Abstract
Introduction
The novel coronavirus (CoV) disease 2019 (COVID-19) is a viral infection that causes Severe Acute Respiratory Syndrome. It is believed that early reports of COVID-19 cases were noticed in December 2019 and soon after became a global public health emergency. It is advised that COVID-19 transmits through human to human contact and in most cases it remains asymptomatic. Several approaches are being utilized to control the outbreak of this fatal viral disease. microRNAs (miRNAs) are known signature therapeutic tool for the viral diseases; they are small non-coding RNAs that target the mRNAs to inhibit their post-transcriptional expression, therefore, impeding their functions, thus can serve as watchdogs or micromanagers in the cells.
Areas covered
This review work delineated COVID-19 and its association with SARS-CoV and MERS-CoV, the possible role of miRNAs in the pathogenesis of COVID-19, and therapeutic potential of microRNAs and their effective delivery to treat COVID 19.
Expert opinion
This review highlighted the importance of various miRNAs and their potential role in fighting with this pandemic as therapeutic molecules utilizing nanotechnology.
Keywords: SARS-CoV-2, COVID-19, miRNA, nanotherapeutics, nanoparticles
1. Introduction
A novel Coronavirus Disease 2019 (COVID-19) belongs to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and has been discovered in humans for the first time [1]. Coronavirus has been linked to a category of pathogens that impacts the respiratory system of humans. According to World Health Organization (WHO), COVID-19 has symptoms ranging from common cold to high fever, and to trouble in breathing. In severe infection conditions, COVID-19 can be an acute respiratory syndrome which leads to kidney failure, and progresses to death [2–4]. Prior to SARS-CoV-2, two major known coronaviruses, Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) were discovered [5]. COVID-19 has been reported to emerge from Wuhan, Hubei, China, in the end of December 2019 when few patients were admitted to the hospitals diagnosed with unexplained pneumonia and later this spread was suspected to be associated with the wet market in Wuhan, China [6,7].
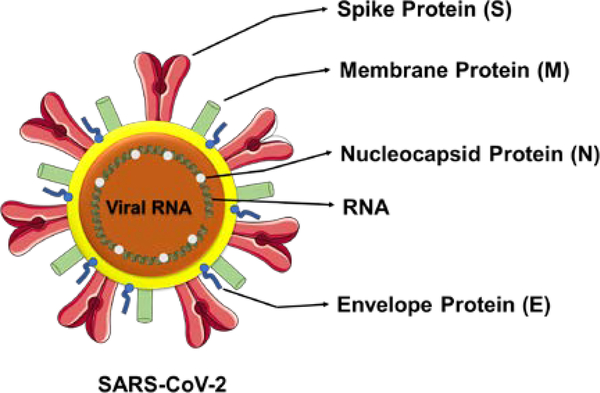
The SARS-CoV-2 belongs to the β-subfamily of coronaviruses similar to SARS-CoV and MERS-CoV [8,9]. Among all other 7 types of coronaviruses, β subtype is the most fatal [8]. Coronaviruses were first identified by Tyrell and Bynoe in 1966 as positive single stranded, enveloped RNA viruses with a genomic size of 26–32 kb, that can infect both mammals and humans [10]. Through literature, it has been considered that SARS-CoV-2 consists of four structural proteins (the spike, membrane, envelope, and nucleocapsid) and RNA viral genome (Figure 1). The whole structure is about 60–140 nm in diameter. The structure of SARS-CoV-2 is considered as a core-shell morphology.
Figure 1.
Ultra-structure and morphological representation of SARS-CoV-2. The representation is for visualization purpose only and not exact for scale and comparison.
Coronaviruses also replicate in the host cytoplasm, like other RNA viruses [11]. Recently, published reports suggested that SARS-CoV-2 was originally transmitted from bats [12,13] and the whole genome of human SARS-CoV-2 was found to be almost 96% identical to the coronavirus of a bat [9]. Bats were reportedly acclaimed as the natural reservoir for other coronaviruses, such as, SARS-CoV, MERS-CoV, HCoV-NL63, and HCoV-229E [14–16].
Most COVID-19 patients were reported to have some other underlying co-morbidity factors, such as, diabetes, respiratory health condition, cardiovascular condition, hypertension, and cancer [3]. According to many reports, the median age of COVID-19 confirmed cases/patients was around 60 years and older, and more than half the population was male. The average incubation time for the virus is ~ 5 days [3,17].
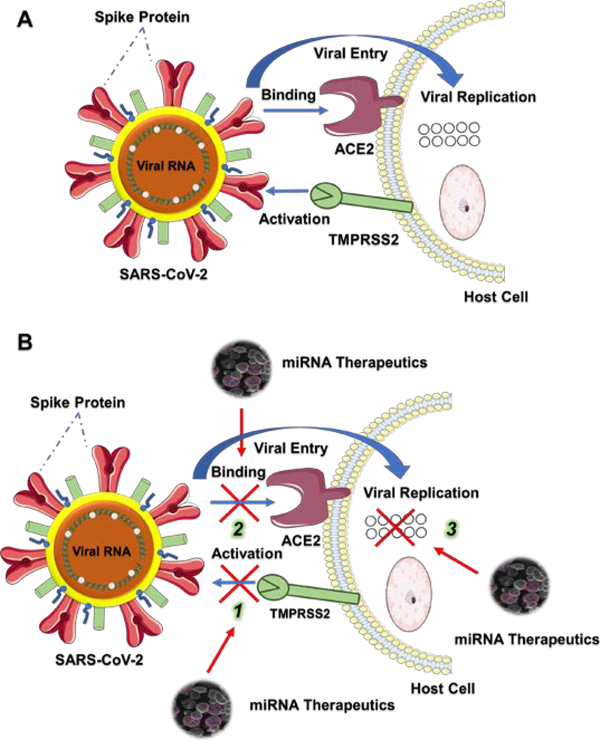
The most favorable route attributing to the transmission of this SARS-CoV-2 virus spread is human to human contact [18,19] (Figure 2A). Upon the transmission, viral particles bind to the host cell receptor and then get fused with the cell membrane. Being a respiratory infection, primarily, SARS-CoV-2 was evident to target the airway and lung epithelial cells. Growing evidences argue that receptor binding domain of SARS-CoV-2 spike protein gets activated after its cleavage by Transmembrane Serine Protease 2 (TMPRSS2) and binds to the Angiotensin Converting Enzyme 2 (ACE2) receptor of the host cells (Figure 2A) [20–22]. Therefore, we hypothesized that identification and successful delivery of certain microRNAs that are involved in blocking the binding or activation with ACE2 or TMPRSS2 is highly rewarding for the prevention and management of COVID-19 (Figure 2B). More detailed information on these aspects are provided in the forthcoming sections.
