Abstract
The tumor microenvironment encompasses a complex cellular network that includes cancer-associated fibroblasts, inflammatory cells, neo-vessels, and an extracellular matrix enriched in angiogenic growth factors. Decorin is one of the main components of the tumor stroma, but it is not expressed by cancer cells. Lack of this proteoglycan correlates with down-regulation of E-cadherin and induction of β-catenin signaling. In this study, we investigated the role of a decorin-deficient tumor microenvironment in colon carcinoma progression and metastasis. We utilized an established model of colitis-associated cancer by administering Azoxymethane/Dextran sodium sulfate to adult wild-type and Dcn−/− mice. We discovered that after 12 weeks, all the animals developed intestinal tumors independently of their genotype. However, the number of intestinal neoplasms was significantly higher in the Dcn−/− microenvironment vis-à-vis wild-type mice. Mechanistically, we found that under unchallenged basal conditions, the intestinal epithelium of the Dcn−/− mice showed a significant increase in the protein levels of epithelial-mesenchymal transition associated factors including Snail, Slug, Twist, and MMP2. In comparison, in the colitis-associated cancer evoked in the Dcn−/− mice, we found that intercellular adhesion molecule 1 (ICAM-1) was also significantly increased, in parallel with epithelial-mesenchymal transition signaling pathway-related factors. Furthermore, a combined Celecoxib/decorin treatment revealed a promising therapeutic efficacy in treating human colorectal cancer cells, in decorin-deficient animals. Collectively, our results shed light on colorectal cancer progression and provide a protein-based therapy, i.e., treatment using recombinant decorin, to target the tumor microenvironment.
Keywords: Decorin, tumor microenvironment, EMT, AOM/DS, colorectal cancer, Celecoxib
Introduction
Colorectal Cancer (CRC) is the third most common cancer diagnosed in both men and women in the United States and the third leading cause of cancer-related deaths. The American Cancer Society estimates 100,000 total new colon cancer cases in the United States in 2020 [1]. CRC originates from benign tumors called adenomatous polyps that form on the inner walls of the large intestine. Over time, multiple mutations of critical genes associated with proliferation, tumor suppression, and stem cell maintenance cause a switch to a malignant phenotype, which eventually may extravasate and disseminate to other distal organs. CRC diagnosis often occurs at late stages when cancer cells have already disseminated, and it is often associated with poor survival. Current therapies for CRC remain predominantly focused on directly targeting the tumor epithelial compartment. However, the ability of tumor epithelial cells to survive and proliferate is heavily determined by the complex, reciprocal interactions with the surrounding stromal cells.
The tumor microenvironment plays an important role in tumorigenesis, tumor progression, and drug resistance and may be a target for CRC therapy [2]. As a complex mixture of mesenchymal cells, secreted factors, and extracellular matrix (ECM) constituents, the tumor microenvironment profoundly influences tumor growth and ultimately dictates whether the primary tumor metastasizes, become dormant, or is eradicated [3–5]. The ECM is a non-cellular component that is aberrantly regulated in many types of tumor microenvironments [5]. Since the ECM generally maintains tissue structure and provides mechanical forces in the tumor microenvironment, it has been assumed to act as a physical barrier for the spreading of cancer metastasis while playing a passive role in cancer progression [6]. However, a substantial body of evidence has suggested that ECM remodeling can directly influence many aspects of cancer cell behavior, and its functions and composition have attracted attention in colorectal cancer biology as a new therapeutic strategy. Proteoglycans are heavily glycosylated proteins, major components of the ECM, with various biological functions [7–11]. A major role in affecting tumor cell behavior is played by decorin, a member of the small leucine-rich proteoglycan (SLRP) family with a chondroitin/dermatan sulfate glycosaminoglycan chain covalently attached to a serine residue on the core protein [7,12,13]. Decorin is encoded by a large gene with alternatively-spliced variants in the 5’-untranslated region [14,15] and a complex regulatory system [16,17]. Decorin proteoglycan primarily synthesized by fibroblasts and myofibroblasts, regulates collagen fibrillogenesis both in vitro and in vivo, and is involved in a number of physiological and pathological processes including control of osteogenic stem cells and muscular development, wound healing, myocardial infarction, Lyme disease, tubulo-interstitial fibrosis and diabetic nephropathy, muscular dystrophy, and cancer growth [5,18–38]. The involvement of decorin in cancer development has been shown in decorin knockout mice. Targeted inactivation of the decorin gene in mice exposed to a Western-style high-risk diet is sufficient to cause intestinal tumorigenesis associated with downregulation of p21Waf1, p27Kip1, ITF/Muc2, and E-cadherin with concurrent upregulation of β-catenin signaling in the intestinal epithelial cells [39–41]. Moreover, double knockout mice deficient in both decorin and p53, a critical tumor-suppressor gene, spontaneously and uniformly develop thymic lymphomas from which they succumb within six months [42]. Notably, de novo expression of decorin in human colon carcinoma cells leads to an arrest in the G1 phase, reduced colony formation, and the inability to generate xenografts in SCID mice [43].
Decorin is a potent tumor suppressor proteoglycan, via direct interactions with multiple growth factors, cytokines, and receptor tyrosine kinases (RTK) expressed on the cell surface of cancer cells, that ultimately inhibit tumor growth, proliferation, angiogenesis, migration, and metastasis, while simultaneously promoting tumor cell apoptosis, autophagy in stromal cells, and mitophagy in tumor cells [44–50]. The intricate signaling cascades evoked by decorin are cell type-specific. On tumor cells, decorin causes growth arrest by inducing expression of the cyclin-dependent kinase inhibitors, p21Waf1 [51,52] and p27Kip1 [53]. This action depends on the direct binding of decorin with the epidermal growth factor receptor (EGFR) and Met receptor with consequent downregulation of the oncogenes β-catenin and Myc [54–58]. More recently, a new role of decorin in endothelial cells has been discovered and has opened new avenues of research. Decorin selectively inhibits the PI3K/AKT/mTOR pathway with concurrent activation of pro-autophagic AMPK-mediated signaling cascades. This leads to induction of autophagy in vascular endothelial cells and suppression of blood vessel growth [46,59]. The dual activity of decorin on cancer and stromal cells highlights the versatility of this soluble molecule as a potential adjunct therapeutic that targets different signaling pathways to inhibit cancer growth and spreading. In this context, the fall of E-cadherin levels in Dcn−/− mice suggests a potential link for decorin in regulating epithelialto-mesenchymal transition (EMT), a highly dynamic process by which epithelial cells transition to a mesenchymal phenotype, often associated with tumorigenesis, metastasis, and drug resistance [60]. Of note, EMT generates tumor cells with stem cell properties, and cells at multiple transitional states that express mixed epithelial and mesenchymal genes [61], that cumulatively play a major role in resistance to cancer treatment. Targeting proteins that regulate this oncogenic transition in the tumor microenvironment represents a new direction for the treatment of colorectal cancer [62]. Previously, we discovered that a genetic background constitutively lacking decorin facilitates intestinal tumor formation through disruption of intestinal cell maturation [63]. Further mechanistic studies found a direct interaction between decorin and E-cadherin, where overexpression of decorin can significantly increase the stability of E-cadherin [40]. In this study, we explored the susceptibility of mice to azoxymethane/dextran sodium sulfate (AOM/DSS) carcinogen-induced colorectal cancer formation and metastasis in a decorin deficient microenvironment [64]. We show that loss of decorin promotes EMT and increases the number of chemically induced colorectal neoplasms. Moreover, we found that abnormally high levels of pro-inflammatory COX2 and PGE2 in the intestinal epithelium of Dcn−/− mice favor EMT. These results indicate that recombinant decorin could be used as a novel protein-based agent to combat colorectal cancer, either alone or in combination with established chemotherapeutic agents.
Results
Decorin deficient microenvironment promotes epithelial-mesenchymal transition in experimental colon carcinogenesis
To investigate the role of decorin in colon carcinogenesis, we used an established chemically-induced mouse model of colon carcinogenesis. In this model, the combination of a single intraperitoneal injection of AOM, followed by subsequent exposure to the pro-inflammatory agent DSS dramatically shortens the latency time for induction of CRC [64]. This mouse model recapitulates the neoplastic transformation that occurs in human CRC characterized by a sequential formation of carcinoma in situ-adenoma-carcinoma [64]. A cohort of Dcn+/+ and Dcn−/− mice were given a single intraperitoneal injection of AOM (10 mg/kg) and then exposed to DSS (2% in drinking water). We alternated three cycles of DSS treatments followed by recovery with regular drinking water as reported before [65] (Fig. 1A). At 12 weeks, the mice were euthanized, and colon tumors were collected for analysis. We discovered that the number of colonic neoplasms was significantly higher in the decorin-null mice (Fig. 1B), although the incidence of tumor development did not appreciably vary between the two genetic backgrounds. Moreover, we found that repeat cycles of DSS treatment evoked more weight loss in the Dcn−/− mice (Fig. 1C).
Fig. 1.

