Abstract
Objective:
Fibronectin is a matrix protein that is fragmented during cartilage degradation in osteoarthritis (OA). Treatment of chondrocytes with fibronectin fragments (FN-f) has been used to model OA in vitro, but the system has not been fully characterized. This study sought to define the transcriptional response of chondrocytes to FN-f, and directly compare it to responses traditionally observed in OA.
Design:
Normal human femoral chondrocytes isolated from tissue donors were treated with either FN-f or PBS (control) for 3, 6, or 18 hours. RNA-seq libraries were compared between time-matched FN-f and control samples in order to identify changes in gene expression over time. Differentially expressed genes were compared to a published OA gene set and used for pathway, transcription factor motif, and kinome analysis.
Results:
FN-f treatment resulted in 3,914 differentially expressed genes over the time course. Genes that are up- or downregulated in OA were significantly up- (p < 0.00001) or downregulated (p < 0.0004) in response to FN-f. Early response genes were involved in proinflammatory pathways, whereas many late response genes were involved in ferroptosis. The promoters of upregulated genes were enriched for NF-κB, AP-1, and IRF motifs. Highly upregulated kinases included CAMK1G, IRAK2, and the uncharacterized kinase DYRK3, while growth factor receptors TGFBR2 and FGFR2 were downregulated.
Conclusions:
FN-f treatment of normal human articular chondrocytes recapitulated many key aspects of the OA chondrocyte phenotype. This in vitro model is promising for future OA studies, especially considering its compatibility with genomics and genome-editing techniques.
Keywords: osteoarthritis, chondrocytes, cartilage, fibronectin, RNA-seq
INTRODUCTION
Osteoarthritis (OA) is the most common form of joint disease and affects over 250 million people worldwide, including over 10% of those older than 60 years1. There is no known cure, and treatments are currently limited to symptom management. One major reason for the lack of treatments is an incomplete understanding of the mechanisms that promote OA and its progression. While mouse models and human samples have provided valuable insights into OA biology, new human disease models amenable to manipulation and high-throughput screening would improve our ability to understand and potentially better treat this painful and disabling disease.
OA involves many, if not all, of the tissues that comprise articular joints, with degradation and loss of articular cartilage noted as a central feature2. Studies of potential OA pathways often compare chondrocytes isolated from normal cartilage obtained from various animal species, including humans, to chondrocytes obtained from OA tissue. A limitation, particularly with human tissue, is that the OA chondrocytes are most often isolated from cartilage obtained at the time of joint replacement, resulting in comparisons being made to cells at an advanced stage of disease. Animal models, including mice, have been critical for mechanistic studies, but major differences in genomes, body structures, and OA prevalence limit the relevance to human biology3.
A commonly used option for modeling the chondrocyte OA phenotype has been to stimulate primary cells or cell lines ex vivo with cytokines such as IL-1 or TNFα4. A major limitation of these studies is that the cells are treated with levels of cytokines in the ng/ml range to obtain a desired response, while (at least in the synovial fluid) IL-1 and TNFα are only present in pg/ml amounts5. In addition, recent studies, including failed clinical trials of IL-1 and TNFα inhibition in OA, suggest that multiple pro-inflammatory mediators contribute to OA development, and IL-1 or TNFα may not be the driving factors6–8.
An alternative in vitro model for simulating a chondrocyte OA phenotype utilizes fragments of fibronectin. Fibronectin is an extracellular matrix protein present in cartilage that is upregulated in OA tissue and subsequently degraded by several proteases9,10. Fibronectin fragments (FN-f) of various sizes and at levels in the μM range have been detected in OA cartilage and synovial fluid as well as in cartilage from patients with rheumatoid arthritis11–13. Injection of FN-f into rabbit joints was found to induce cartilage proteoglycan loss, which is a feature of early OA14. Treatment of isolated human chondrocytes or cartilage explants with FN-f has been shown to recapitulate many known features of OA, including production of multiple matrix-degrading enzymes and proinflammatory cytokines found in OA joints9,15,16. While these results demonstrate the value of FN-f treatment for studying OA, the global similarity between FN-f-treated chondrocytes and OA chondrocytes has not been fully explored.
The purpose of this study was to characterize the transcriptional response to acute FN-f stimulation of ex vivo human chondrocytes and to compare this response to those previously observed in OA. The goal was to have a model system where small molecule inhibitors or methods to alter expression of specific genes could be tested in short-term studies. Acute rather than chronic stimulation was also used because primary chondrocytes do not maintain their phenotype in long-term culture. We found that FN-f triggers a robust transcriptional response in primary chondrocytes, which correlates with changes observed during OA. Analysis of gene ontology terms, signaling pathways, and transcription factor motifs revealed that known regulators of OA progression also play a role in the FN-f response, as do a host of genes and pathways that had not previously been implicated in OA. These results support FN-f treatment as a viable model for studying transcriptional control of OA progression and provide a valuable resource for future studies.
METHODS
Sample collection and treatment
Primary articular chondrocytes were isolated by enzymatic digestion from normal human femoral cartilage obtained from three tissue donors, aged 50–61 years and without a history of arthritis, as previously described17. Cells were cultured to confluency in DMEM/F12 media with 10% fetal bovine serum, and then made serum-free for 2 hours prior to treatment with either purified 42 kDa endotoxin-free recombinant FN-f (1 μM in PBS), prepared as previously described, or PBS as a control18. The FN-f used here consists of domains 7–10 in native fibronectin, which contains the RGD cell-binding domain recognized by the α5β1 integrin. After 3, 6, or 18 hours of treatment with FN-f or PBS, media was removed, cultures were quickly rinsed with cold PBS, and RNA was immediately isolated using the RNeasy kit from Qiagen.
RNA-seq library preparation
Prior to library preparation, all RNA samples were analyzed using a Tapestation RNA HS tape to confirm RNA integrity numbers (RIN) within 8.5–10, indicating high-quality, intact RNA. Ribosomal RNA was removed using the New England Biolabs NEBNext rRNA Depletion Kit (Human/Mouse/Rat), and libraries were prepared using the NEBNext Ultra II Directional RNA Library Prep Kit and NEBNext Multiplex Oligos for Illumina. Final libraries were then quantified using a Qubit 4 Fluorometer and run on a Tapestation D1000 HS tape, to confirm average fragment sizes were within 260–320 base pairs and calculate molarity for pooling.
