prediction for mortality above clinical and echocardiographic criteria, it improved prediction of need for invasive mechanical ventilation.
Conclusions
In hospitalized patients with coronavirus disease 2019, a very limited echocardiographic examination is sufficient for outcome prediction. The addition of echocardiography in patients with high-risk MEWS decreases the rate of falsely identifying patients as high risk to die and may improve resource allocation in case of high patient load.
Keywords: COVID-19, FoCUS, Lung ultrasound, Echocardiography, Risk stratification
Abbreviations: COVID-19, Coronavirus disease 2019; FoCUS, Focused cardiac ultrasound; HR, Hazard ratio; LUS, Lung ultrasound; LV, Left ventricular; LVEF, Left ventricular ejection fraction; MEWS, Modified early warning score; PAT, Pulmonic flow acceleration time; RV, Right ventricular; SOFA, Sequential organ failure assessment; SVI, Stroke volume index; TAPSE, Tricuspid annular plane systolic excursion
Both the European Association of Cardiovascular Imaging and the American Society of Echocardiography recognize the prognostic significance and clinical implications of the cardiac complications of coronavirus disease 2019 (COVID-19).1 , 2 Yet both societies recommend limiting the echocardiographic assessment of patients with COVID-19 to a “focused cardiac ultrasound (FoCUS) approach” to reduce the exposure of medical personnel and thus decrease their risk for contamination.2 , 3 Importantly, the suggested FoCUS algorithm in these consensus documents is based on expert opinion and lacks supporting outcome data. Similarly, whereas lung ultrasound (LUS) is increasingly used as a diagnostic tool in critically ill patients,4, 5, 6 little is known about its role in COVID-19.7 Combining LUS with bedside echocardiography allows a rapid and thorough assessment of both the cardiovascular and respiratory status of critically ill patients.8 , 9 We therefore adopted the combined use of bedside echocardiography and LUS during the initial assessment of consecutive patients hospitalized with COVID-19.7 , 10 The aims of the study were (1) to identify echocardiographic and LUS features that are useful in risk stratification of hospitalized patients with COVID-19 and (2) to assess the adjunctive use and added value of these tests, on top of routine clinical parameters and risk scores.
Methods
At the beginning of the COVID-19 pandemic, we initiated a prospective program of performance of LUS and comprehensive echocardiography on admission for all patients presenting with respiratory illness due to coronavirus infection, irrespective of clinical indication, using a predefined step-by-step protocol, as part of a routine patient care protocol. All patients underwent comprehensive LUS combined with echocardiography within 24 h of admission. We studied 200 consecutive adult patients (≥18 years of age) admitted between March 23, 2020, and June 27, 2020, to the Tel Aviv Medical Center because of COVID-19. One hundred of the patients reported here appeared in preliminary publications.7 , 10 All patients had a diagnosis of COVID-19 confirmed by a positive reverse-transcriptase polymerase chain reaction assay for severe acute respiratory syndrome coronavirus-2 in a respiratory tract sample. Demographic data, comorbid conditions, medications, and physical examination and laboratory findings were systematically recorded. Physical examination components included lung and heart auscultation, vital signs, oxygen saturation, and temperature measurements. Patients were risk-stratified according to their COVID-19 modified early warning score (MEWS)11 (Supplemental Table 1) and sequential organ failure assessment (SOFA) score.12 Clinical data were collected on a daily basis. Mortality was ascertained until the end of follow-up to July 15, 2020, beyond hospitalization and irrespective of discharge date, for all patients, by telephone calls, and was complete for all patients. The ethics committee of the Tel Aviv Medical Center approved the study (0196-20-TLV).
Echocardiography
Echocardiography was performed in a standard manner by cardiologists with expertise in echocardiography using a dedicated echocardiographic recorder (CX 50; Philips Medical Systems, Bothell, WA). In accordance with present guidelines,3 the following measures were undertaken to minimize the risk for inadvertent infection: (1) all studies were performed at the designated COVID-19 units; (2) all examinations were performed using small dedicated scanners; (3) personal protection included airborne precautions comprising N-95 masks, fluid-resistant gowns, two sets of gloves, head covers, eye shields, and shoe covers; (4) electrocardiographic monitoring during imaging was omitted, and all measurements were performed offline to reduce exposure time and contamination.13 , 14 Analysis of all echocardiographic findings was performed by a senior cardiologist with expertise in echocardiography. Suboptimal image quality was identified in 22 patients (11%), but all examinations were diagnostic. Left ventricular (LV) diameters and LV ejection fraction (LVEF) were measured as recommended.15 Measurements of mitral inflow included the peak early filling (E-wave) and late diastolic filling (A-wave) velocities and the E/A ratio. Early diastolic mitral septal and lateral annular velocities (e′) were measured in the apical four-chamber view.16 Left atrial volume was calculated using the biplane area-length method at end-systole. Forward stroke volume was calculated from LV outflow tract Doppler time-velocity interval multiplied by LV outflow tract cross-sectional area with subsequent calculation of cardiac output and index. From four-chamber views encompassing the entire right ventricle, end-systolic and end-diastolic right ventricular (RV) areas and the tricuspid annulus were measured. RV function was evaluated by tricuspid annular plane systolic excursion (TAPSE), systolic tricuspid lateral annular velocity (RV S′) measured in the apical four-chamber view, and fractional area change.15 , 17 Hemodynamic right-sided assessment included measurement of the pulmonic flow acceleration time (PAT) velocity to assess pulmonary vascular resistance and estimated right atrial pressure using the inferior vena cava.18 Estimation of systolic pulmonary pressure on the basis of tricuspid regurgitation pressure gradient was possible in 36 patients (18%).