Decorin deficient microenvironment promotes colitis-associated cancer and EMT in the intestinal epithelium. (A) Schematic timeline of the AOM/DSS treatment for the induction of colitis-associated cancer. (B) Twelve weeks after treatment Dcn−/− mice showed a significantly larger number of intestinal carcinomas compared to Dcn+/+ animals, and (C) significant weight loss. (D) Relative mRNA levels of Cox2 and Pge2 in the intestinal tissue of wild-type and Dcn−/− mice. (E-F) The intestinal epithelium of untreated Dcn−/− and Dcn+/+ mice was interrogated for the expression of factors associated with EMT. Dcn−/− mice show a significant increase of the protein levels of Slug and Twist1 and a reduction of E-cadherin when compared to Dcn+/+ mice. All experiments were performed in triplicate. Quantification of the data was expressed as mean ±S.D. *p< 0.05.
Next, we tested the mRNA levels of cyclooxygenase-2 COX2 (Ptgs2) and prostaglandin E2 (Pge2). The rationale for selecting COX2 is based on the fact COX2, an enzyme that catalyzes the rate-limiting step of prostaglandin biosynthesis, is induced in both inflammation and cancer [66,67]. We found a marked increase in the mRNA levels of both Ptgs2 and Pge2 in the neoplastic colonic epithelium of the Dcn−/− mice vis-à-vis wild-type mice (Fig 1D). As COX2 enhances EMT via a Pge2-dependent mechanism [68], we determined the levels of various EMT-associated proteins in the neoplastic colonic epithelium. We discovered that the neoplastic epithelium of the Dcn−/− mice showed a marked decrease in the epithelial marker E-cadherin (Fig. 1 E,F). In contrast, several mesenchymal markers (N-cadherin, Vimentin, α-SMA) and EMT-related markers (Slug and Twist) were up-regulated (Fig 1E–F). Collectively, our findings indicate that a decorin-deficient microenvironment, together with the increased expression of Cox2 and Pge2, favor a pro-tumorigenic milieu by evoking EMT in colon cancer cells.
Decorin deficient microenvironment favors colon cancer progression
Next, we investigated the influence of a decorin-deficient microenvironment on colon carcinoma metastasis using the intestinal epithelium of Dcn−/− mice. To this end, we compared various biomarkers, mRNAs and proteins, at two different time points, 12 and 21 weeks following induction of colitis-associated cancer. We discovered that the mRNA levels of Ptgs2 and Pge2 were significantly elevated at 21 weeks post-induction (p<0.001, Fig. 2A).
Fig. 2.
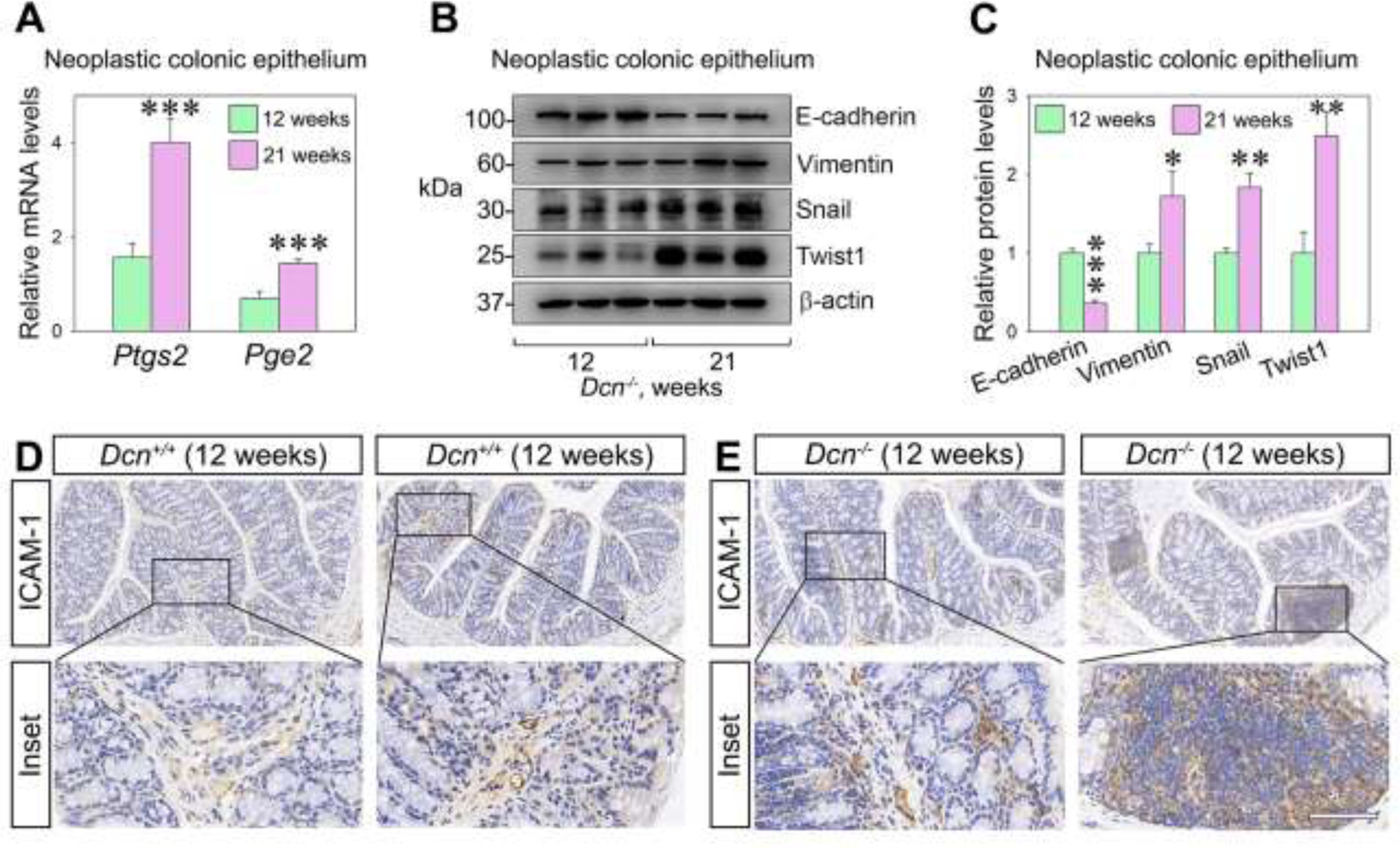
Decorin deficiency favors a pro-metastatic environment in the intestinal epithelium of mice treated with AOM/DSS. (A) Ptgs2 and Pge2 expression in neoplastic colonic epithelium at 12 and 21 weeks post AOM/DSS induction. (B) Representative Western blots showing the expression of metastatic markers in the intestinal epithelial tissue of Dcn−/− mice 12- and 21-weeks post AOM/DSS induction. (C) Quantification of E-cadherin, vimentin, Snail, and Twist 1 in the colonic epithelium of Dcn−/− mice treated with AOM/DSS. (D-E) Representative immune-histochemical analysis depicting ICAM-1 expression in the intestinal epithelium of Dcn−/− and Dcn+/+ mice, 12- and 21-weeks post AOM/DSS induction. Bar, 100 μm. Experiments were performed in triplicate. Data are expressed as mean ±S.D. *p<0.05, **p<0.01, **p<0.001.
We further found that all the protein levels of canonical biomarkers for EMT were markedly accentuated in the 21-week Dcn−/− mice, with a decrease in E-cadherin and a relative increase in vimentin, Snail, and Twist1 (Fig. 2B,C). Next, we investigated the expression of ICAM-1, an intercellular adhesion molecule that mediates cell-to-cell or cell-to-matrix binding, and is known to promote tumor cell proliferation and invasion [69]. We found higher ICAM-1 expression in paraneoplastic tissue from Dcn−/− mice as compared to Dcn+/+ mice, especially after 21 weeks post-induction of the colitis-associated cancer (Fig. 2D,E). Collectively, these findings suggest that: (I) Ptgs2 and Pge2 might be involved in EMT within the tumor microenvironment as well as in the progression of colon cancer, and (II) a decorin-deficient microenvironment is permissive for colon tumorigenesis.
Combination of decorin and Celecoxib inhibits migration and colony formation of colorectal cancer cells
We utilized a combined treatment of decorin and Celecoxib, a non-steroidal anti-inflammatory drug that specifically inhibits COX2 and is commonly used to relieve pain and swelling in a variety of inflammatory conditions [70]. To determine the oncosuppressive function of decorin alone or in combination with Celecoxib, we treated SW480 and LoVo human colorectal cancer cell lines. We found that the proliferation of both SW80 and LoVo was significantly decreased when cells were incubated with increasing concentrations of decorin or Celecoxib and prolonged treatment (up to 72 hours) (Fig. 3A,B). Next, we determined the effects of combining decorin (25 nM) and Celecoxib (40 μM) treatment on cell viability of SW80 colorectal cancer cells. We discovered that the combined treatment was more effective in inhibiting cell growth when compared to individual treatments (Fig. 4A). Moreover, the combination of the two drugs resulted in a significant decrease in colony formation vis-a-vis single incubation with decorin or Celecoxib (Fig. 4B,C). We then questioned if the migration ability of SW480 was impacted by decorin, Celecoxib, or both in a wound-healing assay. The results showed that either recombinant decorin or Celecoxib alone could inhibit the scratch healing ability of SW480 cells. However, combined treatment showed the most significant inhibitory effect on SW480 cell wound healing ability, suggesting a more powerful synergistic effect of the drug combo in inhibiting the migration capability of SW480 cells (p<0.001, Fig. 4D). We then assayed E-cadherin and Snail protein levels and found a marked increase of E-cadherin and with a concurrent decrease of Snail, when cells were treated simultaneously with decorin and Celecoxib (Fig. 4E,F). It is generally accepted that E-cadherin is a Snail target gene that binds to the E-box sequence in the E-cadherin promoter, thereby inhibiting E-cadherin transcription [60]. This ultimately reduces intercellular adhesion prompting invasion and metastasis [40]. In light of this, our findings suggest that the combination of recombinant decorin and Celecoxib could partially inhibit EMT by directly or indirectly inactivating Snail, inducing E-cadherin expression, and decreasing proliferation and migration.
