Abstract
Objective:
To measure the association between market-level promotional payments to urologists by the manufacturers of abiraterone and enzalutamide and national prescribing patterns.
Methods:
A 20% national sample of the 2015 Part D event file was used to identify patients filling their first prescription for abiraterone and enzalutamide and their prescribing physicians. The 2015 Open Payments data was used to characterize promotional payments made to physicians at the market level. Generalized linear models were then used to measure the relationship between market-level payments to urologists and the physician specialty prescribing abiraterone or enzalutamide for the first time
Results:
In 2015, 2318 men filled a prescription for abiraterone or enzalutamide by a urologist or medical oncologist. Increasing market-level promotional payments to urologists for abiraterone or enzalutamide was strongly associated with a urologist prescribing either drug—24.3% vs. 5.8% of those residing in the markets with highest and lowest level of promotional payments to urologists, respectively (p<0.01). Neither the number of urologists residing in a market nor other promotional payment measures (i.e., to medical oncologists for these drugs, or to all physicians for all other drugs) were associated with a urologist prescribing either drug.
Conclusion:
Promotional payments to urologists at the market level are strongly associated with the specialty of the physician prescribing abiraterone or enzalutamide for the first time. Future work should elucidate the effects of the shift in prescribing patterns on quality of care and financial hardship for men with advanced prostate cancer.
Keywords: prostate, cancer, treatment
Introduction
While there is tremendous heterogeneity in the severity of prostate cancer, the lethal form of prostate cancer accounts for 31,000 deaths annually, making it the second most common cause of cancer death among men. Spending for the disease approaches $12 billion annually and is expected to grow by nearly 40% this decade.1 A significant driver of this spending growth relates to the recent proliferation of “targeted” therapies for advanced prostate cancer, which are directed against specific molecules required for tumor growth. These oral agents improve survival,2,3 enhance quality-of-life4–7 and are much better tolerated than the physician-administered alternative, docetaxel.8 Thus, the agents represent a substantial step forward for the management of men with advanced prostate cancer.
Unlike docetaxel, an intravenous chemotherapy with unique certification and privileging requirements that restrict its use primarily to medical oncologists, targeted agents can be prescribed by any physician. The proliferation of these targeted agents has been accompanied by dramatic changes in urologist involvement in the care of men with advanced prostate cancer.9 The benefits of urologist involvement in managing these men may build on the often-longstanding patient-physician relationship. Such strong relationships have the potential to reduce fragmentation, improve compliance, and facilitate crucial conversations about expectations around treatment futility. Further, while medical oncologists manage patients with a wide variety of advanced malignancies, urologists are more focused, which may impart cost and quality benefits hypothesized in other “focused-factory” contexts.10,11 However, unlike medical oncologists, urologists are not always trained to manage important complications of targeted agents (e.g., hypertension, diabetes, and osteoporosis12–15) nor the progressive symptoms of lethal prostate cancer (pain, fatigue, and frailty5,16,17). Moreover, urologists might opt for targeted agents in circumstances where docetaxel chemotherapy is preferred based on randomized trial data. Indeed, pharmaceutical manufacturer payments to urologists to promote use of these drugs, either directly (i.e., gifts) or secondarily (i.e., speaker and consultant fees to opinion leaders), may spur adoption at a pace that outstrips development of expertise needed to ensure appropriate use and management of toxicity.
For these reasons, we performed a study using national Medicare data to assess relationships between promotional payments to urologists by the manufacturers of these drugs and prescribing patterns.
Methods
We used a 20% national sample of the 2015 Part D event file to identify patients aged 66 years or older filling their first prescription for an oral targeted agent (i.e., abiraterone or enzalutamide, which were the only approved agents at the time), and the specialty of the prescribing physician (i.e., urologist or medical oncologist). To ensure that we were assessing first fills, a 4-year “look back” was performed (i.e., from January 1, 2011 through December 31, 2014) to ensure the absence of claims for either drug.
Next, we used Open Payments data18 for 2015 to characterize promotional payments (i.e., not research-based payments) made to urologists by the manufacturers of abiraterone and enzalutamide (Janssen and Astellas, respectively), similar payments made to medical oncologists (to help discern specific targeting of urologists) and all other promotional payments to physicians (as a measure of non-specific structural factors associated with the magnitude of pharmaceutical payments). All three payment measures were aggregated to the level of the healthcare market, defined by the Dartmouth Atlas’ Hospital Referral Region, using the zip code of the physician to which they were made. We chose this market-level approach (as opposed to the physician-level) to capture the broad effects of promotional activities, since payments to key opinion leaders is one strategy that the industry uses to influence prescribing of other physicians within a market. In this way, targeted payments to key leaders within an area may have a multiplier effect. Promotional payments to urologists were then ranked and ordered by the cumulative amount for the year and then sorted into tertiles consisting of equal groups of markets: low (n=96, range $0 - $329), medium (n=97, range $330 to $3,028) and high (n=96, range $3,029 to $189,467). This served as the unit of exposure. To provide further contrast, we also explored differences in prescribing patterns at the margins (i.e., bottom [median $11] vs. top [median $79341] decile of market-level payments to urologists) as a secondary analysis.