Lung Ultrasound
We performed LUS on all patients with COVID-19 using a six-zone method for each lung, including a scan of the anterior, anterolateral, and posterolateral aspects of the thorax. Examinations were performed by cardiologists with expertise in LUS recording and interpretation using the same equipment (CX 50), with the same phased-array probe used for echocardiography. Each LUS examination lasts 2 to 3 min, with the patient supine or semisupine, omitting the need for position change during the examination. A standard point scoring system was used for each region and ultrasound pattern: A-lines (normal reverberation artifacts of the pleural line that when accompanied by lung sliding correspond to normal aeration of the lung) were graded as 0 points, and B-lines (hyperechoic lines vertical to the pleura line, arising from it and reaching the edge of the screen, erasing A-lines), which represent reverberation artifact through edematous interlobular septa or alveoli, were classified as B1 (separated B-lines that correspond to moderate lung aeration loss) and graded as 1 point or as B2 (coalescent B-lines that correspond to severe lung aeration loss) and graded as 2 points. Finally, lung consolidation received 3 points. Thus, a LUS score of 0 was normal, and 36 was the worst score possible19 (Supplemental Figure 1). We also qualitatively documented the presence of pleural thickening and defined a homogenous versus patchy pattern of each examination.
Follow-Up and Outcomes
Clinical follow-up was obtained prospectively. Outcome analysis started at the time of baseline echocardiographic and LUS examination. The study end points were (1) all-cause mortality, (2) need for invasive mechanical ventilation, and (3) the composite event of death or need for invasive mechanical ventilation (excluding patients already invasively mechanically ventilated during the baseline ultrasound examination). Patients who needed invasive mechanical ventilation and eventually died were censored at the time of initiating invasive mechanical ventilation.
Interobserver and Intraobserver Variability
Interobserver and intraobserver variability for stroke volume, TAPSE, and LUS score were determined by a second independent blinded observer, and by the same observer, who measured the parameters ≥1 month apart in 15 randomly selected patients. They were assessed using the Bland-Altman method and the within-subject coefficient of variation, calculated as the ratio of the SD of the measurement difference to the mean value of all measurements.
Statistical Analysis
Continuous normally distributed parameters are presented as mean ± SD and were compared using Student's t test. Non–normally distributed data are presented as median (interquartile range) and were compared using the Wilcoxon rank sum test. Categorical data were compared between groups using the χ2 test or the Fisher exact test and are expressed as numbers and/or percentages. Multiple comparisons for continuous and categorical parameters used the Tukey-Kramer honestly significant difference test and the Bonferroni correction, respectively. The survival estimate was calculated using the Kaplan-Meier method. P values for the pooled analysis of the survival curves were calculated using the log-rank test. Univariate Cox proportional-hazards models for mortality, need for mechanical ventilation, or the composite event as end points allowed the calculation of hazard ratios (HRs) of baseline echocardiographic and LUS parameters. Time of follow-up was calculated between baseline echocardiographic and LUS evaluation and either death, new need for invasive mechanical ventilation, or last date of follow-up. Analysis for survival was obtained for all patients. Analyses for the composite event were done excluding those who were mechanically ventilated at presentation, before baseline echocardiographic and LUS evaluation. To assess the independent echocardiographic and LUS parameters associated with outcomes, we used multivariate Cox proportional-hazard models for the end points. The first step was to group the variables into LV and left atrial, Doppler, RV, and LUS parameters. The second step was to select for each group all the variables with P values < .05 in a univariate analysis. The third step was to assess correlations between the selected variables within each group to avoid collinearity (R 2 > 0.7, P < .0001). In the fourth step, cutoff values for continuous parameters affecting survival were derived using the maximally selected rank statistics method. Although LVEF was not significantly associated with mortality as a continuous variable, because it was previously reported to be associated with mortality and cardiac events,10 it was forced into the Cox hazard model. Covariates were entered in a stepwise forward multivariate analysis. We performed separate analyses for mortality or the composite event adjusted for routine clinical parameters, MEWS or SOFA score. For the routine clinical parameters, we selected only clinical variables that are known associates of adverse events in COVID-19 (age, gender, systolic blood pressure, heart rate, oxygen saturation, and baseline troponin, d-dimer, and brain natriuretic peptide levels). The variables assessing clinical associates were entered first, the echocardiographic significant dichotomous parameters second, and the LUS score last. We performed the same process to assess if a “super-simple” echocardiographic score calculated by multiplying the HRs for LVEF and TAPSE, and multiplying the two together, provides incremental prognostic value. Receiver operating characteristic curve analysis was used to determine the areas under the curve for echocardiographic and LUS models for mortality and the composite event. The statistical significance for the additive value of echocardiographic and LUS parameters were examined using (1) a χ2 test of the log likelihood reduction, (2) the continuous net reclassification improvement, (3) the integrated discrimination improvement, and (4) the Akaike information criterion method. To determine if models incorporating heart or lung imaging improved prediction of outcome and reclassify more individuals with events as high risk (true positive) and/or more individuals without events as low risk (true negative) compared with clinical variables alone, we generated contingency tables for either MEWS alone or the combination of MEWS and either heart or lung imaging, and we calculated sensitivity, specificity, negative predictive value, positive predictive value, and accuracy for each model. A detailed description of the contingency tables is presented in the Supplemental Appendix. CIs for net reclassification improvement and integrated discrimination improvement were calculated using bootstrapping with 2,000 iterations. Model validation was performed using bootstrapping with 2,000 iterations. Original and validation-corrected C statistics were calculated from Somers' D (D xy) statistic using the formula C statistic = (D xy + 1)/2. A detailed description of Somers' D statistic is presented in the Supplemental Appendix. Calculations were performed using R version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The study population consisted of 234 consecutive patients; 34 patients were excluded because they did not undergo cardiac and LUS assessment within the first 24 hours. The reasons for not performing the cardiac and LUS were hospital discharge in <24 hours (21 patients), patient refusal (six patients), and “do not resuscitate/intubate” status (seven patients). Thus, the final study group consisted of 200 patients (Table 1 ). At the time of baseline echocardiographic and LUS evaluation, 133 patients had mild or moderate disease (all with oxygen saturation ≥ 94% on room air), 47 patients had severe disease (need for noninvasive oxygen supplementation), and 20 patients had critical disease (need for mechanical ventilation, vasopressors, or extracorporeal life support).
Table 1.