Figure 2.
Schematic representation of SARS-CoV-2 viral infection and inhibition of its binding, activation, and replication using microRNAs. A. Binding and Fusion of SARS-CoV-2 into the host cell. TMPRSS2 cleaves and activates the spike protein of SARS-CoV-2 which leads the viral spike protein to bind to the host cell membrane receptor ACE2 and get fused into the cell where it replicates and enhances the infection. B. Utilizing miRNA nanotherapeutics, three viral windows can be targeted to inhibit the infection.
2. Association with SARS-CoV and MERS-CoV
COVID-19 has many similarities with the previous two viral infections of its family. Both SARS-CoV and SARS-CoV-2 viruses have originated in China and been linked with animal markets. COVID-19 and MERS both are reported to be asymptomatic in large number of cases [23]. Nonetheless, COVID-19 has also been documented as a contagious and respiratory disease which is another similarity with SARS and MERS [4]. Transmission of all three global health threats has also been via similar means as human to human contacts and, through cough and sneeze droplets in the air [24,25].
Moreover, the receptor binding domain sequence of SARS-CoV-2 is similar to that of SARS-CoV [11]. SARS was a global epidemic exploded in 2003 [26] whereas MERS was the second known coronavirus pandemic and was first reported in 2012 in Saudi Arabia [26]. Reports claimed that these viruses have emerged from bats and camels and transmitted to humans [27,28]. Spread of both viral diseases (SARS and MERS) was worldwide as early clinical features were not clear. MERS was less severe compared to SARS as it did not show global transmission quickly, not even after two years of its first emergence. Previous measures taken to combat SARS and MERS should be reconsidered to better tackle this newer global pandemic, including, maintaining social distancing, proper hygiene, self-quarantine (if any symptoms are present), and isolation (of confirmed positive individuals) [4].
3. miRNAs in SARS, MERS and COVID-19 viral diseases
microRNAs (miRNAs) are about 18–25 nucleotides long, small non-coding RNAs that regulate the target mRNAs post-transcriptionally, thus, serve as watchdogs in the cells [29,30]. In the past recent years, many reports have identified miRNAs as signature biomarkers that play a critical role in various cellular processes, such as, cell proliferation, apoptosis, differentiation, and embryonic development [31]. Dysregulation in the expression status of miRNAs has been associated with several diseases including viral diseases, cancer [31,32], diabetes, schizophrenia [33,34], psoriasis [35], and cardiovascular disease [36].
Growing evidences have suggested a vital role of miRNAs in the pathogenesis and therapeutics of many viral diseases (Table 1), such as Dengue, Influenza, Human Immunodeficiency Virus 1 (HIV-1), Herpes Simplex Viruses (HSV), and Hepatitis C (HCV).A study has reported that miRNA 122 possesses strong anti-HCV characteristics [37]. Based on a bioinformatics study, a group 13 cellular human miRNAs regulate the MERS-CoV. Out of these, only 3 human miRNAs, miRNA 628–5p, miRNA 18a-3p, and miRNA 332–3p have the known biological functions. Remaining 10 human miRNAs (miRNA 6804–3p, miRNA 4289, miRNA 208a-3p, miRNA 510–3p, miRNA 329– 3p, miRNA 548ax, miRNA 3934–5p, miRNA 4474–5p, miRNA 7974, and miRNA 6865–5p) did not have any known role/functions in humans or animals. miRNA 628 and miRNA 332 exhibited remarkable identity with the MERS-CoV viral genome. As of the previously known functions, miRNA 628 has been reported to play tumor suppressive role in glioblastoma where it is crucial for cell proliferation and cell cycle progression [38]. miRNA 18a was also found to critically regulate the genes involved in cellular proliferation, adhesion, and differentiation [39]. On the other hand, miRNA 332 was suggested to be overexpressed in prion disease and crucial for late stage of the disease [40]. Considering the resemblance between MERS and COVID-19, these miRNAs could also be potential targets for COVID-19 therapeutics.
Table 1:
Role of different miRNAs in viral diseases.
| Virus | miRNA | Function | Reference |
|---|---|---|---|
| James Canyon Virus (JCV) | miRNA J1 (Viral) | Downregulates early gene expression | [41] |
| Human Papilomavirus (HPV) | miRNA 203 (Cellular) | Downregulates expression of p63 | [42] |
| SARS | miRNA 17 miRNA 214 (Cellular) |
Facilitates gene replication Helps in immune invasion |
[43] |
| Herpes Simplex Virus (HSV) | miRNA LAT (Viral) | Anti-apoptotic role | [44] |
| Hepatitis C Virus (HCV) | miRNA 122 (Cellular) | Enhances viral replication | [45] |
| Human Immunodeficiency Virus (HIV) | miRNA N367 (Viral) | Reduces LTR transcription | [46] |
| Human Cytomegalovirus (HCMV) | miRNA UL23 (Viral) | Immunomodulation | [47] |
| Simian Virus 40 (SV 40) | miRNA S1 (Viral) | Downregulates early gene expression | [48] |
| Influenza | miRNA 507 (Cellular) | Helps adapting influenza AI (Avian Influenza) to mammalian cells/species via targeting PB2 | [49] |
| BK Virus (BKV) | miRNA B1 (Viral) | Downregulates early gene expression | [50] |
Similarly, many studies have been conducted with SARS regarding its association with miRNAs. Qin et al., have utilized miRNA (Small non-coding RNA) based therapy to reduce the spike gene of SARS-CoV [51]. This strategy can also be utilized for SARS-CoV-2 inhibition as the spike protein of this virus is involved in the binding and fusion to the host cells.