Processing of RNA-Seq libraries
RNA-seq libraries were sequenced to an average depth of approximately 58 million reads per sample (50 bp, paired-end reads) on an Illumina HiSeq 4000 (High Output). Low-quality reads and adapters were trimmed using Trim Galore! (v. 0.4.3), and trimmed reads were then mapped to the hg19 transcriptome and quantified using Salmon (v. 0.8.2)19,20. Both programs were run with default settings.
Identifying differential genes
Gene-level quantifications were summarized from each sample using tximport (v. 1.2.0)21. Differential analysis was conducted in R with DESeq2 (v. 1.22.2) using a design adjusting for donor variability when calculating differences between treatment groups (~ donor + treatment)22. The log2 fold change (L2FC) values were shrunken using the “apeglm” method23. Differential genes were defined as genes with an FDR-adjusted p-value below 0.01 (likelihood ratio test; LRT) and an absolute L2FC above 1 when comparing FN-f treated samples to their time-matched controls.
Temporal clustering of genes
To assign temporal response classes for the 3,914 differential genes, first a z-score was calculated from the variance-stabilized counts, centering the counts in each sample relative to the average counts among all samples for each gene. Then, for each donor and time point, the untreated control score was subtracted from the FN-f treated score for every gene. The difference in z-score was then averaged over the three donors, ultimately providing three values for each gene representing the normalized expression relative to the control at each time point (3, 6, and 18 hours). This matrix was then clustered using k-means clustering with a k of 4. These clusters were labeled “Up Early”, “Up Late”, “Down Early”, and “Down Late” based on their expression relative to untreated controls at each time point.
Comparing with genes differentially expressed in OA cartilage
The previously published RAAK study identified genes that were up- or downregulated in OA-affected cartilage compared to preserved cartilage in the same joint24. To focus on only the genes that exhibited the strongest changes in OA, the genes from the RAAK study were filtered for those with a p-value less than 0.01 and an absolute L2FC of greater than 0.585 (equivalent to 1.5 up- or down-fold). A Mann-Whitney U test was used to determine if the FN-f-induced L2FC of each set of OA-responsive genes were significantly higher or lower, respectively, than the FN-f-induced L2FC of genes outside of each gene set.
GO, KEGG, and transcription factor motif enrichment analysis
The “findMotifs.pl” tool in the HOMER software suite (v. 4.10.4) was used on each cluster of genes in order to identify significantly enriched Gene Ontology (GO) terms (p-value < 0.01), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (p-value < 0.01), and transcription factor motifs (p-value < 1×10−12, per software recommendations)25,26. De novo motifs were compared to known motifs from the HOCOMOCO dataset (v. 11, p-value < 0.001). Motifs with best match scores below 0.9 were classified as “unannotated”, in that they did not appear to have a conclusive known motif match.
Kinome visualization
To identify and visualize protein kinases present in each cluster, cluster assignments were plotted using the human kinome visualization tool, Coral27. Flat text files were created listing the ENSEMBL identifier, k-means cluster, and maximum absolute L2FC among all three time points for each differential gene. These lists were then used to plot both categorical (cluster, encoded in branch/node color) and qualitative data (maximum absolute L2FC, encoded in node size) on the protein kinase tree, originally published by Manning et al28.
Data availability
Data is made publicly available at GEO accession GSE150411, including; raw sequencing data; transcript-level quantification output from Salmon; and a table containing gene-level summaries of read counts in each sample, cluster assignments and FDR-adjusted p-values (LRT) for each gene, as well as L2FC and the difference between FN-f treated and control normalized count z-score at each time point.
RESULTS
FN-f induces global changes in chondrocyte gene expression
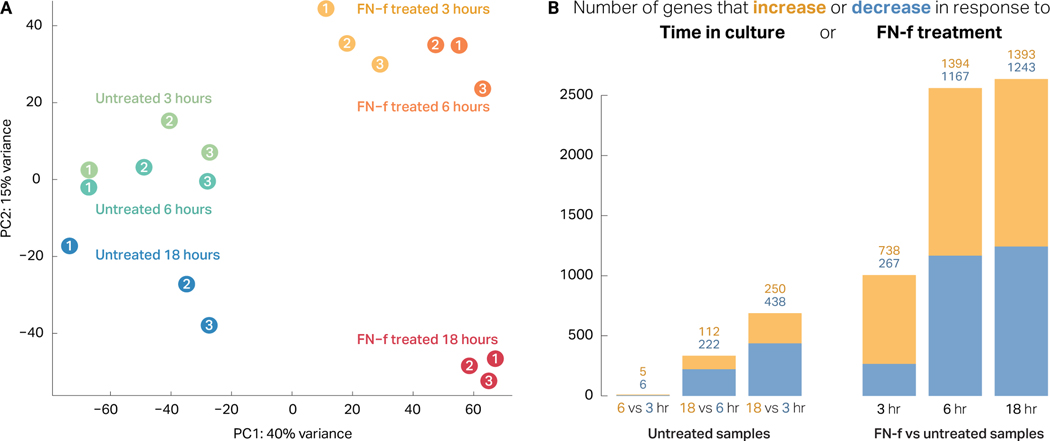
To determine the extent to which FN-f treatment alters transcription in human chondrocytes, we performed a three-point RNA-seq time course of 3, 6, and 18 hours (Fig S1). We used principal component analysis (PCA) of the 18 samples to determine the extent to which each attribute (donor, time in culture, and time treated with FN-f) contributed to transcriptional state. Untreated samples clustered largely by donor rather than by time in culture, suggesting that time in culture had relatively minor impacts on gene expression (Fig 1A). Conversely, samples treated with FN-f clustered by treatment time, suggesting that FN-f treatment alters transcriptional state consistently, independent of the donor of origin. Statistical analysis of differential expression patterns confirmed these results. Untreated samples exhibited 748 differentially expressed genes between time points (FDR < 0.01, absolute L2FC > 1; Fig 1B, Table S1). In contrast, comparison of FN-f-treated samples to their time-matched controls revealed 3,914 genes that changed significantly in response to FN-f treatment in at least one of the three time points (Table S2). Increased treatment time correlated with increased numbers of differentially expressed genes, with 1,005, 2,561, and 2,636 genes affected at 3, 6, and 18 hours, respectively. Together, these results demonstrate that FN-f treatment has a profound effect on transcription that is distinct from the effects of ex vivo culturing, and this effect is robust when accounting for variation in response among biological replicates.