Fig. 3.
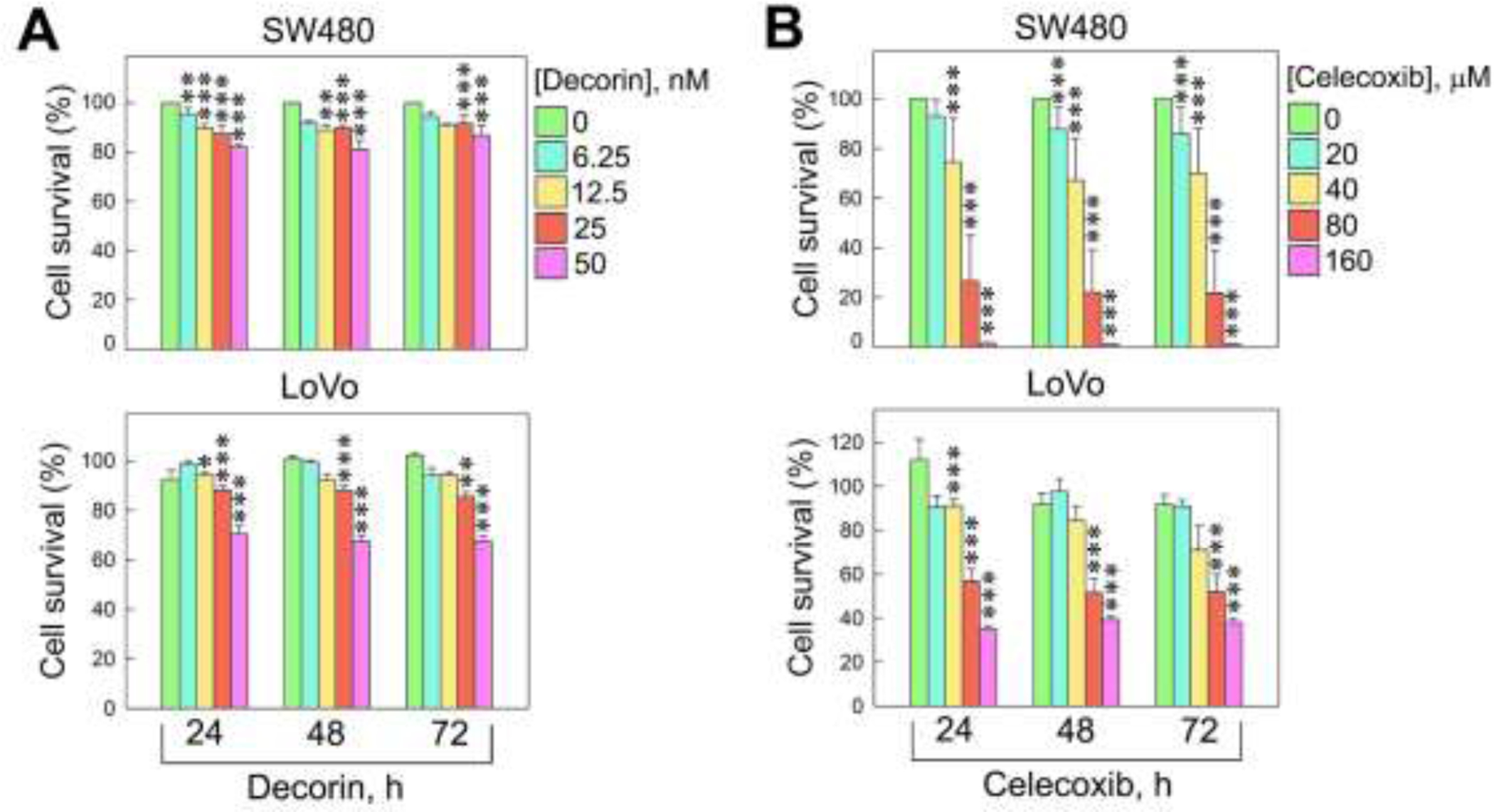
Decorin and Celecoxib inhibit cell proliferation. (A) MTT assay analyzing the effects of various decorin concentrations at 3 time points on the proliferation of SW480 human colorectal adenocarcinoma, and LoVo human metastatic colorectal adenocarcinoma cells. (B) MTT assay of the same cell types treated with Celecoxib at different concentrations over time. These experiments were performed in triplicate. Data are expressed as mean ±S.D. *p<0.05, **p<0.01, **p<0.001
Fig. 4.
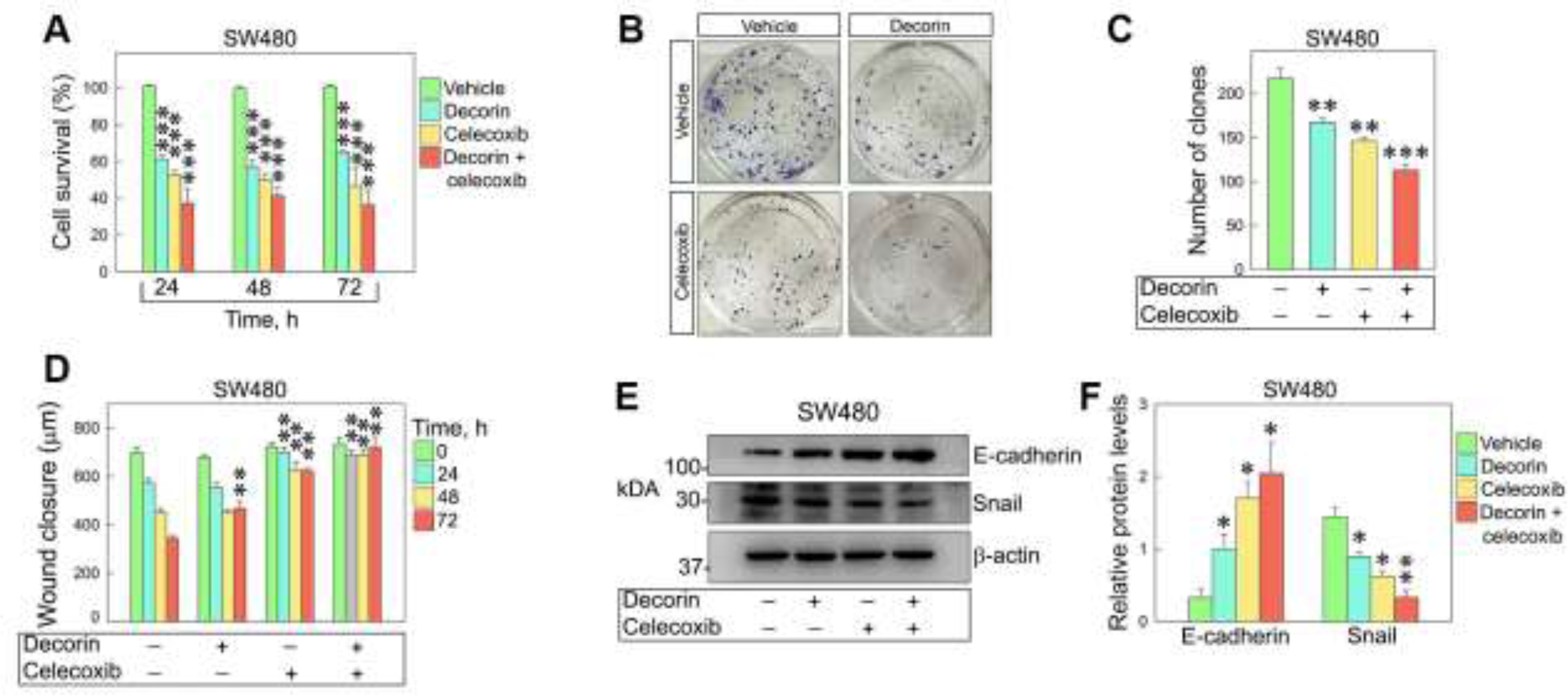
Combined treatment with decorin and Celecoxib inhibits migration and colony formation of colorectal cancer cells. (A) Cell growth inhibition of SW480 treated at three time points with either 25 nM decorin, 40 μM Celecoxib, or both. (B-C) Colony formation assay and relative quantification showing a marked inhibition in the number of colonies after treatment with either decorin, Celecoxib, or both. (D) Quantification of wound-healing assays where SW480 cells were stimulated with decorin, Celecoxib, or both for up to 72 hours. (E-F). Western blot and relative quantification showing the protein levels of E-cadherin and Snail in SW480 cells treated as described. These experiments were performed in triplicate. Data are expressed as mean ±S.D. *p<0.05, **p<0.01, **p<0.001
Decorin and Celecoxib synergistically inhibit epithelial-mesenchymal transition and proliferation of colorectal cancer in mice
We further investigated the therapeutic effect of decorin with or without Celecoxib on colorectal tumorigenesis in vivo. To this end, six-week-old Dcn−/− mice underwent AOM/DSS induction to develop colorectal cancer. The animals were randomized and divided into four treatment groups: vehicle, decorin, Celecoxib, and combined treatment. Celecoxib (20 mg/kg) and decorin (5 mg/kg) were systematically administered starting at week 9 post AOM/DSS induction and lasted for three weeks. At week 12, mice were dissected, and the number of the intestinal tumors quantified. We found that mice treated with either recombinant decorin or Celecoxib developed a smaller number of tumors, ~40% less as compared to vehicle-treated mice (Fig. 5A). Impressively, the number of tumors in mice treated with a combination of decorin and Celecoxib was reduced by ~70% when compared to the control group (Fig. 5A). At the transcriptional level, the relative mRNA levels of Ptgs2 and Pge2 were down-regulated in tumors treated with decorin or Celecoxib. However, tumors treated with the combined therapy showed the most significant transcriptional reduction of Ptgs2 and Pge2 (Fig. 5B).