Analysis
We first contrasted patient and market-level characteristics according to tertiles of promotional payments to urologists for abiraterone or enzalutamide using non-parametric tests. Next, we assessed relationships between our rank ordering of healthcare markets by promotional payments to urologists for abiraterone and enzalutamide with the other 2 payment measures (payments to medical oncologists for abiraterone or enzalutamide, and all other promotional payments to physicians) using Spearman’s Rank-Order Correlation.
Generalized linear models were then used to measure the relationship between promotional payments to urologists and the physician specialty prescribing the targeted agent for the first time, adjusting for patient age and race, and zip code level measures of socioeconomic class19 and urban development. The models were further adjusted for market-level characteristics, including, the number of urologists, promotional payments to medical oncologists for abiraterone or enzalutamide, and all other promotional payments to physicians.
All analyses were carried out using Stata 14 (College Station, TX). Model predicted probabilities were derived using the margins postestimation command in Stata. All tests were two-sided with probability of Type 1 error (alpha) set at 0.05. This study used de-identified administrative claims data and was deemed exempt from review by the institutional review board.
Results
In 2015, 2318 men filled a prescription for abiraterone or enzalutamide by a urologist or medical oncologist. Table 1 describes characteristics of the population stratified by the magnitude of promotional payments to urologists. Compared to those residing in markets associated with the lowest promotional payments to urologists for abiraterone or enzalutamide, men living in markets with the most payments were younger (78.4 vs. 79.2 years, respectively, p=0.02) and were more often from an underrepresented background (25.2% vs. 11.6%, respectively, p<0.01). Further, markets with the most payments to urologists were associated with a greater number of urologists (median 57 vs. 8 for highest and lowest, respectively; p<0.01).
Table 1.
Characteristics of patients receiving abiraterone or enzalutamide in 2015 stratified by extent of promotional payments by pharmaceutical manufacturers to urologists for abiraterone or enzalutamide.
| Characteristic | Promotional payments to urologists for abiraterone and enzalutamide at the level of the Hospital Referral Region | |||
|---|---|---|---|---|
| 1 (lowest) | 2 | 3 (highest) | p-value | |
| No. of patients | 267 | 572 | 1479 | - |
| No. of HRRs | 96 | 97 | 96 | - |
| Patient-level variables | ||||
| Age, mean (SD) | 79.2 (7.7) | 77.7 (7.1) | 78.4 (7.2) | 0.02 |
| Race, % non-white | 11.6 | 19.8 | 25.2 | <0.01 |
| Socioeconomic class, % in lowest tercile | 37.8 | 36.5 | 30.2 | <0.01 |
| % residing in rural area | 33.3 | 21.7 | 10.3 | <0.01 |
| HRR-level variables | ||||
| Promotional payments to urologists in USD, median (IQR) | 135 (160) | 875 (704) | 12310 (47657) | - |
| Number of urologists, median (IQR) | 8 (6) | 20 (15) | 57 (53) | <0.01 |
| Promotional payments to medical oncologists for abiraterone or enzalutamide in USD, median (IQR) | 170 (337) | 761 (1531) | 4566 (15898) | <0.01 |
| All other promotional payments to physicians in USD, median (IQR) | 777044 (859017) | 2514473 (2702469) | 10335238 (17076686) | <0.01 |
As illustrated in Figure 2a, there were 15 markets without any promotional payments to urologists. Among the remaining 291 markets, promotional payments ranged from $4 to $189,467. Holding the rank order of the markets constant, we next characterized promotional payments to medical oncologists for abiraterone or enzalutamide and all other promotional payments to physicians, shown in Figures 2b and 2c, respectively. Market-level payments to urologists were more strongly correlated with all other promotional payments to physicians for other drugs (coefficient = 0.62) than with promotional payments to medical oncologists (coefficient = 0.42) for abiraterone or enzalutamide.
Next, we modeled the relationship between promotional payments to urologists for abiraterone or enzalutamide and patient-level treatment by a urologist with either drug. As shown in Table 2, after adjusting for differences in patient and market characteristics, patients residing in markets with the highest and medium level of promotional payments to urologists had 5.7 and 3.1 times higher odds of the first fill of abiraterone or enzalutamide being prescribed by a urologist. Importantly, neither the number of urologists residing in a market nor either of the other two promotional payment measures (i.e., to medical oncologists for abiraterone or enzalutamide, to physicians for all other drugs) were associated with a first fill of abiraterone or enzalutamide being prescribed by a urologist. As shown in Figure 2, increasing promotional payments to urologists for abiraterone or enzalutamide was strongly associated with a urologist prescribing either drug—24.3% of patients residing in the markets with highest level of promotional payments to urologists had their drug prescribed by a urologist compared with 5.8% of patients living in markets with the lowest level of payments (p<0.01). As part of a secondary analysis, we assessed prescribing patterns at extremes of market-level payments to urologists (i.e., bottom vs. top decile). Consistent with our main analyses, market-level payments to urologists were strongly associated with a first prescription written by a urologist (6.1%% vs. 29.7% in the bottom and top deciles, respectively, p < 0.01).