Baseline characteristics (n = 200)
| Variable | Value |
|---|---|
| Age, y | 64.2 ± 19.2 |
| Gender, male | 121 (60.5) |
| Body surface area, m2 | 2.1 ± 1.9 |
| Ischemic heart disease | 30 (15) |
| Congestive heart failure | 22 (11) |
| Status post coronary artery bypass graft | 10 (5) |
| Atrial fibrillation/flutter | 34 (17) |
| Transient ischemic attack/stroke | 21 (10.5) |
| Chronic obstructive pulmonary disease | 13 (6.5) |
| Diabetes mellitus | 57 (28.5) |
| Hypertension | 109 (54.5) |
| Obesity | 49 (24.5) |
| Chronic medications | |
| Direct oral anticoagulant | 28 (14) |
| Angiotensin-converting enzyme inhibitor | 40 (20) |
| Angiotensin receptor blocker | 26 (13) |
| β-blocker | 52 (26) |
| Systemic corticosteroid | 10 (5) |
| Laboratory values on admission | |
| Hemoglobin, g/dL | 13.1 ± 2 |
| Lymphocytes, 103/μL | 1.2 ± 0.7 |
| Creatinine, mg/dL | 0.89 (0.73–1.22) |
| Blood urea nitrogen, mg/dL | 23.9 ± 19.6 |
| C-reactive protein, mg/L | 72.3 ± 71.1 |
| Troponin I, ng/L | 9 (4–21.5) |
| Troponin I > 28 ng/L | 36 (19) |
| BNP, pg/mL | 45 (18–141) |
| BNP > 80 pg/mL | 56 (31) |
| d-dimer, mg/L | 1.66 ± 1.5 |
| d-dimer > 0.5 mg/L | 138 (72) |
| Physical examination on admission | |
| Lung crackles | 28 (19) |
| Heart rate, beats/min | 85 ± 17 |
| Systolic blood pressure, mm Hg | 134 ± 22 |
| Diastolic blood pressure, mm Hg | 73 ± 15 |
| O2 saturation, % | 95 (90–98) |
| Temperature, °C | 37.1 (36.7–37.9) |
| Chest radiographic findings on admission | |
| Lobar infiltration | 31 (16) |
| Bilateral infiltration | 76 (39) |
| Pleural effusion | 25 (13) |
| Clinical assessment scores on admission | |
| SOFA score | 1 (0–3) |
| MEWS | 4 (2–6) |
Data are expressed as mean ± SD, number (percentage), or median (interquartile range).
BNP, Brain natriuretic peptide.
Univariate Analysis
Results of univariate analysis for mortality and the composite event for echocardiographic parameters are shown in Table 2 . Results of univariate analysis for LUS parameters are presented in Supplemental Table 2. The median follow-up was 59 days, with an interquartile range of 12 to 86 days. A total of 29 patients (14.5%) died, and 43 (21.5%) reached the composite end point. Echocardiographic parameters significantly associated with higher rates of both mortality and the composite end point were LVEF < 50%, stroke volume index (SVI), PAT, and TAPSE. Increased LUS score, presence of pleural effusion, and pleural thickening at baseline LUS were each associated with higher rates of both mortality and the composite end point (Supplemental Figures 2A and 2B). In nested models, the best associate of mortality between the pulmonary parameters was LUS score.
Table 2.
Univariate analysis of echocardiographic prediction of clinical events
| Parameter | Mortality, HR (95% CI) | P | Composite event, HR (95% CI) | P |
|---|---|---|---|---|
| LV parameters | ||||
| LVEF, % | 0.97 (0.95–1.03) | .34 | 0.97 (0.94–1.00) | .08 |
| Dichotomous LVEF < 50% | 2.66 (1.1–5.8) | .03 | 2.39 (1.1–4.8) | .02 |
| LV S′, cm/sec | 0.82 (0.63–1.04) | .10 | 0.92 (0.76–1.10) | .42 |
| LV end-diastolic diameter, mm | 0.96 (0.93–1.00) | .09 | 0.98 (0.95–1.01) | .32 |
| LV end-systolic diameter, mm | 0.96 (0.91–1.01) | .13 | 1.00 (0.95–99) | .91 |
| LV mass index, g/m2 | 1.00 (0.99–1.02) | .54 | 1.00 (0.98–1.01) | .87 |
| Left atrial volume index, mL/m2 | 1.01 (0.98–1.04) | .27 | 1.02 (0.99–1.04) | .12 |
| Doppler parameters | ||||
| E-wave velocity, cm/sec | 0.99 (0.98–1.01) | .90 | 0.99 (0.98–1.01) | .61 |
| A-wave velocity, cm/sec | 1.00 (0.98–1.01) | .99 | 1.00 (0.99–1.02) | .31 |
| E/A ratio | 0.98 (0.31–2.1) | .98 | 0.53 (0.16–1.3) | .19 |
| e′ septal, cm/sec | 0.86 (0.69–1.05) | .15 | 0.93 (0.79–1.08) | .38 |
| e′ lateral, cm/sec | 0.90 (0.78–1.04) | .16 | 0.89 (0.79–1.00) | .06 |
| E/e′ average ratio | 1.02 (0.95–1.07) | .59 | 1.00 (0.95–1.05) | .82 |
| Right atrial pressure, mm Hg | 0.94 (0.83–1.05) | .34 | 1.03 (0.95–1.11) | .44 |
| SVI, per 10 mL/m2 | 0.54 (0.36–0.82) | .004 | 0.59 (0.41–0.85) | .004 |
| Dichotomous SVI ≤ 23.0, ≤27.4 mL/m2 | 3.72 (1.69–8.39) | .001 | 3.6 (1.8–7.1) | .0004 |
| PAT, per 10 msec | 0.87 (0.75–0.99) | .05 | 0.81 (0.71–0.92) | .007 |
| Dichotomous PAT < 77, 90 ms | 2.74 (1.26–6.26) | .01 | 4.6 (2.0–12.3) | <.0001 |
| RV parameters | ||||
| RV end-diastolic area index, cm2/m2 | 0.91 (0.79–1.06) | .25 | 0.95 (0.83–1.08) | .45 |
| RV end-systolic area index, cm2/m2 | 0.88 (0.70–1.08) | .27 | 1.04 (0.88–1.18) | .61 |
| RV fractional area change, % | 1.02 (0.98–1.06) | .17 | 0.99 (0.96–1.02) | .86 |
| TAPSE, per cm | 0.24 (0.12–0.48) | <.0001 | 0.30 (0.16–0.55) | .0001 |
| Dichotomous TAPSE < 1.9, 1.6 cm | 4.1 (1.93–9.0) | .0003 | 3.4 (1.7–6.7) | .0001 |
| RV S′, cm/sec | 0.83 (0.72–0.96) | .01 | 0.87 (0.78–0.98) | .02 |
| Tei index | 1.48 (0.56–2.5) | .33 | 1.46 (0.62–2.4) | .31 |
Multivariate Analysis
SVI and TAPSE were the only echocardiographic parameters independently associated with mortality (Table 3 ). The addition of LUS score consecutively to the echocardiographic multivariate analysis for mortality resulted in improved prediction (Akaike information criterion decreased from 218 to 216, P = .01). SVI, TAPSE, and PAT were the only echocardiographic parameters independently associated with the composite event. The addition of LUS score to the echocardiographic multivariate analysis for the composite event resulted in improved prediction (Akaike information criterion decreased from 303 to 292, P < .0001).
