Abstract
Background
NGS has become a first-line tool for diagnosis of PID. However, patient access remains limited due to restricted insurance coverage and a lack of guidelines addressing use of targeted panels vs WES.
Objectives
To compare targeted next generation sequencing (NGS) with whole exome sequencing (WES) in a global population of patients with primary immunodeficiency (PID).
Methods
This is a longitudinal study of 878 patients with likely PID sequenced between 2010 and 2020. The majority of patients (n=780) were first sequenced using a 264 gene panel. This was followed by WES in selected cases if a candidate gene was not found. A subset of patients (n=98) were selected for a WES-only pipeline if the history was atypical for genes within the targeted panel.
Results
Disease-causing variants were identified in 498 of the 878 probands (56%), encompassing 152 distinct monogenic disorders. Sixteen patients had disorders that were novel at the time of sequencing (1.8%). Diagnostic yield in patients sequenced by targeted panel was 56% (433 of 780 patients) with subsequent WES leading to an additional 18 diagnoses (overall diagnostic yield 58%, 451 of 780 patients). The WES-only approach had a diagnostic yield of 45% (45 of 98 patients), reflecting that these cases had less common clinical and laboratory phenotypes. Cost analysis, based on current commercial WES and targeted panel prices, demonstrated savings ranging from $300-$950 with a WES-only approach, depending on diagnostic yield.
Discussion
Advantages of WES over targeted NGS include simplified workflow, reduced overall cost, and the potential for identification of novel diseases.
Keywords: next generation sequencing, targeted panel, whole exome sequencing, primary immunodeficiency, genomics
Capsule Summary
NGS of 878 probands with immunodeficiency demonstrates the efficacy of WES as a first-line diagnostic test over targeted panels due to increased diagnostic yield and lower cost.
Background
Primary immunodeficiencies (PID), are a heterogenous group of congenital disorders that affect the development and/or function of the immune system.1,2 The introduction of next-generation sequencing (NGS) accelerated the discovery of monogenic causes of PID over the past 10 years from fewer than 200 in 2009 to more than 400 in 2019.3 Genetic testing, previously reserved for a relatively small number of clinical scenarios, has thus become a first-line diagnostic tool for most patients with suspected PID.4 However, patient access to NGS testing remains limited due to restricted insurance coverage of these tests and a lack of guidelines addressing use of targeted panels vs whole exome sequencing (WES) in patients with PID.4,5 Additionally, prior studies limited to patients from one or a few countries may have had diagnostic yields influenced by population-specific founder mutations and/or cultural practices of consanguinity.6 Thus, there is a need for studies comparing outcomes from these two technologies in a global population of patients with PID.
