Abstract
Studies in rodents have shown that interactions between cholecystokinin (CCK) and the endogenous cannabinoid system in the basolateral nuclear complex of the amygdala (BNC) modulate anxiety-like behavior and fear learning/expression. One of the main cell types implicated is a CCK-immunoreactive (CCK+) basket cell that innervates the somata of pyramidal projection neurons (PNs) and expresses the type 1 cannabinoid receptor (CB1R) in its axon terminals. Although numerous studies have elucidated the anatomy and physiology of these CCK+/CB1R+ interneurons in rodents, it has not been determined if they exist in primates. The present investigation used immunohistochemical techniques in the monkey to answer this question. It was found that the monkey BNC, as in rodents, has a very high density of CB1R+ axons, including CB1R+ axon terminals that form basket-like plexuses contacting somata of PNs. These axons, as well as axons in the neuropil, exhibit extensive colocalization of CCK and CB1R. These findings suggest that the same synaptic mechanisms involved in CCK-CB1R interactions in rodents may also apply to primates, and that therapies that target the cannabinoid system in the BNC may be useful for treating fear and anxiety in human patients.
Keywords: immunohistochemistry, primate, basolateral amygdala, cholecystokinin, cannabinoids, fear expression
1. Introduction
The basolateral nuclear complex of the amygdala (BNC) is a critical hub for fear and extinction learning. Recent studies in rodents have begun to elucidate the roles of different BNC cell types in these mnemonic functions. There are two main types of neurons in the BNC: glutamatergic pyramidal projection neurons (PNs), and GABAergic non-pyramidal neurons that are mainly interneurons (INs) [1]. Immunohistochemical studies in rodents suggest that the BNC contains several distinct subpopulations of INs that can be distinguished on the basis of their expression of calcium-binding proteins, neuropeptides, and receptors. These separate IN subpopulations play discrete roles in the intrinsic circuitry of the BNC by innervating distinct compartments of PNs and/or other INs, and by having different inputs/receptors and electrophysiological properties [1-4].
One important IN subpopulation in the rodent BNC consists of CCK-immunoreactive (CCK+) basket cells that provide a perisomatic innervation of PNs [5]. Along with parvalbumin-positive basket cells and axoaxonic cells, these CCK+ basket cells (CCKBCs) can powerfully regulate PN spiking [6]. CCKBCs are unique in that they express very high levels of the type 1 cannabinoid receptor (CB1R) [5, 7-11]. Studies in rodents suggest that activation of CB1Rs in the axon terminals of CCKBCs by endocannabinoids released from postsynaptic PN somata, or by cannabinergic drugs such as delta-9 tetrahydrocannabinol, results in decreased GABA and CCK release from basket cell terminals [8, 12-13]. Interactions between CCK and the endogenous cannabinoid system in the BNC are critical for fear expression and extinction [14, 15]. Since many anxiety disorders, including PTSD, are associated with disruptions in fear expression/extinction, it has been suggested that therapies that target the cannabinoid system in the BNC may be useful for treating anxiety disorders in human patients [14, 16]. However, it remains to be determined whether the BNC in human and non-human primates contains CCKBCs that express CB1Rs. To answer that question dual-labeling immunohistochemistry was used on the present study to examine possible expression of CB1Rs in CCK+ axon terminals in the BNC of the monkey.
2. Materials and Methods
2.1. Tissue preparation
These immunohistochemical experiments were performed on amygdalas obtained from three rhesus monkeys (Macaca mulatta) housed at Yerkes National Primate Research Center at Emory University, Atlanta, Georgia. Animals were deeply anesthetized with an overdose of sodium pentobarbital (100 mg/kg), and then perfused with one of two fixatives: (1) a mixture of 4% paraformaldehyde/0.2% glutaraldehyde/0.2% picric acid (Zamboni’s fixative) in phosphate buffer (0.1 M, pH 7.4; PB), or (2) 4% paraformaldehyde in PB (Table 1). Coronal blocks through the amygdala were cryoprotected in 35% sucrose in PB and frozen until processed for immunohistochemistry. The care of the animals and all anesthesia and euthanasia procedures in this study were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Emory University (Emory IACUC #s 115-98Y and 246-2001Y). All efforts were made to minimize animal suffering and to use the minimum number of animals necessary to produce reliable scientific data.
Table 1.