Fig. 5.
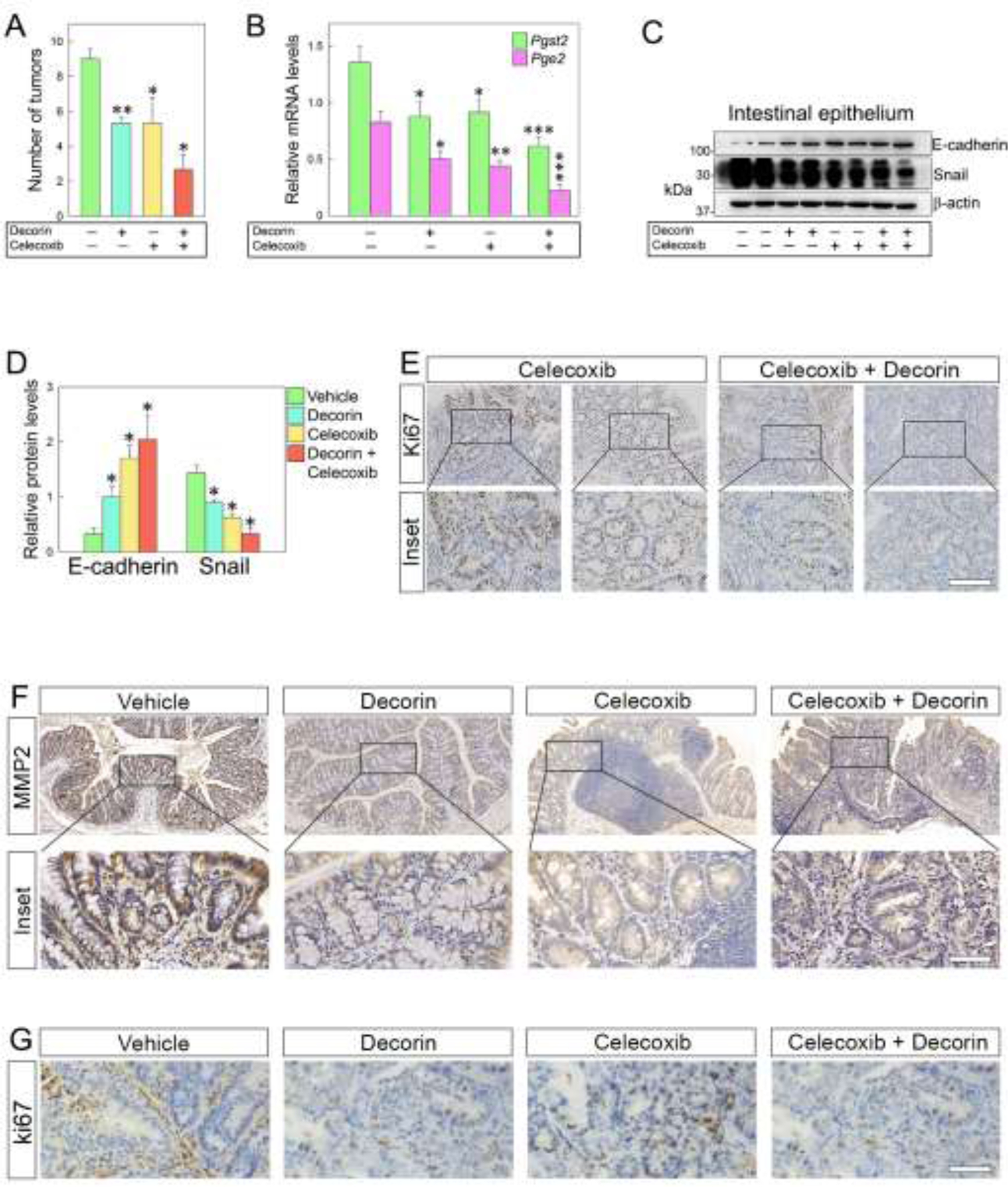
Combined treatment with decorin and Celecoxib significantly inhibits epithelial mesenchymal transition and proliferation of colorectal cancer cells in Dcn−/− mice. (A) Tumor numbers of mice bearing tumors and treated with decorin, Celecoxib, or both. Mice treated with both therapeutic agents show a ~70% reduction in the number of tumors compared to the vehicle-treated group. (B) Relative mRNA levels of Ptgs2 and Pge2 in the neoplastic intestinal epithelium following treatment with decorin and Celecoxib. (C) Western blots showing E-cadherin and Snail levels in intestinal epithelial cells after treatment with decorin, Celecoxib, or both, and (D) relative quantification. (E) Immuno-histochemical detection of the proliferative marker Ki67 in Dcn−/− mice at 12 weeks after AOM/DSS induction. (F-G) Immuno-histochemical detection of MMP2 and Ki67 respectively in the mouse intestinal epithelium following combined treatment with decorin and Celecoxib. The experiments were performed in triplicate. Data are expressed as mean ±S.D. *p< 0.05, **p0.01, **p< 0.001.
We next analyzed the protein levels of E-cadherin and Snail in the intestinal epithelial cells of the treated mice. In line with our previous findings, the protein levels of E-cadherin were significantly up-regulated. In contrast, Snail was markedly down-regulated in the intestinal epithelium of mice treated with the combined treatment (Fig. 5C,D). Moreover, Ki67, an important proliferation marker, was also significantly down-regulated in mice treated with both decorin and Celecoxib (Fig 5E).
It is well established that low expression of matrix metalloproteinases can reduce the turnover of ECM constituents, further preventing the occurrence of EMT and generating negative feedback during the malignant transformation of cells [71]. We, therefore, performed immune-histochemical analyses to investigate the association between matrix metalloproteinase 2 (MMP2) and EMT in the decorin-deficient microenvironment. Treatment with decorin plus Celecoxib significantly decreased the levels of MMP2 compared to vehicle-treated tissue (Fig 5F) as well as the levels of Ki67 (Fig. 5G). Thus, the current data indicate that the synergistic effects of decorin and Celecoxib could retard EMT by downregulating MMP2, providing a novel, protein-based treatment for colorectal cancer.
Discussion
Decorin, a critical component of the tumor microenvironment, has become considerably attractive in translational medicine primarily because of its well-established anti-oncogenic and anti-metastatic activity and lack of cytotoxicity [12,44,48,72–74]. A large number of in vitro and in vivo studies have confirmed that decorin functions as a tumor repressor via interactions with multiple growth factors, cytokines, and cell surface receptors [20]. Initial evidence suggesting an oncosuppressive role for decorin came from a preliminary analysis of decorin knockout mice [63]. Genetic deletion of decorin in mice induces the spontaneous formation of colorectal tumors [63]. Further studies established a direct interaction between decorin and E-cadherin in colon cancer, where over-expressing decorin significantly increased the stability of E-cadherin [40]. Moreover, decorin binds to Activin C in colorectal cancer, leading to degradation of this oncogene [75]. In a colorectal tumor model, intratumoral injection of an oncolytic adenovirus encoding decorin inhibits tumor growth and metastatic spreading to the lungs by downregulation of both angiogenesis and EMT markers in the tumors [76].
In this study, we examined the mechanism of decorin anti-oncogenic activity in the microenvironment of colorectal cancer utilizing the AOM/DSS carcinogen-induced colorectal cancer model [64] where endogenous decorin was genetically ablated. Our results suggest that loss of decorin in the tumor microenvironment promotes carcinogenesis of intestinal epithelium following induction of a colitis-associated tumorigenesis protocol. These results are closely linked and support the notion that lack of decorin in a p53-deficient background promotes lymphomagenesis [42]. In this case, mice double null for the tumor suppressor gene p53 and decorin showed a faster rate of tumor development and succumbed almost uniformly to thymic lymphomas within six months, with a mean survival age of four months [42].