Table 2.
Association between promotional payments to urologists for abiraterone or enzalutamide and patient-level treatment by a urologist with either drug.
| Characteristic | Treatment by a Urologist | ||
|---|---|---|---|
| Odds Ratio | 95%CI | p-value | |
| Patient-level variables | |||
| Age (per year) | 1.03 | 1.01–1.04 | <0.01 |
| Non-white race | 1.06 | 0.80–1.39 | 0.69 |
| Socioeconomic class (lowest vs. highest tertile) | 0.99 | 0.77–1.27 | 0.93 |
| Rural | 0.76 | 0.53–1.08 | 0.12 |
| HRR-level variables | |||
| High | 5.66 | 3.01–10.63 | <0.01 |
| Number of urologists (per 1) | 1.00 | 0.99–1.00 | 0.33 |
| High | 0.71 | 0.43–1.18 | 0.19 |
| All other promotional payments to physicians (per ($10,000) | 1.00 | 1.00–1.00 | 0.96 |
Figure 2.
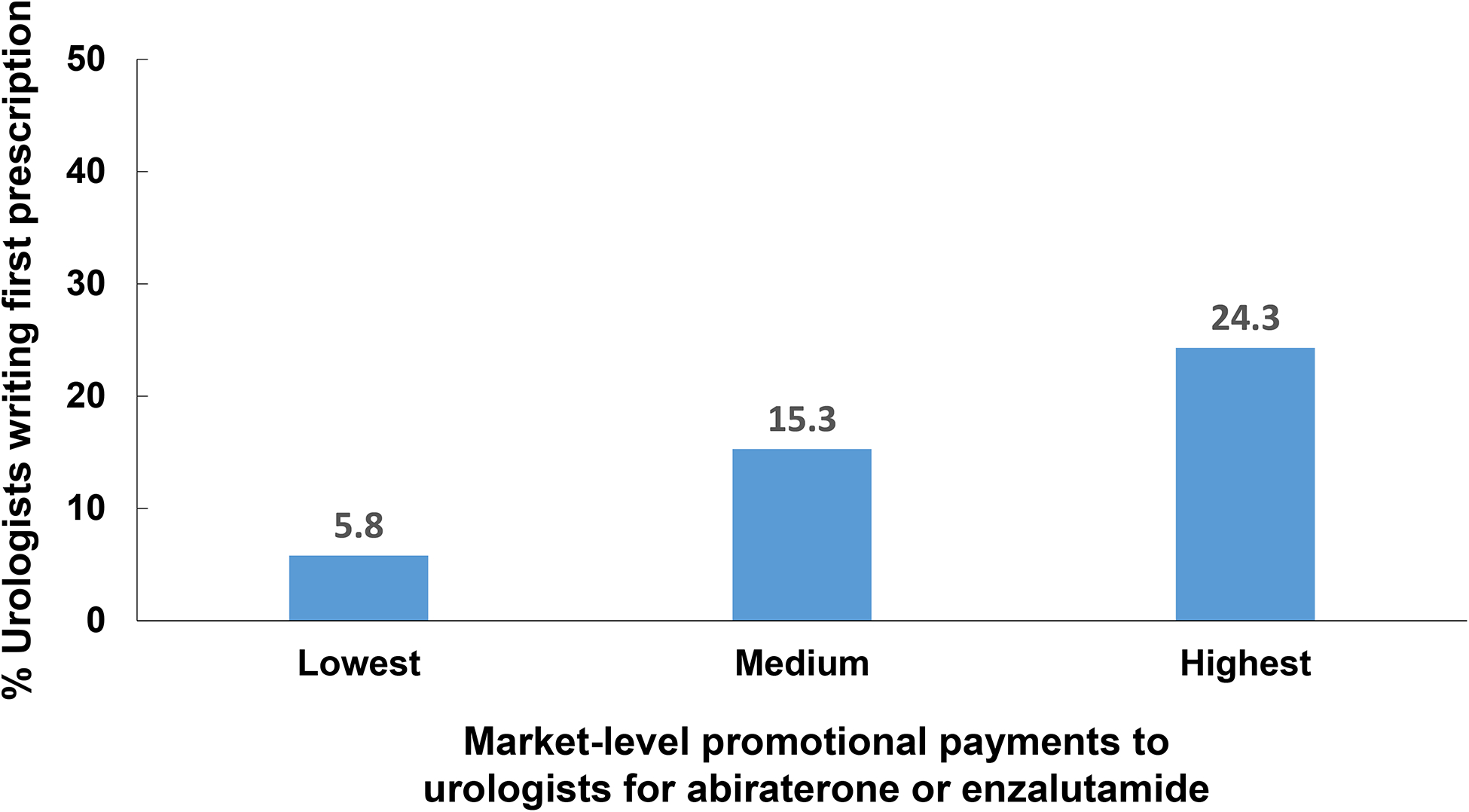
Percentage of first prescription fills written by urologists according to promotional payments to urologists by pharmaceutical manufacturers, adjusted for patient age, race, zip code level measures of socioeconomic class and urban development, number of urologists per HRR, promotional payments to medical oncologists per HRR, and all other promotional payments to physicians per HRR.
