Abstract
Introduction:
Different methods have resulted in variable Z-scores for echocardiographic measurements. Using the measurements from 3215 healthy North American children in the Pediatric Heart Network (PHN) echocardiographic Z-score database, we compared the PHN model with previously published Z-score models.
Methods:
Z-scores were derived for cardiovascular measurements using four models (PHN, Boston, Italy, Detroit). Model comparisons were performed by evaluating 1) overlaid graphs of measurement versus body surface area (BSA) with curves at Z = −2, 0, +2; 2) scatterplots of PHN versus other Z-scores with correlation coefficients; 3) Bland-Altman plots of PHN versus other Z-scores; and 4) comparison of median Z-scores for each model.
Results:
For most measurements, PHN Z-score curves were similar to Boston and Italian curves but diverged from Detroit curves at high BSAs. Correlation coefficients were high when comparing the PHN model with the others, highest with Boston (mean 0.99) and lowest with Detroit (mean 0.90). Scatterplots suggested systematic differences despite high correlations. Bland-Altman plots also revealed poor agreement at both extremes of size and a systematic bias for most when comparing PHN against Italian and Detroit Z-scores. There were statistically significant differences when comparing median Z-scores between the PHN and other models.
Conclusion:
Z-scores from the multicenter PHN model correlated well with previous single center models, especially the Boston model, which also had a large sample size and similar methodology. The Detroit Z-scores diverged from the PHN Z-scores at high BSAs, possibly because there were more subjects in this category in the PHN database. Despite excellent correlation, significant differences in Z-scores between the PHN model and others were seen for many measurements. This is important when comparing publications using different models and for clinical care, particularly when Z-score thresholds are used to guide diagnosis and management.
Keywords: Z-scores, cardiovascular growth, echocardiographic quantification
Background
Echocardiographic Z-scores based on body size are used to normalize the sizes of cardiovascular structures in growing children.1–12 They represent the number of standard deviations a particular cardiovascular measurement is from the mean value for the measurement at any given body size, thereby providing the best approach to determine normal reference values for the sizes of cardiovascular structures in the pediatric population.13,14 The Pediatric Heart Network (PHN) recently established a robust normative database of the most common two-dimensional echocardiographic measurements in 3215 healthy and racially diverse North American children.1 This multicenter study revealed that Z-score models based on body surface area (BSA) for these measurements are not affected by age, sex, race, or ethnicity.
Published allometric models evaluating the relationship between cardiovascular growth and total body growth frequently yield different Z-scores for a single measurement in the same patient since they use heterogeneous measurement performance and normalization methodologies.15–19 The most popular models used in clinical practice derived regression equations to characterize this relationship using a mathematical transformation of the body size parameter, the raw measurement value, or both. 1,2,6,20 For example, the PHN1 and Boston20 models used an exponential transformation of BSA and no transformation of the measurement value for the regression equations of most of the parameters (Table 1). The Italian model2 involved logarithmic transformations of both BSA and the measurement value, whereas the Detroit model6 involved logarithmic transformations of the measurement value and polynomial transformations of BSA.
Table 1:
Characteristics of the models used for comparison.
| Model Characteristics | ||||
|---|---|---|---|---|
| Z-Score Model | PHN1 | Boston20 | Italy2 | Detroit6 |
| Reference | Lopez, 2017 | Boston Z-Scores* | Cantinotti, 2017 | Pettersen, 2008 |
| Model Regression# | y = m×BSAα | y = m×BSAα (y = β + m×BSAα for the coronary arteries) | ln(y) = b + (m×ln[BSA]) | ln(y) = b + (m1×BSA) + (m2×BSA2) + (m3×BSA3) |
| Data Source | 19 centers | 1 center | 1 center | 1 center |
| Sample Size | 3215 | >2000 | 1151 | 782 |
| Study Population | 1) Weight-forlength Z <2 if <2 years old 2) BMI < 95th percentile if ≥2 years old 3) Gestational age ≥37 weeks 4) No structural or congenital heart disease 5) No systemic disorder with cardiovascular manifestations 6) No family history of left heart disease or cardiomyopathy | 1) Weight >2.5 kg 2) BSA >0.18 m2 3) −2 > BMI Z > 2 4) No premature babies 5) No structural or congenital heart disease 6) No systemic disorder with cardiovascular manifestations 7) No family history of left heart disease or cardiomyopathy |
1) Weight-forlength Z <2 if <2 years old 2) BMI < 95th percentile if ≥2 years old 3) Premature babies included 4) No structural or congenital heart disease 5) No systemic disorder with cardiovascular manifestations 6) No family history of genetic cardiac disease | 1) No obese subjects 2) BSA <2 m2 3) No reference to gestational age or BMI 4) No structural or congenital heart disease 5) No systemic disorder with cardiovascular manifestations 6) No family history of genetic cardiac disease |
| Race Data | Multiracial | Not reported | Caucasian | Not reported |
| Observers | 2 | Multiple | 1 | Multiple |
(BMI = body mass index, BSA = body surface area, PHN = Pediatric Heart Network).
The original publication from Boston involved only 496 children (8), but the regression equations used for comparison in this study were based on data from >2000 children with variable sample sizes for each parameter.19
y = measurement value; b = intercept; m, m1, m2, m3 = slope values.