Macaques used in this study.
| Animal # | Species | Age, Sex | Fixative |
|---|---|---|---|
| M58 | Rhesus | 7.9 y.o., male | 4% Paraformaldehyde |
| M102 | Rhesus | 2.8 y.o., male | Zamboni’s |
| M60 | Rhesus | 3.9, y.o., female | Zamboni’s |
Blocks containing the amygdala were thawed and placed in descending concentrations of sucrose in PB over 2-3 days. The amygdalas were excised and 50 μm coronal sections were cut on a vibratome (Leica VT 1200; Nussloch, Germany). Sections were incubated in 1% borohydride in PB for 30 min, and then rinsed thoroughly for 45-60 m in several changes of PB. A one-in-ten series of sections through each amygdala was processed for localization of CB1R using immunoperoxidase methods, and another series was used for dual-localization of CB1R with CCK using immunofluorescence. In addition, a one-in-ten series of sections through each amygdala was Nissl-stained (cresyl violet) so that borders of amygdalar nuclei could be recognized in neighboring sections processed for immunohistochemistry, using blood vessels and fiber bundles as landmarks. Nuclei of the macaque amygdala were identified based on the description of Price and coworkers [17].
2.2. Immunoperoxidase experiments
Localization of CB1Rs in the monkey BNC was performed using the avidin-biotin immunoperoxidase (ABC) technique. The primary antibody to CB1R (1:500; catalog # CB1-Rb-Af380; Frontier Institute Co., Shinkonishi, Ishikari City, Hokkaido, Japan; RRID AB_2571591) was raised in rabbit. The diluent for all antibodies in this study was a mixture of 3% normal goat serum, 1% bovine serum albumin, and 0.4% Triton X-100 in 0.05M phosphate buffered saline (PBS, pH 7.4). Coronal sections at regular intervals through the rostrocaudal extent of the amygdala were incubated in primary antibody for 24 h at 4° C and then processed for the avidin-biotin immunoperoxidase technique using a biotinylated goat anti-rabbit secondary antibody (1:400), and an Elite ABC kit (Vector Laboratories, Burlingame, CA). DAB (3, 3'-diaminobenzidine-4HCl, Sigma) was used as a chromogen to generate a brown reaction product. Following the immunohistochemical procedures, sections were mounted on gelatinized slides, dried overnight, dehydrated in descending ethanols, cleared in xylene (Fisher Scientific, Pittsburgh, PA), and coverslipped with DPX (Sigma). Sections were analyzed using an Olympus X51 microscope, and digital light micrographs were taken with an Olympus DP2-BSW camera system. Brightness and contrast were adjusted using Photoshop CS2 software.
2.3. Dual-labeling Immunofluorescence experiments
These experiments used a cocktail consisting of the same rabbit anti-CB1R antibody used in the immunoperoxidase experiments (1:500) and a mouse anti-CCK antibody (1:2000, antibody #9303 obtained from CURE-UCLA, Los Angeles, CA; RRID AB_2314185). Coronal sections at regular intervals through the rostrocaudal extent of the amygdala were incubated in a primary antibody cocktail for 24 hrs at 4° C. Sections were then rinsed in three changes of PBS (10 min each), and then incubated in a cocktail of Alexa Fluor 546-labeled goat anti-mouse IgG (1:400; Thermo Fisher Scientific, West Columbia, SC) and Alexa Fluor 488-labeled goat anti-rabbit IgG (1:400; Thermo Fisher Scientific), or a cocktail of Alexa Fluor 546-labeled goat anti-rabbit IgG (1:400 Thermo Fisher Scientific) and Alexa Fluor 488-labeled goat anti-mouse IgG (1:400; Thermo Fisher Scientific), for 3 h at room temperature. Sections were then rinsed in PBS and PB (10 min each), mounted on glass slides, and coverslipped using Vectashield HardSet Antifade mounting medium (Vector Laboratories, Burlingame, CA). In addition, some sections were incubated in a fluorescent Nissl stain (1:300; NeuroTrace 640/660 deep red; Thermo Fisher) before coverslipping to examine possible contacts of CB1R+ and/or CCK+ axon terminals with somata. Sections were examined with a Zeiss LSM 510 Meta confocal microscope.