Studies even suggested that some plant miRNAs are also identical with human miRNAs as they share similar genomic sequences [52]. One plant-based miRNA, pab-miRNA 11409d found in gymnosperm Picea abies (L), showed sequence similarity with 3’ of SARS-CoV-2 spike gene (NCBI Accession number LC528233.1), suggesting that it may be implemented to cure the COVID-19 [53]. Anti-viral miRNAs present in the host cells are also of great significance as they act as a crucial regulator of immune response via targeting viral gene replication and expression during the viral infections. A geographical (USA, Wuhan, Italy, India and Nepal) genomic study on COVID-19 reported 6 anti-viral host cell miRNAs specific to SARS-CoV-2 such as hsa-let 7a (targets Non Structural Protein), hsa-miRNA 101 (targets Non Structural Protein), hsa-miRNA 126 (targets Nucleocapsid), hsa-miRNA 23b (targets Spike protein), hsa-miRNA 378 (targets Nucleocapsid), and hsa-miRNA 98 (targets Spike protein) [54].
Patients suffering with diabetic and cardiac diseases who are on ACE2 enhancement drugs (inhibitors and blockers that increase the expression of ACE2 receptor), are more prone to be infected with SARS-CoV-2 [55], and ACE2 was found to be regulated by hsa-miRNA 27b [54,56], therefore, these findings further notify of an important correlation between hsa-miRNA 27b and SARS-CoV-2. It is also important to note that miRNA 27b is associated with Indian origin variant genome of SARS-CoV-2 [54]. Viral protein replication/synthesis occurs in the host cell and miRNAs inhibit the target mRNA translation into the protein, therefore, miRNAs can be utilized as a therapeutic tool for viral diseases [57–59].
Considering the importance of miRNAs, it becomes imperative to identify the miRNAs regulating the pathogenesis of COVID-19 and/or other coronavirus diseases. Another method to control COVID-19 and/or other two coronavirus diseases is, to utilize completely complementary miRNAs (cc miRNA) that can target the viral gene and inhibit its post-transcriptional expression. The cc miRNAs (modified to 25–27 nucleotides), namely, ID02510.3p-miRNA, ID00448.3p-miRNA, miRNA 3154, miRNA 7114–5p, miRNA 5197–3p, ID02750.3p-miRNA, and ID01851.5p-miRNA showed a strong binding with the SARS-CoV-2 viral genome, suggestively [60].
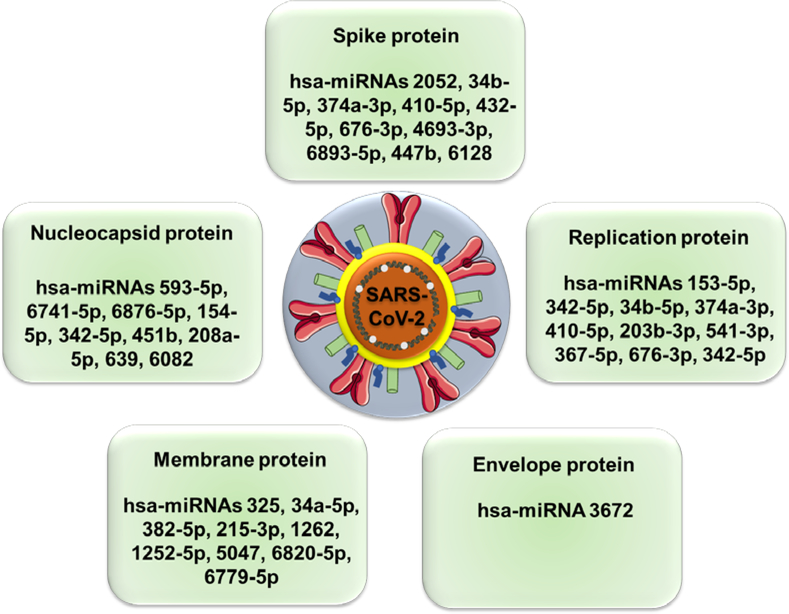
The izMiR (miRNA prediction software) and PANTHER, bioinformatics based classification systems, identified the possible mature viral and host cell miRNA candidates that could play a crucial role in SARS-CoV-2 infection [61]. According to this study, SARS-CoV-2 genes (Spike, Envelope, Membrane, Nucleocapsid, ORF1ab, ORF3a, ORF6, ORF8, ORF7a and ORF10) were targeted by multiple human miRNAs (Figure 3) and these miRNAs already have previously known roles in multiple viral diseases, to illustrate, ORF1ab and ORF3a, two SARS-CoV-2 viral genes, were shown to be targeted by hsa-miRNA 203b-3p which was reported to suppress viral replication in Influenza [62].
Figure 3.
A comprehensive list of host cell miRNAs that target different SARS-CoV-2 proteins.
According to a bioinformatics based study, the spike protein of the virus was shown to be targeted by 67 different human miRNAs whereas this computational prediction indicated that ORF1ab gene was targeted by 369 miRNAs [61]. On the other hand, 18 SARS-CoV-2 viral miRNAs were also identified in this study with their possible host cell target genes. Majority of these target genes were transcription factors and mediators of RNA polymerase II, mainly TAF 4, TAF 5, TAF 7L, SOX 11, TCF 4, TFDP 2, TRPS 1, BRF 1 and MED 1, MED 9, MED 12L, MED 19, respectively. Some signal transducers such as STAT 1 and STAT 5B were also reported among the predicted target genes of viral miRNAs.