Table 3.
Multivariate analysis of echocardiographic and LUS prediction of clinical events
| Variable | Univariate analysis, HR (95% CI) | Multivariate analysis, HR (95% CI) |
|---|---|---|
| Mortality: echocardiography | ||
| LVEF | 2.66 (1.1–5.8), P = .03 | 0.73 (0.22–2.03), P = .55 |
| SVI | 3.72 (1.69–8.39), P = .01 | 2.12 (1.001–5.23), P = .05 |
| PAT | 2.74 (1.26–6.26), P = .01 | 2.31 (0.84–6.7), P = .10 |
| TAPSE | 4.1 (1.93–9.0), P = .0003 | 4.3 (1.68–11.6), P = .002 |
| χ2 for model | 23.9 | |
| P value for model | <.0001 | |
| AIC | 218 | |
| Mortality: echocardiography and LUS | ||
| LVEF | 0.70 (0.22–2.00), P = .52 | |
| SVI | 3.09 (1.3–7.35), P = .01 | |
| PAT | 2.25 (0.83–6.6), P = .11 | |
| TAPSE | 3.21 (1.32–7.82), P = .01 | |
| LUS score | 2.78 (1.27–5.82), P = .01 | 2.38 (1.01–5.61), P = .04 |
| χ2 for model | 24.8 | |
| P value for model | <.0001 | |
| AIC | 216 | |
| P value for log likelihood | .01 | |
| Mechanical ventilation: echocardiography | ||
| LVEF | 0.96 (0.93–1.007), P = .09 | 0.95 (0.88–1.04), P = .28 |
| SVI | 0.96 (0.91–1.02), P = .21 | 0.97 (0.89–1.04), P = .43 |
| PAT | 0.97 (0.95–0.99), P = .01 | 0.97 (0.94–0.99), P = .01 |
| TAPSE | 0.52 (0.21–1.28), P = .15 | 0.98 (0.28–3.3), P = .90 |
| χ2 for model | 8.7 | |
| P value for model | .06 | |
| AIC | 91.4 | |
| Mechanical ventilation: echocardiography and LUS | ||
| LVEF | 1.00 (0.97-1.04), P = .64 | |
| SVI | 0.99 (0.97-1.04), P = .45 | |
| PAT | 0.99 (0.98–1.001), P = .07 | |
| TAPSE | 0.77 (0.49–1.05), P = .09 | |
| LUS score | 3.5 (1.4–8.9), P = .006 | 1.03 (1.04–1.06), P = .007 |
| χ2 for model | 17.8 | |
| P value for model | .003 | |
| AIC | 84.5 | |
| P value for log likelihood | .001 | |
| Composite event: echocardiography | ||
| LVEF | 2.39 (1.1–4.8), P = .02 | 0.82 (0.32–2.06), P = .66 |
| SVI | 3.6 (1.8–7.1), P = .0004 | 3.52 (1.63–7.59), P = .001 |
| PAT | 4.6 (2.0–12.3), P < .0001 | 2.41 (1.01–5.9), P = .04 |
| TAPSE | 3.4 (1.7–6.7), P < .0001 | 1.99 (1.29–6.8), P = .01 |
| χ2 for model | 22.6 | |
| P value for model | <.0001 | |
| AIC | 303 | |
| Composite event: echocardiography and LUS | ||
| LVEF | 0.95 (0.33–2.43), P = .92 | |
| SVI | 2.39 (1.04–5.46), P = .04 | |
| PAT | 2.06 (0.85–5.15), P = .11 | |
| TAPSE | 2.47 (1.07–5.73), P = .03 | |
| LUS score | 3.03 (1.33–6.82), P = .009 | |
| χ2 for model | 29.5 | |
| P value for model | <.0001 | |
| AIC | 292 | |
| P value for log likelihood | <.0001 | |
Bold indicates statistical significance.
AIC, Akaike information criterion.
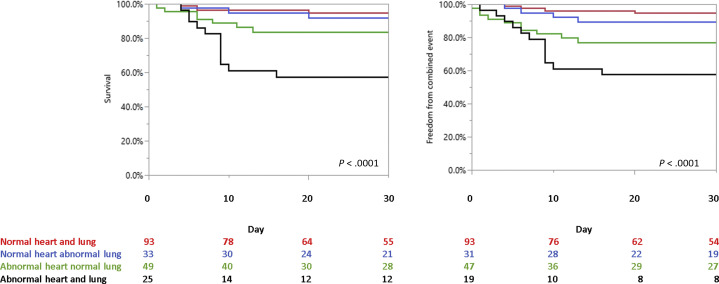
The prevalence of abnormal TAPSE, LVEF, and SVI were 18%, 14%, and 28%, respectively. The remaining 126 patients (63%) did not have any of the echocardiographic parameters associated with adverse outcomes. Outcome of patients stratified into those with normal or abnormal findings on cardiac or LUS are described in Figure 1 and in the supplemental results.
Figure 1.