Mechanistically, the intestinal epithelium of decorin null mice displays in basal, unchallenged conditions, a more mesenchymal-like phenotype characterized by the expression of various EMT-associated markers. This pro-tumorigenic phenotype explains why decorin null mice, when treated with AOM/DSS, develop a more significant number of intestinal carcinomas vis-à-vis wild-type animals. Therapeutically, synergistic administration of recombinant decorin with the potent COX2 inhibitor, Celecoxib, decreases the number of tumors in vivo and downregulates the expression of different cancer hallmarks associated with invasion and metastatic spreading of colorectal cancer cells both in vitro and in vivo. In the tumor microenvironment, malignant cells interact with inflammatory and stromal cells to maintain conditions germane for tumor growth and metastatic spreading [71]. One of the novel findings of our in vivo studies is that a genetic background lacking decorin in the tumor microenvironment favors epithelial-mesenchymal transition, a condition that contributes to tissue repair, but also promotes carcinogenesis through a variety of mechanisms [60]. EMT endows cells with pro-migratory and invasive properties, evokes stem cell phenotypes, prevents apoptosis and senescence, and contributes to immunosuppression and drug resistance [77–79]. The EMT-induced acquisition of cell motility and contractile proprieties of epithelial cells allows the development of distant metastases, which are ultimately the leading cause of death in colorectal cancer patients. This cell differentiation and behavioral shift is regulated by a set of key transcription factors, loss of the adhesion molecule E-cadherin, and concomittant up-regulation of N-cadherin, a mesenchymal cell marker. Additionally, Snail, Slug, and Twist 1 are other factors linked to EMT whose expression is regulated transcriptionally in cancer metastases [80]. Indeed, a recent study has identified a group of fifty-one gene pairs related to EMT that could reliably predict a risk of relapse in patients with stage II colorectal cancers [81].
It is well established that the conversion of epithelial cells to mesenchymal cells encompasses deep phenotypic changes that include loss of cell-cell adhesion molecules and the concurrent loss of cell polarity and the acquisition of pro-migratory properties [60]. Specifically, a reduction of E-cadherin and an increase in N-cadherin expression are defining characteristics of EMT. In this study, we found that the protein levels of the epithelial marker E-cadherin were significantly down-regulated. In contrast, mesenchymal markers (N-cadherin, Vimentin, α-SMA), EMT-related proteins (Snail, Slug, Twist), and ICAM-1 were markedly up-regulated in intestinal epithelial cells of Dcn−/− mice. Importantly, this process occurs at early stages of tumor progression as we observed it at 12 weeks post AOM/DSS induction. This newly described function of decorin in regulating the tumor microenvironment, clearly corroborates a critical oncosuppressive role for this proteoglycan that can also directly target tumor cells via binding to several RTKs to inhibit cancer growth [82,83]. Moreover, overexpression of decorin enhances survival in dystrophic epidermolysis bullosa, through inhibition of transforming growth factor beta 1 (TGFβ1) signaling [84], further enhancing the therapeutic effects that decorin or other small leucine rich proteoglycans can have in counteracting cancer and other diseases [85]. The recent characterization of the matrisome [86] and delineation of the tumor matrisome from diverse histo-genetic backgrounds [87–89] has unraveled important proteomic signatures, provided a platform for novel therapies, and furthered our understanding of therapeutic resistance. Our data strongly support the hypothesis that the decorin deficient microenvironment favors EMT and eventually leads to colorectal cancer development and distant spreading.
Several preclinical, clinical, and epidemiological studies have shown a chemo-preventive potential for a class of drugs known as non-steroidal anti-inflammatory drugs (NSAIDs) [90]. NSAIDs, such as cyclooxygenase inhibitors, either alone or in combination with other therapies can reduce the risk of many types of malignancies including colon, lung, breast, and prostate cancer [91,92]. Celecoxib, a commonly used and specific COX2 inhibitor, has been reported to contrast the growth of colon cancer. Under hypoxic conditions, treatment with Celecoxib can prevent EMT, thus inhibiting colon cancer invasion and metastasis [93]. Compared to wild type mice, Dcn−/− mice showed a significant increase of Ptgs2 and Pge2 mRNAs at both 12- and 21-weeks post AOM/DSS induction, with a dramatic increase at the later time point. Furthermore, we investigated the therapeutic effects of recombinant decorin and Celecoxib individually or in combination to inhibit tumor growth in our in vivo mouse model. The findings were striking as the invasion and metastasis of colorectal cancer in Dcn−/− mice could be dramatically inhibited by the combined use of decorin and Celecoxib. Previous studies have shown that Celecoxib can enhance the anti-cancer effect of cisplatin compounds and induce anoikis through PI3K/Akt signaling in osteosarcoma [92]. Moreover, decorin inhibits this pathway in endothelial cells via direct binding to vascular endothelial growth factor receptor 2 (VEGFR2) [59]. More effective therapeutic combinations such as multiple cytotoxic agents and targeted therapies have increased the overall survival of patients with metastatic colorectal cancer [94]. The current study sheds light on the potential use of both decorin and Celecoxib in treating colorectal cancer invasion and metastasis.
In conclusion, we investigated the role of a decorin deficient microenvironment in colorectal cancer invasion and metastasis in vitro and in vivo. The results suggest that the decorin deficient microenvironment favors metastasis of colorectal cancer, which is associated with induction of EMT conducive for driving a pro-tumorigenic phenotype. In light of this, effective administration of recombinant decorin core in combination with a well-tolerated NSAID, such as Celecoxib, could serve as a potential adjuvant protein therapy in the fight against colorectal cancer.
Materials and Methods
Chemicals and reagents
Azoxymethane (AOM) (Cat# A5486) was purchased from Sigma-Aldrich Inc., USA. Dextran sulfate sodium (DSS, Cat#160110) was obtained from MP Inc., USA. Human recombinant Decorin (Cat# 10189-H08H) was purchased from Sino Biological Inc., China and reconstituted in PBS according to manufacturer’s instruction and used at final concentrations of 0, 250, 500, 1000 and 2000 ng/ml. Celecoxib (Cat# S1261) was purchased from Selleckchemo (Houston, TX, USA). For in vitro analysis, Celecoxib was dissolved in 0.1% DMSO to make a 100 mM stock solution and was then diluted with culture medium at final concentrations of 0, 20, 40, 80 and 160 μM before treatment. For in vivo analysis, Celecoxib was dissolved in mixture solution contain 0.8 % DMSO, 12 % PEG 300 and 2% Tween 80 to make 20 mg/kg stock solutions. The concentration of DMSO was less than 0.1% of drug treatment. (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was from Sigma–Aldrich, Inc., Ronkonkoma, NY. Primary antibodies used include E-cadherin (CST, Cat#3195), N-cadherin (CST, Cat#14215), vimentin (Abcam, Cat#ab92547), α-SMA (Abcam, Cat#ab5694), Snail (Sangon Biotech, Cat#D221239), Slug (Sangon Biotech, Cat#D 121235), MMP2 (Sangon Biotech, Cat#), Twist (Abcam, Cat#ab50887) and β-actin (Sangon Biotech, Cat#AB10024).
Animals
Dcn−/− and Dcn+/+ with an age of 6–8 weeks were selected for experiments after genotyping through genomic DNA extraction from tail tissue. Mice were housed in cages (three to five per cage) and allowed access to food and water ad libitum with room temperature of 22 ± 1°C and 50 ± 5% humidity under a 12 hour light–dark cycle (7:00 am – 7:00 pm). All the procedures were followed the National Institutes of Health regulations for the care and use of animals, and approved by Liaoning University of Traditional Chinese Medicine ethics committee.
Establishment of colitis-induced colorectal mice model
Each mouse (18–22 gm) was given a single intraperitoneal injection AOM of 10 mg/kg (mice body weight) and then went through three cycles of 2% DSS in drinking water for a week, following by two weeks of normal drinking water. Mice were divided into groups and sacrificed at 12, and 21 weeks following AOM administration. Number and size of tumors were calculated in the intestinal tract of mice. Tumor tissues and normal intestinal tissues were dissected and fixed with 4% paraformaldehyde (PFA) used for further histopathology and immunohistochemistry analysis. Intestinal epithelial cells were extracted from intestinal tissues using 15 mM EDTA buffer according to the quick perfusion method for the isolation of intact epithelium from mouse intestine described by Dr. Cheng [95]. Tumor and normal intestinal tissues were snap frozen in liquid nitrogen at the time of dissection for RNA and others analysis.
Cell cultures
Human colorectal cancer cell lines SW480 and LoVo cells were obtained from CHI Scientific, Inc., China and were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Thermo Fisher Scientific Inc., Waltham, MA) supplemented with 10% (v/v) fetal bovine serum (Dalian Meilun Biology, Meilun Bio, China) and antibiotics (10,000 U/ml penicillin, 10 mg/ml streptomycin) (Hyclone, Thermo Fisher Scientific Inc.,Waltham, MA). Cells were incubated at 37°C in 5% CO2 humidified atmosphere [96,97].
Western blot analysis
Whole cells were collected and lysed in RIPA buffer (50 mM Tris-HCl (pH 7.5), 0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate, 150 mM NaCl) with phenyl-methylsulphonyl fluoride (PMSF,1 mM) (Beijing Dingguo Changsheng Biotechnology Co., Ltd.). The supernatant was subjected to protein assay using the BCA protein assay kit (Thermo Fisher Scientific Co., Ltd, USA) after centrifugation at 14000 g/min for 15 min at 4°C. The amount of protein 20 ng per sample was resolved by SDS-PAGE using 10% gel and the protein bands were subsequently transferred onto nitrocellulose (NC) membranes (Sangon Biotech Co., Ltd.). The membrane was blocked with TBST buffer (20 mM Tris, 150 mM NaCl, 0.1% Tween 20 and adjust pH with HCl to pH 7.4–7.6) containing 5% BSA for 0.5 hour and then incubated with primary antibodies in blocking solution at room temperature for 3 hours or overnight at 4 °C. After washing with TBST buffer three times, the membranes were incubated with secondary antibodies tagged with Horseradish Peroxidase (HRP) for 2 hours at room temperature. Then the bands were detected by a detection system (Amersham Life Science, USA) with enhanced chemiluminescence (ECL) reagent.