Results and Discussion
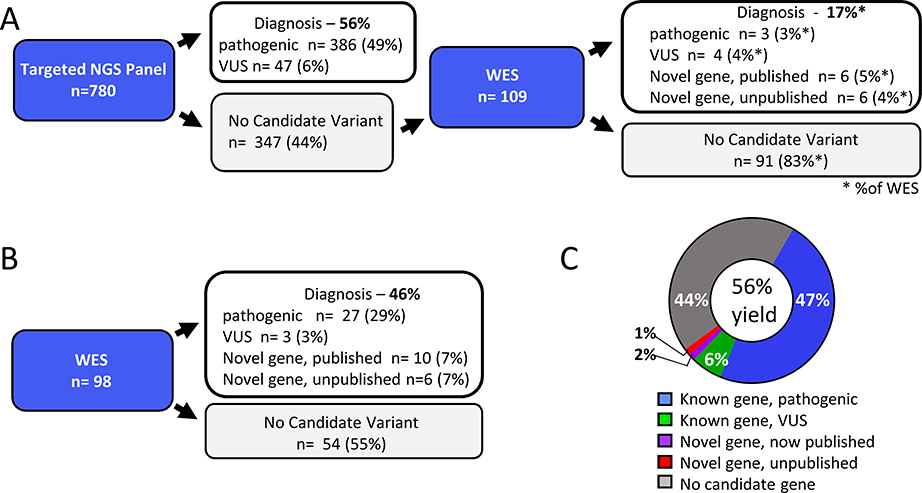
The age of patients ranged from 1 month to 72 years, with a median of four years. Sixty percent (n=529/878) of patients were male, reflecting the incidence of X-linked PIDs.6 Consanguinity, determined either by family history or revealed by genetic analysis, was found in 63% (n=551/878) of probands (Table 1). Two sequencing approaches were utilized. In the first approach, 780 patients were studied with a 264 gene NGS panel (Table S1). Of the 347 patients in whom no candidate variants were identified by the NGS panel, 109 (31%) were selected for WES because they had infectious, autoimmune, or autoinflammatory sequelae requiring hospitalization. A second, WES-only approach was used for 98 “challenging cases,” defined as clinical and laboratory phenotypes atypical for any of the disorders associated with the 264 gene panel (Fig 1A & B). Variants were classified as “pathogenic,” “likely pathogenic,” or “variant of uncertain significance (VUS)” per American College of Medical Genetics and Genomics (ACMG) guidelines.7
Table 1:
Patient Demographics
| Total number of probands | 878 |
| Male | 529 (60%) |
| Female | 349 (40%) |
| Age range (years) | 0.1 – 72 |
| Median age (years) | 4 |
| Pediatric (<18 years) | 795 (91%) |
| Adult (=>18 years) | 83 (9%) |
| Non-consanguineous | 327 (37%) |
| Consanguineous | 551 (63%) |
| Based on family history | 507 (58%) |
| Denied consanguinity but evidence on genetic analysis | 44 (5%) |
Fig 1.
A, B. NGS pipelines. Targeted NGS panel followed by WES (A) WES only (B). C. Overall diagnostic yield. Legend indicates variants meeting ACMG criteria for “pathogenic” or “likely pathogenic” mutation (pathogenic), VUS in genes that strongly fit the clinical phenotype (VUS), and published and non-published variants in novel genes at the time of sequencing.
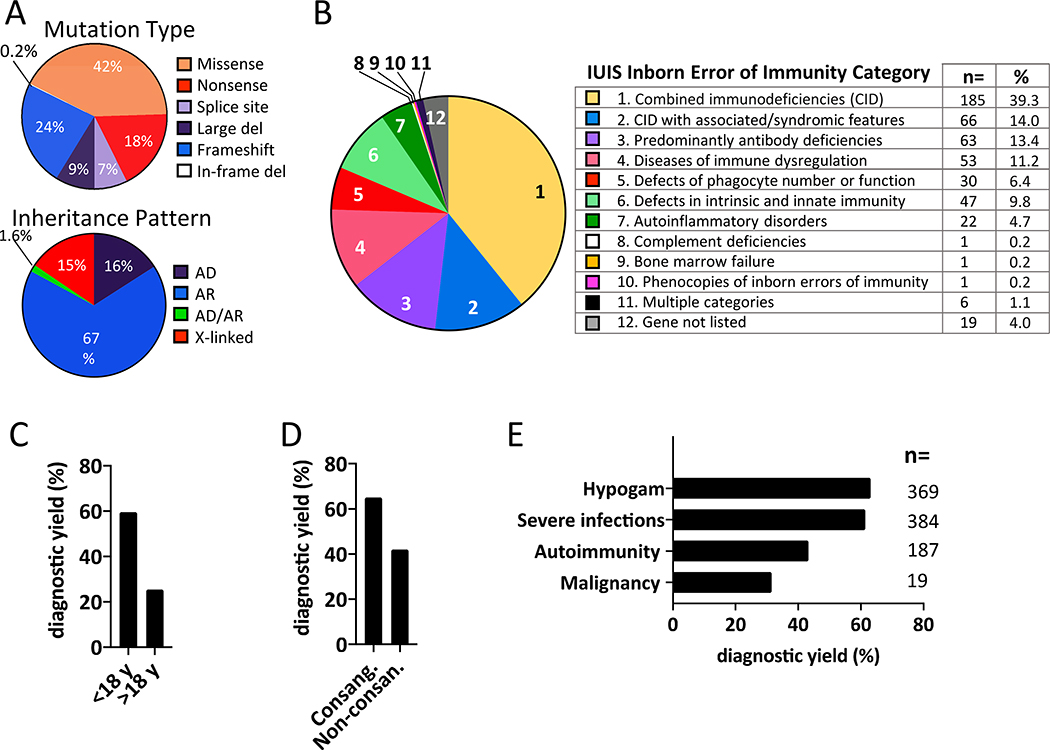
The diagnostic yield of the NGS panel was 56% (433 of 780 patients); subsequent WES of 109 unsolved cases identified an additional 18 diagnoses, for a total diagnostic yield of 58% (451 of 780) (Fig 1A, Table S2). The diagnostic yield of the WES-only approach was 45% (45 of 98 patients), reflecting the fact that this approach was utilized only for challenging cases with unusual clinical and laboratory phenotypes. Collectively, we identified disease-causing variants in 498 of the 878 probands (56%), encompassing 152 distinct monogenic disorders (Fig. 1C, Tables S2 & S3). Variants in known genes that met ACMG criteria for “pathogenic” or “likely pathogenic” classification were found in 416 patients (47%). There were 54 additional patients (6%) with a VUS concordant with the patient’s clinical phenotype. An example of this is a patient with T−B+NK− severe combined immunodeficiency (SCID) with a missense