Because these Z-score models are frequently used in both pediatric clinical practice and research studies, it is important to recognize the differences among the models as well as the limitations associated with using multiple models for a specific clinical scenario or research investigation. A recent study performed a theoretical statistical comparison of the PHN model to the Boston, Italian, and Detroit models and found differences in the predicted ranges of normal values for the sizes of 12 cardiovascular structures.21 Using actual echocardiographic measurements from healthy, normal children in the PHN database, we sought to compare and contrast the Boston, Italian, and Detroit models with the newly published PHN model by calculating the Z-scores based on each model for all the measurements in the database.
Methods
PHN Echocardiographic Z-Score Database
The multicenter PHN Echocardiographic Z-score Project collected demographic data and high-quality echocardiograms performed as standard of care from a study population of 3215 healthy children ≤18 years of age. All subjects had documentation of height, weight, sex, race, and ethnicity, and enrollment involved systematic sampling across the full range of age, sex, and race. A core laboratory measured pre-specified echocardiographic diameters, widths, and lengths using the strict pediatric quantification guidelines from the American Society of Echocardiography,22 and the data coordinating center used these values to calculate areas, volumes, mass, and ratios (Table 2).
Table 2:
Measurements and calculations performed in the Pediatric Heart Network Echocardiogram Z-score model (Column 1) and in the other three models (Columns 2 – 4).
| Measurements and Calculations for all 4 Models | |||
|---|---|---|---|
| PHN | Boston | Italy | Detroit |
| ANN | ANN | ANN | ANN |
| ROOT | ROOT | ROOT | ROOT |
| STJ | STJ | STJ | STJ |
| AAO | AAO | AAO | |
| ARCHPROX | ARCHPROX | ARCHPROX | |
| ARCHDIST | ARCHDIST | ARCHDIST | ARCHDIST |
| ISTH | ISTH | ISTH | ISTH |
| LMCA | LMCA | ||
| LAD | LAD | ||
| RCA | RCA | ||
| PVSAX | |||
| PVLAX | PVLAX | PVLAX | PVLAX |
| MPA | MPA | MPA | MPA |
| RPA | RPA | RPA | RPA |
| LPA | LPA | LPA | LPA |
| MVLAT | MVLAT | MVLAT | MVLAT |
| MVAP | MVAP | ||
| MVA | MVA | ||
| TVLAT | TVLAT | TVLAT | TVLAT |
| TVAP | TVAP | ||
| TVA | TVA | ||
| LVEDD | LVEDD | LVEDD | |
| LVESD | LVESD | LVESD | |
| LVPWT | LVPWT | LVPWT | |
| LVST | LVST | LVST | |
| LVEDV | LVEDV | ||
| LVEDVEPI | LVEDVEPI | ||
| LVESV | LVESV | ||
| LVM | LVM | ||
| LVMVR | LVMVR | ||
| LVTDR | LVTDR | ||
| LVSI | LVSI | ||
(AAO = ascending aortic diameter, ANN = aortic annular diameter, ARCHDIST = distal transverse aortic diameter, ARCHPROX = proximal transverse aortic arch diameter, ISTH = aortic isthmus diameter, LAD = left anterior descending coronary artery, LMCA = left main coronary artery diameter, LPA = left pulmonary artery diameter, LVEDD = left ventricular end-diastolic dimension, LVEDV = endocardial left ventricular end-diastolic volume, LVEDVEPI = epicardial left ventricular end-diastolic volume, LVESD = left ventricular end-systolic dimension, LVESV = endocardial left ventricular end-systolic volume, LVM = left ventricular mass, LVMVR = left ventricular mass-to-volume ratio, LVPWT = left ventricular end-diastolic posterior wall thickness, LVSI = left ventricular sphericity index, LVST = left ventricular end-diastolic septal thickness, LVTDR = left ventricular thickness-to-dimension ratio, MPA = main pulmonary artery diameter, MVA = mitral annular area, MVAP = anteroposterior mitral annular diameter, MVLAT = lateral mitral annular diameter, PHN = Pediatric Heart Network, PVLAX = long-axis pulmonary annular diameter, PVSAX = short-axis pulmonary annular diameter, RCA = right coronary artery diameter, ROOT = aortic root diameter, RPA = right pulmonary artery diameter, STJ = aortic sinotubular junction diameter, TVA = tricuspid annular area, TVAP = anteroposterior tricuspid annular diameter, TVLAT = lateral tricuspid annular diameter).
Z-Score Derivation
Z-scores were derived from the raw measurements recorded for all parameters in the PHN normative database using the four models (PHN, Boston, Italy, Detroit). The latest version of the Boston model published in June 2020 was used for comparison.20 The Detroit model did not include subjects with a BSA >2 m2, so Z-scores based on this model were not calculated for subjects in the PHN database with a BSA >2 m2. Because the regression equations and Z-score calculations for left ventricular (LV) end-systolic dimension and volume were not published for the PHN model, Z-scores for these parameters were derived and evaluated for clinically significant effects of age, sex, race, and ethnicity using the PHN methodology to index parameter values to BSA.1 This approach determined the appropriate BSA transformation (BSAα) to index the parameter value (parameter/BSAα), after which the mean value and standard deviation (SD) of the indexed parameter value were used in the following equation:
Clinically significant measurement differences were defined as those that exceeded the reproducibility of the echocardiographic measurement.