Figure 1. FN-f treatment induces a robust transcriptional response in human chondrocytes.
(A) Principal component analysis (PCA) of each sample (colors indicate condition, numbers indicate donor) reveals a distinct separation between FN-f-treated and untreated samples along the first principle component (explaining 40% variance). Additionally, FN-f-treated samples cluster by length of treatment rather than by donor, whereas untreated samples cluster more based on donor (particularly along PC1). (B) Bar plots depicting the number of genes that exhibit significant differences in expression due to time in culture (left) or FN-f treatment (right; FDR > 0.01, absolute L2FC > 1). Yellow and blue bars represent the number of genes that increase or decrease in each comparison. Above each bar, the exact number of up- and downregulated genes are labelled in yellow and blue, respectively. (Left) A bar plot depicting the number of genes that change significantly between untreated control samples reveals that time in culture has only a minimal impact on gene expression. (Right) A bar plot depicting the number of genes that change significantly in FN-f-treated samples compared to time-matched controls demonstrates that FN-f induces a robust transcriptional response in chondrocytes.
Investigation of specific differential genes revealed expected changes for many known regulators of OA. Genes upregulated in response to FN-f included cytokines and chemokines such as CXCL2 (178-fold), LIF (147-fold), and IL6 (292-fold). Interleukin-1β (IL1B), a proinflammatory cytokine with elevated expression in OA chondrocytes29,30, was upregulated at all time points, peaking at 110-fold. Upregulated genes also included proteases such as MMP13 and MMP10 (both 11-fold). Matrix metallopeptidase 13 (MMP13) is an enzyme that degrades type II collagen and is thought to play a critical role in cartilage degradation in OA12,15,31–34. Interestingly, among the downregulated genes were the collagen-binding integrins ITGA10 and ITGA11, which decreased by 3.6- and 3.4-fold, respectively. Additionally, the BMP and WNT antagonists GREM1 and DKK1, which are downregulated in osteoarthritic cartilage, decreased by 40-fold and 5.8-fold, respectively35.
FN-f treatment induces transcriptional changes similar to OA
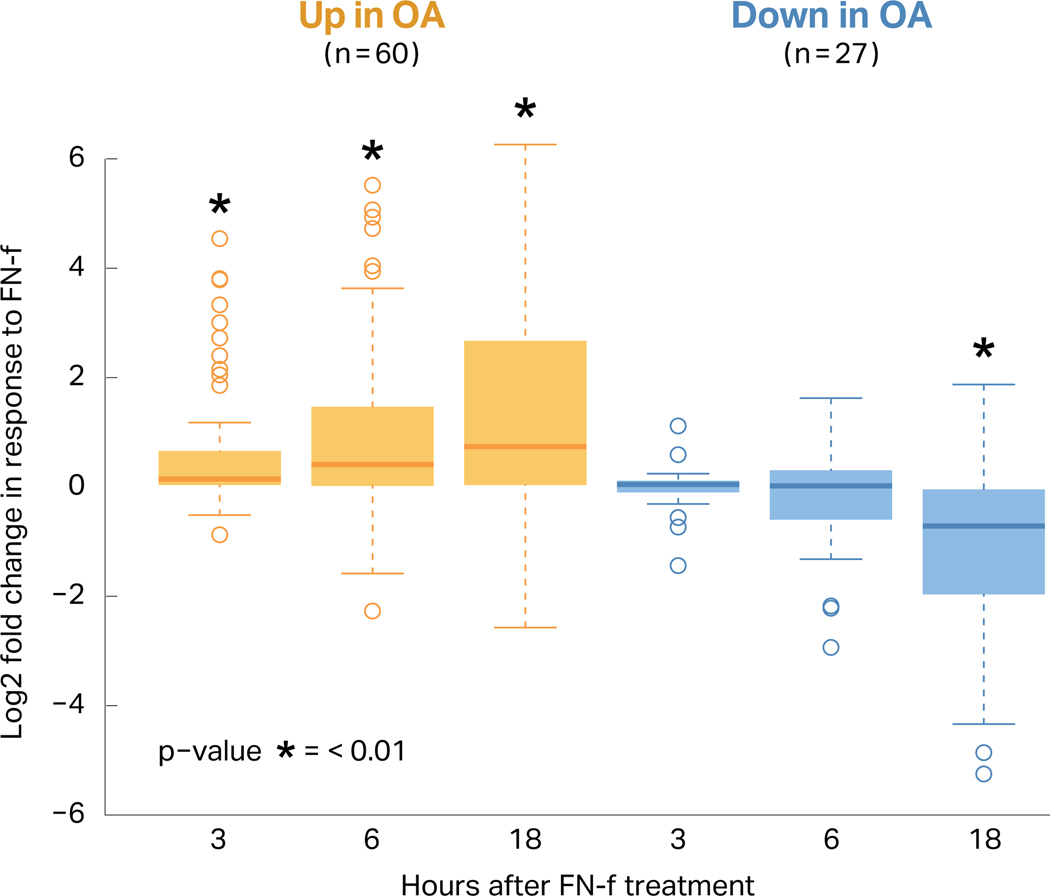
Changes in gene expression in response to FN-f were compared to the differential expression reported in chondrocytes isolated from OA and preserved tissue in the RAAK study24 using a subset of differential genes with the strongest effects (p-value < 0.01, absolute L2FC > 0.585) (Table S3; Fig 2). Genes upregulated in the OA tissue were also upregulated in response to FN-f treatment, and these effects grew more pronounced with longer exposure to FN-f (Fig 2; Mann-Whitney U test, Bonferroni-adjusted p-value < 0.01). Genes that were downregulated in OA tissue were similarly downregulated in response to FN-f, though only exhibiting statistical significance after 18 hours of treatment. These results suggest that FN-f induces similar transcriptional changes to those found in OA chondrocytes.