Outcome in patients with COVID-19 stratified according to cardiac and LUS evaluation. (A) Overall survival in patients with COVID-19 comparing patients with “good lung” (LUS score ≤ 18) and “good heart” (red line), “good lung” (LUS score ≤ 18), and “bad heart” (at least one cardiac parameter associated with adverse outcome; blue line); “bad lung” (LUS score > 18) with “good heart” (no cardiac parameter associated with adverse outcome; green line), and “bad lung” (LUS score > 18) combined with “bad heart” (black line). (B) Freedom from the composite event of mortality or need for invasive mechanical ventilation in patients with COVID-19 comparing patients with “good lung” (LUS score ≤ 18) and “good heart” (red line), “good lung” (LUS score ≤ 18), and “bad heart” (at least one cardiac parameter associated with adverse outcome; blue line); “bad lung” (LUS score > 18) with “good heart” (no cardiac parameter associated with adverse outcome; green line), and “bad lung” (LUS score > 18) combined with “bad heart” (black line).
Added Value of Combined Lung and Echocardiographic Evaluation to Clinical Scores
Stepwise analyses evaluating the significant echocardiographic and LUS parameters and either preselected combination of clinical parameters or existing clinical risk scores (SOFA score and MEWS) are presented in Table 4 . Addition of TAPSE and SVI improved the prediction of mortality when added to SOFA score or clinical variables but not when added to MEWS. LUS did not have additive predictive value for mortality on top of clinical and echocardiographic parameters. The results of contingency tables for models incorporating MEWS with or without echocardiography or LUS are shown in Table 5 . The addition of echocardiography, so patients were categorized as high risk only if having both high-risk MEWS and high-risk imaging features, reclassified more individuals without events as low risk and improved specificity, positive predictive value, and accuracy of the models compared with MEWS alone. The addition of either LUS or echocardiography, so patients were categorized as high risk if having either high-risk MEWS or high-risk imaging features, reclassified more individuals with events as high risk, increasing sensitivity, but came with the expense of decreasing accuracy, specificity, and positive predictive value.
Table 4.
Stepwise multivariate analysis of echocardiographic, LUS, and clinical scores for prediction of clinical events
| Variable | Model with SOFA | Model with MEWS | Model with clinical parameters∗ | Simple model with SOFA | Simple model with MEWS | Simple model with clinical parameters∗ |
|---|---|---|---|---|---|---|
| Mortality | ||||||
| TAPSE | 3.38 (1.43 to 7.97) | 2.22 (1.01 to 5.94) | 8.45 (1.98 to 36.05) | |||
| LVEF | 0.83 (0.29 to 2.32) | 0.43 (0.13 to 1.49) | 0.79 (0.19 to 3.32) | |||
| SVI | 2.58 (1.01 to 6.56) | 2.14 (0.81 to 5.69) | 5.13 (1.57 to 16.82) | |||
| LUS | 1.78 (0.69 to 4.56) | 1.42 (0.60 to 3.6) | 0.7 (0.18 to 2.72) | 1.68 (0.72 to 3.9) | 1.34 (0.56 to 3.2) | 1.26 (0.5 to 3.2) |
| “Super-simple” echocardiographic risk score | 1.28 (1.04 to 1.57) | 1.15 (0.97 to 1.45) | 1.24 (0.99 to 1.6) | |||
| SOFA | 1.1 (1.001 to 1.22) | 1.14 (1.03 to 1.25) | ||||
| MEWS | 1.26 (1.12 to 1.41) | 1.22 (1.10 to 1.36) | ||||
| AUC for score or clinical alone | 0.76 | 0.79 | 0.86 | 0.76 | 0.79 | 0.86 |
| AUC for combined model | 0.80 | 0.82 | 0.9 | 0.78 | 0.80 | 0.88 |
| AIC for score or clinical alone | 217 | 185 | 143 | 217 | 185 | 143 |
| AIC for combined model | 214 | 183 | 134 | 215 | 184 | 139 |
| IDI | 0.10 (0.02 to 0.2), P = .006 | 0.07 (0.004 to 0.16), P = .02 | 0.19 (0.06 to 0.3), P = .009 | 0.18 (0.05 to 0.323), P = .004 | 0.09 (0.026 to 0.19), P = .003 | 0.07 (0.004 to 0.16), P = .004 |
| NRI | 0.5 (0.3 to 0.7), P = .009 | 0.48 (0.05 to 0.6), P = .02 | 0.5 (0.13 to 0.7), P = .02 | 0.57 (0.36 to 0.75), P = .005 | 0.44 (−0.04 to 0.68), P = .08 | 0.58 (0.05 to 0.68), P = .02 |
| Log likelihood difference | χ2 = 11.1, P = .001 | χ2 = 6.51, P = .01 | χ2 = 17.7, P < .001 | χ2 = 14.3, P < .001 | χ2 = 6.99, P = .0008 | χ2 = 19.0, P < .001 |
| Composite event | ||||||
| TAPSE | 1.55 (0.68 to 3.54) | 1.02 (1.01 to 2.54) | 1.68 (0.47 to 5.97) | |||
| LVEF | 0.86 (0.34 to 2.19) | 0.44 (0.15 to 1.31) | 1.24 (0.35 to 4.47) | |||
| SVI | 2.49 (1.14 to 5.42) | 2.18 (1.001 to 4.92) | 4.05 (1.68 to 9.7) | |||
| LUS | 2.17 (1.07 to 4.42) | 1.28 (0.57 to 2.84) | 2.57 (1.003 to 6.7) | 1.87 (0.86 to 4.3) | 1.47 (0.7 to 3.3) | 1.56 (0.68 to 3.6) |
| Simple heart score | 3.15 (1.25 to 7.7) | 1.09 (0.88 to 1.32) | 1.19 (1.0002 to 1.51) | |||
| SOFA | 1.18 (1.1 to 1.27) | 1.24 (1.04 to 1.46) | ||||
| MEWS | 1.33 (1.19 to 1.46) | 1.29 (1.17 to 1.42) | ||||
| AUC for score or clinical alone | 0.83 | 0.83 | 0.75 | 0.83 | 0.83 | 0.75 |
| AUC for combined model | 0.85 | 0.85 | 0.80 | 0.85 | 0.85 | 0.81 |
| AIC for score or clinical alone | 280 | 292 | 201 | 280 | 292 | 201 |
| AIC for combined model | 277 | 230 | 193 | 276 | 256 | 194 |
| IDI | 0.06 (0.002 to 0.16), P = .03 | 0.03 (−0.002 to 0.12), P = .07 | 0.11 (0.025 to 0.23), P = .007 | 0.11 (0.02 to 0.23), P = .02 | 0.03 (−0.003 to 0.13), P = .10 | 0.14 (0.04 to 0.25), P = .005 |
| NRI | 0.4 (0.11 to 0.62), P = .02 | 0.4 (−0.07 to 0.6), P = .07 | 0.46 (0.05 to 0.62), P = .03 | 0.4 (0.2 to 0.6), P = .02 | 0.4 (−0.3 to 0.7), P = .17 | 0.43 (0.04 to 0.66), P = .05 |
| Log likelihood difference | χ2 = 6.96, P = .008 | χ2 = 4.24, P = .03 | χ2 = 14.3, P < .001 | χ2 = 11.6, P = .001 | χ2 = 2.82, P = .09 | χ2 = 18.7, P < .001 |
AIC, Akaike information criterion; AUC, area under the receiver operating characteristic curve; IDI, integrated discrimination improvement; NRI, net reclassification improvement.