RNA extraction and qRT-PCR
Total RNA isolated from intestinal tissues with TRIzol Reagent Kit (ComWin Biotech Co., Ltd., Beijing, China) was subjected to quantitative reverse transcription-PCR. One μg of RNA was reverse transcribed using the PrimeScript™ RT reagent Kit with gDNA Eraser (Takara Bio Inc., Ltd., Japan). For mRNA analyses, resulting cDNAs were analyzed in triplicate SYBR® Premix Ex Taq™ II (Takara Bio Inc., Ltd., Japan) on Applied Biosystems 7500 Real-Time PCR system. Relative mRNA expression level was calculated by 2−△(Ct-Cc) where Ct and Cc are the mean threshold cycle differences after normalizing to β-actin values. Primers used for quantitative PCR (qRT-PCR) are as follows: mCOX2 5’-TGAGCAACTATTCCAAACCAG-3’, 3’-GCACGTAGTCTTCGATCACTATC-5’; mPGE2 5’-ACTTCCACTCCCTGCCCTAT-3’, 3’-GTTGCAAGCTGTCTCCTTCC-5’
Immunohistochemistry
For histological analysis, paraffin sections of intestinal para-carcinoma tissue were deparaffinized with xylene and hydrated with a series of graded alcohol washes essentially as described before [98,99] Antigen retrieval was accomplished using a pressure cooker in 10 mM citrate buffer followed by washing with TBST (pH7.6). Sections were incubated in a 3% hydrogen peroxide solution at room temperature for 20 min in the dark followed by washing with PBS and then blocking in 3% BSA for 30 min. Primary antibodies were incubated overnight at 4°C. Biotinylated secondary antibody was incubated at room temperature for 1 hour. Samples were developed with diaminobenzidine (DAB) and samples counterstained with hematoxylin and mounted with Neutral balsam.
Cell growth analysis (MTT assay)
The growth status of SW480 and LoVo cells after treatment with recombinant decorin or with Celecoxib 24, 48 and 72 hours later was determined using a modified MTT assays, as described before [100]. Briefly, following treatment, the medium of the wells was removed, and cells were incubated with MTT solution (0.25 mg/ml) for 4h at 37°C, then the liquid was aspirated and crystal in the bottom of wells was solved with 100 μl of DMSO for 10 minutes. The cell viability was calculated according to the absorbance using a microplate reader.
Scratch-wound migration and colony formation assays
To investigate colon cancer cell migration, a single scratch wound was created using a 200μl sterile pipette tip in 70% confluence SW480 cells before treatment with/without recombinant Decorin or/and Celecoxib. Cells were washed with PBS and refreshed with medium and incubated at 37°C for 24, 48 and 72 hours, respectively. The initial gap length (0 hour) and the residual gap length (24, 48, 72 hours) after the scratch were calculated using photomicrographs.
For the colony formation assay, SW480 cells were seeded in 12-well plates with density 100 cells/well after treatment with recombinant decorin alone or with Celecoxib 24 hours, and continue to grow for 2 more weeks. After 14 days, colonies were washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 30 min, and stained with 0.5% Giemsa. The number of colonies was counted under an inverted microscope (≥ 50 cells as one clone).
Statistical analysis
Data were expressed as mean ± SEM, and p < 0.05 was considered as statistically significant. All experiments were performed at least in triplicates and statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA).
Highlights.
We have established a chemically-induced colon carcinogenesis in control and decorin null mice
The number of intestinal neoplasms is markedly increased in the decorin-null mice
The decorin-deficient background favors epithelial-mesenchymal transition and metastasis
Combined treatment with Celecoxib and decorin is a promising therapy for colorectal cancer
Acknowledgments
We thank Thomas Neill for critically reading of the manuscript. This study was funded by National Nature Science foundation of China (Grants#: 81472821) LiaoNing Revitalization Talents Program (#XLYC 1807058), in part by National Institutes of Health grant CA39481 (to RVI), and partially supported by Key Grant of Education department of Liaoning Province (LZD201904).
Abbreviations used:
- AOM/DSS
Azoxymethane /Dextran sodium sulfate
- EMT
epithelial-mesenchymal transition
- ICAM-1
intercellular adhesion molecule 1, also known as CD54
- CRC
colorectal cancer
- COX2
cyclooxygenase 2
- PGE2
prostaglandin E2
- TGFβ1
transforming growth factor beta 1
- VEGFR2
vascular endothelial growth factor receptor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no conflict of interest
References
- [1].Nyman MC, Sainio AO, Pennanen MM, Lund RJ, Vuorikoski S, Sundström JT, Järveläinen HT, Decorin in human colon cancer: Localization in vivo and effect on cancer cell behavior in vitro, J. Histochem. Cytochem 63 (2015) 710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Goldoni S, Iozzo RV, Tumor microenvironment: Modulation by decorin and related molecules harboring leucine-rich tandem motifs, Int. J. Cancer 123 (2008) 2473–2479. [DOI] [PubMed] [Google Scholar]
- [3].Iozzo RV, Cohen I, Altered proteoglycan gene expression and the tumor stroma, Experientia 49 (1993) 447–455. [DOI] [PubMed] [Google Scholar]
- [4].Iozzo RV, Proteoglycans and neoplasia, Cancer Metastasis Rev 7 (1988) 39–50. [DOI] [PubMed] [Google Scholar]
- [5].Iozzo RV, Gubbiotti MA, Extracellular matrix: The driving force of mammalian diseases, Matrix Biol 71–72 (2018) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK, Extracellular matrix structure, Adv. Drug Deliv. Rev 97 (2016) 4–27. [DOI] [PubMed] [Google Scholar]
- [7].Iozzo RV, Schaefer L, Proteoglycan form and function: A comprehensive nomenclature of proteoglycans, Matrix Biol 42 (2015) 11–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Iozzo RV, Karamanos N, Proteoglycans in health and disease: emerging concepts and future directions, FEBS J 277 (2010) 3863. [DOI] [PubMed] [Google Scholar]
- [9].Ferdous Z, Wei VM, Iozzo RV, Höök M, Grande-Allen KJ, Decorin-transforming growth factor-ß interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices, J. Biol. Chem 282 (2007) 35887–35898. [DOI] [PubMed] [Google Scholar]
- [10].Weis SM, Zimmerman SD, Shah M, Covell JW, Omens JH, Ross J Jr., Dalton N, Jones Y, Reed CC, Iozzo RV, McCulloch AD, A role for decorin in the remodeling of myocardial infarction, Matrix Biol 24 (2005) 313–324. [DOI] [PubMed] [Google Scholar]
- [11].Suhovskih AV, Aidagulova SV, Kashuba VI, Grigorieva EV, Proteoglycans as potential microenvironmental biomarkers for colon cancer, Cell Tissue Res 361 (2015) 833–844. [DOI] [PubMed] [Google Scholar]
- [12].Neill T, Schaefer L, Iozzo RV, Decorin as a multivalent therapeutic agent against cancer, Adv. Drug Deliv. Rev 97 (2016) 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Goldoni S, Owens RT, McQuillan DJ, Shriver Z, Sasisekharan R, Birk DE, Campbell S, Iozzo RV, Biologically active decorin is a monomer in solution, J. Biol. Chem 279 (2004) 6606–6612. [DOI] [PubMed] [Google Scholar]
- [14].Danielson KG, Fazzio A, Cohen I, Cannizzaro LA, Eichstetter I, Iozzo RV, The human decorin gene: intron-exon organization, discovery of two alternatively spliced exons in the 5’ untranslated region, and mapping of the gene to chromosome 12q23, Genomics 15 (1993) 146–160. [DOI] [PubMed] [Google Scholar]
- [15].Iozzo RV, Matrix proteoglycans: from molecular design to cellular function, Annu. Rev. Biochem 67 (1998) 609–652. [DOI] [PubMed] [Google Scholar]
- [16].Iozzo RV, Danielson KG, Transcriptional and post-transcriptional control of proteoglycan gene expression, Progr. Nucl. Acids Res. Mol. Biol 62 (1999) 19–53. [DOI] [PubMed] [Google Scholar]
- [17].Mauviel A, Santra M, Chen YQ, Uitto J, Iozzo RV, Transcriptional regulation of decorin gene expression. Induction by quiescence and repression by tumor necrosis factor-α, J. Biol. Chem 270 (1995) 11692–11700. [DOI] [PubMed] [Google Scholar]
- [18].Schaefer L, Tredup C, Gubbiotti MA, Iozzo RV, Proteoglycan neofunctions: regulation of inflammation and autophagy in cancer biology, FEBS J 284 (2017) 10–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gubbiotti MA, Vallet SD, Ricard-Blum S, Iozzo RV, Decorin interacting network: A comprehensive analysis of decorin-binding partners and their versatile functions, Matrix Biol 55 (2016) 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Karamanos NK, Piperigkou Z, Theocharis AD, Watanabe H, Franchi M, Baud S, Brezillon S, Gotte M, Passi A, Vigetti D, Ricard-Blum S, Sanderson RD, Neill T, Iozzo RV, Proteoglycan Chemical Diversity Drives Multifunctional Cell Regulation and Therapeutics, Chem. Rev 118 (2018) 9152–9232. [DOI] [PubMed] [Google Scholar]