Statistical Analysis
The measurements available for comparison among the models are listed in Table 2. A comparison of Z-scores from the PHN model against each of the other models was performed for all parameters using four different analytic methods. The first was a visual comparison of overlaid graphs with non-transformed BSA values on the x-axis and non-transformed measurement values on the y-axis showing curves at Z-score = −2, 0, and +2 for the PHN model versus each of the other models. The second involved a visual comparison using scatterplots of the PHN Z-scores versus the Z-scores derived from each of the other models. The Pearson correlation coefficients for each of these comparisons were then calculated. The third involved Bland-Altman plots comparing the PHN Z-scores to those using the other models. In the final method, median Z-scores for all parameters using each of the models were calculated. The PHN median Z-scores were then compared to the median Z-scores based on the other models, and statistically significant differences were determined using the Kruskal-Wallis test.
Results
Characteristics of the Z-Score Models
The PHN study involved subjects from 19 centers, whereas the other three models used data from single centers (Table 1). The PHN model involved 3215 children, whereas the sample sizes for the other models ranged from 782 to >2000 children (the original Boston publication involved only 496 children,8 but the regression equations used for comparison in this study were based on data from >2000 children20). The PHN study collected data from nearly equal numbers of subjects in three race categories (White, African American, and Other); the Italian model involved only Caucasian subjects; and race data were not reported for the Boston or Detroit models. Measurements were performed by two observers for the PHN model, a single observer in the Italian model, and multiple observers in the Boston and Detroit models. Z-scores using the PHN model were determined for LV end-systolic dimension and volume (Table 3). Residual effects of BSA, age, sex, race, and ethnicity were found to be clinically insignificant for this analysis.
Table 3:
Components of Z-score calculations for two new parameters using the PHN methodology that have not been previously published.
| Z-Score Calculation Elements Not Previously Published* | |||
|---|---|---|---|
| Parameter | α | Mean Indexed Value | SD |
| LVESD (cm) | 0.5 | 2.675 | 0.352 |
| LVESV (ml) | 1.3 | 25.76 | 5.551 |
(LVESD = left ventricular end-systolic dimension, LVESV = left ventricular end-systolic volume, SD = standard deviation).
The other Z-score calculation elements have been previously published1 and are available at http://www.pediatricheartnetwork.org/ResourcesPublications/EchoZ-Scores.aspx; Z-scores are calculated as Z=[(Parameter/BSAα) – Mean]/SD.
Comparisons of Z-Score Curves
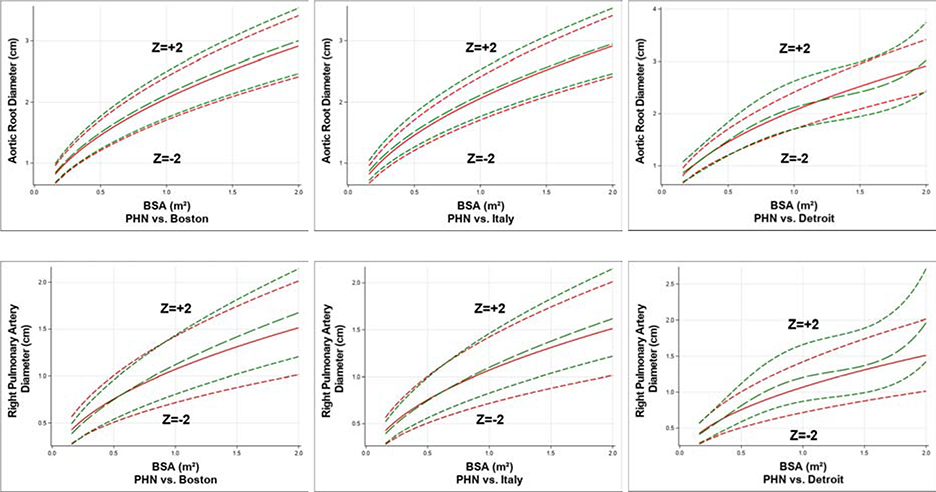
For most parameters, the curves at Z-score = −2, 0, and +2 for the PHN model were generally similar to equivalent curves for the Boston and Italian models, but they diverged from equivalent curves for the Detroit model at higher BSA values. Figure 1 displays two examples of these comparisons for the aortic root and the right pulmonary artery, and Supplementary Appendix 1 displays the comparison for all of the study parameters.