Clinical parameters: age, gender, systolic blood pressure, heart rate, oxygen saturation, and baseline troponin, d-dimer, and brain natriuretic peptide levels.
Table 5.
Contingency tables for models incorporating MEWS with or without echocardiography or LUS
| Died | Survived | All | |
|---|---|---|---|
| Mortality with MEWS alone | |||
| High risk | 24 | 62 | 86 |
| Low risk | 5 | 109 | 114 |
| Sensitivity 83%, specificity 63%, NPV 96%, PPV 28%, accuracy 66% | |||
| Mortality MEWS with echocardiography specific | |||
| High risk | 20 | 22 | 42 |
| Low risk | 9 | 149 | 158 |
| Sensitivity 69%, specificity 87%, NPV 94%, PPV 48%, accuracy 85% | |||
| Mortality MEWS with LUS Specific | |||
| High risk | 15 | 32 | 47 |
| Low risk | 14 | 139 | 153 |
| Sensitivity 52%, specificity 81%, NPV 91%, PPV 32%, accuracy 77% | |||
| Mortality MEWS with echocardiography sensitive | |||
| High risk | 25 | 93 | 118 |
| Low risk | 4 | 78 | 82 |
| Sensitivity 86%, specificity 46%, NPV 95%, PPV 21%, accuracy 52% | |||
| Mortality MEWS with LUS sensitive | |||
| High risk | 26 | 82 | 108 |
| Low risk | 3 | 89 | 92 |
| Sensitivity 89%, specificity 52%, NPV 97%, PPV 24%, accuracy 58% | |||
| Mechanically ventilated | No mechanical ventilation | All | |
|---|---|---|---|
| Mechanical ventilation MEWS alone | |||
| High risk | 16 | 70 | 86 |
| Low risk | 8 | 106 | 114 |
| Sensitivity 66%, specificity 60%, NPV 93%, PPV 13%, accuracy 61% | |||
| Mechanical ventilation MEWS with echocardiography specific | |||
| High risk | 8 | 34 | 42 |
| Low risk | 16 | 142 | 158 |
| Sensitivity 33%, specificity 81%, NPV 89%, PPV 19%, accuracy 75% | |||
| Mechanical ventilation MEWS with LUS specific | |||
| High risk | 11 | 36 | 47 |
| Low risk | 13 | 140 | 153 |
| Sensitivity 46%, specificity 79%, NPV 91%, PPV 23%, accuracy 75% | |||
| Mechanical ventilation MEWS with echocardiography sensitive | |||
| High risk | 19 | 99 | 118 |
| Low risk | 5 | 77 | 82 |
| Sensitivity 79%, specificity 44%, NPV 94%, PPV 16%, accuracy 48% | |||
| Mechanical ventilation MEWS with LUS sensitive | |||
| High risk | 20 | 88 | 108 |
| Low risk | 4 | 88 | 92 |
| Sensitivity 83%, specificity 50%, NPV 96%, PPV 18%, accuracy 54% | |||
NPV, Negative predictive value; PPV, positive predictive value.
“Super-Simple” Echocardiography Risk Score
We assessed a simplified echocardiographic approach using a calculated “super-simple” echocardiographic risk score. The “super-simple” score was valuable, significantly associated with mortality (HR, 1.36; 95% CI, 1.16–1.59; χ2 = 13.9; P = .0002), and with the composite event (HR, 1.41; 95% CI, 1.19–1.66; χ2 = 15.0; P = .0001). However, it was inferior to the complete echocardiographic examination including Doppler data both for mortality (HR, 4.90; 95% CI, 2.25 to 11.8; χ2 = 16.9; P < .0001; log likelihood for nested models, P = .005) and for the composite event (HR, 3.85; 95% CI, 2.05 to 7.6; χ2 = 17.9; P < .0001, log likelihood for nested models, P = .005).
We evaluated different models using the “super-simple” risk score, LUS, and either preselected clinical parameters or existing clinical risk scores (SOFA score and MEWS; Table 4). Addition of the “super-simple” risk score showed significant improvement of models for mortality on top of SOFA score, MEWS, or selected clinical parameters and a significant improvement of models for the composite event on top of either SOFA score or selected clinical parameters, but not on top of MEWS.
C statistics of the multivariate models including MEWS and either the “super simple” or the complete echocardiography score were highly predictive both of mortality and the composite event. For the models with complete echocardiography score, the C statistic was 0.79 (95% CI, 0.68–0.89) for mortality and 0.79 (95% CI, 0.69–0.88) for the composite event. For the models with “super simple” echocardiography score, the C statistic was 0.78 (95% CI, 0.66–0.88) for mortality and 0.79 (95% CI, 0.70–0.88) for the composite event. Using a bootstrapping validation technique, with 2,000 iterations, showed similar C statistics for the validated models. For the models with complete echocardiography score, the C statistic was 0.79 (95% CI, 0.78–0.79) for mortality and 0.79 (95% CI, 0.78–0.79) for the composite event. For the models with “super simple” echocardiography score, the C statistics was 0.78 (95% CI, 0.77–0.78) for mortality and 0.78 (95% CI, 0.76–0.79) for the composite event.
