Neutrophils are the most abundant white blood cell and are an important part of the innate immune system’s defense against opportunistic infections.(1) When evaluating patient susceptibility to infection, both the number and function of neutrophils are clinically important, as neutropenic patients and non-neutropenic patients with primary neutrophil disorders are at greater risk of developing invasive fungal and bacterial infections. However, examples exist of non-neutropenic individuals without a known neutrophil disorder who suffer from repeat or multiple infections and thus, are suspected to harbor an uncharacterized defect in their neutrophil function. Currently, the functional capacity of neutrophils is rarely tested outside of research laboratories. In this case we demonstrate that ex vivo functional assessment of neutrophils can be used to identify such defects and can ultimately aid in better understanding the in vivo host response to invasive pathogens.
Here, we describe a patient with multiple recurrent infections over a prolonged period, months after receiving an autologous hematopoietic stem cell transplant, who was found to have a neutrophil function defect that was successfully treated with cytokine augmentation. The patient is a 22-year-old woman with history of chronic gastroparesis and orthostatic hypotension related to an autoimmune small fiber neuropathy. Prior to transplant, she underwent gastric neurostimulator and jejunostomy-tube placements and was trialed on immunomodulatory agents, including systemic corticosteroids, intravenous immunoglobulin, and anti-lymphocyte therapy, without success. Her course was complicated by worsening nausea and emesis prompting initiation of parenteral nutrition, as well as frequent Clostridium difficile colitis requiring fecal microbiota transplantation. At age 20, she underwent autologous hematopoietic stem cell transplant in an attempt to control autoimmunity, which yielded some improvement in her refractory nausea and vomiting. Eight months after her transplant, the patient developed a central line-associated bloodstream infection. Since that founding infection and despite adequate antimicrobial therapy, she has been hospitalized nearly continuously for recurrent fungemia and bacteremia, mainly with Candida spp. and enteric bacteria, often seen in immunosuppressed hosts. Though patients are at higher risk of infection post-transplant, immune reconstitution is expected within several months, with myeloid lineages arising before lymphoid lineages. Her protracted course eight months post-transplant raised suspicion for an immunodeficiency. Standard clinical immunology diagnostics including respiratory burst assay, flow cytometric analysis of white blood cells, immunoglobulin profiling, T-cell functional assays, and HIV testing, were unrevealing, prompting further investigation into the functional capacity of her neutrophils.
After obtaining informed consent, neutrophils were isolated from blood by negative selection using the EasySep Direct Human Neutrophil Isolation Kit (STEMCELL Technologies). Functional profiling of neutrophils, compared to healthy controls, revealed a reduced ability of the patient’s neutrophils to control C. albicans (Figure 1). Neutrophils were independently primed with four cytokines including granulocyte- (G) and granulocyte-macrophage- (GM) colony-stimulating factor (CSF), interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) to determine the effect of cytokine augmentation on the patient’s neutrophils. Following co-culture with neutrophils, the number of remaining live C. albicans was determined using a viability assay (Figure 1, panel D). Swarming, assessed using time-lapse microscopy of C. albicans clusters, measured complex multicellular neutrophil responses including migration, killing, and cell-to-cell communication. (Figure 1, panel A-C).(2)
Figure 1.
Ex vivo patient neutrophil profiling. Micrograph area of Candida at rest (Panel A) and germinated (Panel B). Following co-incubation with neutrophils, fungal growth area was quantified (pixel2) (Panel C). Percent killing following Candida and neutrophil co-incubation calculated using PMN plus yeast over yeast alone (Panel D). As indicated, neutrophils primed with G-CSF, GM-CSF, IFN-γ, and TNF-α. Significance p<0.05 by Mann-Whitney.