Figure 1:
Comparisons of curves at Z-score = −2, 0, and +2 for the PHN model (red lines) versus similar curves for the Boston, Italy, and Detroit models (green lines) for aortic root diameter and right pulmonary artery diameter. (BSA = body surface area, PHN = Pediatric Heart Network)
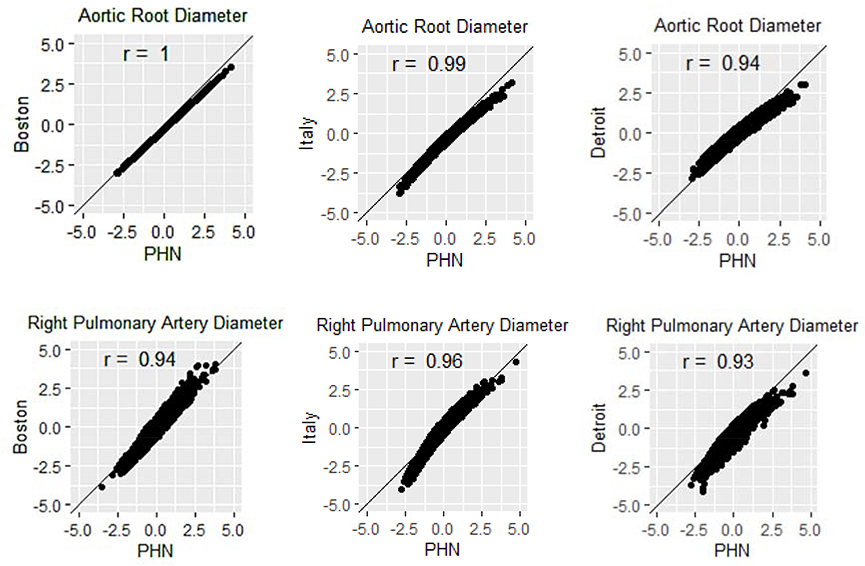
Scatterplots and Correlation Coefficients
Scatterplots comparing the PHN Z-scores against the Z-scores derived using the comparison models revealed high correlation coefficients (0.82 – 1.00) but suggested systematic differences in some measures. Figure 2 displays two examples of scatterplots for the aortic root and right pulmonary artery, and Supplementary Appendix 2 displays the scatterplots for all study parameters. Among all comparisons, the mean correlation coefficient was highest for PHN versus the Boston model (mean 0.99, range 0.94 – 1.00), followed by PHN versus the Italian model (mean 0.98, range 0.95 – 0.99), and lowest for PHN versus the Detroit model (mean 0.92, range 0.82 – 0.96).
Figure 2:
Scatterplots comparing the PHN Z-scores versus the Boston, Italian, and Detroit Z-scores for the aortic root diameter and the right pulmonary artery diameter. (PHN = Pediatric Heart Network)
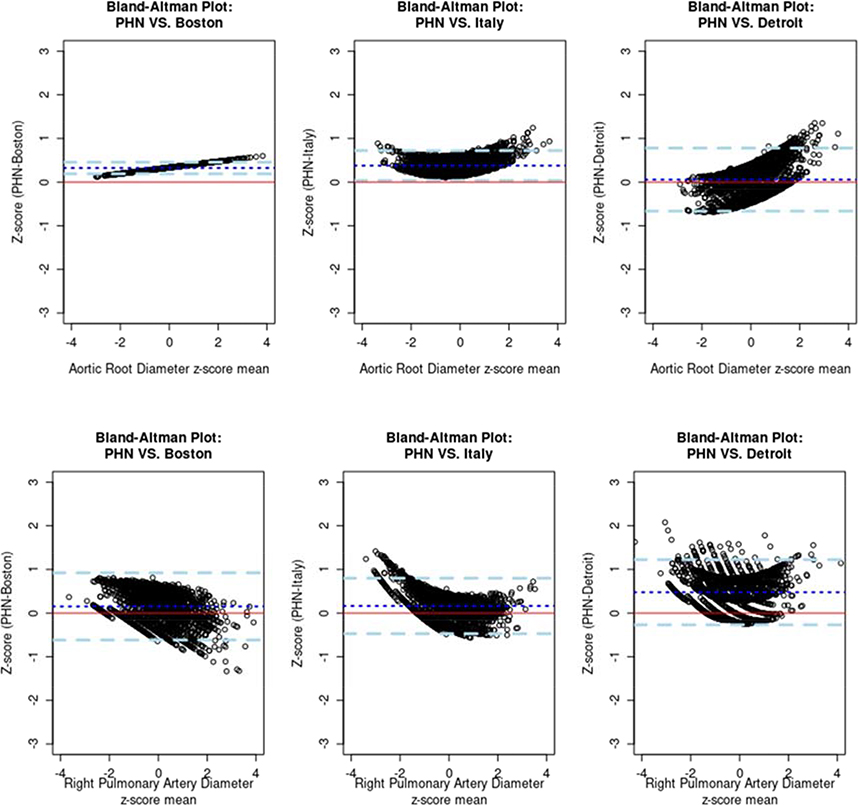
Bland-Altman Plots
Comparison of the PHN Z-scores to the Boston Z-scores revealed the best agreement (both models used the exponential approach), but differences in Z-scores tended to either increase or decrease linearly with increasing size. In contrast, the plots comparing the PHN Z-scores to the Italian and Detroit Z-scores revealed a parabolic or convex shape instead of a linear shape for most measurements. Figure 3 displays two examples of Bland-Altman plots for the aortic root and right pulmonary artery, and Supplementary Appendix 3 displays the Bland-Altman plots for all study parameters.