Interobserver and Intraobserver Variability
Results for inter- and intraobserver variability are presented in Supplemental Table 4.
Discussion
We analyzed the predictive value of combined echocardiography and LUS in hospitalized patients with COVID-19. Our main findings are as follows: (1) in hospitalized patients with COVID-19, several echocardiographic (TAPSE and SVI) and LUS (LUS score) parameters at admission are univariate predictors of mortality and need for invasive mechanical ventilation; (2) a very limited echocardiographic examination is sufficient to develop a strategy of risk stratification; and (3) LUS may be useful for identifying individuals at risk for mechanical ventilation but not useful for mortality prediction above and beyond clinical and echocardiographic criteria.
Echocardiographic Evaluation in Patients with COVID-19
Although the American and European societies recognize the importance of echocardiographic assessment of patients with COVID-19, the amount of data collected prospectively is limited to several reports.20, 21, 22, 23, 24, 25, 26 Half of the patients in the present cohort appeared in our previous publication,10 in which we reported on the echocardiographic results of the first 100 patients with COVID-19 admitted to our institution. Doubling the number of studied patients, the addition of LUS parameters, and the longer follow-up period in the present study allow us to evaluate the independent predictive ability of echocardiography combined with LUS and clinical parameters for mortality or need for invasive mechanical ventilation. The only echocardiographic parameters associated with adverse outcome in nonadjusted analyses are LVEF, SVI, PAT, and TAPSE. The cutoff values for TAPSE and LVEF were within the lower normal range and thus unlikely to be discriminatory in other populations. However, because of the heightened adrenergic tone in patients with respiratory failure, a “lower normal range” TAPSE or LVEF may reflect early cardiac deterioration.
Combined Echocardiographic and LUS Evaluation
At our center, the same cardiologist performing the echocardiographic assessment also routinely performs LUS. We show that survival drops with an abnormal LUS score, in line with those achieved by chest computed tomography.9 , 27 , 28 This study is the first to combine results of LUS with echocardiographic evaluation. We show that neither echocardiography nor LUS significantly improved the sensitivity or negative predictive value of MEWS for mortality. Echocardiography or LUS adds little in the further identification of those at high risk on top of MEWS. However, MEWS alone has low specificity and very low positive predictive value, reflecting a large number of patients falsely identified as at high risk for dying by the MEWS. Once echocardiography is added, and patients are recategorized as high risk only if having both high-risk MEWS and high-risk echocardiographic features, the specificity increases by approximately 25%, and positive predictive value almost doubles. In simple words, the addition of echocardiography in patients with high-risk MEWS decreases the rate of falsely identifying patients as high risk to die by approximately two thirds. In clinical terms, it seems that performing echocardiography in patients with low-risk MEWS adds very little in the further identification of those at high risk, but addition of echocardiography in patients with high-risk MEWS may allow us to properly identify patients with lower risk for death and to improve the resource allocation in case of high patient load.
As to the role of LUS, it did not have incremental prognostic value in terms of mortality to the clinical scores. As to its role in prediction for need of mechanical ventilation, although sensitivity increased from 66% to 83%, because of the small number of patients requiring mechanical ventilation in the present cohort, the role of LUS in the further identification of those at high risk for mechanical ventilation on top of MEWS will need further studies with a larger patient population. For now, because of the extended time of exposure of the sonographer, and the superiority of MEWS, we believe that the incremental value of the LUS is limited, and thus it should be performed only when clinically indicated.
Focused Cardiac Ultrasound
Recent documents published by the European Association of Cardiovascular Imaging and the American Society of Echocardiography have recommended a FoCUS approach in patients with COVID-19.2 , 3 As these guidelines were based on expert opinion rather than on outcome data, we aimed to assess whether an even more limited approach is sufficient. We found that an optimal model including only two echocardiographic parameters, TAPSE and SVI, provides information that is potentially valuable for clinical management. Recent advances in ultrasound technology have led to the miniaturization of machines to the size of a mobile phone, which do not provide spectral Doppler functions.29 We show that in the context of patients with COVID-19, assessment of LVEF and TAPSE from the four-chamber view alone carries significant prognostic data. Nevertheless, because the echocardiographic model including Doppler data was superior to the “super-simple” approach, we recommend the first and not the latter, if possible.
Study Limitations
Our study included only patients with COVID-19 who were hospitalized. The fact that only a minority of patients with COVID-19 are admitted to the hospital may lead to overestimation of the severity of echocardiographic and LUS pathology in COVID-19. Seven patients were excluded because they had “do not resuscitate/intubate” orders and thus received palliative care and died shortly after admission. This limitation might create an opposite bias resulting in underestimation of echocardiographic or LUS manifestations in patients with COVID-19. In view of the small number of events, the algorithms presented in this report are liable to overfitting; thus their prognostic value will need external validation before clinical use. Echocardiography was performed by cardiologists with expertise in echocardiography using a mobile system and not a pocket-size handheld device. Thus, our hypothesis regarding the use of handheld devices and very limited examinations by noncardiologists should serve as incentive to explore the issue of “super-simple” echocardiographic examinations in patients with COVID-19 in larger prospective series. The fact that in some cases, echocardiographic and LUS parameters were measured by the cardiologist caring for the patient may lead to bias.
Conclusion
We describe a cohort of combined echocardiographic and LUS studies in patients with COVID-19. To achieve maximal clinical value for risk stratification, a very limited echocardiographic examination in patients with high-risk clinical criteria is sufficient. LUS is possibly useful for identifying individuals at risk for mechanical ventilation but not useful for prediction of mortality above clinical and echocardiographic criteria. Importantly, the MEWS provides most of the prognostic information needed for risk assessment.
Footnotes
Conflicts of interest: None.
Drs. Szekely and Lichter contributed equally to this work.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.echo.2021.02.003.