CHAC1 and RAD9A are two crucial proteins for apoptosis [63] and found to be targeted by two SARS-CoV-2 viral miRNAs, namely as, miRNA MD2–5p and miRNA 147–3p [64]. In addition, miRNA 66–3p was identified to target the transcription enhancer of TNF-α, a very well-known cytokine [64]. TMPRSS2 has been linked to activate the spike protein of SARS-CoV-2, therefore, promotes the infection [22]. This activator gene had been predicted to be targeted by miRNA 147–3p in the gut. This study further identified two more viral miRNAs as miRNA 198–3p and miRNA 359–5p that target and enhance the activity of Adenosine Deaminases Acting on RNA (ADAR) and non-muscle myosin heavy chain 9 (MYH9), respectively [64].
A PubMed search with the keywords “miRNA and SARS-CoV-2” (https://pubmed.ncbi.nlm.nih.gov/?term=miRNA+and+SARS-CoV-2) indicated about 20 peer-reviewed studies (data accessed on August 3, 2020). Out of these, we discussed the articles which are highly important and provide a new direction to the field of miRNAs in COVID-19 research. Guterres et al., [65] screened 60 SARS-CoV-2 genomes for the possible identification of seed sponges which may be involved in binding with the human miRNA, thus, preventing the interaction with their native targets. This study extracted a perfect match with 11 nucleotides encompassing the seed region. Additionally, this study demonstrated that there are 34 and 45 miRNAs for positive-sense and negative-sense viral RNA, respectively, that can strongly bind to certain key SARS-CoV-2 genes. Through the Kyoto Encyclopedia of Genes and Genomes pathway analysis prediction study [66], 7 important miRNAs (miRNAs 8066, 5197, 3611, 3934–3p, 1307–3p, 3691–3p and 1468–5p) were identified that are perfectly linked with host responses and virus pathogenicity. Another study of a sequence analysis of SARS-CoV-2 genome has identified that out of 10, 3 targets have been lost. This includes miR-197–5p [67].
Considering the significance of ACE2 and TMPRSS2 in SARS-CoV-2 infection, we also performed a TargetScan (http://www.targetscan.org/vert_72/) search for the miRNAs that could target these two receptors, and the analyses revealed a list of miRNAs such as, hsa-miRNA 200b-3p, hsa-miRNA 200c-3p and miRNA 429 for ACE2, and hsa-let 7c-5p, hsa-miRNA 98–5p, hsa-let 7f-5p, hsa-let 7a-5p, hsa-let 7g-5p, hsa-let 7b-5p, hsa-miRNA 4458, hsa-let 7e-5p, hsa-let 7i-5p, hsa-let 7d-5p and hsa-miRNA 4500 for TMPRSS2. According to this search result, these miRNAs appear to directly target ACE2 and TMPRSS2, hence, could be utilized as potent therapeutic molecules to regulate key proteins that are required for viral contraction and its entry to the host airway/lung epithelial cells.
4. Therapeutic potential of miRNAs for COVID-19
Viruses cannot replicate on their own, thus, they utilize the host cell environment for their replication. One of the many strategies that viruses use, is modifying the host cell miRNA for their favor [49]. SARS-CoV viral infection in Bronchioalveolar Stem Cells (BASCs) was reported to modulate the differentiation of BASCs for its successful replication as it cannot replicate in the well differentiated cells [43]. In the host cell, SARS-CoV modulated the expression of various miRNAs such as miRNA 17, miRNA 574, miRNA 214, and moreover the spike and nucleocapsid proteins of this virus inhibited the expression of miRNA 98 and miRNA 223, respectively, to control the cellular differentiation in BASCs [43].
On the other hand, some host cell miRNAs modulate the target viral gene expression in the immune response as to defend the cells [68]. Signifying to the relationship between miRNAs and viral infection, miRNAs appear to be potential biomarkers and therapeutic targets in viral diseases. Trobaugh and Klimstra [69] have delineated the various aspects of the interaction between RNA viruses and cellular miRNAs, including factors influencing miRNA-RNA virus interaction, miRNA interactions with the RNA viral genome, miRNA-mediated stabilization of RNA virus genomes, modulation of host miRNA levels during viral infections, miRNA-mediated changes in protein expression that alter host responses in infection and promote viral replication, and maintenance of miRNA-binding sites in the RNA virus genome. This review discusses, how “miRNAs can affect RNA virus replication and pathogenesis through direct binding to the RNA virus genome or through virus-mediated changes in the host transcriptome”. Additionally, this study documents the direct and indirect interactions between cellular miRNAs and RNA viruses.
Currently, in the lack of COVID-19 treatments and vaccines which will take time to be developed, it is important to note that discovering miRNA-based therapies can be an attractive option for controlling the replication of the virus. Host cell miRNAs that were predicted to target SARS-CoV-2 viral genes, warrant further research in cell lines and animal models. ACE2 and TMPRSS2 are two important receptors that were reported to facilitate the activation and binding of SARS-CoV-2, and its entry to the host cell. miRNAs that are associated with these two receptors could act as a therapeutic modality for this virus. To illustrate, host miRNA 27b regulates ACE2 and viral miRNA 147–3p targets TMPRSS2, if these therapeutic miRNAs can be delivered to the cells, attachment of SARS-CoV-2 spike protein and these receptors can be inhibited, therefore, contraction of viral infection can be mitigated or minimized (Figure 2B). Additionally, miRNAs from our TargetScan search seem to be directly targeting these two genes, herein, enhanced or restored expression of the listed miRNAs in the host cell could suppress the expression/presence of ACE2 and TMPRSS2, which will result in the inhibition of SARS-CoV-2 viral entry and infection in the host cell.