Figure 3:
Bland-Altman plots comparing the PHN Z-scores versus the Boston, Italian, and Detroit Z-scores for the aortic root diameter and the right pulmonary artery diameter. The red line is the 0-reference line and the blue dashed lines are the 95% confidence interval around the mean difference (dark blue dotted line). (PHN = Pediatric Heart Network)
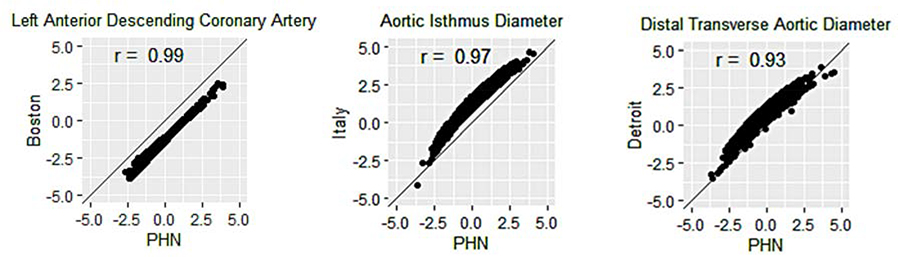
Comparison of Median Z-scores
Given the large sample size in the PHN normative database, almost all of the median Z-score comparisons between the PHN model and the other models revealed statistically significant differences (Table 4). The left anterior descending coronary artery (LAD) diameter in the Boston model, aortic isthmus diameter in the Italian model, and distal aortic arch diameter in the Detroit model had the largest median Z-score differences when compared with the PHN model (Figure 4). The large difference in median LAD Z-scores between the PHN and Boston models existed despite a correlation coefficient of 0.99. In fact, further evaluation of this difference revealed the following mathematical relationship via linear regression modeling: ZLAD(PHN) = ZLAD(Boston) + 1.3; in other words, the PHN Z-scores were systematically 1.3 points higher than the Boston Z-scores for the LAD.
Table 4:
Median Z-scores for all the parameters used for comparison among the different models with red values designating the median Z-scores that are significantly different from the corresponding median PHN Z-scores (p < 0.05).
| Median Z-Scores | ||||
|---|---|---|---|---|
| Parameter | PHN | Boston | Italy | Detroit |
| ANN | 0.05 | −0.51 | −0.54 | −0.12 |
| ROOT | −0.03 | −0.35 | −0.35 | −0.04 |
| STJ | −0.01 | −0.28 | 0.33 | 0.21 |
| AAO | −0.03 | −0.90 | −0.74 | NA |
| ARCHPROX | <−0.01 | NA | −0.08 | −0.31 |
| ARCHDIST | 0.01 | −0.29 | 0.34 | 0.77 |
| ISTH | −0.03 | 0.09 | 1.23 | 0.38 |
| LMCA | −0.08 | −0.04 | NA | NA |
| LAD | −0.03 | −1.33 | NA | NA |
| RCA | −0.11 | −0.22 | NA | NA |
| PVSAX | −0.02 | NA | NA | NA |
| PVLAX | <0.01 | 0.10 | 0.92 | 0.68 |
| MPA | <0.01 | −0.43 | −0.41 | −0.04 |
| RPA | −0.04 | −0.20 | −0.11 | −0.41 |
| LPA | 0.02 | 0.00 | 0.24 | 0.65 |
| MVAP | −0.02 | 0.55 | NA | NA |
| MVLAT | −0.02 | 0.10 | −0.74 | −0.57 |
| MVA | −0.04 | 0.36 | NA | NA |
| TVAP | <−0.01 | 0.15 | NA | NA |
| TVLAT | 0.01 | 0.18 | −0.26 | −0.51 |
| TVA | −0.04 | 0.20 | NA | NA |
| LVEDD | −0.01 | −0.25 | NA | 0.02 |
| LVESD | −0.03 | 0.23 | NA | 0.73 |
| LVPWT | −0.13 | −0.60 | NA | 0.12 |
| LVST | −0.14 | −1.03 | NA | −0.36 |
| LVEDV | −0.05 | −0.82 | NA | NA |
| LVEDVEPI | −0.05 | −0.88 | NA | NA |
| LVESV | −0.07 | 0.00 | NA | NA |
| LVM | −0.07 | −0.66 | NA | NA |
| LVMVR | −0.13 | 0.08 | NA | NA |
| LVTDR | −0.12 | −0.68 | NA | NA |
| LVSI | −0.03 | −0.11 | NA | NA |
(AAO = ascending aortic diameter, ANN = aortic annular diameter, ARCHDIST = distal transverse aortic diameter, ARCHPROX = proximal transverse aortic arch diameter, ISTH = aortic isthmus diameter, LAD = left anterior descending coronary artery, LMCA = left main coronary artery diameter, LPA = left pulmonary artery diameter, LVEDD = left ventricular end-diastolic dimension, LVEDV = endocardial left ventricular end-diastolic volume, LVEDVEPI = epicardial left ventricular end-diastolic volume, LVESD = left ventricular end-systolic dimension, LVESV = endocardial left ventricular end-systolic volume, LVM = left ventricular mass, LVMVR = left ventricular mass-to-volume ratio, LVPWT = left ventricular end-diastolic posterior wall thickness, LVSI = left ventricular sphericity index, LVST = left ventricular end-diastolic septal thickness, LVTDR = left ventricular thickness-to-dimension ratio, MPA = main pulmonary artery diameter, MVA = mitral annular area, MVAP = anteroposterior mitral annular diameter, MVLAT = lateral mitral annular diameter, PHN = Pediatric Heart Network, PVLAX = long-axis pulmonary annular diameter, PVSAX = short-axis pulmonary annular diameter, RCA = right coronary artery diameter, ROOT = aortic root diameter, RPA = right pulmonary artery diameter, STJ = aortic sinotubular junction diameter, TVA = tricuspid annular area, TVAP = anteroposterior tricuspid annular diameter, TVLAT = lateral tricuspid annular diameter).