Figure 2. FN-f treatment induces changes similar to osteoarthritis.
The previously published RAAK study compared osteoarthritis cartilage to preserved cartilage in the same joint, and of the differential genes reported, 60 were upregulated and 27 were downregulated with a high degree of change and significance (FDR p-value < 0.01, absolute L2FC > 0.585). The boxplot shows the log2 fold change between FN-f treated and control samples at each time point for both the up- (yellow) and downregulated (blue) genes. These genes were ranked by fold change and compared to a ranked list of fold changes for genes outside of the OA set (* = Mann-Whitney p-values < 0.01).
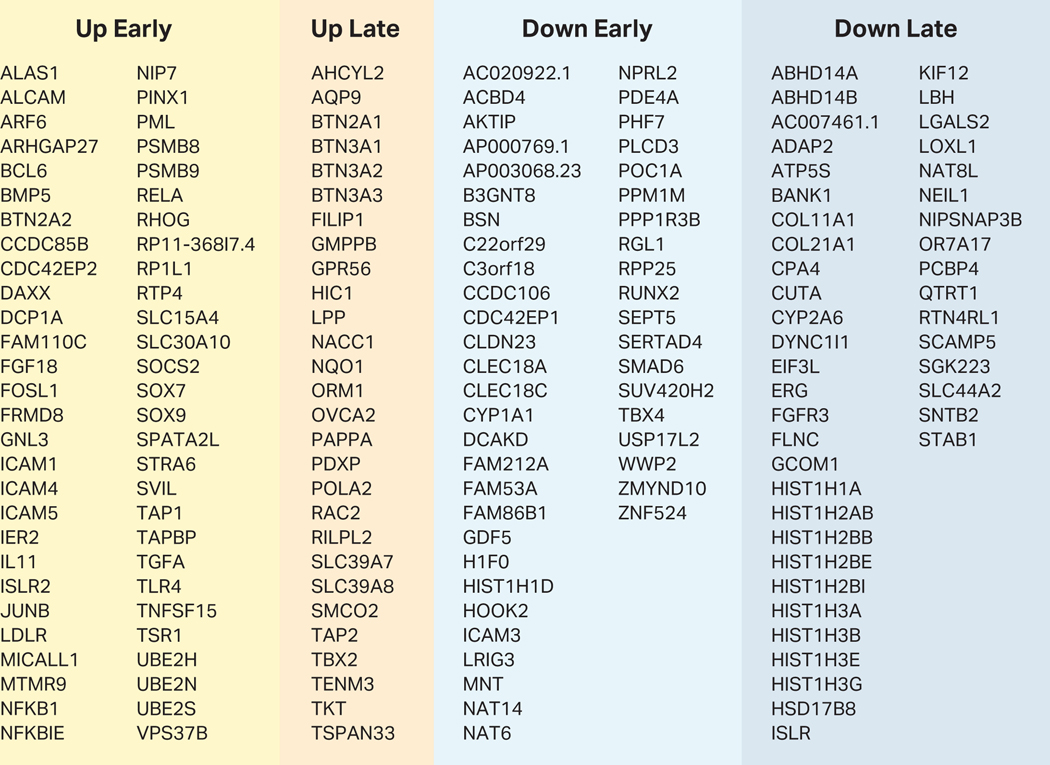
To further evaluate FN-f treatment as a model of the OA chondrocyte phenotype, we intersected our differentially expressed genes with 64 OA-associated variants that have been recently identified through Genome Wide Association Studies (GWAS)36. These single-nucleotide polymorphisms (SNPs) represent loci with genotypes statistically associated with the OA phenotype, but most occur in non-coding regions of the genome. This suggests that the SNPs directly impact regulatory regions, and the gene(s) that they affect—which in turn promote the OA phenotype—could be up to hundreds of thousands of base pairs away, mediated through short- or long-range regulatory interactions37–42. In order to identify genes that could be affected by these variants, we overlapped the SNP coordinates with our differentially expressed genes and found that 175 of the differential genes identified in this study were within 400 Kb of a GWAS SNP. These genes are summarized in Figure 3 and include many genes with previously reported connections to OA, such as NFKB143, SOX744, IL1145, GDF546,47, FGF1848, TNFSF15, and NKX3–249. While differential genes were not enriched near OA GWAS loci (permutation test p-value = 0.61), the genes within this range represent a preliminary identification of target genes potentially affected by OA-associated genetic variants.
Figure 3. FN-f responsive genes near osteoarthritis GWAS loci.
This table lists all differential genes that change in response to FN-f-treatment and that have a transcriptional start site within 400 Kb of a GWAS SNP as identified in Tachmazidou et al, 201936. Each gene is listed under the appropriate FN-f response class (as determined by k-means clustering of all differential genes).