2.4. Antibody specificity
The CB1R antibody was raised in rabbit to the C-terminal 31 amino acids of mouse CB1R protein and was affinity-purified with the antigen polypeptide. Immunoblots using the CB1R antibody detected a single protein band at the expected 52 kDA line in mouse brain, preadsorption with CB1R eliminated immunohistochemical staining in mouse brain, and no staining was seen in CB1R knockout mice [9, 18, and manufacturers information]. As in previous studies in the mouse amygdala using this antibody [9]: (1) CB1R immunoreactivity in the monkey amygdala was confined to axons and axon terminals, including many contacting somata of BNC pyramidal cells, and (2) staining intensity in the BNC was stronger than in most other amygdalar nuclei such as the central nucleus. The finding of no obvious staining differences in the monkey BNC versus the mouse BNC, along with its well-documented specificity in mouse, strongly suggests that the antibody recognizes only the CB1R protein in both species. The CCK antibody was raised in mouse against gastrin but recognizes CCK due to homologies in the terminal pentapeptide shared by these peptides. It has been used extensively in CCK localization studies in the amygdala and other brain regions. In our lab preadsorption of this antibody with CCK-8 (25 μg/ml; Sigma) abolished all immunostaining in the rat brain [7].
To examine method specificity in the present study control sections were processed with one of the two primary antibodies omitted. In all cases only the color of the corresponding secondary fluorescent antibody was observed, and only on the appropriate channel. These results indicate that the secondary antibodies were specific for rabbit or mouse IgGs, and that there was no “crosstalk” between the red and green channels.
3. Results
3.1. Immunoperoxidase experiments
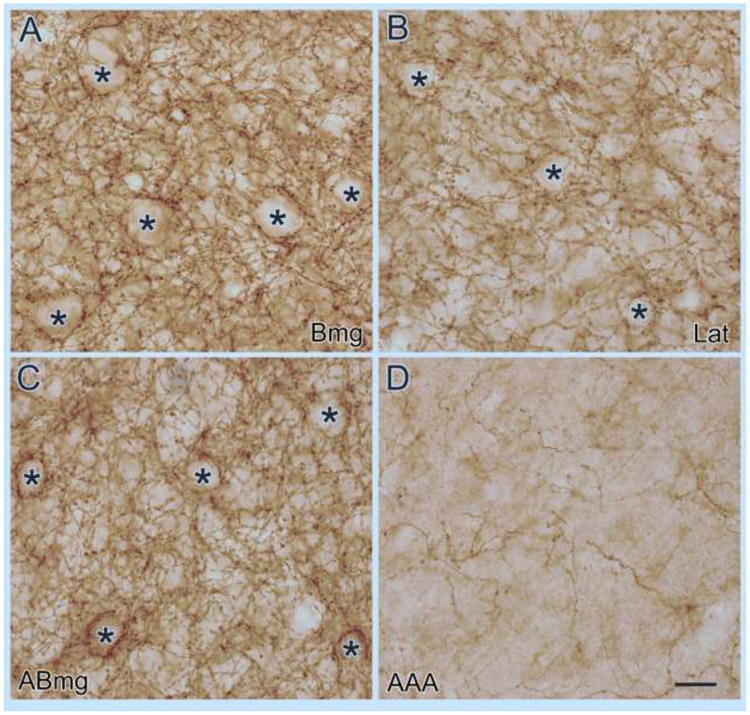
CB1R immunoreactivity (CB1R-ir) was very dense in all nuclei of the monkey BNC (Fig. 1). CB1R-ir was also very dense in the periamygdaloid cortex superficial to the BNC, but less dense in the cortical nucleus, medial nucleus, central nucleus, and the anterior amygdaloid area. Within the BNC CB1R-ir was less dense in the lateral nucleus than in the basal and accessory basal nuclei. Higher power examination revealed that all CB1R-ir in the BNC, and all other brain regions at the level of the amygdala, was in the form of a dense plexus of axons; no CB1R immunoreactive (CB1R+) somata were observed. CB1R+ axons in the BNC were located in the neuropil, as well as forming basket-like plexuses that contacted unstained somata (Fig. 2). The size and shape of the unstained somata indicated that most were PNs. Varicosities along the axons, most likely corresponding to axon terminals, were 0.5-1.0 μm in diameter. Perisomatic baskets were prominent in most portions of the BNC, but less conspicuous in the lateral nucleus (Fig. 2).
Fig. 1.
