Abstract
Background:
Experience incorporating frailty and functional metrics in the transplant evaluation process is limited. We hypothesized that simple tests correlate with kidney transplant listing outcomes.
Methods:
Frailty metrics, treadmill ability, pedometer data, troponin T, and brain natriuretic peptide were collected on 375 consecutive kidney transplant evaluations between July 2015 and December 2018. Patients initially denied were compared with those listed or deferred. Frailty metrics included handgrip, chair sit-stand, up-and-go, chair sit-reach, and questions related to exhaustion.
Results:
A total of 95 (25%) patients were initially denied. Those denied were older, diabetic, or had higher body mass indexes. Frailty metrics including chair sit-stand, up-and-go, chair sit-reach, grip strength, and exhaustion; biochemical markers troponin and brain natriuretic peptide; and pedometer and treadmill ability were all significantly associated with denial (P < .001). The best order three model combining parsimony and predictiveness included treadmill ability, exhaustion, and troponin. The most predictive pedometer model also included exhaustion and up-and-go. The best order three model excluding biochemical markers, pedometer, and treadmill results included up-and-go, exhaustion, and chair sit-reach.
Conclusion:
Outcomes after on-site kidney transplant evaluation strongly correlated with the results of common clinical and functional frailty metrics.
TOC summary
A combination of simple readily available frailty measures, wearable fitness device, or treadmill ability can effectively stratify patients before an on-site kidney transplant evaluation. This could be used to increase healthcare system efficiency, facilitate patient preparation for successful transplant evaluation, and enhance telemedicine applications.
Introduction
The Veterans Affairs (VA) kidney transplant system operates as a spoke-and-hub model often serving as an option for veterans with demographic or socioeconomic factors that are obstacles to transplant in the private sector.1 Approximately one-third of all VA transplant referrals and about 44% of our own patients travel 500 or more miles for transplant evaluation.2 The standard process for referral involves collation of key clinical information by the referring center. Screening this information leads to an on-site evaluation or denial at the screening level. Packet creation and travel are costly, and unfortunately a significant proportion of patients are deemed unsuitable for transplant after packet or on-site evaluation. Frailty and poor functional status: (1) are common reasons for transplant listing denial; (2) may correlate with psychologic profile, cognitive ability, and comorbidity; and (3) provide insights that would enhance the transplant screening process.
Frailty assessment has been suggested as a tool for candidate selection, resource allocation, and prognostication in cardiovascular and other surgery.3-5 Frailty, functional ability, and certain biochemical values6,7 are commonly incorporated into transplant evaluations8 as they are known to correlate with outcomes in renal failure and in transplant patients.9 These outcomes include mortality among dialysis patients,10,11 pretransplant and posttransplant mortality, and other transplant-related complications.8,12 However, most centers lack a standardized tool or method for frailty assessment. Also, patient self-assessment of frailty and subjective frailty evaluation by providers in dialysis patients are known to be frequently inaccurate.13,14 Historically our experience also mirrored that of other centers in that our frailty assessment was variable, subjective, and clinician dependent.12
To study emerging prognostic trends from the transplant literature and objectify the functional evaluation of our patients, we began to collect a set of measured functional metrics on all patients undergoing transplant evaluation. With this current study, we retrospectively correlated initial on-site transplant evaluation listing outcomes with functional metrics and biochemical values to establish what best correlated with patients we determined were suitable for listing to create normative values to better allow us to incorporate the information into practice. We were most interested in the factors that might identify patients for immediate denial and separate them from the other groups that were selected to continue in the evaluation process. We sought to create models that excluded certain demographic factors, such as race, underlying diagnosis, age, and body mass index (BMI), that might introduce certain biases in the evaluation.
We hypothesized that easily collected or simply performed metrics may enable the identification of patients at most risk for transplant denial at an earlier phase in the process. This is a relevant area to examine because of the recent mandate to increase screening of all dialysis patients for transplant, to demonstrate the increased need for telemedicine, and to focus on prehabilitation.
Methods
Study population
Following institutional review board approval through the Iowa City VA Health Care System and University of Iowa, we performed a retrospective analysis of patients undergoing a first-time kidney transplant evaluation at the Iowa City VA Health Care System between January 1, 2015, and December 31, 2018. Patients were categorized according to their initial on-site transplant evaluation outcome as falling into 1 of 3 groups: listed, denied, or deferred.
Demographic factors that were studied include: patient age, sex, race (as self-reported to and categorized by the VA), BMI, length of time on dialysis at the time of the evaluation, smoking history (yes/no), any past diagnosis (yes/no) of diabetes and hypertension (regardless of the cause of renal failure), and cause of renal failure.
Measured factors
All measured factors were assessed at the Iowa City VA at the time of the on-site transplant evaluation.