Figure 4:
Parameters with the Largest Median Z-score Difference for Each Comparison with the PHN Model. (PHN = Pediatric Heart Network)
Discussion
Z-scores are critical in the assessment of cardiovascular sizes among growing children. Z-scores based on measurements made in different populations and calculated from mathematically different models are often used in the same clinical scenario or research project without accounting for the differences in the populations and the models. The Z-scores derived from the multicenter PHN model based on a large, diverse pediatric population correlated well with Z-scores derived from commonly used single center models. This is despite the fact that the PHN dataset was used specifically to develop the PHN model, whereas data from different populations were used to develop the other models, thereby affording the PHN model an advantageous goodness of fit for the study database. For most parameters, curves depicting increasing raw measurement values with increasing BSA that corresponded to Z-scores of −2, 0, and +2 were similar among the models, particularly at lower BSA values. Equivalent curves diverged at higher BSA values, particularly for the PHN versus Detroit comparison, likely related to mathematical differences in the approach to fitting the regression equations and to the availability of more subjects with high BSA values in the PHN database.
However, despite excellent correlations, the Z-scores derived from the four models exhibited some discrepancies. There were significant differences among the models for many parameters, as highlighted by the scatterplots, the Bland-Altman plots, and the comparison of median Z-scores. In other words, systematic differences existed among the Z-score models. The etiology for these differences is likely multifactorial. Heterogeneous echocardiographic protocols may be present at each individual center and for single versus multiple observers, especially if some of the data used to develop the model were obtained prior to the publication of the pediatric quantification guidelines by the American Society of Echocardiography.22 An example of protocol differences involves the right pulmonary artery diameter, which was measured in a parasternal short-axis view for three of the models1,2,8 and in a suprasternal short-axis view for the fourth model.6 Secondly, there may have been subtle differences in the inclusion and exclusion criteria for each population, such as the extent to which normal, healthy individuals were identified for inclusion in the study; this factor could potentially lead to variable study populations and hence different Z-scores. Finally, variable Z-score modeling methodology using different mathematical approaches to fitting regression equations will lead to different Z-scores, whether the model involves transformation of the body size parameter and/or transformation of the measurement value as well as exponential versus logarithmic versus polynomial transformation of the parameters. It is also important to note that the biggest differences in median Z-scores were found for the smallest structures, namely the LAD, distal aortic arch, and aortic isthmus. Although they are not a cause of the systematic bias discussed above, these differences suggest that variability and random error for smaller structures are higher, especially with the improving but still finite spatial resolution of available echocardiographic technology.
The visual comparisons of the Z-score curves and the correlation comparisons showed that similarities in Z-scores are most common between the PHN and Boston models. Although the original publication describing the Boston model involved only 496 subjects,8 updates to the Boston Z-scores and regression equations have become available periodically over the years as more subjects were included in the Boston database,20 allowing for a specific measurement in the same individual to have different Boston Z-scores depending on the timing of the measurement. This is in contrast to established and unchanging regression equations from the other three published models. The PHN model and the most recent Boston model had the largest sample sizes, and both used the same normalization method with exponential transformation of BSA for most parameters. In addition, the rigorous inclusion of subjects at lower and higher BSA values likely contributed to better modeling across the full range of body sizes encountered in the pediatric population. When comparing the PHN Z-scores against the Italian and Detroit Z-scores, the differences in sampling as well as the different mathematical approaches likely explain the divergence of Z-scores at the extremes of size in the Bland-Altman plots. This is particularly important since the impact of many clinical decisions involving Z-scores is usually more significant at the extremes of cardiovascular size where the degree of hypoplasia or enlargement is likely to affect the plan of care.
It is difficult to know which of the four models is best suited for routine clinical use in a pediatric echocardiography laboratory. The PHN measurements were made by only two observers in the core laboratory whereas the Boston measurements were made by multiple observers during routine evaluation at their center. Studies with multiple observers are more representative of “real world” clinical practice and associated with wider confidence intervals, whereas studies with fewer observers in a multicenter study avoid the systematic error that occurs from different center-specific measurement methodologies. In fact, the differences in methodologies may play the more important role in distinguishing the Z-score models. Center-specific measurement methodologies could not be evaluated, however, since the multicenter PHN model included only core laboratory measurements. In addition, no measurements made as routine practice from any individual center were collected. Future studies evaluating the effects of center-specific practices would be valuable to further elucidate the etiology for variable Z-scores from different models.