Low power photomicrograph of the dorsal two-thirds of the monkey amygdala in a coronal section stained for CB1R (immunoperoxidase technique). Note the high density of CB1R-immunoreactivity in the lateral nucleus (Lat), basal magnocellular nucleus (Bmg), and the magnocellular and parvicellular subdivisions of the accessory basal nuclei (ABmg and ABpc) of the BNC, compared to the anterior amygdaloid area (AAA) and anterior cortical nucleus (Coa). Scale bar = 500 μm.
Fig. 2.
Higher power photomicrographs of Bmg (A), Lat (B), ABmg (C), and the AAA (D) taken from the section shown in Fig. 1. Note the high density of CB1R+ axons and terminals in the BNC (A-C) compared to the AAA (D). CB1R+ axons and terminals in the BNC are found both in the neuropil, and forming basket-like plexuses surrounding presumptive PNs (asterisks). Scale bar = 20 μm.
3.2. Immunofluorescence experiments
As in a previous study, the BNC contained a low density of CCK+ somata, as well as CCK+ punctae that were the size and shape of axon terminals [19]. As in the immunoperoxidase preparations, CB1R-ir was found in axons and terminal-like varicosities along the axons. Dual-labeling for CB1R and CCK revealed extensive colocalization in presumptive axon terminals in the BNC (Fig. 3). CB1R+/CCK+, CB1R−/CCK+, and CB1R+/CCK− axon terminals were observed in the neuropil, as well as forming baskets around presumptive PNs. Because no third marker specific for axon terminals was used in this study, no attempt to count single- and double-labeled axon terminals was attempted.
Fig. 3.
Colocalization of CB1R and CCK in a subset of BNC axons. A and B) CB1R+ axons (red) and CCK+ axons (green) in the neuropil of the Bmg (A) and the lateral nucleus (B). Yellow indicates colocalization of CB1R with CCK. C and D) CB1R+ axons (green) and CCK+ axons (red) in Bmg (C) and the lateral nucleus (D) as seen in z-stacks of seven 1-μm-thick optical sections (total thickness of 7 μm for each image). Neuronal somata (and glial cells) are stained blue using a fluorescent Nissl stain. Yellow indicates colocalization of CB1R with CCK. Large neuronal somata representing presumptive PNs (arrows) are contacted by basket-like plexuses of CB1R+, CCK+, and CB1R+/CCK+ axon terminals. Similar terminals are also seen in the neuropil. Scale bar in D = 5 μm (A-C are at the same magnification).
4. Discussion
A previous study employing autoradiographic receptor binding and positron emission tomography techniques to investigate the distribution of CB1Rs in the monkey brain did not mention findings in the amygdala, but did suggest that the overall distribution pattern of CB1Rs in the monkey brain was consistent with that seen in other species [20]. The present investigation demonstrates that there is a very high density of CB1Rs in the BNC of the monkey, and that CCK+ axon terminals in the neuropil, as well as forming pericellular baskets contacting somata, express CB1Rs. Since colocalization of CCK and CB1Rs in axon terminals is also seen in the rodent BNC, it seems likely that the same interactions of CCK and the endogenous cannabinoid system in the BNC also apply to the primate BNC, and that these interactions may be critical for fear expression and extinction in primates, including humans.
Consistent with our finding of high levels of CB1R immunoreactivity in the primate BNC, a high density of CB1Rs and CB1R mRNA in the BNC was seen in the human amygdala in autoradiographic binding studies and in situ hybridization studies, respectively [21, 22]. A previous immunoperoxidase investigation in the monkey reported high levels of CB1R in both PN and IN somata in the BNC, but stained almost no axon terminals [23]. Likewise, using the same antibody, we saw negligible axonal staining in the BNC of the rat [7]. This discrepancy may be due to the use of different antibodies and forms of the receptor protein in somata versus axons. The CB1R antibody used by Ong and Mackie [23] was raised against the N-terminal 77 amino acids of the cloned rat CB1R whereas the antibody used in the present study was raised against the C-terminal 31 amino acids of mouse CB1R.
The expression of CB1Rs in the axon terminals of CCKBCs of both monkey and rodents suggests that similar synaptic mechanisms may exist in both species. In rodents axon terminals of CCK+/CB1R+ basket cells form symmetrical (inhibitory) synapses with PN somata that exhibit the molecular machinery required for endocannabinoid signaling [8-10]. Thus, the somatic region apposed to these CCK+ axon terminals expressed diacylglyerol lipase (DGL), the enzyme that synthesizes the endocannabinoid 2-arachidonoylglycerol (2-AG), whereas the CCK+ axon terminals expressed CB1Rs and monoacylglyerol lipase (MGL; the enzyme that degrades 2-AG) [9, 10].