In the field of medicine, miRNAs have been considered as promising biomarker(s) and novel target(s) for therapeutic approaches. miRNAs as therapeutics [70] have extensively been reviewed and documented for their key roles and recent advances in cancer and other diseases (pulmonary and cardiac disorders, asthma, pneumonia, cardiac fibrosis, and so on). However, in virology, the landscape of miRNAs as diagnostic and interventional medicine is still an unexplored area of research. The major issues with miRNA based viral research/therapy are, the delivery of miRNAs to the target cells/tissues, poor circulation/half-life (as miRNAs are highly unstable), and the toxicity associated with conventional delivery vehicles. Simple chemical modification can serve as a medium to improve stability of miRNAs, but it may not provide in vivo and clinical translation. On the other hand, various viral vectors (adenoviral, retroviral, and lentiviral vectors) have been widely applied to the preclinical and clinical purposes. However, their poor miRNA loading efficacy, off-target toxicity, and immunogenicity significantly hamper their use. Thus, the development of non-viral delivery vectors is considered to be a suitable option for effective delivery of microRNAs [71].
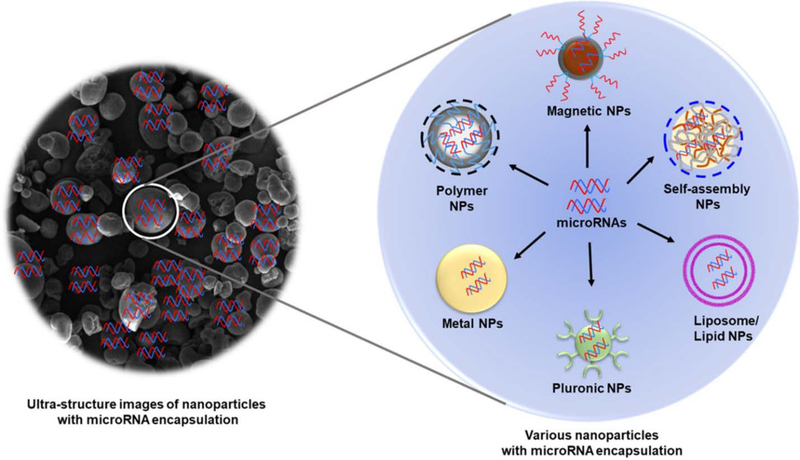
Nanoparticle based delivery has been reported to tackle these above-mentioned obstacles related to miRNA therapeutics [72,73]. Nanotherapeutics are one of the most favorable platforms to effectively deliver the miRNAs to the host cells pertaining to their tiny size and low molecular weight. Various nanoparticle carriers have been suggested for the successful miRNA delivery mainly inorganic nanoparticles, polymeric particles, lipid nanoparticles and others [74]. Among all these non-viral delivery systems, lipid-based carriers (complexed or encapsulated miRNAs inside the lipoplex/liposome membrane-like surface) are the widely employed category. Currently, this type of formulations (lipofectamine, SiPORT, and DharmaFECT) [71,75] are already being used for in vitro and in vivo applications. On the other hand, polymer-based delivery systems, such as PEI, PLGA, or other biodegradable polymers-based carriers not only offer biocompatibility but also minimal toxicity and efficient delivery. Nanoparticle cores protect the naked miRNA from its degradation, thus, provide the enhanced circulation in the system. Also, due to the nanometer size range of nanocarriers, targeted delivery of miRNAs is achieved [76]. Our lab has the expertise in generating nanoformulations as we have successfully developed several miRNA-based nanotherapeutics in the past (Figure 4) [77,78]. A relatively new concept of using exosomes or endosomal vesicles (extracellular vesicles) as delivery vehicles, is also another option to successfully deliver miRNAs [79]. Hence, utilization of miRNA with nanoparticles-based delivery, has the great potential of becoming a novel therapeutic approach to tackle COVID-19 pandemic.
Figure 4.
A schematic representation of different nanosystems that could be utilized for successful miRNA nanotherapy.
5. Concluding remarks
World Health Organization has declared the COVID-19, a global pandemic in February 2020, which has caused more than 4 million confirmed positive cases and more than quarter million deaths globally as of May 8, 2020. COVID-19 has been reported in humans for the first time, therefore, development of effective therapies is an unmet clinical need. Many research groups have been focusing on understanding the genomic profile of the virus and utilizing the repurposed drugs to treat COVID-19. A very few reports, so far, have reported the miRNAs as therapeutic molecules for this disease. miRNAs control the post-transcriptional expression of target mRNA genes, therefore, have reported to play an important role in the pathogenesis of many viral diseases including SARS-CoV and MERS-CoV infections. In this review, we have shed the light on various important and previously reported miRNAs in coronavirus diseases and, also how they can be beneficial for combating this invisible enemy to protect the human race. This should be an active area for future research to conduct detailed in vitro and in vivo experimental procedures for the clinical translation of miRNAs into COVID-19 therapeutics.
6. Expert opinion
Since the first emergence of SARS-CoV-2 in the later part of December 2019, more than 18.2 million people have been impacted globally with this viral infection. As of now, there is no FDA approved treatment modality available but to slow-down the spread of the pandemic and control the number of infected people, some agencies allowed repurposing of few drugs including hydroxychloroquine, chloroquine, remedesivir, and other HIV drugs under Emergency Use Authorization (EUA). Along with these, many other treatments are under investigation ranging from anti-viral drugs to plasma therapy and antibody drugs. As the preventative measures, many vaccine candidates are also being tested (13 under clinical trials and more than 100 in preclinical studies) (clinicaltrials.gov). In order to propose an adequately feasible treatment option for SARS-CoV-2, researchers should focus on understanding the components involving in the pathogenesis of the viral disease.
miRNAs have emerged as newer group of components playing a crucial role in the pathogenesis of many diseases including viral diseases with clinically relevant therapeutic applications. In this review, we have highlighted several miRNAs that have identified for SARS-CoV-2 using bioinformatic studies. These miRNAs are, however, subjected to further in vitro and in vivo research, but still provide essential information on their possible role in SARS-CoV-2 infection. As miRNAs regulate the expression of target genes (mRNA), changing the expression status of miRNAs by overexpressing or knocking them down, can result in desired therapeutic outcomes. Viral diseases can have dual functionality of miRNAs as there could be viral miRNAs and host cell miRNAs. Host cell miRNAs could either inhibit the viral genome or enhance the viral genome replication upon the interaction. Despite having these advantages of miRNA therapeutics, delivery of naked miRNAs is often associated with rapid degradation and non-specific target effects which can be overcome by nanotechnology-based approach.