FN-f triggers both early- and late-response genes
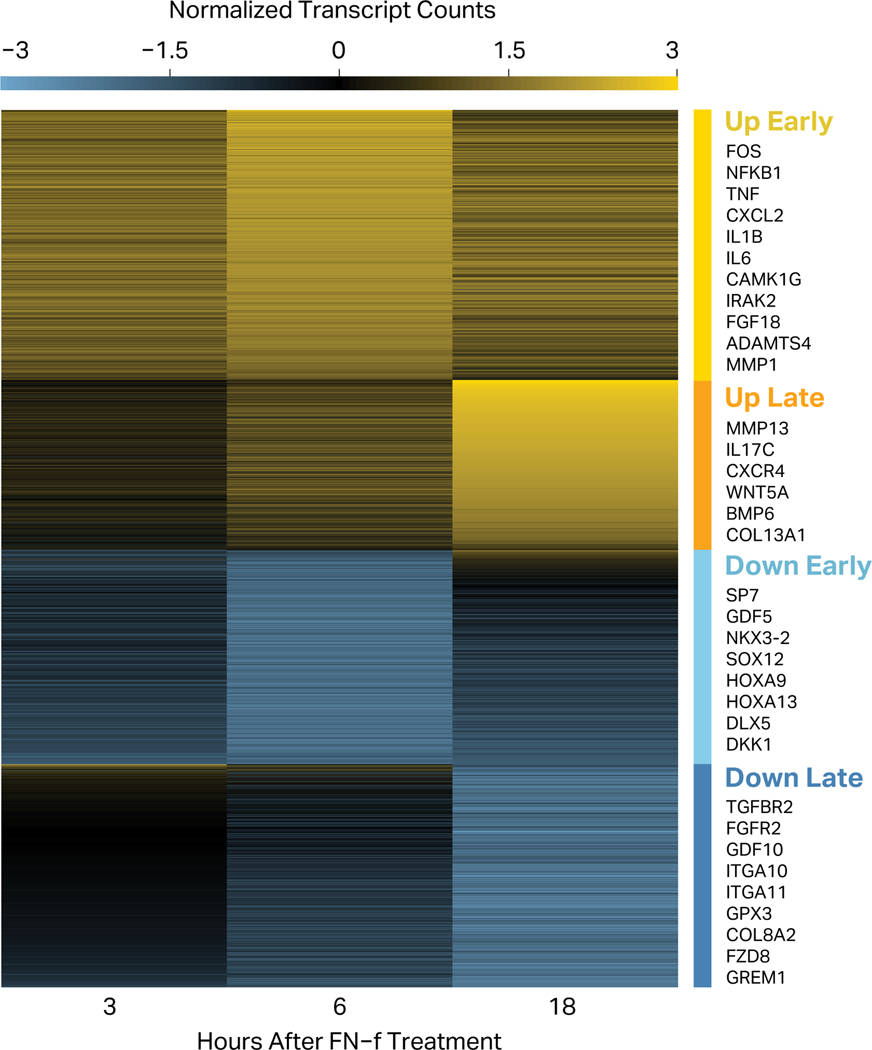
To investigate the temporal patterns of transcriptional changes in response to FN-f, we performed k-means clustering of differentially expressed genes (Fig 4). This revealed four distinct temporal patterns. Early-response genes (both up- and downregulated) exhibited changes in expression as early as 3 hours but generally peaked at 6 hours of FN-f treatment. This response class includes many upregulated genes that have been previously implicated in OA, including: AP-1 components FOS and JUN30,50; interleukins such as IL1B, IL11, IL6 and IL829,32,51–55; interleukin-receptor-associated kinases IRAK2 and IRAK3; NOD2 and RIPK256; aggrecanases ADAMTS1 and ADAMTS430,51,57,58; metalloproteinase MMP150,51; chemokines CXCL1, CXCL2, and CXCL316; NGF24; LIF and TNF53,59,60; TNFAIP6 and TNFRSF11B24; and PTGES24. Among genes that decreased early were: transcription factor SP7, known to be downregulated by TNF61; WNT antagonist DKK135; and regulators of differentiation such as DLX5 and SOX1262,63. Late-response genes showed maximum absolute L2FC after 18 hours of FN-f treatment. Many of these genes have also been implicated in OA and/or matrix remodeling, including: MMP10 and MMP1315,51,56,64; CD55 and PAPPA24; interleukins such as IL17C51,52,55; chemokine receptor CXCR465; signaling protein WNT5A66–68; bone morphogenetic protein BMP657,69,70; IL1 receptor antagonist IL1RN; and collagens COL13A1 and COL7A157,71. Among the genes downregulated at later time points were: cartilage-specific integrin ɑ10β1 (ITGA10), as well as integrin ɑ11β1 (ITGA11); BMP antagonist GREM135,72; WNT receptor FZD873; differentiation factor GDF1057; and oxidative defense gene GPX374. These results highlight the value of looking at FN-f response across a time course as it both reveals transient events that are not observed at every time point, and provides insight into the temporal order and possibly even causal relationships between regulatory events.
Figure 4. FN-f treatment regulates both early- and late-response genes.
The genes that changed significantly in response to fibronectin fragment treatment were clustered according to their difference in z-score normalized counts between FN-f-treated and untreated samples at each time point. This separated the differential genes into four classes: “Up Early” (yellow; n=1,205), “Up Late” (orange; n=759), “Down Early” (light blue; n=956), and “Down Late” (dark blue; n=994). Selected genes are highlighted in each cluster.
We investigated whether the length of genes—and the corresponding time it would take to transcribe them—could account for the difference in response time. While the mean gene length for the late-response genes was significantly longer than that of early-response genes (Mann-Whitney U test, p-value = 1.3 × 10−11; Fig S2), the two distributions exhibited substantial overlap. Therefore, gene length is not likely to be the primary determinant of early versus late response.
FN-f induces transcription of proinflammatory genes and pathways
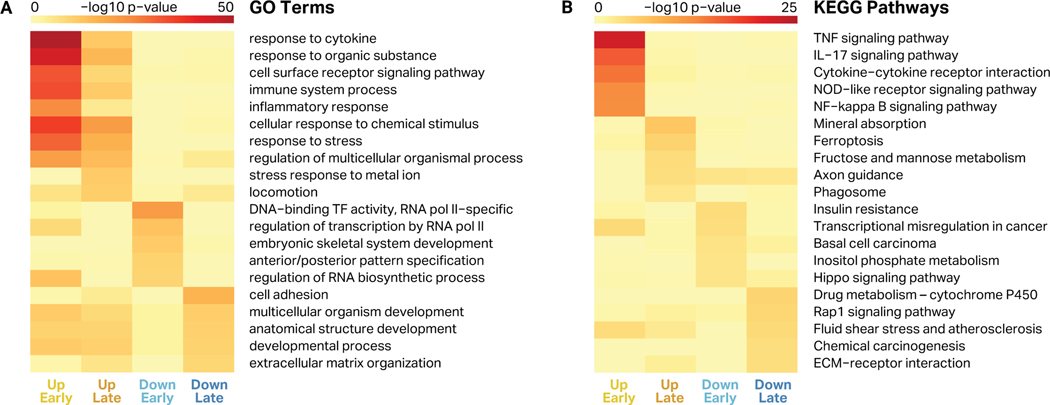
To understand the likely phenotypic impact of the changes induced by FN-f, we performed Gene Ontology (GO) enrichment analysis for the genes in each of the four clusters (Fig 5A, Table S4). Both early- and late-response genes that were upregulated in response to FN-f were strongly enriched for proinflammatory biological processes including “response to cytokine” and “immune system process”. Genes in these categories include NF-κB subunits, chemokine receptors, interleukins, MAP kinases, TNF ligands, and other proinflammatory cytokines. This is consistent with previous studies that have demonstrated that FN-f treatment stimulates a proinflammatory response via MAP kinases and NF-κB signaling15,16,31,53,75, as well as the established role of inflammation in the progression of OA30,43,51,52,55,59,60,76,77. Genes downregulated in response to FN-f were more weakly enriched for GO terms for development, transcriptional regulation, and cell adhesion, and included HOX genes, TGFBR2, and COL8A2, among others.