Source: Authors’ analysis of national Part D Medicare data, 2015.
Notes: P<0.01 for comparison between groups.
Discussion
Promotional payments for abiraterone or enzalutamide to urologists were monotonically associated with the likelihood of urologists prescribing these drugs. Patients residing in regions with the highest payments were more than four times more likely to receive their prescription from a urologist compared with those living in regions with the lowest payments. Importantly, this relationship was independent of the number of urologists practicing within a market. Further, payments to other physicians treating advanced prostate cancer (i.e., medical oncologists) and general promotional payment levels do not explain urologist’s more frequent prescribing of these drugs in markets with higher levels of promotional payments to urologists.
Important work in this area by Dr. Bandari and colleagues examined this issue of industry payments to prescribers of abiraterone and enzalutamide and prescription counts of these drugs.20 They found no direct relationship for abiraterone and a weak association for enzalutamide, suggesting differences between the two drugs might be due to differences in the ease of implementation (i.e., abiraterone requires more rigorous follow-up for adverse events because of the need to concurrently administer prednisone). Our study looks at this issue differently using a market-level approach, which allows us also to examine the indirect effects of payments to key opinion leaders on prescribing patterns of their colleagues within the same market. Indeed, the findings of this study and others20 collectively support the possibility of a multiplier effect in which promotional payments have limited direct implications (i.e., on prescribing patterns of the physician to which they are made) but significant indirect effects (i.e., on the prescribing of these drugs by other urologists within the same market).
The use of targeted agents by urologists has grown dramatically, with the number of moderate to high users increasing from 98 in 2013 to 671 in 2016.9 Unlike docetaxel, which has unique certification and privileging requirements, targeted agents can be prescribed by any physician. The Large Urology Group Practice Association21 and leaders in urology22–24 have advocated for urologists to broaden their scope of practice to care for these men through the end-of-life. The American Urological Association, the leading professional society for urologists, has supported this expansion in practice scope through educational workshops at its annual meeting and dissemination of clinical care guidelines.25 Entry into this space may be partially motivated by economics, as revenue from drug delivery can be a profit center for some physician groups21 although the implications for patients are unclear. This possibility is further enhanced by prior studies supporting the responsiveness of some urologists to financial incentives embedded in the delivery of pharmaceuticals.26–28
Local symptoms (e.g., urinary obstruction, bladder hemorrhage) are common at the end-of-life, and more than 50% of those dying from prostate cancer undergo a urologic procedure for palliation.29 There are several potential benefits to increasing urologist involvement in the management of these men. Building on the usually longstanding relationship dating back to the time of initial diagnosis of prostate cancer, this may facilitate better adherence to treatment, decrease care fragmentation and ease the initiation of difficult conversations around end-of-life care. Also, while medical oncologists manage patients with a wide variety of advanced malignancies, urologists are more focused, which may impart cost and quality benefits hypothesized in other “focused-factory” contexts.10,11 Finally, there may be improvements in access to care to these important agents.
However, unlike medical oncologists, urologists are not trained to manage important complications of targeted agents (e.g., hypertension, diabetes, and osteoporosis12–15), which frequently do not fall under the purview of typical urological care. For example, prior work has shown that use of bone density imaging in the context of androgen deprivation therapy, designed to detect those at risk of fracture and guide therapy, was less common among men managed by urologists versus those cared for by a medical oncologist (2.8% vs. 9.6%).30 Also, medical oncologists may be more comfortable managing the progressive symptoms of lethal prostate cancer (pain, fatigue, and frailty5,16,17). Further, the broad focus and the volume that it implies, equips medical oncology practices with the necessary infrastructure to handle the administrative complexities associated with filling (e.g., prior authorizations, counseling, securing financial assistance) and delivering these drugs efficiently. Finally, while management by medical oncologists is strongly associated with transitioning to hospice,5,16,17 surgeons are less timely with such referrals.31–33
These findings should be considered in the context of limitations. First, the study design precludes a causal relationship between promotional payments to urologists and urologists prescribing either abiraterone or enzalutamide. Thus, these promotional payments could represent either an incentive or a reward. However, it’s notable that promotional payments to medical oncologists for these drugs were not associated with the specialty of the physician prescribing the drug, suggesting pharmaceutical companies may be targeting distinct markets that may have potential as growth opportunities. Second, as this data used Part D data, we did not adjust for patient comorbidity, which may affect the specialty of the physician prescribing one of these two drugs for the first time (i.e., patients with more comorbidity may be more likely to be managed by medical oncologists). However, as patient comorbidity is unlikely to be related to the magnitude of promotional payments to urologists in a market, it would not necessarily be a confounder in the analysis. Third, we did not examine the impact of the prescribing physician specialty on the care of men with advanced prostate cancer, though as noted above, there are several arguments supporting the potential for both positive and negative effects.