The overall idea of developing nanoformulations of the SARS-CoV-2 related miRNAs is to deliver these miRNAs successfully and safely to the cells to exert their therapeutic effects. This review has proposed multifaceted targeting approach for SARS-CoV-2 utilizing miRNA nanotherapy. Activation of SARS-CoV-2 spike protein upon the cleavage by TMPRSS2 is the first event of viral infection which further leads the cleaved spike protein to get fused with ACE2 membrane receptor of the host cells. Therefore, miRNAs that are specific to activation and binding of spike protein are of great therapeutic potential. Like other delivery systems, identification of suitable miRNA nanoformulations with predetermined loading efficacy, sustained release, and targeting characteristics, is highly warranted. In addition, miRNAs that could inhibit the viral replication in the host cell, could also be enveloped in the nanoparticles to suppress the viral load.
Other than therapeutic purpose, nanoparticles-based miRNAs could also be used in the form of nano-vaccines for the prevention from SARS-CoV-2. Nano-vaccines have several benefits over traditional vaccines as they are specific for infection site and have minimal to no off-target effects. Additionally, nano-vaccines can be developed as nasal spray/drops. In case of SARS-CoV-2 infection, nasal spray nano-vaccine appears to be a more reliable modality as it can directly activate the immune response in the respiratory tract including nasal passage as well as the lungs which are the primary contraction sites for SARS-CoV-2 viral infection, this also indicates the direct and specific delivery of miRNAs in the targeted sites. It is important to develop a unique and multi-disciplinary team of pharmaceutical and nanomedicine experts, and basic and clinical scientists, for the effective clinical translation of miRNA nanoformulations. However, applying already existing nanomedicine technology has also revived the interest for effective implementation of miRNA delivery for COVID-19. Oral delivery of therapeutics is always an easy step towards developing the medicines [71]. In the case of oral delivery of miRNAs, miRNA availability is highly challenging due to the nucleic acid degradation in gastric environment. Therefore, developing formulations that are suitable and can be applied to oral delivery of miRNAs is highly sought. Literature has identified that chitosan [80], mannose modified chitosan [81], bovine milk derived exosomes [82], bovine lactoferrin [83], lipidic [84], and PLGA-based nanoformulations [85] are employed for effective oral delivery of miRNAs/ therapeutics. Hence, developing oral nanoformulation(s) of miRNAs is achievable and is highly recommended for its successful implementation into the clinic.
Article highlights.
The novel coronavirus disease 2019 (COVID-19) is caused due to SARS-CoV-2 infection which has been declared a pandemic.
The importance of miRNAs in the pathogenesis of this viral disease is summarized.
Currently, there is no approved treatment or vaccine for COVID-19 and miRNAs can become a potential therapeutic tool.
This work delineates the overall idea of developing nanoformulation(s) of the SARS-CoV-2 related miRNAs.
Multifaceted targeting approaches are required for miRNA nanotherapy for effectively tackling SARS-CoV-2.
The nanoparticles-based miRNAs could also be used in the form of nano-vaccines for the prevention from SARS-CoV-2.
Acknowledgments
Funding
The study was partially supported by the National Institute of Health of United States of America (R01 CA210192, R01 CA206069, R01 CA204552), Faculty Stat up fund from UTRGV (to M.M.Y., M.J., and S.C.C.), and Herb Kosten Foundation.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Chang L, Yan Y, Wang L. Coronavirus Disease 2019: Coronaviruses and Blood Safety. Transfusion medicine reviews, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren LL, Wang YM, Wu ZQ et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chinese medical journal, 133(9), 1015–1024 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England), 395(10223), 497–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peeri NC, Shrestha N, Rahman MS et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? International journal of epidemiology, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. Journal of autoimmunity, 109, 102433 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. Journal of travel medicine, 27(2) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. Journal of medical virology, 92(4), 401–402 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu Y, McCauley J. GISAID: Global initiative on sharing all influenza data - from vision to reality. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin, 22(13) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P, Yang XL, Wang XG et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyrrell DA, Bynoe ML. Cultivation of viruses from a high proportion of patients with colds. Lancet (London, England), 1(7428), 76–77 (1966). [DOI] [PubMed] [Google Scholar]
- 11.Hasan MM, Akter R, Ullah MS, Abedin MJ, Ullah GM, Hossain MZ. A Computational Approach for Predicting Role of Human MicroRNAs in MERS-CoV Genome. Advances in bioinformatics, 2014, 967946 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovanetti M, Benvenuto D, Angeletti S, Ciccozzi M. The first two cases of 2019-nCoV in Italy: Where they come from? Journal of medical virology, 92(5), 518–521 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paraskevis D, Kostaki EG, Magiorkinis G, Panayiotakopoulos G, Sourvinos G, Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases, 79, 104212 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee A, Kulcsar K, Misra V, Frieman M, Mossman K. Bats and Coronaviruses. Viruses, 11(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Shi Z, Yu M et al. Bats are natural reservoirs of SARS-like coronaviruses. Science (New York, N.Y.), 310(5748), 676–679 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Hampton T Bats may be SARS reservoir. Jama, 294(18), 2291 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Guan X, Wu P et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med, 382(13), 1199–1207 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlos WG, Dela Cruz CS, Cao B, Pasnick S, Jamil S. Novel Wuhan (2019-nCoV) Coronavirus. American journal of respiratory and critical care medicine, 201(4), P7–p8 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Wu P, Hao X, Lau EHY et al. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin, 25(3) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. Journal of virology, 94(7) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaimes JA, Millet JK, Stout AE, André NM, Whittaker GR. A Tale of Two Viruses: The Distinct Spike Glycoproteins of Feline Coronaviruses. Viruses, 12(1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuyama S, Nao N, Shirato K et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proceedings of the National Academy of Sciences of the United States of America, 117(13), 7001–7003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothe C, Schunk M, Sothmann P et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med, 382(10), 970–971 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nassar MS, Bakhrebah MA, Meo SA, Alsuabeyl MS, Zaher WA. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: epidemiology, pathogenesis and clinical characteristics. European review for medical and pharmacological sciences, 22(15), 4956–4961 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Malave A, Elamin EM. Severe Acute Respiratory Syndrome (SARS)-Lessons for Future Pandemics. The virtual mentor : VM, 12(9), 719–725 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Lin MH, Chuang SJ, Chen CC et al. Structural and functional characterization of MERS coronavirus papain-like protease. Journal of biomedical science, 21(1), 54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azhar EI, El-Kafrawy SA, Farraj SA et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med, 370(26), 2499–2505 (2014). [DOI] [PubMed] [Google Scholar]
- 28.van Doremalen N, Miazgowicz KL, Milne-Price S et al. Host species restriction of Middle East respiratory syndrome coronavirus through its receptor, dipeptidyl peptidase 4. Journal of virology, 88(16), 9220–9232 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ambros V The functions of animal microRNAs. Nature, 431(7006), 350–355 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 116(2), 281–297 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nature reviews. Cancer, 6(4), 259–269 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Serban M, Ghiorghiu I, Craciunescu I et al. Spontaneous echo contrast of unexpected etiology. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology, 7(3), 257–259 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Miao C, Chang J, Zhang G, Fang Y. MicroRNAs in type 1 diabetes: new research progress and potential directions. Biochemistry and cell biology = Biochimie et biologie cellulaire, 96(5), 498–506 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Perkins DO, Jeffries CD, Jarskog LF et al. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome biology, 8(2), R27 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonkoly E, Wei T, Janson PC et al. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PloS one, 2(7), e610 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latronico MV, Catalucci D, Condorelli G. Emerging role of microRNAs in cardiovascular biology. Circulation research, 101(12), 1225–1236 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Janssen HL, Reesink HW, Lawitz EJ et al. Treatment of HCV infection by targeting microRNA. N Engl J Med, 368(18), 1685–1694 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Xu J, Chen H et al. Comprehensive analysis of the functional microRNA–mRNA regulatory network identifies miRNA signatures associated with glioma malignant progression. Nucleic acids research, 41(22), e203–e203 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui FM, Li JX, Chen Q et al. Radon-induced alterations in micro-RNA expression profiles in transformed BEAS2B cells. Journal of toxicology and environmental health. Part A, 76(2), 107–119 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Montag J, Hitt R, Opitz L, Schulz-Schaeffer WJ, Hunsmann G, Motzkus D. Upregulation of miRNA hsa-miR-342–3p in experimental and idiopathic prion disease. Molecular neurodegeneration, 4, 36 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seo GJ, Fink LH, O’Hara B, Atwood WJ, Sullivan CS. Evolutionarily conserved function of a viral microRNA. Journal of virology, 82(20), 9823–9828 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melar-New M, Laimins LA. Human papillomaviruses modulate expression of microRNA 203 upon epithelial differentiation to control levels of p63 proteins. Journal of virology, 84(10), 5212–5221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin C, Wang J, Wei Q et al. An animal model of SARS produced by infection of Macaca mulatta with SARS coronavirus. The Journal of pathology, 206(3), 251–259 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta A, Gartner JJ, Sethupathy P, Hatzigeorgiou AG, Fraser NW. Anti-apoptotic function of a microRNA encoded by the HSV-1 latency-associated transcript. Nature, 442(7098), 82–85 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Ura S, Honda M, Yamashita T et al. Differential microRNA expression between hepatitis B and hepatitis C leading disease progression to hepatocellular carcinoma. Hepatology (Baltimore, Md.), 49(4), 1098–1112 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Omoto S, Fujii YR. Regulation of human immunodeficiency virus 1 transcription by nef microRNA. The Journal of general virology, 86(Pt 3), 751–755 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Dunn W, Trang P, Zhong Q, Yang E, van Belle C, Liu F. Human cytomegalovirus expresses novel microRNAs during productive viral infection. Cellular microbiology, 7(11), 1684–1695 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Sullivan CS, Grundhoff AT, Tevethia S, Pipas JM, Ganem D. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature, 435(7042), 682–686 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Scaria V, Hariharan M, Maiti S, Pillai B, Brahmachari SK. Host-virus interaction: a new role for microRNAs. Retrovirology, 3, 68 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seo GJ, Chen CJ, Sullivan CS. Merkel cell polyomavirus encodes a microRNA with the ability to autoregulate viral gene expression. Virology, 383(2), 183–187 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Qin ZL, Zhao P, Zhang XL et al. Silencing of SARS-CoV spike gene by small interfering RNA in HEK 293T cells. Biochem Biophys Res Commun, 324(4), 1186–1193 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rebolledo-Mendez JD, Vaishnav RA, Cooper NG, Friedland RP. Cross-kingdom sequence similarities between human micro-RNAs and plant viruses. Communicative & integrative biology, 6(5), e24951 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaschetto LM. A putative 20-nt miRNA in COVID-19 aligns to both the forward and reverse complementary strands of hsa-mir-8055 involved in T-cell response. (2020). [Google Scholar]