mutation in JAK3 deemed pathogenic by in silico prediction algorithms (Fig 1C, Table S2). Sixteen probands (1.8%) had a mutation in a novel gene that has been subsequently published (Fig 1A&B, Table S3). An additional 12 genes are under investigation as likely candidates for novel disorders. Missense mutations (n = 207, 42%) were the most common variant (Fig 2A top panel, Table S2). The majority of diagnoses (67%) were autosomal recessive and 51% of diagnosed patients had combined immunodeficiency, but variants spanning all categories of inheritance and types of PID were identified (Fig 2 A, B, and Tables S2 & S3).3 As found in prior reports, patients under age 18, and patients with a history of consanguinity had a significantly higher diagnostic yield than adult patients (Fig 2C&D).6 Life-threatening infections and laboratory evidence of antibody deficiency were correlated with increased diagnostic yield (61% and 63% respectively) (Fig 2E). The diagnostic yield was lower in patients with a history of autoimmunity (43%) or malignancy (32%), reflecting the multigenic or non-germline etiology frequently associated with these conditions (Fig 2E).8,9
Fig 2.
A. Mutation type (top panel) and inheritance pattern (bottom panel). B. Percent of patients in each IUIS disease category. C. Diagnostic yield in pediatric patients vs adults. D. Diagnostic yield in consanguineous vs non-consanguineous patients. E. Diagnostic yield based on features of clinical history.
While cost and reimbursement barriers continue to exist for both targeted panels and WES, these barriers are greater for WES, despite having a higher diagnostic yield and the narrowing price differential between these technologies.10,11 When this study was initiated, targeted panels were significantly less expensive than WES, leading to the tiered approach that we adopted for the majority of the cases studied (Fig 1A). However, based on current pricing, WES is now less expensive than this tiered approach. Applying current commercial costs to our cohort, which had a diagnostic yield of 56% for the targeted NGS panel, a WES-only strategy would have saved $300 in diagnostic testing costs per patient (Table 2). This includes the fractional cost of variant confirmation, which is typically included in the cost of clinical-grade NGS. We note that the high rate of consanguinity, and the young age of our patient population contributed to a diagnostic yield that is higher than what may be expected in most clinical settings.12 Cost analysis based on a lower diagnostic yield of 30%, as has been documented in prior studies,12 yields a savings of $950 per patient (Table 2). While this presumes that any patient with an unrevealing targeted panel should proceed to WES, we feel that this is necessary as targeted panels are quickly rendered out of date by advances in gene discovery, and are not amenable to reanalysis of unsolved cases (Fig 1A).11
Table 2.