Biochemical markers
Blood for biochemical tests was drawn before functional tests. Cardiac troponin T and N-terminal pro-brain natriuretic peptide (BNP) drawn at the time of evaluation were immunoassayed in our clinical lab, using electrochemiluminescence on a Roche Cobas 6000 analyzer (Roche Diagnostics, Indianapolis, IN, USA). The troponin T reference range was 0.00 to 0.03 ng/mL, with a critical level defined as > 0.09ng/mL. BNP reference ranges were age (by decade) and sex adjusted. For males and females respectively, the 95th percentile for BNP levels in pg/mL were as follows: ages 45–54 y, 138 and 192; ages 55–64 y, 177 and 226; ages 65–74 y, 229 and 353; and ages ≥75 y, 852 and 624.
Frailty metrics
Four functional metrics were collected, including handgrip, 30-s chair sit-stand, chair sit-reach, and timed up-and-go. Each metric was measured as described eslewhere.14-16 Briefly, handgrip was measured in kilograms on a calibrated Jamar Dynamometer (JLW Instruments, Chicago, IL, USA) in the right and left hands and a mean value was calculated.17 The number of times that a patient rose to the full standing position within a 30-s period was recorded in the sit-stand test. For the sit and reach test, the gap in centimeters between the middle fingertip and toes of an extended leg was measured with the patient reaching with both arms extended, in the seated position, at the edge of the chair. Ability to touch the toes or go beyond was recorded as zero. In the up-and-go test the patient was timed while proceeding as quickly as possible from the seated position around a cone positioned 8 feet from the chair and returning to the seated position.
Two self-reported answers to questions from the Center of Epidemiological Studies Depression Scale and a question on change in nutritional status (shrinking) were recorded. These included the following: (1) In how many days in the past week (0–7) was everything you did an effort? (2) In how many days in the past week (0–7) you could not get going? and (3) Was there a 10-lb or more weight loss in the past year (yes/no)?18
Treadmill and pedometer testing
Treadmill and other functional frailty measurements were made on non-dialysis days (in the case of hemodialysis) or before dialysis if conducted on a dialysis day.
Symptom limited treadmill testing was performed on a GE Health care T-2100 treadmill (GE Medical Systems Milwaukee, WI, USA) according to the modified Bruce or Naughton protocols as described elsewhere.19 Early in the data collection phase, the modified Bruce or Naughton was used, but with experience we evolved to exclusively use the Naughton protocol, because we found that its more graded acceleration into stress gave the renal failure patients a better opportunity to achieve their maximal plateau. Treadmill data that were recorded included maximal metabolic equivalents of task (METS) level achieved, total time on treadmill, and reason for stopping the test. For this analysis only METS level was analyzed.
An EKHO Two pedometer (EKHO; Dallas, TX, USA) was calibrated for each patient per manufacturer instructions. Patients were instructed, to wear the pedometer all the time while awake. Pedometer “on” and “off” times were recorded. These data were measured as steps taken and calculated distance (miles) per time.
At the time of the multidisciplinary listing conference, the team was blinded to all metrics except for treadmill, troponin T, and BNP results.
Statistical analysis
Initial assessments of demographic factors and frailty measures were stratified by patients who were initially denied and patients who were initially accepted or deferred. Assessments of differences between continuous measures with normal and non-normal distributions were made using the two-sample t and the Wilcoxon rank-sum tests, respectively. Differences for categorical measures were assessed using Pearson χ2 statistic.
Under the logistic regression framework, univariate models were fit to screen for demographic and frailty variables that may significantly impact listing decision. Those predictors that were significant at the α = 0.1 level were considered for multivariate analysis. To assess which variables best explain the listing decision outcome while maintaining a parsimonious model, all models with predictor subsets of order three were constructed. Model fits were assessed using the Akaike information criterion,20 with smaller values indicating a more appropriate model specification. Top models were identified and assessed further for interaction effects between the predictors. Estimates for predictors in the top models were compared with determine the relative magnitude of their effect on listing decision. Estimated probabilities of listing decision were generated over the range of values for the predictors in the top models to identify patients who are at extremely low or high risk of denial. To assess whether each of the main pedometer, treadmill, and frailty measures is independently associated with denial of listing, models for all eight subset combinations of controls (age, diabetes, BMI) were fit so any changes in the magnitude, direction , or significance of the main measure estimates could be identified.
Results
Study population
Among 375 consecutive patients evaluated for kidney transplant, 95 (25%) were initially denied. Compared with candidates who were initially listed or deferred for transplant, those denied were older (62.6 y vs 60.2 y, P = .0106), diabetic (76.8% vs 60.2%, P = .0042), and had a higher BMI (31.2 vs 29.6, P = .0033). Race, sex, and hypertension diagnosis at the time of evaluation, time on dialysis, and smoking history were not associated with the listing decision. The underlying cause of renal failure also correlated with the evaluation decision (Table I).