Figure 5. Gene ontology terms and KEGG pathways enriched in response to FN-f.
For every cluster of differential genes, five representative GO terms (A) and KEGG pathways (B) are shown, based on enrichment p-value and redundancy with other terms. Heatmap color represents the -log10 of the enrichment p-value for each term or pathway for genes in each cluster (up early, up late, down early, and down late).
To determine pathways that were affected by FN-f treatment, we identified Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways that were enriched in each of the four gene sets (Fig 5B, Table S5). Early-response upregulated genes were enriched for TNF and NF-κB pathways, both of which have been implicated in OA and even targeted for therapeutic OA treatments43,60,78. Intriguingly, late-response upregulated genes were strongly enriched for the ferroptosis pathway, a form of programmed cell death dependent on iron and accumulation of lipid peroxides induced by reactive oxygen species, which have been implicated in OA79. The late-response upregulated genes involved in ferroptosis included ACSL1, ACSL4, ACSL5, GCLM, SLC39A8, SLC7A11, TFRC, HMOX1, and FTH1. Downregulated genes were also weakly enriched for Rap1 and Hippo signaling pathways, as well as ECM-receptor and carcinogenesis genes.
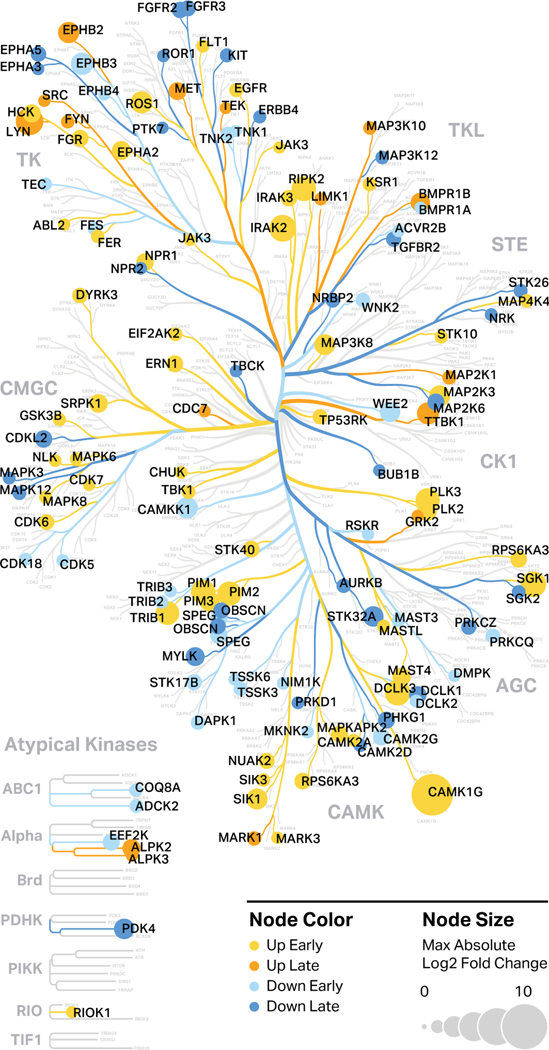
To visualize how specific kinases were regulated in response to FN-f, we generated kinome tree maps using the human kinase visualization tool Coral (Fig 6). Kinase branches and nodes were colored to indicate time course clusters. This analysis identified many kinases with suspected roles in OA progression, including the p38 pathway member MAP2K3, which was upregulated 9.3-fold in response to FN-f53,80. In contrast, we found that TGFBR2 was downregulated 3.4-fold in response to FN-f, which is consistent with recent findings that decreased TGFBR2 was correlated with increased OA severity in mice81. These studies also uncovered a number of kinases that have not been previously implicated in OA or chondrocyte dysfunction. For example, DYRK3, which was upregulated 3.6-fold in response to FN-f, is relatively poorly annotated and has thus been characterized as part of the “dark kinome”. DYRK3 and other understudied kinases identified in this study provide novel targets for further study with regard to their involvement in OA.
Figure 6. Protein kinases transcriptionally regulated by FN-f.
Protein kinases that are differentially expressed in response to FN-f treatment were highlighted in a human kinome map generated by Coral. Color represents the temporal response class of the genes as determined by k-means clustering, while node size represents the maximum absolute L2FC between time-matched FN-f-treated and control samples among the three time points.