This patient has a dysfunctional neutrophil baseline, which improved upon priming with G-CSF and GM-CSF, but not IFN-γ or TNF-α (Figure 1). This defect was only observed in functional assays and lacks a molecular mechanism at this time. Though we cannot determine whether this neutrophil dysfunction existed prior to, or resulted from, the transplant given the absence of pre-transplant data, the pattern of infections post-transplant suggests a remarkable change in her immune response. Recent data indicates that prophylactic or therapeutic administration of cytokines can result in clinically meaningful prevention and treatment of fungal infections in non-neutropenic patients.(3),(4) Stimulating growth factors and inflammatory cytokines including G-CSF, GM-CSF, and IFN-γ have been shown to “prime” neutrophil function allowing for enhanced responses to bacterial, and fungal pathogens such as C. albicans.(5),(6) G-CSF, GM-CSF, and IFN-γ are used clinically in neutropenic patients, individuals with known primary immune disorders such as Chronic Granulomatous Disease, and those at high risk for infection such as trauma victims.(7),(8),(9) Recent data corroborate these cytokines’ anti-fungal role in non-neutropenic hosts at high risk for infection such as cirrhotic patients (unpublished data).
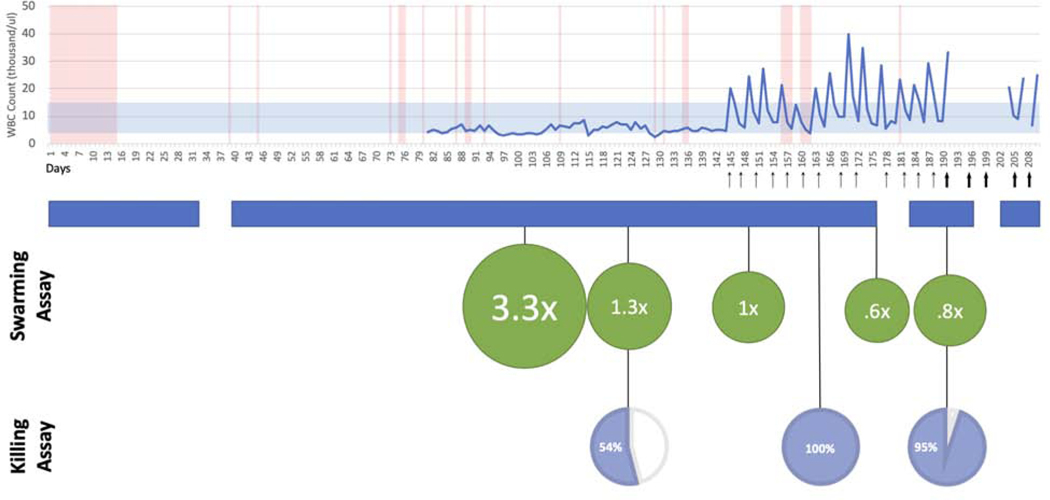
After establishing baseline ex vivo neutrophil function and following multi-disciplinary discussions and institutional review board approval, G-CSF was administered at a dose of 300 micrograms subcutaneously twice per week. G-CSF was chosen due to its effectiveness in enhancing neutrophil function as demonstrated in profiling assays as well as significant prior clinical safety in patients. Following cytokine augmentation, the neutrophil assays were performed repeatedly demonstrating restoration of neutrophil function (Figure 2). Swarming data indicated that the initial neutrophil defect observed in baseline functional assessment was markedly improved. Repeated killing and swarming assays indicated that the pathogen control by the patient’s neutrophils had improved and was equivalent to normal, healthy controls.
Figure 2.
Clinical timeline of infections indicating instances of positive blood culture (red vertical bars) overlaid with WBC (blue line). Arrows indicate G-CSF administered (360mcg, thin arrow, 480mcg, thicker arrow). Inpatient admission periods (blue banner). Green circles represent ratio of C. albicans growth in presence of patient neutrophils to healthy neutrophils. Patient neutrophil killing as a percentage of healthy control (pie chart).
Clinically, the patient began to exhibit fewer infections and was able to be discharged from the hospital. After three continuous months of in-patient hospitalization, following the administration of G-CSF, the patient experienced her longest period free from infection. At the time of submission, the patient continues to receive weekly G-CSF augmentation (total of six months of treatment) without serious side effects. While bloodstream infections continue to occur, presumably due to intermittent bacterial translocation through her gastrointestinal tract, the overall frequency and severity of infections has decreased since starting G-CSF.