Table I.
Study population of patients evaluated for transplant, stratified by those listed and deferred versus those denied at initial on-site evaluation
| Initial listed/deferred (N = 280) | Initial denial (N = 95) | P value | |
|---|---|---|---|
| Age, years mean (SD) | 60.2 (9.4) | 62.6 (9.8) | .0106 |
| Sex, % male | 96.1 | 97.9 | .5296 |
| Race, % | .9044 | ||
| White | 58.2 | 56.8 | |
| Black/African American | 31.1 | 32.6 | |
| Hispanic | 8.6 | 7.4 | |
| Other | 2.1 | 3.2 | |
| BMI, mean (SD) | 29.6 (4.4) | 31.2 (5.0) | .0033 |
| Days on dialysis, median (IQR) | 567 (227–1247) | 500 (250–1188) | .3872 |
| Previous smoking,% | 67.9 | 75.8 | .1562 |
| Current smoking,% | 6.5 | 8.4 | .4957 |
| HTN, % | 97.1 | 97.9 | >.9999 |
| DM, % | 60.2 | 76.8 | .0042 |
| Cause of renal failure, (%) | .0001 | ||
| Diabetes | 132 (47.14) | 59 (62.11) | |
| Hypertension | 46 (16.43) | 21 (22.11) | |
| FSGS | 27 (9.64) | 5 (5.26) | |
| PKD | 22 (7.86) | 2 (2.11) | |
| Primary urologic | 9 (3.21) | 2 (2.11) | |
| Glomerulonephritis | 8 (2.86) | 2 (2.11) | |
| IgA | 6 (2.14) | 1 (1.05) | |
| Lupus nephritis | 3 (1.07) | 0 | |
| Other | 27 (9.64) | 3 (3.16) |
BMI, body mass index; BNP, brain natriuretic peptide; DM, diabetes mellitus; FSGS, focal and segmental glomerulosclerosis; HTN, hypertension; IgA, immunoglobulin A nephropathy; IQR, interquartile range; PKD, polycystic kidney disease.
Biochemical markers
Average troponin T and BNP were both significantly lower among listed and deferred patients compared with those denied. The average and interquartile differences between the two groups are presented in Table II.
Table II.
Results of frailty testing stratified by those listed and deferred versus those denied at initial on site evaluation
| N | Initial list/defer |
Initial denial |
P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
280 |
95 |
|||||||||
| Measure | Mean | SD | Median | Q1 | Q3 | Mean | SD | Median | Q1 | Q3 | |
| Troponin T (ng/mL) | 0.062 | 0.08 | 0.03 | 0.01 | 0.08 | 0.09 | 0.096 | 0.06 | 0.03 | 0.12 | .0003 |
| BNP (pg/mL) | 4474 | 9577 | 1575 | 542 | 3238 | 9409 | 16101 | 3067 | 945 | 8217 | .0001 |
| Mean handgrip (kg) | 31.64 | 9.48 | 31 | 25 | 37 | 27.38 | 7.83 | 27 | 23.25 | 32.5 | .0004 |
| Right handgrip (kg) | 32.46 | 9.64 | 32 | 25 | 39 | 28.49 | 8.86 | 28.5 | 24 | 34 | .0027 |
| Left handgrip (kg) | 30.73 | 10.1 | 30 | 24 | 36 | 26.27 | 7.79 | 26 | 22 | 30 | .0001 |
| Sit-stand (reps/30s) | 14.76 | 5.94 | 14 | 11 | 18 | 11.53 | 5.07 | 12 | 8 | 14 | <.0001 |
| Chair sit-reach (cm) | 4.56 | 7.82 | 0 | 0 | 6 | 7.62 | 9.75 | 6 | 0 | 10 | .0007 |
| Up-and-go | 5.65 | 1.89 | 5.2 | 4.3 | 6.5 | 7.58 | 3.85 | 6.5 | 5.3 | 8.7 | <.0001 |
| 10-lb weight loss (%) | 33.6 | 27.4 | .3083 | ||||||||
| Exhausted 1* >0 (%) | 30.7 | 55.8 | <.0001 | ||||||||
| Exhausted 2† >0 (%) | 24.3 | 43.2 | .0007 | ||||||||
| Treadmill (METS) | 5.72 | 2.21 | 5.4 | 4.3 | 7.2 | 3.55 | 1.69 | 3.35 | 2.2 | 4.8 | <.0001 |
| Pedometer steps/day | 3,790 | 2,350 | 3,500 | 2,020 | 5,130 | 2,910 | 1,980 | 2,620 | 1,600 | 4,000 | .0013 |
| Pedometer miles/day | 3.29 | 2.37 | 2.87 | 1.44 | 4.56 | 2.1 | 1.68 | 1.7 | 0.83 | 2.9 | <.0001 |
Exhaustion question 1: How many days in the past week (0–7) did you feel that everything you did was an effort?