- [21].Iozzo RV, Sanderson RD, Proteoglycans in cancer biology, tumour microenvironment and angiogenesis, J. Cell. Mol. Med 15 (2011) 1013–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schaefer L, Macakova K, Raslik I, Micegova M, Gröne H-J, Schönherr E, Robenek H, Echtermeyer FG, Grässel S, Bruckner P, Schaefer RM, Iozzo RV, Kresse H, Absence of decorin adversely influences tubulointerstitial fibrosis of the obstructed kidney by enhanced apoptosis and increased inflammatory reaction, Am. J. Pathol 160 (2002) 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV, Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility, J. Cell Biol 136 (1997) 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Iozzo RV, Buraschi S, Genua M, Xu S-Q, Solomides CC, Peiper SC, Gomella LG, Owens RT, Morrione A, Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling, J. Biol. Chem 286 (2011) 34712–34721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schaefer L, Iozzo RV, Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction, J. Biol. Chem 283 (2008) 21305–21309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zoeller JJ, Pimtong W, Corby H, Goldoni S, Iozzo AE, Owens RT, Ho S-Y, Iozzo RV, A central role for decorin during vertebrate convergent extension, J. Biol. Chem 284 (2009) 11728–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fetting JL, Guay JA, Karolak MJ, Iozzo RV, Adams DC, Maridas DE, Brown AC, Oxburgh L, FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney, Development 141 (2014) 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schaefer L, Iozzo RV, Small leucine-rich proteoglycans, at the crossroad of cancer growth and inflammation, Curr. Opin. Genet. Dev 22 (2012) 56–57. [DOI] [PubMed] [Google Scholar]
- [29].Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ, Decorin expression is important for age-related changes in tendon structure and mechanical properties, Matrix Biol 32 (2013) 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhão JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, Schaefer L, Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and microRNA-21, Sci. Signal 4 (2011) ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moreth K, Iozzo RV, Schaefer L, Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation, Cell Cycle 11 (2012) 2084–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Theocharis AD, Skandalis SS, Neill T, Multhaupt HA, Hubo M, Frey H, Gopal S, Gomes A, Afratis N, Lim HC, Couchman JR, Filmus J, Ralph DS, Schaefer L, Iozzo RV, Karamanos NK, Insights into the key roles of proteoglycans in breast cancer biology and translational medicine, Biochim. Biophys. Acta 1855 (2015) 276–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Baghy K, Iozzo RV, Kovalszky I, Decorin-TGFβ axis in hepatic fibrosis and cirrhosis, J. Histochem. Cytochem 60 (2012) 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Baghy K, Horváth Z, Regõs E, Kiss K, Schaff Z, Iozzo RV, Kovalszky I, Decorin interferes with platelet-derived growth factor receptor signaling in experimental hepatocarcinogenesis, FEBS J 280 (2013) 2150–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Robinson KA, Sun M, Barnum CE, Weiss SN, Huegel J, Shetye SS, Lin L, Saez D, Adams SM, Iozzo RV, Soslowsky LJ, Birk DE, Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons, Matrix Biol 64 (2017) 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rousselle P, Montmasson M, Garnier C, Extracellular matrix contribution to skin wound re epithelialization, Matrix Biol 75–76 (2019) 12–26. [DOI] [PubMed] [Google Scholar]
- [37].Godwin ARF, Singh M, Lockhart-Cairns MP, Alanazi YF, Cain SA, Baldock C, The role of fibrillin and microfibril binding proteins in elastin and elastic fibre assembly, Matrix Biol 84 (2019) 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Theocharis AD, Manou D, Karamanos NK, The extracellular matrix as a multitasking player in disease, FEBS J 286 (2019) 2830–2869. [DOI] [PubMed] [Google Scholar]
- [39].Horvath Z, Kovalszky I, Fullar A, Kiss K, Schaff Z, Iozzo RV, Baghy K, Decorin deficiency promotes hepatic carcinogenesis, Matrix Biol 35 (2014) 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bi X, Pohl NM, Yang GR, Gou Y, Guzman G, Kajdacsy-Balla A, Iozzo RV, Yang W, Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice, Carcinogenesis 33 (2012) 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Baghy K, Dezsó K, László V, Fullár A, Péterfia B, Paku S, Nagy P, Schaff Z, Iozzo RV, Kovalszky I, Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice, Lab. Invest 91 (2011) 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B, Eichstetter I, Cooperative action of germline mutations in decorin and p53 accelerates lymphoma tumorigenesis, Proc. Natl. Acad. Sci. USA 96 (1999) 3092–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Santra M, Skorski T, Calabretta B, Lattime EC, Iozzo RV, De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells, Proc. Natl. Acad. Sci. USA 92 (1995) 7016–7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Goldoni S, Seidler DG, Heath J, Fassan M, Baffa R, Thakur ML, Owens RA, McQuillan DJ, Iozzo RV, An anti-metastatic role for decorin in breast cancer, Am. J. Pathol 173 (2008) 844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Neill T, Torres AT, Buraschi S, Iozzo RV, Decorin has an appetite for endothelial cell autophagy, Autophagy 9 (2013) 1626–1628. [DOI] [PubMed] [Google Scholar]
- [46].Buraschi S, Neill T, Goyal A, Poluzzi C, Smythies J, Owens RT, Schaefer L, Torres A, Iozzo RV, Decorin causes autophagy in endothelial cells via Peg3, Proc. Natl. Acad. Sci. U. S. A 110 (2013) E2582–E2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Neill T, Schaefer L, Iozzo RV, Decoding the matrix: Instructive roles of proteoglycan receptors, Biochemistry 54 (2015) 4583–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Neill T, Schaefer L, Iozzo RV, An oncosuppressive role for decorin, Mol. Cell. Oncol 2 (2015) e975645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Torres A, Gubbiotti MA, Iozzo RV, Decorin-inducible Peg3 Evokes Beclin 1-mediated Autophagy and Thrombospondin 1-mediated Angiostasis, J. Biol Chem 292 (2017) 5055–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Neill T, Sharpe C, Owens RT, Iozzo RV, Decorin-Evoked Paternally Expressed Gene 3 (PEG3) is an Upstream Regulator of the Transcription Factor EB (TFEB) in Endothelial Cell Autophagy, J. Biol Chem 292 (2017) 16211–16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Csordás G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, Nugent MA, Hajnóczky G, Iozzo RV, Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo, J. Biol. Chem 275 (2000) 32879–32887. [DOI] [PubMed] [Google Scholar]
- [52].Santra M, Mann DM, Mercer EW, Skorski T, Calabretta B, Iozzo RV, Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin-dependent kinases, J. Clin. Invest 100 (1997) 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Xaus J, Comalada M, Cardó M, Valledor AF, Celada A, Decorin inhibits macrophage colony-stimulating factor proliferation of macrophages and enhances cell survival through induction of p27Kip1 and p21Waf1, Blood 98 (2001) 2124–2133. [DOI] [PubMed] [Google Scholar]
- [54].Santra M, Reed CC, Iozzo RV, Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping with but distinct from the EGF-binding epitope, J. Biol. Chem 277 (2002) 35671–35681. [DOI] [PubMed] [Google Scholar]
- [55].Santra M, Eichstetter I, Iozzo RV, An anti-oncogenic role for decorin: downregulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells, J. Biol. Chem 275 (2000) 35153–35161. [DOI] [PubMed] [Google Scholar]
- [56].Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV, Decorin is a novel antagonistic ligand of the Met receptor, J. Cell Biol 185 (2009) 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Buraschi S, Pal N, Tyler-Rubinstein N, Owens RT, Neill T, Iozzo RV, Decorin antagonizes Met receptor activity and downregulates β-catenin and Myc levels, J. Biol. Chem 285 (2010) 42075–42085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Neill T, Painter H, Buraschi S, Owens RT, Lisanti MP, Schaefer L, Iozzo RV, Decorin antagonizes the angiogenic network. Concurrent inhibition of Met, hypoxia inducible factor-1α and vascular endothelial growth factor A and induction of thrombospondin-1 and TIMP3, J. Biol. Chem 287 (2012) 5492–5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Goyal A, Neill T, Owens RT, Schaefer L, Iozzo RV, Decorin activates AMPK, an energy sensor kinase, to induce autophagy in endothelial cells, Matrix Biol 34 (2014) 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Thiery JP, Acloque H, Huang RY, Nieto MA, Epithelial-mesenchymal transitions in development and disease, Cell 139 (2009) 871–890. [DOI] [PubMed] [Google Scholar]
- [61].Plaks V, Kong N, Werb Z, The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells?, Cell Stem Cell 16 (2015) 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cecchin E, De ME, Ecca F, Toffoli G, Host genetic profiling to increase drug safety in colorectal cancer from discovery to implementation, Drug Resist. Updat 39 (2018) 18–40. [DOI] [PubMed] [Google Scholar]