Promotional payments to urologists at the market level are strongly associated with the specialty of the physician prescribing abiraterone or enzalutamide for the first time. Future work should elucidate the effects of the shift in prescribing patterns on quality of care and financial hardship for men with advanced prostate cancer.
Figure 1.
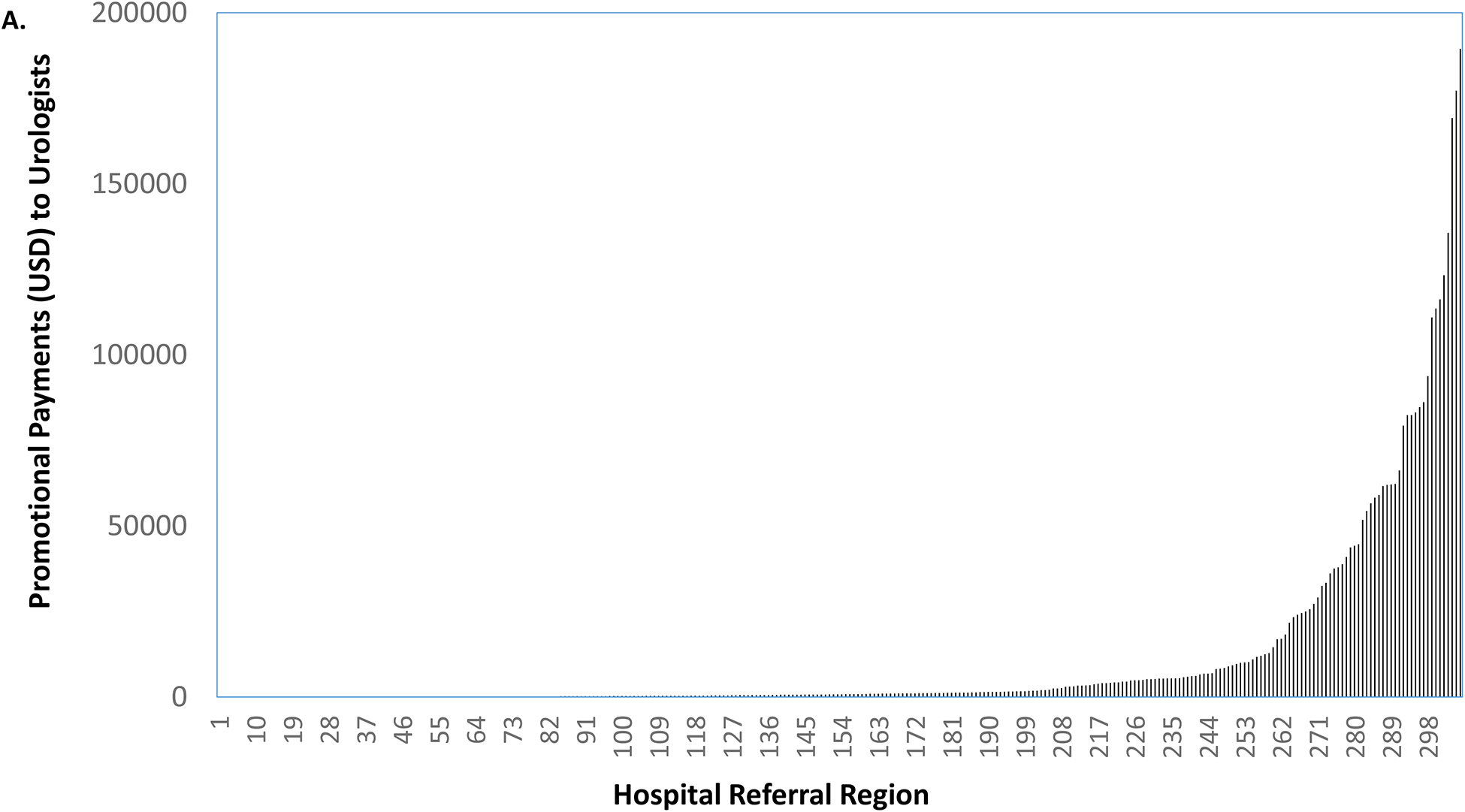
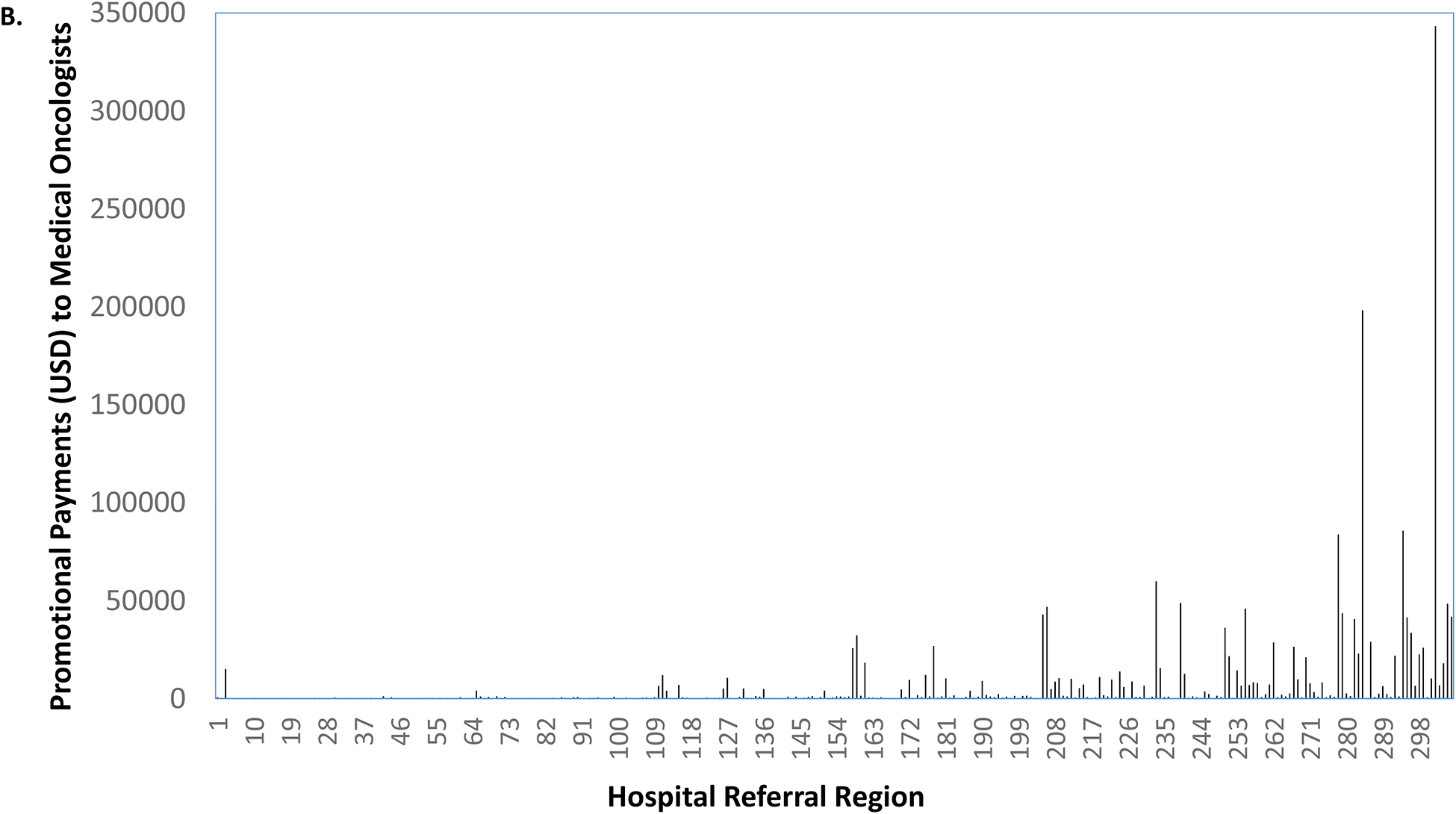
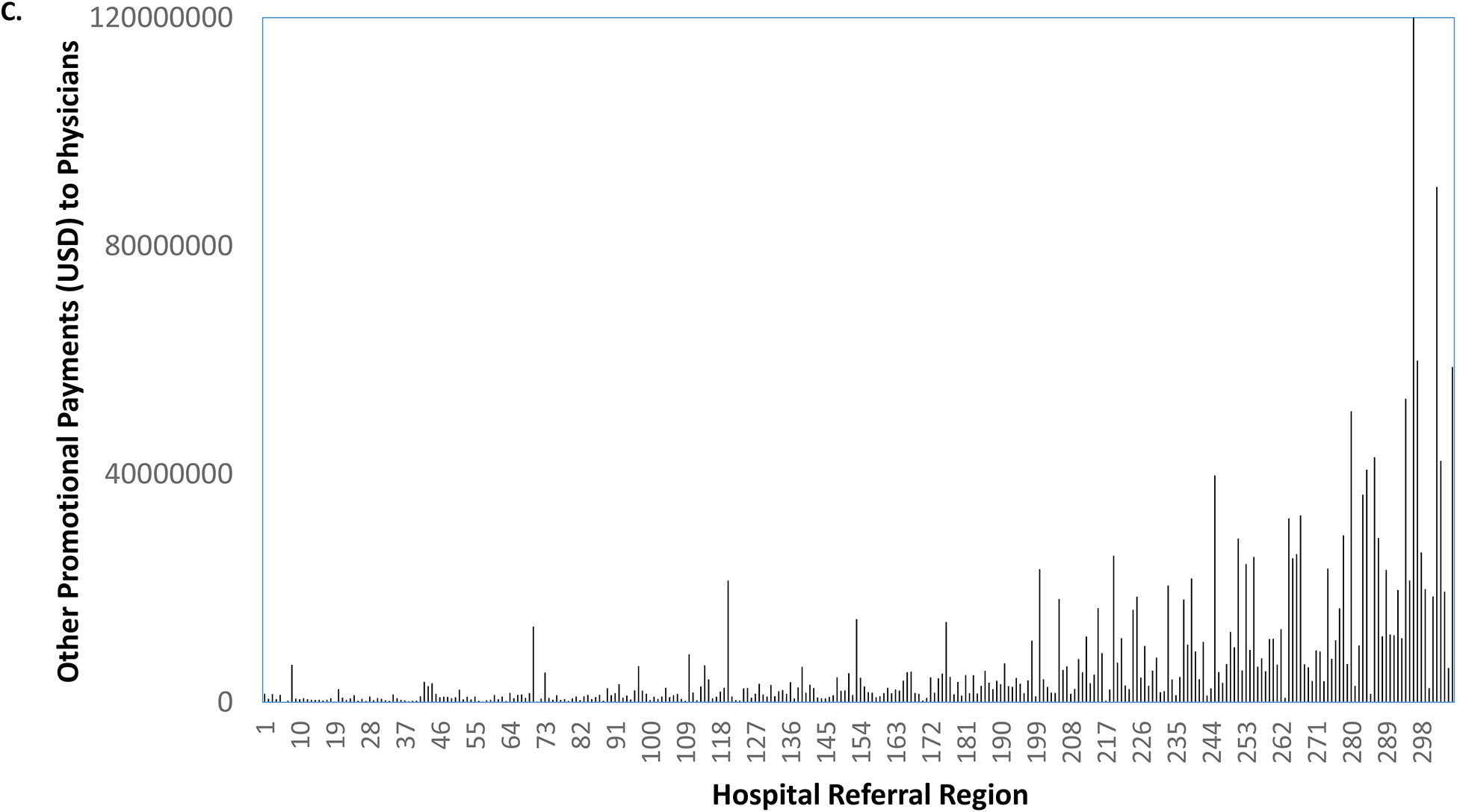
Promotional payments to urologists for abiraterone or enzalutamide by HRR, sorted by rank (a). Holding the rank order constant, promotional payments to medical oncologists for abiraterone or enzalutamide (b), and all other promotional payments to all physicians (c).
Source: Authors’ analysis of Medicare Open Payments data, 2015.
Notes: Market-level payments to urologists were more strongly correlated with all other promotional payments to physicians (coefficient = 0.62) than with promotional payments to medical oncologists (coefficient = 0.42).
Funding and disclosure:
This work was supported by research funding from the AHRQ (R01 HS025707) to BKH and VBS. The authors have no additional competing interests.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the United States government.
Bibliography
- 1.NCI. Prostate cancer costs of care projections. 2016. https://costprojections.cancer.gov/graph.php. Accessed May 4, 2016.
- 2.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. [DOI] [PubMed] [Google Scholar]