Exhaustion question 2: How many days in the past week (0–7) did you feel that you could not get going?
Frailty measures
The up-and-go and 30-s chair sit-stand were the most significant physical measures that were associated with denial on univariate analysis (P < .0001). Handgrip strength and chair sit-reach also reached levels of significance (P < .001). Self-reported levels of exhaustion, particularly the question asking how many days per week “I could not get going,” also were significant predictors of denial (Table II). Self-reported weight loss did not correlate with listing decision.
Treadmill and pedometer testing
Listed and deferred patients performed significantly better on the treadmill compared with denied patients, 5.7 METS (standard deviation [SD] ± 2.2) versus 3.5 (SD ± 1.7, P < .0001). Steps per time and distance per time both demonstrated differences between transplant evaluation outcomes (Table II). Because steps per time was a more generalizable measure than distance per time, it was selected as the pedometer measurement for use in subsequent modeling.
Correlation coefficients
Moderate correlation was observed between pedometer activity and both treadmill ability (R = 0.468, P < .0001) and up-and-go (R = 0.354, P < .001). Treadmill ability strongly correlated with results of the up-and-go, (R = 0.584, P < .0001). A strong correlation was observed between troponin T and BNP (R = 0.52, P < .0001). Weak correlations were observed between treadmill ability and troponin T (R = −0.19) and BNP (R = −0.26). Similarly, there were weak correlations between pedometer results and troponin T (R = −0.16) and BNP (R = −0.23[data not presented]).
Development of the prediction models
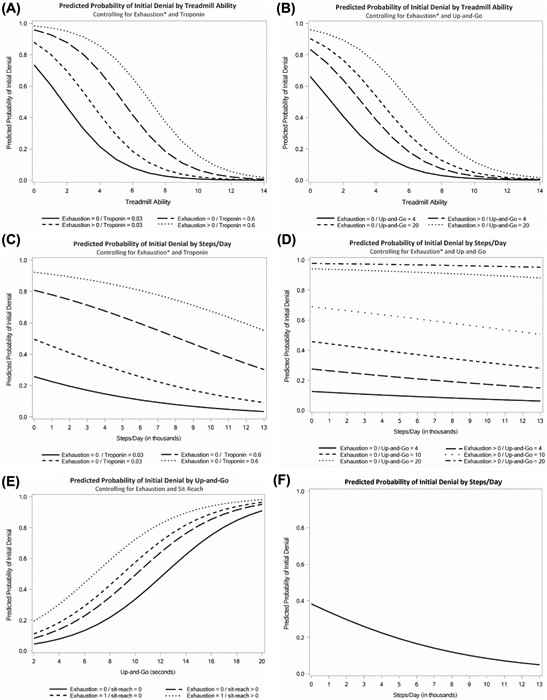
Multivariate logistic regression analysis demonstrated that treadmill ability, steps per day, troponin T, up-and-go, chair sit-reach, and being unable to get going at least 1 day per week were associated with transplant evaluation outcome and used in subsequent modeling. To best balance parsimony with predictiveness, we created multivariate models of order three, using all possible combinations of significant univariate factors (Table III). To further analyze the effect of each predictor set on initial listing status, predicted probability plots were produced at fixed values of 2 model predictors and plotted against the strongest continuous variable in that model to provide an indication of the relative contribution of the individual metrics to the overall model (Fig 1). The best model included treadmill ability, the self-reported question asking how many days per week “I could not get going,” and troponin T, (Table III, model 1). When max treadmill ability was equal to 2 METS, up to 95% of the candidates were predicted to be denied for transplant when they could not get going 1 or more days per week and the troponin T was equal to 0.6 ng/mL. On the other hand, between 84.4% and 99.2% of patients were predicted to be accepted for transplant when METS was greater than or equal to 10, depending on the values of the covariates (Fig 1, A).
Table III.