The costs of tiered NGS (targeted panel followed by WES in unsolved cases) vs WES-only approach will differ, based on the pretest probability of monogenic PIDs within the population.
| Comparative costs of NGS in a population with a high prevalence of monogenic PIDs: In a highly consanguineous population, such as the one used in this study, a monogenic cause of PID is more likely to be found by NGS. For these types of populations, we have used the diagnostic yield found in our study population (56%). | ||
| Tiered approach | WES-only | |
| NGS panel $1700 per patient | 100 patients × $1700 | Not applicable |
| WES $2500 per patient | 44 patients × $2500 | 100 patients × $2500 |
| Final cost per patient | $2,800 | $2,500 |
| Savings per patient | $2800 – $2500 = $300 | |
| Comparative costs of NGS in a population with a lower prevalence of monogenic PIDs. Previously published studies have achieved a diagnostic yield of 25 – 45% with NGS in non-consanguineous populations.1,2,10,11 For this population, we have used a NGS diagnostic yield of 30%. | ||
| Tiered approach | WES-only | |
| NGS panel $1700 per patient | 100 patients × $1700 | Not applicable |
| WES $2500 per patient | 70 patients × $2500 | 100 patients × 2500 |
| Final cost per patient | $3,450 | $2,500 |
| Savings per patient | $3450 – $2500 = $950 | |
In specific circumstances, targeted panels may be more feasible. As targeted panels produce a lower volume of data, there are reduced costs for data storage.5 Panels also typically offer faster turnaround time for routine testing: under four weeks for targeted panels compared to about three months for routine clinical-grade exome sequencing. Accordingly, in cases where laboratory data is highly consistent with a defect in particular gene or well-defined group of genes, targeted panels are a reasonable first-line test. Such cases would include X-linked agammaglobulinemia, CD40 ligand deficiency, Wisckott-Aldrich syndrome, autosomal dominant hyper-IgE syndrome, and ataxia telangiectasia. Targeted sequencing panels can offer 200x-800x coverage, which is much deeper than the 50x-100x coverage typical of clinical-grade exome sequencing. This is of particular utility for the identification of a somatic variant affecting a small percentage of cells; however, the difference in performance between targeted panel and clinical-grade WES has been shown to be negligible for the identification of germline variants.13 Different panels can vary dramatically in number of genes covered, thus leading to variability in cost. However, both targeted sequencing and WES will suffer from reduced accuracy when lower cost is prioritized over sufficient depth of coverage.
We did not find a strong candidate variant in 44% of patients studied. Although sequencing technologies have improved in the past decade, the major limitations of both targeted and exome sequencing approaches have remained constant. Both depend on the accuracy of the probes utilized to capture the target region of interest. The technologies are not typically used to detect deep intronic mutations, mutations within 5’ or 3’ untranslated regions or polyadenylation signal, mutations within genes that have highly homologous pseudogenes, and chromosomal deletions, duplications or rearrangements, a subject that has been delineated in previously publications.5 Long-read WGS, which is not yet widely available for clinical use, has increased diagnostic capability for all of the above scenarios, but comes at the expense of a massive increase in data storage and processing.5 Alternatively, targeted sequencing of non-coding regions known to contain more frequent pathogenic variants can be used to complement WES. Chromosomal microarrays and comparative genomic hybridization are used to increase the detection of structural variations, such as changes in copy number variation or unbalanced translocations. Trio analysis, in which patients and parents are analyzed simultaneously, was not used in this study given the increased cost and because candidate variants identified by WES can be rapidly Sanger sequenced in parents if needed. As costs continue to drop, the simplified workflow inherent in trio analyses will be easier to justify.
For this study, data was analyzed by a core group of physicians and scientists, thus standardizing the analysis. Importantly, our findings were discussed in detail with the referring collaborators. One advantage of such close collaboration is increased access to information regarding disease prevalence in particular geographic locations, thus increasing clinical suspicion for pathogenic variants common to specific regions. The recent expansion of telemedicine will facilitate global collaborations, thus enabling broader access to such collaboration.
Our study tests the relative merits of targeted NGS panels and WES in one of the largest cohorts of patients with PID. We show that a WES-only approach has the advantages of reduced cost, simplified workflow, and the potential for the identification of novel diseases – all of which are amplified by the decreasing cost of WES and the increasing the number of inborn errors of immunity. For detailed methods, please see the Methods section in this article’s Online Repository at www.jacionline.org.