- 54.Sardar R, Satish D, Birla S, Gupta D. Comparative analyses of SAR-CoV2 genomes from different geographical locations and other coronavirus family genomes reveals unique features potentially consequential to host-virus interaction and pathogenesis. 2020.2003.2021.001586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet. Respiratory medicine, 8(4), e21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen LJ, Xu R, Yu HM, Chang Q, Zhong JC. The ACE2/Apelin Signaling, MicroRNAs, and Hypertension. International journal of hypertension, 2015, 896861 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartel DP. Metazoan MicroRNAs. Cell, 173(1), 20–51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nature reviews. Genetics, 11(9), 597–610 (2010). [DOI] [PubMed] [Google Scholar]
- 59.Zhdanov VP. Intracellular miRNA or siRNA delivery and function. Bio Systems, 171, 20–25 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Rakhmetullina A, Ivashchenko A, Akimniyazova A, Aisina D, Pyrkova A. The miRNA Complexes Against Coronaviruses COVID-19, SARS-CoV, And MERS-CoV. (2020). [Google Scholar]
- 61.Saçar Demirci MD, Adan A. Computational analysis of microRNA-mediated interactions in SARS-CoV-2 infection. 2020.2003.2015.992438 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang S, Li J, Li J et al. Up-regulation of microRNA-203 in influenza A virus infection inhibits viral replication by targeting DR1. Sci Rep, 8(1), 6797 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komatsu K, Miyashita T, Hang H et al. Human homologue of S. pombe Rad9 interacts with BCL-2/BCL-xL and promotes apoptosis. Nature cell biology, 2(1), 1–6 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Liu Z, Wang J, Xu Y et al. Implications of the virus-encoded miRNA and host miRNA in the pathogenicity of SARS-CoV-2. (2020). [Google Scholar]
- 65.Guterres A, de Azeredo Lima CH, Miranda RL, Gadelha MR. What is the potential function of microRNAs as biomarkers and therapeutic targets in COVID-19? Infection, Genetics and Evolution, 85, 104417 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arisan ED, Dart A, Grant GH et al. The Prediction of miRNAs in SARS-CoV-2 Genomes: hsa-miR Databases Identify 7 Key miRs Linked to Host Responses and Virus Pathogenicity-Related KEGG Pathways Significant for Comorbidities. Viruses, 12(6) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hosseini Rad Sm A, McLellan AD. Implications of SARS-CoV-2 Mutations for Genomic RNA Structure and Host microRNA Targeting. International journal of molecular sciences, 21(13) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moens U Silencing viral microRNA as a novel antiviral therapy? Journal of biomedicine & biotechnology, 2009, 419539 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trobaugh DW, Klimstra WB. MicroRNA Regulation of RNA Virus Replication and Pathogenesis. Trends Mol Med, 23(1), 80–93 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nature reviews. Drug discovery, 16(3), 203–222 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Fu Y, Chen J, Huang Z. Recent progress in microRNA-based delivery systems for the treatment of human disease. ExRNA, 1(1), 24 (2019). [Google Scholar]
- 72.Lee SWL, Paoletti C, Campisi M et al. MicroRNA delivery through nanoparticles. J Control Release, 313, 80–95 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernandez-Piñeiro I, Badiola I, Sanchez A. Nanocarriers for microRNA delivery in cancer medicine. Biotechnology advances, 35(3), 350–360 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Bai Z, Wei J, Yu C et al. Non-viral nanocarriers for intracellular delivery of microRNA therapeutics. Journal of materials chemistry. B, 7(8), 1209–1225 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Yang N An overview of viral and nonviral delivery systems for microRNA. Int J Pharm Investig, 5(4), 179–181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ganju A, Khan S, Hafeez BB et al. miRNA nanotherapeutics for cancer. Drug Discov Today, 22(2), 424–432 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Setua S, Khan S, Yallapu MM et al. Restitution of Tumor Suppressor MicroRNA-145 Using Magnetic Nanoformulation for Pancreatic Cancer Therapy. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract, 21(1), 94–105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagesh PKB, Chowdhury P, Hatami E et al. miRNA-205 Nanoformulation Sensitizes Prostate Cancer Cells to Chemotherapy. Cancers, 10(9) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baumann V, Winkler J. miRNA-based therapies: strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents. Future Med Chem, 6(17), 1967–1984 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martirosyan A, Olesen MJ, Howard KA. Chitosan-based nanoparticles for mucosal delivery of RNAi therapeutics. Advances in genetics, 88, 325–352 (2014). [DOI] [PubMed] [Google Scholar]
- 81.Deng F, He S, Cui S et al. A Molecular Targeted Immunotherapeutic Strategy for Ulcerative Colitis via Dual-targeting Nanoparticles Delivering miR-146b to Intestinal Macrophages. Journal of Crohn’s & colitis, 13(4), 482–494 (2019). [DOI] [PubMed] [Google Scholar]
- 82.Arntz OJ, Pieters BC, Oliveira MC et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Molecular nutrition & food research, 59(9), 1701–1712 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Mahidhara G, Kanwar RK, Roy K, Kanwar JR. Oral administration of iron-saturated bovine lactoferrin-loaded ceramic nanocapsules for breast cancer therapy and influence on iron and calcium metabolism. International journal of nanomedicine, 10, 4081–4098 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beuzelin D, Pitard B, Kaeffer B. Oral Delivery of miRNA With Lipidic Aminoglycoside Derivatives in the Breastfed Rat. Frontiers in physiology, 10, 1037 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zou D, Ganugula R, Arora M, Nabity MB, Sheikh-Hamad D, Kumar M. Oral delivery of nanoparticle urolithin A normalizes cellular stress and improves survival in mouse model of cisplatin-induced AKI. American journal of physiology. Renal physiology, 317(5), F1255–f1264 (2019). [DOI] [PubMed] [Google Scholar]