Supplementary data
References
- 1.Kirkpatrick J.N., Mitchell C., Taub C., Kort S., Hung J., Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American College of Cardiology. J Am Coll Cardiol. 2020;75:3078–3084. doi: 10.1016/j.jacc.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skulstad H., Cosyns B., Popescu B.A., Galderisi M., Salvo G.D., Donal E. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020;21:592–598. doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkpatrick J.N., Mitchell C., Taub C., Kort S., Hung J., Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American College of Cardiology. J Am Soc Echocardiogr. 2020;33:648–653. doi: 10.1016/j.echo.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Man M.A., Dantes E., Domokos Hancu B., Bondor C.I., Ruscovan A., Parau A. Correlation between transthoracic lung ultrasound score and HRCT features in patients with interstitial lung diseases. J Clin Med. 2019;8:1199. doi: 10.3390/jcm8081199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xirouchaki N., Kondili E., Prinianakis G., Malliotakis P., Georgopoulos D. Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40:57–65. doi: 10.1007/s00134-013-3133-3. [DOI] [PubMed] [Google Scholar]
- 6.Volpicelli G., Elbarbary M., Blaivas M., Lichtenstein D.A., Mathis G., Kirkpatrick A.W. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 7.Lichter Y., Topilsky Y., Taieb P., Banai A., Hochstadt A., Merdler I. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020:1–11. doi: 10.1007/s00134-020-06212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corradi F., Brusasco C., Pelosi P. Chest ultrasound in acute respiratory distress syndrome. Curr Opin Crit Care. 2014;20:98–103. doi: 10.1097/MCC.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 9.Vetrugno L., Bove T., Orso D., Barbariol F., Bassi F., Boero E. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography. 2020;37:625–627. doi: 10.1111/echo.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szekely Y., Lichter Y., Taieb P., Banai A., Hochstadt A., Merdler I. Spectrum of cardiac manifestations in COVID-19. Circulation. 2020;142:342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao X., Wang B., Kang Y. Novel coronavirus infection during the 2019–2020 epidemic: preparing intensive care units-the experience in Sichuan Province, China. Intensive Care Med. 2020;46:357–360. doi: 10.1007/s00134-020-05954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambden S., Laterre P.F., Levy M.M., Francois B. The SOFA score—development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019;23:374. doi: 10.1186/s13054-019-2663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell C., Collins K., Hua L., McClanahan C., Shea E., Umland M. Specific considerations for sonographers when performing echocardiography during the 2019 novel coronavirus outbreak: supplement to the American Society of Echocardiography statement. J Am Soc Echocardiogr. 2020;33:654–657. doi: 10.1016/j.echo.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johri A.M., Galen B., Kirkpatrick J.N., Lanspa M., Mulvagh S., Thamman R. ASE statement on point-of-care ultrasound during the 2019 novel coronavirus pandemic. J Am Soc Echocardiogr. 2020;33:670–673. doi: 10.1016/j.echo.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Nagueh S.F., Smiseth O.A., Appleton C.P., Byrd B.F., III, Dokainish H., Edvardsen T. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 17.Topilsky Y., Khanna A.D., Oh J.K., Nishimura R.A., Enriquez-Sarano M., Jeon Y.B. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation. 2011;123:1929–1939. doi: 10.1161/CIRCULATIONAHA.110.991018. [DOI] [PubMed] [Google Scholar]
- 18.Kitabatake A., Inoue M., Asao M., Masuyama T., Tanouchi J., Morita T. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation. 1983;68:302–309. doi: 10.1161/01.cir.68.2.302. [DOI] [PubMed] [Google Scholar]
- 19.Bouhemad B., Mongodi S., Via G., Rouquette I. Ultrasound for “lung monitoring” of ventilated patients. Anesthesiology. 2015;122:437–447. doi: 10.1097/ALN.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 20.Jain S.S., Liu Q., Raikhelkar J., Fried J., Elias P., Poterucha T.J. Indications for and findings on transthoracic echocardiography in COVID-19. J Am Soc Echocardiogr. 2020;33:1278–1284. doi: 10.1016/j.echo.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L., Wang B., Zhou J., Kirkpatrick J., Xie M., Johri A.M. Bedside focused cardiac ultrasound in COVID-19 from the Wuhan epicenter: the role of cardiac point-of-care ultrasound, limited transthoracic echocardiography, and critical care echocardiography. J Am Soc Echocardiogr. 2020;33:676–682. doi: 10.1016/j.echo.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beyls C., Bohbot Y., Huette P., Abou-Arab O., Mahjoub Y. Tricuspid longitudinal annular displacement for the assessment of right ventricular systolic dysfunction during prone positioning in patients with COVID-19. J Am Soc Echocardiogr. 2020;33:1055–1057. doi: 10.1016/j.echo.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Churchill T.W., Bertrand P.B., Bernard S., Namasivayam M., Churchill J., Crousillat D. Echocardiographic features of COVID-19 illness and association with cardiac biomarkers. J Am Soc Echocardiogr. 2020;33:1053–1054. doi: 10.1016/j.echo.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sud K., Vogel B., Bohra C., Garg V., Talebi S., Lerakis S. Echocardiographic findings in patients with COVID-19 with significant myocardial injury. J Am Soc Echocardiogr. 2020;33:1054–1055. doi: 10.1016/j.echo.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMahon S.R., De Francis G., Schwartz S., Duvall W.L., Arora B., Silverman D.I. Tablet-based limited echocardiography to reduce sonographer scan and decontamination time during the COVID-19 pandemic. J Am Soc Echocardiogr. 2020;33:895–899. doi: 10.1016/j.echo.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatraju P.K., Ghassemieh B.J., Nichols M. COVID-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Q., Wang Y., Huang S., Liu S., Zhou Z., Zhang S. Multicenter cohort study demonstrates more consolidation in upper lungs on initial CT increases the risk of adverse clinical outcome in COVID-19 patients. Theranostics. 2020;10:5641–5648. doi: 10.7150/thno.46465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mongodi S., Orlando A., Arisi E., Tavazzi G., Santangelo E., Caneva L. Lung ultrasound in patients with acute respiratory failure reduces conventional imaging and health care provider exposure to COVID-19. Ultrasound Med Biol. 2020;46:2090–2093. doi: 10.1016/j.ultrasmedbio.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardim N., Dalen H., Voigt J.-U., Ionescu A., Price S., Neskovic A.N. The use of handheld ultrasound devices: a position statement of the European Association of Cardiovascular Imaging (2018 update) Eur Heart J Cardiovasc Imaging. 2019;20:245–252. doi: 10.1093/ehjci/jey145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.