Predictive models of transplant evaluation outcome
| Model | Label | OR | OR 95% CI lower bound | Or 95% CI upper bound | P value | AIC |
|---|---|---|---|---|---|---|
| MET | 0.56 | 0.47 | 0.6 | <.0001 | ||
| 1 | Exhaustion question 2* >0 | 2.63 | 1.44 | 4.83 | .0018 | 283.7 |
| Troponin (per 0.1) | 1.45 | 1.02 | 2.05 | .0365 | ||
| MET | 0.59 | 0.49 | 0.72 | <.0001 | ||
| 2 | Exhaustion question 2 >0 | 2.58 | 1.41 | 4.72 | .0022 | 285.1 |
| 8-feet up-and-go | 1.10 | 0.98 | 1.24 | .1115 | ||
| Steps/day (per 1,000) | 0.94 | 0.82 | 1.08 | .4040 | ||
| 3 | Exhaustion question 2 >0 | 2.63 | 1.49 | 4.64 | .0009 | 320.0 |
| 8-feet up-and-go | 1.34 | 1.18 | 1.53 | <.0001 | ||
| Steps/day (per 1,000) | 0.84 | 0.73 | 0.96 | .0116 | ||
| 4 | Exhaustion question 2 >0 | 2.83 | 1.64 | 4.91 | .0002 | 337.6 |
| Troponin (per 0.1) | 1.55 | 1.13 | 2.13 | .0073 | ||
| 8-feet up-and-go | 1.33 | 1.18 | 1.50 | <.0001 | ||
| 5 | Exhaustion question 2 >0 | 2.46 | 1.42 | 4.28 | .0016 | 336.2 |
| Chair sit-reach | 1.03 | 0.10 | 1.06 | .069 |
Exhaustion question 2: How many days in the past week (0–7) did you feel that you could not get going?
Fig 1.
Predicted probability plots were created at fixed values of 2 model predictors and plotted against the strongest continuous variable in that model to provide an indication of the relative contribution of the individual metrics to the overall model. (A) Probability of denial by treadmill ability, controlling for exhaustion and Troponin T. (B) Probability of denial by treadmill ability, controlling for exhaustion and up-and-go. (C) Probability of denial by pedometer ability (steps per day), controlling for exhaustion and Troponin T. (D) Probability of denial by pedometer ability (steps per day), controlling for exhaustion and up-and-go. (E) Probability of denial, utilizing metrics not dependent on special equipment or lab testing, chair sit-reach, and exhaustion plotted against up-and-go. (F) Probability of denial by pedometer ability (steps per day) alone.
Another goal of our study was to test whether pedometer data could be modeled to predict transplant evaluation outcome. Therefore, additional models were created combining significant multivariate factors coupled with pedometer steps per day. The best multivariate model of order three that included steps per time also included the self-reported question asking how many days per week “I could not get going” and up-and-go, (Table III, model 3). When the patient took 1,000 steps per day, between 11.9% and 97.5% were predicted to be denied for transplant, a range that demonstrates the strong influence of the up-and-go test in this model (Fig 1, D). Examination of the Fig 1, D demonstrates that pedometer results exerted only a small effect when the up-and-go test was at either end of its range and for the most part improved the prediction of listing outcome when the covariates (exhaustion question and up-and-go) were in the intermediate range. For instance, in model 3, when up-and-go was equal to 4 s, only between 11.9% and 14.9% of patients were denied. Model 4 (Fig 1, C) compares an analogous pedometer model with the best treadmill model. Compared with model 3, model 4 (Fig 1, C) shows a stronger effect of pedometer ability when combined with the exhaustion question and troponin T. Model 4 predicts that, when only 1,000 steps per day was combined with any amount of exhaustion and troponin T of 0.6 ng/mL, 77.9-90.9% of candidates were denied (Fig 1, C).
Considering the potential need to employ predictive modeling without the use of devices, lab tests, or special equipment, models of order three excluding biochemical markers, pedometer and treadmill results were created. The best model combining parsimony and predictiveness included up-and-go, exhaustion question 2, and chair sit-reach (Table III, model 5). Figure 1, E demonstrates that using this model, between 5.9% and 24.4% were predicted to be denied when up-and-go was 3 s, compared with 75.2% to 94% when up-and-go was at least 16 s, depending on the associated covariates.
In model 1, for every single point increase in METS, the odds of denial went down by 40%–46%. In model 4, for every increase in 1,000 steps per day, the odds of denial went down by 16%. When comparing the magnitude of estimates, an increase of 3,345 steps per day was required to match the effect of a 1-MET increase on the treadmill. In model 5 for every 1-s decrease in timed up-and-go, the odds of denial decreased by 25%. Every 1-s decrease in the up-and-go test was equivalent to a 0.516 increase in METS.
A goal of our work was to fit models that did not depend on patient age, BMI, or underlying diagnosis. To assess whether each of the main pedometer, treadmill, and frailty measures is independently associated with denial of listing, models for all eight subset combinations of controls (age, diabetes, BMI) were fit so any changes in the magnitude, direction, or significance of the main measure estimates could be identified. No set of age, BMI, and/or diabetes changed the magnitude or significance of the relationships between the pedometer, treadmill, or frailty measures and initial denial (data not presented).
Discussion
In this single-center series of patients prospectively tested at the time of kidney transplant evaluation, we observed that potential for listing strongly correlated with common determinants of frailty including chair sit-stand, up-and-go, chair sit-reach, handgrip strength, and exhaustion. Using modeling based on frailty and functional metrics, we sought to create a set of simple tools that rely more on ability than on age or pre-existing diagnoses so they could be an important adjunct to holistic screening of patients for transplant. The predictors we used are inexpensive, easily performed, and widely accessible. Accurate assessment of ability is particularly interesting to us as our program evaluates patients who are generally older and have a higher incidence of diabetes compared with the typical US transplant program. Our best model included treadmill ability, a question about exhaustion, and troponin T.