Transcriptional drivers of FN-f response include NF-κB and AP-1
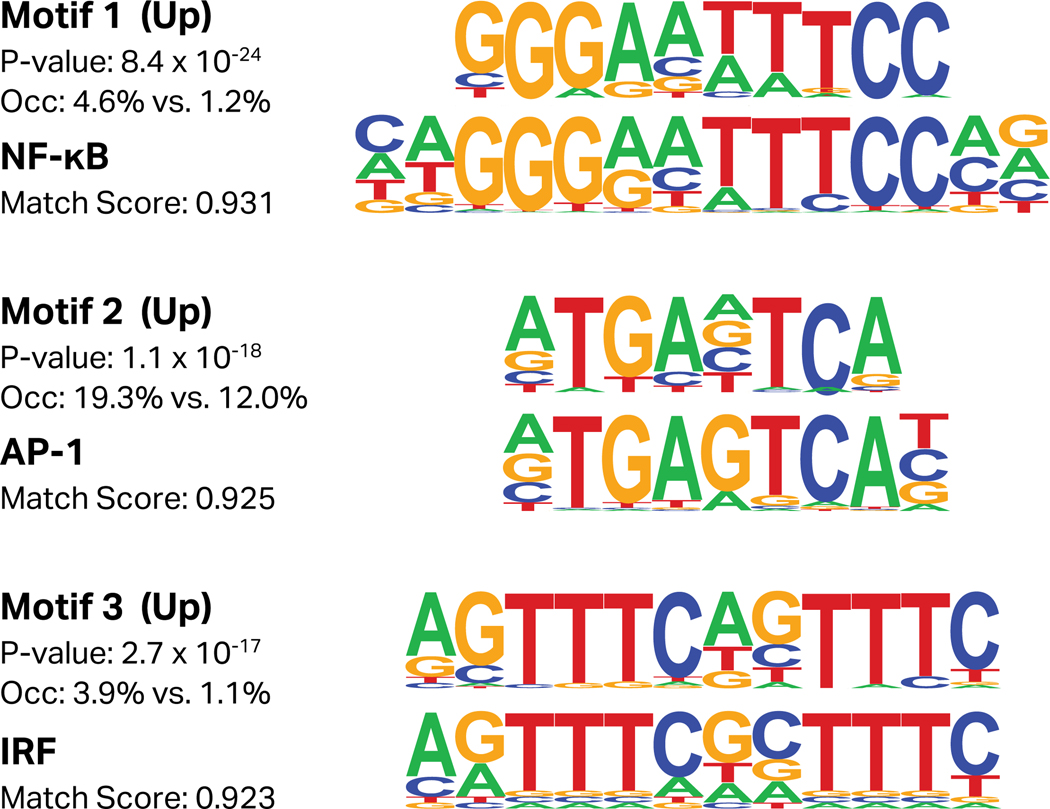
To determine the transcription factors responsible for the global transcriptional changes induced by FN-f treatment, we used the HOMER software suite to identify de novo transcription factor motifs enriched in the promoters of up- or downregulated genes (Fig 7). Upregulated genes exhibited a strong enrichment for NF-κB, Activator Protein 1 (AP-1), and interferon regulatory factor (IRF) binding motifs. In addition to NF-κB, AP-1 and IRF-8 have both been shown to contribute to OA and cartilage matrix degradation30,82. These results are also consistent with our findings that many AP-1 and NF-κB subunit genes are upregulated early in response to FN-f.
Figure 7. Transcription factor motifs enriched in promoters of genes upregulated by FN-f.
Transcription factor motifs were identified de novo from the promoters of genes either upregulated or downregulated by FN-f, as determined by their k-means clustering assignment. Each de novo motif (top) lists the p-value of enrichment, and the percent of promoters occupied by the motif both within the gene set and in the background. The bottom motifs are the best match from the HOCOMOCO database, and are reported along with the match score as determined by HOMER. Only factors with enrichment p-values below 1×10−12 and a match score of 0.90 or higher to a known motif are reported here. The best matches for the top three motifs among upregulated genes are NF-κB (TF65_HUMAN.H11MO.0.A), Activator Protein 1 (AP-1; FOSB_HUMAN.H11MO.0.A), and interferon regulatory factor (IRF; IRF9_HUMAN.H11MO.0.C).
This analysis also revealed the enrichment of several unannotated motifs in the promoters of up- and downregulated genes (Fig S3). The presence of these de novo motifs may suggest that transcription factors with currently uncharacterized motifs also play a role in FN-f response. Additional investigation of the proteomic and phosphoproteomic landscape of chondrocytes responding to FN-f treatment may help to further characterize this model system and identify other key regulators in this response, which could also play a role in OA progression.
DISCUSSION
Developing cell culture systems that model key aspects of diseases can be incredibly valuable for deciphering mechanisms and testing therapeutic interventions, particularly when high-throughput screens are necessary. Using a fragment of the matrix protein fibronectin, we investigated a human cell-culture model of the acute chondrocyte response to cartilage matrix breakdown, a key trigger of OA. Our transcriptome-wide analysis confirmed a similarity between this system and changes observed in OA tissue, suggesting that this is a powerful system with which to study the OA chondrocyte phenotype. We classified over a thousand FN-f-responsive genes by their direction and timing of regulation and confirmed that many genes and pathways upregulated in response to FN-f have previously been characterized as a part of the OA phenotype. This includes inflammatory cytokines and chemokines such as IL1B and CXC ligands, matrix-degrading proteinases such as MMP13, and members of the NF-κB signaling pathway.
The presence of early- and late-response gene clusters in this system is reminiscent of primary and secondary responses observed in other systems, such as the inflammatory response in immune cells83–88 and the growth factor response promoting differentiation and proliferation89. Variation in response times can stem from differences in genomic and regulatory features, including the degree to which products are regulated at transcriptional, post-transcriptional and translational levels, the baseline differences in RNA polymerase II occupancy at transcription start sites, and the dependency of target gene regulation on epigenetic modifications89. Further exploration of these and other regulatory mechanisms in this system may provide a deeper understanding of how chondrocytes are phenotypically altered in OA.
This study also revealed many genes and transcription factors that have not been previously associated with OA. One example is the finding of over-representation of the ferroptosis pathway in the upregulated late-response genes. Ferroptosis is a relatively recently described mechanism of cell death that involves iron and excessive levels of lipid peroxides generated by oxidation of lipids79. Ferroptosis can result from disturbances in the glutathione-dependent antioxidant system, release of excessive reactive oxygen species (ROS) from the mitochondria, and oxidation of lipids by lipoxygenases and cyclooxygenases90. Previous studies have demonstrated that FN-f treatment of chondrocytes generates ROS that regulate signaling involved in MMP expression18. Although ferroptosis per se has not been described in OA cartilage, studies have demonstrated lipid peroxidation91, glutathione oxidation92, mitochondrial dysfunction93, and increased activity of lipoxygenases and cyclooxygenases94, indicating ferroptosis could contribute to chondrocyte death in OA cartilage. This finding may also be relevant to osteoarthritis associated with hemochromatosis where excessive iron is present95.