- 3.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. New England Journal of Medicine. 2011;364(21):1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harland S, Staffurth J, Molina A, et al. Effect of abiraterone acetate treatment on the quality of life of patients with metastatic castration-resistant prostate cancer after failure of docetaxel chemotherapy. Eur J Cancer. 2013;49(17):3648–3657. [DOI] [PubMed] [Google Scholar]
- 5.Basch E, Autio K, Ryan CJ, et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naive men with metastatic castration-resistant prostate cancer: patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol. 2013;14(12):1193–1199. [DOI] [PubMed] [Google Scholar]
- 6.Cella D, Ivanescu C, Holmstrom S, Bui CN, Spalding J, Fizazi K. Impact of enzalutamide on quality of life in men with metastatic castration-resistant prostate cancer after chemotherapy: additional analyses from the AFFIRM randomized clinical trial. Ann Oncol. 2015;26(1):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fizazi K, Scher HI, Miller K, et al. Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol. 2014;15(10):1147–1156. [DOI] [PubMed] [Google Scholar]
- 8.Tonyali S, Haberal HB, Sogutdelen E. Toxicity, Adverse Events, and Quality of Life Associated with the Treatment of Metastatic Castration-Resistant Prostate Cancer. Curr Urol. 2017;10(4):169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caram MEV, Kaufman SR, Modi PK, et al. Adoption of Abiraterone and Enzalutamide by Urologists. Urology. 2019(131):176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casalino LP, Devers KJ, Brewster LR. Focused factories? Physician-owned specialty facilities. Health Affairs 2003;22(6):56–67. [DOI] [PubMed] [Google Scholar]