Frailty, the diminished capacity to recover from stressor events, contributes distinctive prognostic significance aside from individual disabilities, comorbidities, and age.21 Compared with published normal values, listed and deferred patients tended to perform in the average range for frailty testing; whereas denied patients performed below average and trended toward the bottom decile, indicating that frailty may have been prevalent among denied patients. Consistent with earlier reports, listing trended toward lower age and less comorbidity. However, in our study, frailty metrics were more closely associated with the multidisciplinary team’s listing decision than with demographic factors such as chronologic age, diabetes mellitus, and BMI. The importance of frailty assessment during transplant evaluation as a method to avoid age bias in candidate selection was pointed out by McAdams-DeMarco et al.22 They found that although frailty increased with age, frailty was prevalent in young patients and plateaued after age 55 y.22 Similarly, Alfaadhel et al10 found that frailty was more closely associated with mortality risk among dialysis patients than with age.
Although duration of hemodialysis is known to be associated with transplant outcomes, our results demonstrated that frailty assessment is more important than dialysis period when considering the probability of being listed. Observing a large dialysis cohort with a mean dialysis duration of 5.2 y, Lee et al23 also found that dialysis period was not associated with frailty and that frailty was more significantly associated with hospitalizations and death on dialysis. Lee et al23 also demonstrated that although comorbidities were associated with frailty, frailty remained independently associated with long-term outcomes even after adjustment for comorbidities like diabetes. Similarly, in our series, comorbidities including diabetes were prevalent but less strongly associated with transplant evaluation outcome than functional and frailty measures.
Walking speed is consistently demonstrated to be an important frailty component and is a strong correlate of frailty and mortality among kidney transplant recipients.22,24 Similarly, treadmill ability is well known to correlate with mortality.25 Therefore, the strong correlation between treadmill and up-and-go testing and contribution to our models is not surprising. However, differences in modeling suggest that despite significant overlap, the ability measured by each test is different. The up-and-go test may better test balance, and the treadmill may be better suited to measuring stamina. Compared with treadmill testing, pedometer activity may provide a more accurate measure of a patient’s innate activity level but could lead to an underestimation of a patient’s maximal ability.26
Wearable fitness tracking (WFT) devices including pedometers and accelerometers have been used for medical prognostication and frailty assessment.27 Steps per day was selectively used in our report as patients and care givers are more familiar with this metric and may be able to better relate to prehabilitation goals in terms of steps than of distance. In addition to allowing an objective assessment of patient progress and adherence, WFTs may enhance self-awareness of sedentary time, motivating an increase in physical activity, allowing patients to compete online or with family or perhaps with dialysis center-based communities.27 Our results suggest that pedometer measures may be especially useful as an inclusion tool rather than as an exclusion tool. A WFT may be a useful tool for motivating denied patients toward the goal of listing by setting goals the center knows correlate with a high probability of listing.
Similar to other reports, both of the Center of Epidemiological Studies Depression Scale-based questions on exhaustion correlated with the transplant evaluation outcome and highlight an interplay between mental and physical frailty.28,29 Mental health concerns are known to be prevalent in veteran populations and are associated with worse outcomes.30,31 Among our cohort, 49% had a psychiatric diagnosis, with depression (31%), post-traumatic stress disorder (19%), and anxiety (16%) being most common. This compares with published reports of mental health diagnoses in 48% of veterans who were transplant recipients32 and depression in 43% of patients on dialysis, respectively.33
Elevated troponin markers were also associated with an increased likelihood of denial. The absolute difference in troponin T levels between the list/deferred and the deny groups was only 0.03, suggesting that this is a precise measure correlated with the evaluation decision. However, given the small magnitude of this difference, it is unclear how exactly troponin elevation may factor into the decision. Literature supports that patients with higher preoperative troponin levels have a higher 30-day mortality after noncardiac surgeries. Particularly, there is evidence that elevated troponin at transplant evaluation confers an increased risk of cardiovascular mortality among renal transplant patients. 34 This appears to be more pronounced among the elderly, where it has been independently associated with mortality (hazard ratio: 5.34, 95% confidence interval: 1.25–22.92).34 This experience may holistically impact the decision to list or deny, even if troponin levels are not specifically viewed as criteria for listing. Similarly, our data demonstrated a correlation between elevations in BNP and listing decision. Although less data are present in the published literature regarding this relationship, elevated BNP has been correlated with mortality and cardiovascular outcomes in stable kidney transplant recipients.7