Lastly, it is not unusual for Z-score thresholds to be used in clinical practice guidelines, which are based on expert consensus and/or scientific evidence. Examples of recommended Z-score thresholds for treatment can be found for LV end-systolic diameters in patients with aortic regurgitation,23 aortic root diameters in Marfan syndrome,24 and coronary artery diameters in Kawasaki disease.25 It is important to remember that there are few if any outcomes data that have established true inflection points or boundaries of uncertainty or confidence in the continuum of risk for these clinical scenarios, highlighting the limitations of Z-score thresholds for single variables in clinical guidelines. In addition, the difference of 1.3 noted for the LAD Z-scores when comparing the PHN and Boston models is an example of why it is important to choose the correct Z-score model when making clinical decisions for patients with Kawasaki disease. Because the studies cited in the guidelines establishing thresholds for treatment in this population used the Boston Z-scores,25 the Boston Z-scores should be used to guide therapeutic decisions in Kawasaki disease (assuming that the regression equations for the LAD have not changed significantly since publication of the guidelines). In other words, the same Z-score model on which outcome studies and guidelines are based should be used to make clinical decisions until such a time when outcomes utilizing the PHN Z-scores have been reported.
Conclusions
Z-scores from the PHN model correlated well with Z-scores from the comparison models, particularly for the Boston Z-scores, likely related to similar mathematical methods to fit the data in regression models and to larger sample sizes. However, significant differences in Z-scores were noted for many parameters, highlighting the fact that different modeling methodologies result in different Z-scores for the same measurement in the same patient, especially at the upper and lower extremes of BSA values. These differences influence the proportion of patients who fall outside the range of Z-score thresholds used to determine risk and to make clinical decisions. This is an important consideration for clinical care and research studies, especially when comparing publications and echocardiograms using different Z-score models. Clinicians should avoid using more than one Z-score model when making clinical decisions or determining trends over time in the care of a particular patient. Consistency is also important when researchers use Z-scores in studies to assess clinical impact, associations, and risk in a particular patient population.
Supplementary Material
Highlights.
Published Z-score models involve variable populations and normalization approaches.
Different models result in different Z-scores for the same measurement and person.
The rigorous multicenter PHN Z-score model compares favorably to other models.
Systematic differences among Z-scores should be considered in clinical decisions.
The same Z-score model should be used for practice guidelines and decision making.
Acknowledgments:
Brenda Ni, MPH, for preliminary statistical support.
Funding: The study was supported by grants (HL068270, HL068290, HL109673, HL109737, HL109741, HL109741, HL109743, HL109777, HL109778, HL109781, HL109816, HL109818) from the National Heart, Lung, and Blood Institute, NIH. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute.
Abbreviations:
- AAO
ascending aortic diameter
- ANN
aortic annular diameter
- ARCHDIST
distal transverse aortic diameter
- ARCHPROX
proximal transverse aortic arch diameter
- BSA
body surface area
- ISTH
aortic isthmus diameter
- LAD
left anterior descending coronary artery
- LMCA
left main coronary artery diameter
- LPA
left pulmonary artery diameter
- LV
left ventricular
- LVEDD
left ventricular end-diastolic dimension
- LVEDV
endocardial left ventricular end-diastolic volume
- LVEDVEPI
epicardial left ventricular end-diastolic volume
- LVESD
left ventricular end-systolic dimension
- LVESV
endocardial left ventricular end-systolic volume
- LVM
left ventricular mass
- LVMVR
left ventricular mass-to-volume ratio
- LVPWT
left ventricular end-diastolic posterior wall thickness
- LVSI
left ventricular sphericity index
- LVST
left ventricular end-diastolic septal thickness
- LVTDR
left ventricular thickness-to-dimension ratio
- MPA
main pulmonary artery diameter
- MVA
mitral annular area
- MVAP
anteroposterior mitral annular diameter
- MVLAT
lateral mitral annular diameter
- PHN
Pediatric Heart Network
- PVLAX
long-axis pulmonary annular diameter
- PVSAX
short-axis pulmonary annular diameter
- RCA
right coronary artery diameter
- ROOT
aortic root diameter
- RPA
right pulmonary artery diameter
- SD
standard deviation
- STJ
aortic sinotubular junction diameter
- TVA
tricuspid annular area
- TVAP
anteroposterior tricuspid annular diameter
- TVLAT
lateral tricuspid annular diameter
Footnotes