- 11.Shactman D. Specialty hospitals, ambulatory surgery centers, and general hospitals: charting a wise public policy course. Health Affairs 2005;24(3):868–73. [DOI] [PubMed] [Google Scholar]
- 12.Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. Journal of the National Cancer Institute. 2010;102(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. Journal of Clinical Oncology. 2006;24(27):4448–4456. [DOI] [PubMed] [Google Scholar]
- 14.Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. New England Journal of Medicine. 2005;352(2):154–164. [DOI] [PubMed] [Google Scholar]
- 15.Ross RW, Small EJ. Osteoporosis in men treated with androgen deprivation therapy for prostate cancer. Journal of Urology. 2002;167(5):1952–1956. [PubMed] [Google Scholar]
- 16.Holmstrom S, Naidoo S, Turnbull J, Hawryluk E, Paty J, Morlock R. Symptoms and Impacts in Metastatic Castration-Resistant Prostate Cancer: Qualitative Findings from Patient and Physician Interviews. Patient. 2019;12(1):57–67. [DOI] [PubMed] [Google Scholar]
- 17.Sartor O, Flood E, Beusterien K, et al. Health-related quality of life in advanced prostate cancer and its treatments: biochemical failure and metastatic disease populations. Clin Genitourin Cancer. 2015;13(2):101–112. [DOI] [PubMed] [Google Scholar]
- 18.CMS. Open Payments. https://www.cms.gov/openpayments/. Published 2019. Accessed October 23, 2019.