While this case of immunomodulation through cytokine augmentation suggests an innovative approach for G-CSF in non-neutropenic patients with undefined neutrophil defects, larger, placebo-controlled clinical studies are necessary to corroborate these findings. Long-term monitoring and assessment are critical to determine any untoward effects of prolonged cytokine exposure and if such a treatment can provide lasting protection against infection. This patient’s neutrophil data suggest a larger role for immune profiling studies, such as killing and swarming assays, to establish functional baselines and identify previously unknown defects in patients with suspected immune dysregulation. Functionally profiling neutrophils from immune dysregulated patients can aid in pinpointing novel therapeutic strategies for those at highest risk of infection.
Clinical Implications Statement:
Ex vivo functional neutrophil profiling of a patient with multiple recurrent infections revealed an undiscovered neutrophil defect in controlling pathogenic fungi. Cytokine augmentation with G-CSF restored function and reduced incidence of infection.
Acknowledgments:
Alex Hopke is the recipient of a postdoctoral fellowship from Shriners Hospital for Children. This work was supported by grants from the National Institute of General Medical Sciences (GM092804) and the Shriners Hospitals for Children (to D. Irimia) and, in part, National Institute of Allergy and Infectious Diseases, RO1 AI132638 (to M. Mansour). Microfabrication was conducted at the BioMEMS Research Center, supported by a grant from the National Institute of Biomedical Imaging and Bioengineering (EB002503).
Footnotes
Conflict of Interest
M.K.M. reports consulting to Celularity, GenMark, SmartPharm, Pulsethera, Vericel, Globe Life House; research support from ThermoFisher Scientific, Genentech. No other authors report conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Schaffner A, Davis CE, Schaffner T, Markert M, Douglas H, Braude AI. In vitro susceptibility of fungi to killing by neutrophil granulocytes discriminates between primary pathogenicity and opportunism. J Clin Invest. 1986;78(2):511–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hopke A, Scherer A, Kreuzburg S, Abers MS, Zerbe CS, Dinauer MC, et al. Neutrophil swarming delays the growth of clusters of pathogenic fungi. Nat Commun [Internet]. 2020;11(1):2031 Available from: http://www.nature.com/articles/s41467-020-15834-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nami S, Aghebati-Maleki A, Morovati H, Aghebati-Maleki L. Current antifungal drugs and immunotherapeutic approaches as promising strategies to treatment of fungal diseases. Biomed Pharmacother [Internet]. 2019;110(November 2018):857–68. Available from: 10.1016/j.biopha.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 4.Scriven JE, Tenforde MW, Levitz SM, Jarvis JN. Modulating host immune responses to fight invasive fungal infections. Curr Opin Microbiol. 2017;40:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swain SD, Rohn TT, Quinn MT. Neutrophil priming in host defense: Role of oxidants as priming agents. Antioxidants Redox Signal. 2002;4(1):69–83. [DOI] [PubMed] [Google Scholar]
- 6.Antachopoulos C, Roilides E. Cytokines and fungal infections. Br J Haematol. 2005;129(5):583–96. [DOI] [PubMed] [Google Scholar]
- 7.Komrokji RS, Lyman GH. The colony-stimulating factors: use to prevent and treat neutropenia and its complications. Expert Opin Biol Ther [Internet]. 2004. December 1;4(12):1897–910. Available from: 10.1517/14712598.4.12.1897 [DOI] [PubMed] [Google Scholar]
- 8.Slack MA, Thomsen IP. Prevention of infectious complications in patients with chronic granulomatous disease. J Pediatric Infect Dis Soc. 2018;7(Suppl 1):S25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohannon JK, Luan L, Hernandez A, Afzal A, Guo Y, Patil NK, et al. Role of G-CSF in monophosphoryl lipid A-mediated augmentation of neutrophil functions after burn injury. J Leukoc Biol. 2016;99(4):629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]