Alternative explanations exist for these correlations. Indeed, pretransplant troponin T has been associated with other factors that impact listing decision, including diabetes mellitus, ejection fraction, and 6-min walk time. The last deserves special note, because troponin T and BNP are associated with frailty in general. In addition, troponin T and BNP are both associated with decreased estimated glomerular filtration rate, which further confounds interpretation.35
This work is limited by the bias inherent in retrospective analysis. Also, we were unable to include patients who were denied at the packet level before on-site evaluation. Inclusion of these packet-level denials would most likely have resulted in evaluating patients with worse functional ability. Because the multidisciplinary listing committee was not blinded to the results of the treadmill ability, troponin T, and BNP results, these results could have influenced decisions or led to additional testing that could impact the listing decision. However, up-and-go and pedometer testing, which overlap with the domain tested by the treadmill, were still predictive. Cardiopulmonary exercise testing is known to provide more accurate prognostic information than simple treadmill testing and has been used to predict outcomes in liver transplant and other surgical candidates but it is costlier and more difficult to perform and obtain.36 Also, pedometer step-counting error increases at extremes of walking speed and this is particularly relevant in frail patients who may have slower walking speed.37 Our VA transplant population differs from other transplant cohorts in being older, most of them being male, and more of them having diabetes. These and other factors have differential effects on frailty. However, because the Fried scale (upon which our observations are for the most part based is the most widely used frailty instrument and has been validated across various patient subsets, our results should be broadly comparable with other populations. Also, in our modeling we sought to reduce the confounding effect that could have been introduced by including the presence of age and comorbidities in our measures, which should strengthen the applicability of our methods across various transplant communities.
In conclusion, our outcomes after on-site kidney transplant evaluation strongly correlated with the results of several common clinical and functional frailty metrics. Automation of the transplant evaluation is improbable and perhaps unwise; however, special processes may be built around understanding which patients are highly likely or unlikely to succeed in becoming listed. Future work may clarify whether frailty testing may: (1) help eliminate bias against listing older patients for transplant based on age alone, (2) enable prehabilitation at an earlier point in the evaluation process, and (3) provide a tool for motivating patients to remain fit while awaiting transplant. Finally, testing functional metrics could potentially strengthen the accuracy of a transplant evaluation performed via telemedicine as well as allow streamlining of the follow-up transplant re-evaluation.
Acknowledgments
Funding/Support
This study was supported in part by The University of Iowa Clinical and Translational Science Award—NIH (UL1TR002537).
Footnotes
Conflict of interest/Disclosure
Daniel Katz, MD, receives commercial research support for immunosuppression drug trials from Bristol Myers Squibb. All other authors have no conflicts to disclose.
Accepted for presentation at the Central Surgical Association meeting.
References
- 1.Myaskovsky L, Kendall K, Li X, et al. Unexpected race and ethnicity differences in the US National Veterans Affairs Kidney Transplant Program. Transplantation. 2019;103:2701–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunnar W The VA transplant program: A rebuttal to criticism and a look to the future. Am J Transplant. 2019;19:1288–1295. [DOI] [PubMed] [Google Scholar]
- 3.Hewitt J, Long S, Carter B, Bach S, McCarthy K, Clegg A. The prevalence of frailty and its association with clinical outcomes in general surgery: a systematic review and meta-analysis. Age Ageing. 2018;47:793–800. [DOI] [PubMed] [Google Scholar]
- 4.Baldasseroni S, Pratesi A, Orso F, et al. Role of frailty on risk stratification in cardiac surgery and procedures. Adv Exp Med Biol. 2020;1216:99–113. [DOI] [PubMed] [Google Scholar]
- 5.Ketelaers SHJ, Fahim M, Rutten HJT, Smits AB, Orsini RG. When and how should surgery be performed in senior colorectal cancer patients? Eur J Surg Oncol. 2020;46:326–332. [DOI] [PubMed] [Google Scholar]