There is evidence that GABA and CCK released at axosomatic synapses of CCKBCs activate GABAAα1-containing and CCK-2 receptors (CCK2Rs), respectively [10]. In contrast to GABA, the activation of CCK2Rs at these synapses increases neuronal excitability through membrane depolarization [24]. As at other cannabinergic synapses, depolarization of postsynaptic PNs synthesizes and releases endocannabinoids which suppress GABA and CCK release in presynaptic CCK+ terminals by activating presynaptic CB1Rs in a retrograde manner [8, 9, 12, 13, 25].
Interactions of CCK and endocannabinoids in the BNC modulate fear expression and extinction in rodents [14, 15]. CCK causes panic attacks in humans [26] and increases in anxiety-like behavior and fear expression in rodents [14]. CCK in the BNC is anxiogenic due to its excitation of PNs via CCK2Rs [24]. Extinction is associated with increases in the synthesis of two endocannabinoids in the BNC, 2-AG and anandamide [13]. Investigations by Ressler’s group suggest that endocannabinoids released from activated PNs suppress CCK release from presynaptic CCK terminals by activating CB1Rs, which in turn decreases the normal activation of PNs via CCK2Rs [14, 15]. This weakening of PN excitation appears to dampen fear behavior and enhance fear extinction. Since many anxiety disorders, including PTSD, are associated with disruptions in fear learning and/or extinction, it has been suggested that therapies that target the cannabinoid system in the BNC may be useful for treating anxiety disorders in human patients [14, 16].
Highlights.
CB1R immunoreactivity is very dense in the monkey basolateral nuclear complex (BNC)
Many CB1R+ axons formed basket-like plexuses around pyramidal cell somata
There is extensive colocalization of CB1R and CCK in axon terminals
These data suggest interactions between CB1Rs and CCK in the primate BNC
Acknowledgements
The author is grateful to Dr. E. Chris Muly (Emory University and Yerkes National Primate Research Center, Atlanta, Georgia) for the generous donation of the monkey amygdalas. The technical assistance of Grace Jones is greatly appreciated. This work was supported by the National Institutes of Health Grant R01MH104638 to A.J. McDonald and D.D. Mott, and a Yerkes Center Grant (P51OD011132).
Footnotes
Declaration of Competing Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability statement
The data that support the findings of this study are available from the author upon reasonable request.
References
- [1].McDonald AJ, Functional neuroanatomy of the basolateral amygdala: neurons, neurotransmitters, and circuits in: Urban JH and Rosenkranz JA (Eds.), Handbook of amygdala structure and function, Academic Press, San Diego, 2020, pp. 1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Spampanato J, Polepalli J, Sah P, Interneurons in the basolateral amygdala. Neuropharmacology 60 (2011) 765–773. [DOI] [PubMed] [Google Scholar]
- [3].Capogna M, GABAergic cell type diversity in the basolateral amygdala. Curr Opin Neurobiol. 26 (2014) 110–116. [DOI] [PubMed] [Google Scholar]
- [4].Krabbe S, Gründemann J, Lüthi A, Amygdala inhibitory circuits regulate associative fear conditioning. Biol Psychiatry. 83 (2018) 800–809. [DOI] [PubMed] [Google Scholar]
- [5].Vereczki VK, Veres JM, Müller K, Nagy GA, Rácz B, Barsy B, Hájos N, Synaptic organization of perisomatic GABAergic inputs onto the principal cells of the mouse basolateral amygdala. Front Neuroanat.10 (2016) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Veres JM, Nagy GA, Hájos N, Perisomatic GABAergic synapses of basket cells effectively control principal neuron activity in amygdala networks. Elife. 2017. January 6;6 pii: e20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McDonald AJ, Mascagni F, Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin containing interneurons. Neuroscience 107 (2001) 641–652. [DOI] [PubMed] [Google Scholar]