- 19.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease.[see comment]. New England Journal of Medicine 2001;345(2):99–106. [DOI] [PubMed] [Google Scholar]
- 20.Bandari J, Ayyash OM, Turner RM 2nd, Jacobs BL, Davies BJ. The lack of a relationship between physician payments from drug manufacturers and Medicare claims for abiraterone and enzalutamide. Cancer 2017;123(22):4356–4362. [DOI] [PubMed] [Google Scholar]
- 21.Albala DM, Kirsh GM, Shore ND. Advanced prostate cancer in large group practices. Reviews in Urology. 2016;18(4):226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford ED. The role of the urologist in treating patients with hormone-refractory prostate cancer. Reviews in Urology. 2003;5 Suppl 2:S48–52. [PMC free article] [PubMed] [Google Scholar]
- 23.Sartor AO, Fitzpatrick JM. Urologists and oncologists: adapting to a new treatment paradigm in castration-resistant prostate cancer (CRPC). BJU International. 2012;110(3):328–335. [DOI] [PubMed] [Google Scholar]
- 24.Shore ND. Chemotherapy for prostate cancer: when should a urologist refer a patient to a medical oncologist? Prostate Cancer Prostatic Dis. 2013;16(1):1–6. [DOI] [PubMed] [Google Scholar]
- 25.American Urological Association. Castration-Resistant Prostate Cancer. https://www.auanet.org/guidelines/prostate-cancer-castration-resistant-guideline. Published 2018. Accessed April 14, 2019.
- 26.Elliott SP, Jarosek SL, Wilt TJ, Virnig BA. Reduction in physician reimbursement and use of hormone therapy in prostate cancer. Journal of the National Cancer Institute. 2010;102(24):1826–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKoy JM, Lyons EA, Obadina E, et al. Caveat medicus: consequences of federal investigations of marketing activities of pharmaceutical suppliers of prostate cancer drugs. Journal of Clinical Oncology. 2005;23(34):8894–8905. [DOI] [PubMed] [Google Scholar]
- 28.Shahinian VB, Kuo YF, Gilbert SM, Freeman JL, Orihuela E, Goodwin JS. Reimbursement policy and androgen-deprivation therapy for prostate cancer. New England Journal of Medicine. 2010;363:1822–1832. [DOI] [PubMed] [Google Scholar]
- 29.Dinan MA, Li Y, Zhang Y, et al. Resource Use in the Last Year of Life Among Patients Who Died With Versus of Prostate Cancer. Clin Genitourin Cancer. 2016;14(1):28–37.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahinian VB, Kuo Y-F. Patterns of bone mineral density testing in men receiving androgen deprivation for prostate cancer. J Gen Intern Med. 2013;28(11):1440–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CW, Vitous CA, Silveira MJ, et al. Delays in Palliative Care Referral Among Surgical Patients: Perspectives of Surgical Residents Across the State of Michigan. J Pain Symptom Manage. 2019;57(6):1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suwanabol PA, Kanters AE, Reichstein AC, et al. Characterizing the Role of U.S. Surgeons in the Provision of Palliative Care: A Systematic Review and Mixed-Methods Meta-Synthesis. J Pain Symptom Manage. 2018;55(4):1196–1215.e1195. [DOI] [PubMed] [Google Scholar]
- 33.Suwanabol PA, Reichstein AC, Suzer-Gurtekin ZT, et al. Surgeons’ Perceived Barriers to Palliative and End-of-Life Care: A Mixed Methods Study of a Surgical Society. J Palliat Med. 2018;21(6):780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]