- 6.McAdams-DeMarco MA, Ying H, Thomas AG, et al. Frailty, inflammatory markers, and waitlist mortality among patients with end-stage renal disease in a prospective cohort study. Transplantation. 2018;102:1740–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarolim P, Claggett BL, Conrad MJ, et al. B-type natriuretic peptide and cardiac troponin I are associated with adverse outcomes in stable kidney transplant recipients. Transplantation. 2017;101:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harhay MN, Rao MK, Woodside KJ, et al. An overview of frailty in kidney transplantation: measurement, management and future considerations [e-pub ahead of print] Nephrol Dial Transplant. 2020. 10.1093/ndt/gfaa016. Accessed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haugen CE, Chu NM, Ying H, et al. Frailty and access to kidney transplantation. Clin J Am Soc Nephrol. 2019;14:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alfaadhel TA, Soroka SD, Kiberd BA, Landry D, Moorhouse P, Tennankore KK. Frailty and mortality in dialysis: evaluation of a clinical frailty scale. Clin J Am Soc Nephrol. 2015;10:832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pugh J, Aggett J, Goodland A, et al. Frailty and comorbidity are independent predictors of outcome in patients referred for pre-dialysis education. Clin Kidney J. 2016;9:324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobashigawa J, Dadhania D, Bhorade S, et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant. 2019;19:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salter ML, Gupta N, Massie AB, et al. Perceived frailty and measured frailty among adults undergoing hemodialysis: a cross-sectional analysis. BMC Geriatr. 2015;15:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 15.Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 16.Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70:113–119. [DOI] [PubMed] [Google Scholar]
- 17.Garonzik-Wang JM, Govindan P, Grinnan JW, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147:190–193. [DOI] [PubMed] [Google Scholar]
- 18.Radloff L The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 19.Naughton J, Sevelius G, Balke B. Physiological responses of normal and pathological subjects to a modified work capacity test. J Sports Med Phys Fitness. 1963;3:201–207. [PubMed] [Google Scholar]
- 20.Akaike H Information theory and an extension of the maximum likelihood principle In: Petrov BN, Csaki F, eds. 2nd International Symposium on Information Theory. Budapest: Akademia Kiado; 1973:267–281. [Google Scholar]
- 21.Harhay MN, Reese PP. Frailty and cognitive deficits limit access to kidney transplantation: unfair or unavoidable? Clin J Am Soc Nephrol. 2019;14:493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAdams-DeMarco MA, Ying H, Olorundare I, et al. Individual frailty components and mortality in kidney transplant recipients. Transplantation. 2017;101:2126–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SY, Yang DH, Hwang E, et al. The prevalence, association, and clinical outcomes of frailty in maintenance dialysis patients. J Ren Nutr. 2017;27:106–112. [DOI] [PubMed] [Google Scholar]
- 24.White DK, Neogi T, Nevitt MC, et al. Trajectories of gait speed predict mortality in well-functioning older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2013;68:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrell SW, DeFina LF, Radford NB, et al. Relevance of fitness to mortality risk in men receiving contemporary medical care. J Am Coll Cardiol. 2020;75:1538–1547. [DOI] [PubMed] [Google Scholar]
- 26.Thorup CB, Andreasen JJ, Sørensen EE, Grønkjær M, Dinesen BI, Hansen J. Accuracy of a step counter during treadmill and daily life walking by healthy adults and patients with cardiac disease. BMJ Open. 2017;7, e011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rumer KK, Saraswathula A, Melcher ML. Prehabilitation in our most frail surgical patients: are wearable fitness devices the next frontier? Curr Opin Organ Transplant. 2016;21:188–193. [DOI] [PubMed] [Google Scholar]
- 28.Lopes AA, Lantz B, Morgenstern H, et al. Associations of self-reported physical activity types and levels with quality of life, depression symptoms, and mortality in hemodialysis patients: The DOPPS. Clin J Am Soc Nephrol. 2014;9:1702–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konel JM, Warsame F, Ying H, et al. Depressive symptoms, frailty, and adverse outcomes among kidney transplant recipients. Clin Transplant. 2018;32:e13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trivedi RB, Post EP, Sun H, et al. Prevalence, comorbidity, and prognosis of mental health among US Veterans. Am J Public Health. 2015;105:2564–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novak M, Molnar MZ, Szeifert L, et al. Depressive symptoms and mortality in patients after kidney transplantation: a prospective prevalent cohort study. Psychosom Med. 2010;72:527–534. [DOI] [PubMed] [Google Scholar]
- 32.Evans LD, Stock EM, Zeber JE, et al. Posttransplantation outcomes in veterans with serious mental illness. Transplantation. 2015;99:e57–e65. [DOI] [PubMed] [Google Scholar]
- 33.Lopes AA, Albert JM, Young EW, et al. Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int. 2004;66:2047–2053. [DOI] [PubMed] [Google Scholar]
- 34.Firth C, Shamoun F, Cha S, et al. Cardiac troponin T risk stratification model predicts all-cause mortality following kidney transplant. Am J Nephrol. 2018;48:242–250. [DOI] [PubMed] [Google Scholar]
- 35.Devine PA, Courtney AE, Maxwell AP. Cardiovascular risk in renal transplant recipients. J Nephrol. 2019;32:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulubay G, Ulasli S, Kupeli E, Yilmaz E, Sezgin A, Haberal M. Value of exercise testing to estimate post-operative complications and mortality in solid organ recipients: a preliminary study. Ann Transplant. 2010;15:11–20. [PubMed] [Google Scholar]
- 37.Park W, Lee VJ, Ku B, Tanaka H. Effect of walking speed and placement position interactions in determining the accuracy of various newer pedometers. J Exerc Sci Fitness. 2014;12:31–37. [Google Scholar]