Declaration of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lopez L, Colan S, Stylianou M, Granger S, Trachtenberg F, Frommelt P, et al. Relationship of Echocardiographic Z Scores Adjusted for Body Surface Area to Age, Sex, Race, and Ethnicity: The Pediatric Heart Network Normal Echocardiogram Database. Circ Cardiovasc Imaging 2017;10(11):e006979. doi: 10.1161/CIRCIMAGING.117.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantinotti M, Giordano R, Scalese M, Murzi B, Assanta N, Spadoni I, et al. Nomograms for two-dimensional echocardiography derived valvular and arterial dimensions in Caucasian children. J Cardiol 2017;69:208–15. doi: 10.1016/j.jjcc.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Bhatla P, Nielsen JC, Ko HH, Doucette J, Lytrivi ID, Srivastava S. Normal values of left atrial volume in pediatric age group using a validated allometric model. Circ Cardiovasc Imaging 2012;5:791–6. doi: 10.1016/j.echo.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Lytrivi ID, Bhatla P, Ko HH, Yau J, Geiger MK, Walsh R, et al. Normal values for left ventricular volume in infants and young children by the echocardiographic subxiphoid five-sixth area by length (bullet) method. J Am Soc Echocardiogr 2011;24:214–8. doi: 10.1016/j.echo.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Olivieri L, Arling B, Friberg M, Sable C. Coronary artery Z score regression equations and calculators derived from a large heterogeneous population of children undergoing echocardiography. J Am Soc Echocardiogr 2009;22:159–64. doi: 10.1016/j.echo.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. J Am Soc Echocardiogr 2008;21:922–34. doi: 10.1016/j.echo.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Foster BJ, Mackie AS, Mitsnefes M, Ali H, Mamber S, Colan SD. A novel method of expressing left ventricular mass relative to body size in children. Circulation 2008;117:2769–75. doi: 10.1161/CIRCULATIONAHA.107.741157. [DOI] [PubMed] [Google Scholar]
- 8.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol 2005;99:445–57. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 9.Tacy TA, Vermilion RP, Ludomirsky A. Range of normal valve annulus size in neonates. Am J Cardiol 1995;75:541–3. doi: 10.1016/s0002-9149(99)80605-5. [DOI] [PubMed] [Google Scholar]
- 10.Gutgesell HP, Rembold CM. Growth of the human heart relative to body surface area. Am J Cardiol 1990;65:662–8. doi: 10.1016/0002-9149(90)91048-b. [DOI] [PubMed] [Google Scholar]
- 11.Hanseus K, Bjorkhem G, Lundstrom NR. Dimensions of cardiac chambers and great vessels by cross-sectional echocardiography in infants and children. Pediatr Cardiol 1988;9:7–15. doi: 10.1007/BF02279877. [DOI] [PubMed] [Google Scholar]
- 12.King DH, Smith EO, Huhta JC, Gutgesell HP. Mitral and tricuspid valve anular diameter in normal children determined by two-dimensional echocardiography. Am J Cardiol 1985;55:787–9. doi: 10.1016/0002-9149(85)90157-2. [DOI] [PubMed] [Google Scholar]
- 13.Chubb H, Simpson JM. The use of Z-scores in paediatric cardiology. Ann Pediatr Cardiol 2012;5:179–84. doi: 10.4103/0974-2069.99622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colan SD. The why and how of Z scores. J Am Soc Echocardiogr 2013;26:38–40. doi: 10.1016/j.echo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Cantinotti M, Scalese M, Molinaro S, Murzi B, Passino C. Limitations of current echocardiographic nomograms for left ventricular, valvular, and arterial dimensions in children: a critical review. J Am Soc Echocardiogr 2012;25:142–52. doi: 10.1016/j.echo.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Mawad W, Drolet C, Dahdah N, Dallaire F. A review and critique of the statistical methods used to generate reference values in pediatric echocardiography. J Am Soc Echocardiogr 2013;26:29–37. doi: 10.1016/j.echo.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Cantinotti M, Kutty S, Franchi E, Paterni M, Scalese M, Iervasi G, et al. Pediatric echocardiographic nomograms: What has been done and what still needs to be done. Trends Cardiovasc Med 2017;27:336–49. doi: 10.1016/j.tcm.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Simpson JM, Chubb H. Do We Finally Have the A to Z of Z Scores? Circ Cardiovasc Imaging 2017;10(11):e007191. doi: 10.1161/CIRCIMAGING.117.007191. [DOI] [PubMed] [Google Scholar]
- 19.Cantinotti M, Scalese M, Franchi E, Corana G, Viacav C, Assanta N, et al. Why use percentiles and not Z scores to calculate pediatric echocardiographic nomograms? The need for a uniform approach to data normalization. J Am Soc Echocardiog 2018;31:1068–70. doi: 10.1016/j.echo.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Boston Children’s Hospital Z-Score Calculator: http://zscore.chboston.org/ (Last access July 2020). [Google Scholar]
- 21.Cantinotti M, Scalese M, Giordano R, Assanta N, Marchese P, Franchi E, et al. A statistical comparison of reproducibility in current pediatric two-dimensional echocardiographic nomograms. Pediatr Res 2020. (epub ahead of print). doi: 10.1038/s41390-020-0900-z. [DOI] [PubMed] [Google Scholar]
- 22.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010;23:465–95; quiz 576–7. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Selamet Tierney ES, Gal D, Gauvreau K, Zhou J, Soluk Y, McElhinney DB, et al. Echocardiographic predictors of left ventricular dysfunction after aortic valve surgery in children with chronic aortic regurgitation. Congenit Heart Dis 2013;8:308–15. doi: 10.1111/chd.12009. [DOI] [PubMed] [Google Scholar]
- 24.Hoskoppal A, Menon S, Trachtenberg F, Burns KM, De Backer J, Gelb BD, et al. Predictors of Rapid Aortic Root Dilation and Referral for Aortic Surgery in Marfan Syndrome. Pediatr Cardiol 2018;39:1453–61. doi: 10.1007/s00246-018-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son MBF, Gauvreau K, Tremoulet AH, Lo M, Baker AL, de Ferranti S, et al. Risk Model Development and Validation for Prediction of Coronary Artery Aneurysms in Kawasaki Disease in a North American Population. J Am Heart Assoc 2019;8(11):e011319. doi: 10.1161/JAHA.118.011319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.