Supplementary Material
Key Message.
This longitudinal study compared targeted panel sequencing and whole exome sequencing in 878 probands from 28 countries.
Disease-causing variants were identified in 498 of the 878 probands (56%), encompassing 152 distinct monogenic disorders and 16 patients with novel disorders.
Compared to targeted panels, WES has the advantages of reduced cost, simplified workflow, and the potential for the identification of novel diseases.
Acknowledgments
Supported by: 1K08AI116979-01 (J.C.), Eleanor and Miles Shore 50th Anniversary Fellowship Award (C.D.P.), 1R01AI139633-01 (R.S.G), the Samara Turkel Foundation (R.S.G), and the Perkin Fund (R.S.G).
Abbreviations
- ACMG
American College of Medical Genetics and Genomics
- IUIS
International Union of Immunological Societies
- NGS
next generation sequencing
- PID
primary immunodeficiency
- VUS
variant of uncertain significance
- WES
whole exome sequencing
Footnotes
Conflict of interest: The spouse of C.D.P. analyzes next generation sequencing for Quest Diagnostics. All other authors declare that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rudilla F, Franco-Jarava C, Martínez-Gallo M, Garcia-Prat M, Martín-Nalda A, Rivière J, et al. Expanding the Clinical and Genetic Spectra of Primary Immunodeficiency-Related Disorders With Clinical Exome Sequencing: Expected and Unexpected Findings. Front Immunol 2019;10:2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan TY, Dillon OJ, Stark Z, Schofield D, Alam K, Shrestha R, et al. Diagnostic impact and cost-effectiveness of whole-exome sequencing for ambulant children with suspected monogenic conditions. JAMA Pediatr 2017;171:855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol 2020;40:24–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heimall J Now Is the Time to Use Molecular Gene Testing for the Diagnosis of Primary Immune Deficiencies. J Allergy Clin Immunol Pract 2019;7:833–8. [DOI] [PubMed] [Google Scholar]
- 5.Chinn IK, Chan AY, Chen K, Chou J, Dorsey MJ, Hajjar J, et al. Diagnostic interpretation of genetic studies in patients with primary immunodeficiency diseases: A working group report of the Primary Immunodeficiency Diseases Committee of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 2020;145:46–69. [DOI] [PubMed] [Google Scholar]
- 6.Modell V, Knaus M, Modell F, Roifman C, Orange J, Notarangelo LD. Global overview of primary immunodeficiencies: a report from Jeffrey Modell Centers worldwide focused on diagnosis, treatment, and discovery. Immunol Res 2014;60:132–44. [DOI] [PubMed] [Google Scholar]
- 7.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marson A, Housley WJ, Hafler DA, Marson A, Housley WJ, Hafler DA. Genetic basis of autoimmunity. J Clin Invest 2015;125:2234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortaz E, Tabarsi P, Mansouri D, Khosravi A, Garssen J, Velayati A, et al. Cancers related to immunodeficiencies: Update and perspectives. Front Immunol 2016;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reuter CM, Kohler JN, Bonner D, Zastrow D, Fernandez L, Dries A, et al. Yield of whole exome sequencing in undiagnosed patients facing insurance coverage barriers to genetic testing. J Genet Couns 2019;28:1107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillon OJ, Lunke S, Stark Z, Yeung A, Thorne N, Gaff C, et al. Exome sequencing has higher diagnostic yield compared to simulated disease-specific panels in children with suspected monogenic disorders. Eur J Hum Genet 2018;26:644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cifaldi C, Brigida I, Barzaghi F, Zoccolillo M, Ferradini V, Petricone D, et al. Targeted NGS platforms for genetic screening and gene discovery in primary immunodeficiencies. Front Immunol 2019;10:1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaDuca H, Farwell KD, Vuong H, Lu HM, Mu W, Shahmirzadi L, et al. Exome sequencing covers >98% of mutations identified on targeted next generation sequencing panels. PLoS One 2017;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.