- [63].Bi X, Tong C, Dokendorff A, Banroft L, Gallagher L, Guzman-Hartman G, Iozzo RV, Augenlicht LH, Yang W, Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation, Carcinogenesis 29 (2008) 1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].De RM, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Signori E, Fazio VM, The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies, J. Carcinog 10 (2011) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Parang B, Barrett CW, Williams CS, AOM/DSS Model of Colitis-Associated Cancer, Methods Mol. Biol 1422 (2016) 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhang W, Zhang C, Tian W, Qin J, Chen J, Zhang Q, Fang L, Zheng J, Efficacy of an Oncolytic Adenovirus Driven by a Chimeric Promoter and Armed with Decorin Against Renal Cell Carcinoma, Hum. Gene Ther 31 (2020) 651–663. [DOI] [PubMed] [Google Scholar]
- [67].Jarvinen TAH, Pemmari T, Systemically Administered, Target-Specific, Multi-Functional Therapeutic Recombinant Proteins in Regenerative Medicine, Nanomaterials. (Basel) 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Neil JR, Johnson KM, Nemenoff RA, Schiemann WP, Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-beta through a PGE2-dependent mechanisms, Carcinogenesis 29 (2008) 2227–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Rosette C, Roth RB, Oeth P, Braun A, Kammerer S, Ekblom J, Denissenko MF, Role of ICAM1 in invasion of human breast cancer cells, Carcinogenesis 26 (2005) 943–950. [DOI] [PubMed] [Google Scholar]
- [70].Strasser-Weippl K, Higgins MJ, Chapman JW, Ingle JN, Sledge GW, Budd GT, Ellis MJ, Pritchard KI, Clemons MJ, Badovinac-Crnjevic T, Han L, Gelmon KA, Rabaglio M, Elliott C, Shepherd LE, Goss PE, Effects of Celecoxib and Low-dose Aspirin on Outcomes in Adjuvant Aromatase Inhibitor-Treated Patients: CCTG MA.27, J. Natl. Cancer Inst 110 (2018) 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Karamanos NK, Theocharis AD, Neill T, Iozzo RV, Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases, Matrix Biol 75–76 (2019) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RA, McQuillan DJ, Iozzo RV, Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation, J. Biol. Chem 281 (2006) 26408–26418. [DOI] [PubMed] [Google Scholar]
- [73].Xu W, Neill T, Yang Y, Hu Z, Cleveland E, Wu Y, Hutten R, Xiao X, Stock SR, Shevrin D, Kaul K, Brendler C, Iozzo RV, Seth P, The systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer, Gene Therapy 22 (2015) 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yang Y, Xu WW, Neill T, Hu Z, Wang CH, Xiao X, Stock S, Guise T, Yun CO, Brendler CB, Iozzo RV, Seth P, Systemic Delivery of an Oncolytic Adenovirus Expressing Decorin for the Treatment of Breast Cancer Bone Metastases, Hum. Gene Ther 26 (2015) 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bi X, Xia X, Fan D, Mu T, Zhang Q, Iozzo RV, Yang W, Oncogenic activin C interacts with decorin in colorectal cancer in vivo and in vitro, Mol. Carcinog 55 (2015) 1786–1795. [DOI] [PubMed] [Google Scholar]
- [76].Liu Z, Yang Y, Zhang X, Wang H, Xu W, Wang H, Xiao F, Bai Z, Yao H, Ma X, Jin L, Wu C, Seth P, Zhang Z, Wang L, An Oncolytic Adenovirus Encoding Decorin and Granulocyte Macrophage Colony Stimulating Factor Inhibits Tumor Growth in a Colorectal Tumor Model by Targeting Pro-Tumorigenic Signals and via Immune Activation, Hum. Gene Ther 28 (2017) 667–680. [DOI] [PubMed] [Google Scholar]
- [77].Koistinen V, Harkonen K, Karna R, Arasu UT, Oikari S, Rilla K, EMT induced by EGF and wounding activates hyaluronan synthesis machinery and EV shedding in rat primary mesothelial cells, Matrix Biol 63 (2017) 38–54. [DOI] [PubMed] [Google Scholar]
- [78].Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA, Mesenchymal stem cells within tumor stroma promote breast cancer metastasis, Nature 449 (2007) 557–565. [DOI] [PubMed] [Google Scholar]
- [79].Preca BT, Bajdak K, Mock K, Lehmann W, Sundararajan V, Bronsert P, Matzge-Ogi A, Orian-Rousseau V, Brabletz S, Brabletz T, Maurer J, Stemmler MP, A novel ZEB1/HAS2 positive feedback loop promotes EMT in breast cancer, Oncotarget 8 (2017) 11530–11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Marquardt S, Solanki M, Spitschak A, Vera J, Pützer BM, Emerging functional markers for cancer stem cell-based therapies: Understanding signaling networks for targeting metastasis, Semin. Cancer Biol 53 (2018) 90–109. [DOI] [PubMed] [Google Scholar]
- [81].Wang K, Song K, Ma Z, Yao Y, Liu C, Yang J, Xiao H, Zhang J, Zhang Y, Zhao W, Identification of EMT-related high-risk stage II colorectal cancer and characterisation of metastasis-related genes, Br. J. Cancer 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zhang W, Ge Y, Cheng Q, Zhang Q, Fang L, Zheng J, Decorin is a pivotal effector in the extracellular matrix and tumour microenvironment, Oncotarget 9 (2018) 5480–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Buraschi S, Neill T, Iozzo RV, Decorin is a devouring proteoglycan: Remodeling of intracellular catabolism via autophagy and mitophagy, Matrix Biol 75–76 (2019) 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cianfarani F, De DE, Nystrom A, Mastroeni S, Abeni D, Baldini E, Ulisse S, Uva P, Bruckner-Tuderman L, Zambruno G, Castiglia D, Odorisio T, Decorin counteracts disease progression in mice with recessive dystrophic epidermolysis bullosa, Matrix Biol 81 (2019) 3–16. [DOI] [PubMed] [Google Scholar]
- [85].Theocharis AD, Karamanos NK, Proteoglycans remodeling in cancer: Underlying molecular mechanisms, Matrix Biol 75–76 (2019) 220–259. [DOI] [PubMed] [Google Scholar]
- [86].Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO, The extracellular matrix: Tools and insights for the “omics” era, Matrix Biol 49 (2016) 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Naba A, Clauser KR, Mani DR, Carr SA, Hynes RO, Quantitative proteomic profiling of the extracellular matrix of pancreatic islets during the angiogenic switch and insulinoma progression, Sci. Rep 7 (2017) 40495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Izzi V, Lakkala J, Devarajan R, Kääriäinen A, Kolvunen J, Heljasvaara R, Pihlajaniemi T, Pan-Cancer analysis of the expression and regulation of matrisome genes across 32 tumor types, Matrix Biol Plus 1 (2019) 100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Socovich AM, Naba A, The cancer matrisome: From comprehensive characterization to biomarker discovery, Semin. Cell Dev. Biol 89 (2019) 157–166. [DOI] [PubMed] [Google Scholar]
- [90].Mohammed A, Yarla NS, Madka V, Rao CV, Clinically Relevant Anti-Inflammatory Agents for Chemoprevention of Colorectal Cancer: New Perspectives, Int. J. Mol. Sci 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Cummings M, Massey KA, Mappa G, Wilkinson N, Hutson R, Munot S, Saidi S, Nugent D, Broadhead T, Wright AI, Barber S, Nicolaou A, Orsi NM, Integrated eicosanoid lipidomics and gene expression reveal decreased prostaglandin catabolism and increased 5-lipoxygenase expression in aggressive subtypes of endometrial cancer, J. Pathol 247 (2019) 21–34. [DOI] [PubMed] [Google Scholar]
- [92].Liu B, Yan S, Qu L, Zhu J, Celecoxib enhances anticancer effect of cisplatin and induces anoikis in osteosarcoma via PI3K/Akt pathway, Cancer Cell Int 17 (2017) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Dinicola S, Masiello MG, Proietti S, Coluccia P, Fabrizi G, Catizone A, Ricci G, de TG, Bizzarri M, Cucina A, Nicotine increases colon cancer cell migration and invasion through epithelial to mesenchymal transition (EMT): COX-2 involvement, J. Cell Physiol 233 (2018) 4935–4948. [DOI] [PubMed] [Google Scholar]
- [94].Fakih MG, Metastatic colorectal cancer: current state and future directions, J. Clin. Oncol 33 (2015) 1809–1824. [DOI] [PubMed] [Google Scholar]
- [95].Bjerknes M, Cheng H, Methods for the isolation of intact epithelium from the mouse intestine, Anat. Rec 199 (1981) 565–574. [DOI] [PubMed] [Google Scholar]
- [96].Alvarez RJ, Sun MJ, Haverty TP, Iozzo RV, Myers JC, Neilson EG, Biosynthetic and proliferative characteristics of tubulointerstitial fibroblasts probed with paracrine cytokines, Kidney Int 41 (1992) 14–23. [DOI] [PubMed] [Google Scholar]
- [97].Zoeller JJ, McQuillan A, Whitelock J, Ho S-Y, Iozzo RV, A central function for perlecan in skeletal muscle and cardiovascular development, J. Cell Biol 181 (2008) 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Ryynänen M, Ryynänen J, Solberg S, Iozzo RV, Knowlton RG, Uitto J, Genetic linkage of Type VII collagen (COL7A1) to dominant dystrophic epidermolysis bullosa in families with abnormal anchoring fibrils, J. Clin. Invest 89 (1992) 974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Rudnicka L, Varga J, Christiano AM, Iozzo RV, Jimenez SA, Uitto J, Elevated expression of type VII collagen in the skin of patients with systemic sclerosis, J. Clin. Invest 93 (1994) 1709–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bix G, Castello R, Burrows M, Zoeller JJ, Weech M, Iozzo RA, Cardi C, Thakur MT, Barker CA, Camphausen KC, Iozzo RV, Endorepellin in vivo: targeting the tumor vasculature and retarding cancer growth and metabolism, J. Natl. Cancer Inst 98 (2006) 1634–1646. [DOI] [PubMed] [Google Scholar]