- [8].Katona I, Rancz EA, Acsady L, Ledent C, Mackie K K, Hajos N, Freund TF Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission, J Neurosci. 21 (2001) 9506–9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yoshida T, Uchigashima M, Yamasaki M, Katona I, Yamazaki M, Sakimura K, Kano M, Yoshioka M, Watanabe M, Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proc Natl Acad Sci U S A. 108 (2011) 3059–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Omiya Y, Uchigashima M, Konno K, Yamasaki M, Miyazaki T, Yoshida T, Kusumi I, Watanabe M, VGluT3-expressing CCK-positive basket cells construct invaginating synapses enriched with endocannabinoid signaling proteins in particular cortical and cortex-like amygdaloid regions of mouse brains. J Neurosci. 35 (2015) 4215–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rovira-Esteban L, Péterfi Z, Vikór A, Máté Z, Szabó G, Hájos N, Morphological and physiological properties of CCK/CB1R-expressing interneurons in the basal amygdala. Brain Struct Funct. 222 (2017) 3543–3565. [DOI] [PubMed] [Google Scholar]
- [12].Beinfeld MC, Connolly K, Activation of CB1 cannabinoid receptors in rat hippocampal slices inhibits potassium-evoked cholecystokinin release, a possible mechanism contributing to the spatial memory defects produced by cannabinoids. Neurosci Lett 301 (2001) 69–71. [DOI] [PubMed] [Google Scholar]
- [13].Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Grazia Cascio M, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B, The endogenous cannabinoid system controls extinction of aversive memories. Nature. 418 (2002) 530–534. [DOI] [PubMed] [Google Scholar]
- [14].Chhatwal JP, Gutman AR, Maguschak KA, Bowser ME, Yang Y, Davis M, Ressler KJ, Functional interactions between endocannabinoid and CCK neurotransmitter systems may be critical for extinction learning. Neuropsychopharmacology 34 (2009) 509–521. [DOI] [PubMed] [Google Scholar]
- [15].Bowers ME, Ressler KJ, Interaction between the cholecystokinin and endogenous cannabinoid systems in cued fear expression and extinction retention. Neuropsychopharmacol. 40 (2015) 688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chhatwal JP, Ressler KJ (2007) Modulation of fear and anxiety by the endogenous cannabinoid system. CNS Spectr. 12:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Price JL, Russchen FT, Amaral DG, The limbic region. II. The amygdaloid complex in: Bjorklund A, Hokfelt T, Swanson LW, (Eds.) Handbook of chemical neuroanatomy, vol. 5, Elsevier Science Publishers, Amsterdam, 1987, pp. 279–388. [Google Scholar]
- [18].Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M, Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 27 (2007) 3663–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McDonald AJ, Mascagni F, Cholecystokinin immunoreactive neurons in the basolateral amygdala of the rhesus monkey (Macaca mulatta). J Comp Neurol. 527 (2019) 2694–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hamill TG, Lin LS, Hagmann W, Liu P, Jewell J, Sanabria S, Eng W, Ryan C, Fong TM, Connolly B, Vanko A, Hargreaves R, Goulet MT, Burns HB, PET imaging studies in rhesus monkey with the cannabinoid-1 (CB1) receptor ligand [11C]CB-119. Mol Imaging Biol. 11 (2009) 246–252. [DOI] [PubMed] [Google Scholar]
- [21].Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M, Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer's brains. Neuroscience 63 (1994) 637–652. [DOI] [PubMed] [Google Scholar]
- [22].Glass M, Dragunow M, Faull RL RL, Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77 (1997) 299–318. [DOI] [PubMed] [Google Scholar]
- [23].Ong WY, Mackie K, A light and electron microscopic study of the CB1 cannabinoid receptor in primate brain. Neuroscience 92 (1999) 1177–1191. [DOI] [PubMed] [Google Scholar]
- [24].Meis S, Munsch T, Sosulina I, Pape HC, Postsynaptic mechanisms underlying responsiveness of amygdaloid neurons to cholecystokinin are mediated by a transient receptor potential-like current. Mol Cell Neurosci. 35 (2007) 356–367. [DOI] [PubMed] [Google Scholar]
- [25].Azad SC, Eder M, Marsicano G, Lutz B, Zieglgänsberger W, Rammes G, Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learn Mem. 10 (2003) 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].de Montigny C, Cholecystokinin tetrapeptide induces panic-like attacks in healthy volunteers. Preliminary findings. Arch Gen Psychiatry. 46 (1989) 511–517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the author upon reasonable request.