The intersection between FN-f-responsive genes and OA GWAS loci provides a subset of genes that could be affected by OA-associated genomic variants, offering potential targets for follow-up studies. Identifying eQTLs and mapping the three-dimensional chromatin architecture in chondrocytes would allow us to more accurately and specifically identify target genes of OA risk variants. In addition to the NF-κB family, which has been considered as an OA target for quite some time, FGF18 was a FN-f-responsive gene (up early) present in the GWAS dataset. Unlike NF-κB, FGF18, which is an anabolic growth factor, is in clinical trials for knee OA as an intra-articular agent that may promote cartilage growth96. Additional growth factors present in both datasets were the BMP family members GDF5 (down early) and BMP5 (up early) as well as the BMP signaling protein SMAD6 (down early). Consistent with the FN-f-induced chondrocyte phenotype, allelic variation in the GDF5 gene has been associated with reduced expression97. BMP5 is a regulator of bone and cartilage formation during development, but its role in OA is not clear69. SMAD6 is an inhibitor of SMAD1/5, and its overexpression in mice was associated with a reduction in osteophyte formation, suggesting that decreased SMAD6 expression could be detrimental in OA98,99.
While FN-f treatment of ex vivo chondrocytes represents a powerful tool to understand some of the events that promote OA, it does not recapitulate all aspects of OA, nor does it serve to replace animal models nor analysis of human tissue. Osteoarthritis is a complex disease involving multiple tissues and arises due to both genetic and environmental factors. A more complete mechanistic understanding of OA therefore requires orthogonal approaches with offsetting advantages and limitations. This ex vivo FN-f treatment model does, however, fill a valuable gap and provide a flexible and manipulatable system with which to understand the behavior of chondrocytes in both healthy and disease conditions. By combining this system with recent advances in genomics and genome editing (including the ability to edit primary human chondrocytes100), this FN-f model offers incredible promise for study of OA.
Supplementary Material
Table S5. KEGG pathways enriched among FN-f differential response genes
This table includes the full KEGG pathway enrichment results from HOMER. The results from each response class (as determined by k-means clustering) are on a separate tab.
Table S3. Osteoarthritis genes in response to FN-f treatment
This table includes all OA-responsive genes from the RAAK study with a p-value less than 0.01 and an absolute L2FC greater than 0.585 (n=87), and details how they change in response to FN-f treatment. For each gene, we report: the p-value (LRT); the FN-f response class (as determined by k-means clustering of all differential genes); the L2FC between control and FN-f treated samples at each time point; the difference between FN-f treated and control z-score normalized counts at each time point; the raw counts from each sample; and the original p-value and L2FC from the RAAK study24.
Table S4. Gene ontology terms enriched among FN-f differential response genes
This table includes the full gene ontology (GO) term enrichment results from HOMER, for terms within the “biological process” category. The results from each response class (as determined by k-means clustering) are on a separate tab.
Table S2. Differential genes in response 823 to FN-f treatment
This table lists all differential genes (n=3,914) that change in response to FN-f treatment. For each gene, we report: the p-value (LRT); the FN-f response class (as determined by k-means clustering of all differential genes); the L2FC between control and FN-f treated samples at each time point; the difference between FN-f treated and control z-score normalized counts for each time point; and the raw counts for each sample.
Table S1. Differential genes in response to time in culture
This table lists all genes (n=748) that changed significantly due to time in culture. For each gene, we report: the p-value (LRT); the FN-f response class (as determined by k-means clustering of all differential genes); the L2FC between control samples at different time points; L2FC between control and FN-f treated at each time point; the difference between FN-f treated and control z-score normalized counts for each time point; and the raw counts for each sample.
Figure S3. Unannotated de novo motifs identified in differential gene promoters.
When the promoters of differential genes among up- and downregulated genes were analyzed for enriched transcription factor motifs, several had no convincing match to known motifs (motif match score < 0.9). These unannotated de novo motifs represent potential binding sites of transcription factors that have yet to be characterized. For each motif, the motif logo, p-value of enrichment, and cluster in which it was enriched are listed.
Figure S2. Gene length distributions among genes up- and downregulated by FN-f.
Boxplots depicting the lengths of early-response genes (both up and down, n=2,161) and late response genes (both up and down, n=1,753) reveal that late-response genes are statistically significantly longer (Mann-Whitney U test, p-value <0.01). Outliers are excluded from this plot.
Figure S1. Experimental design of fibronectin fragment 848 (FN-f) treatment
Primary articular chondrocytes were isolated from normal human femoral cartilage obtained from three tissue donors aged 50-61 years, each without osteoarthritis. Six samples from each donor were cultured and treated with either 1 ¼M fibronectin fragments (FN-f) or PBS. After 3, 6, or 18 hours, RNA was extracted and used to create RNA-seq libraries. This design allowed for the comparison of gene expression in FN-f-treated samples compared to time-matched untreated controls, accounting for changes occurring as a result of isolation, time in culture, and donor genotype.
ACKNOWLEDGEMENTS
We would like to thank the Gift of Hope Organ and Tissue Donor Network, and the donor families, for providing normal donor tissue. We would like to thank Dr. Arkady Margulis for donor tissue procurement and Mrs. Arnavaz Hakimiyan for technical assistance. We would also like to thank Erika Deoudes for her figure and preprint design.
ROLE OF THE FUNDING SOURCE
This project was supported by grants from the National Institute of Arthritis, Musculoskeletal, and Skin Disease (R37-AR049003), the National Institute on Aging (RO1-AG044034), the National Human Genome Research Institute (R00-HG008662) and the National Institute of General Medical Sciences (R35-GM128645 and T32-GM007092). This project was also supported in part by the Klaus Kuettner Chair for Osteoarthritis Research (SC).
Footnotes
CONFLICT OF INTEREST
The authors certify that they do not have any affiliations with or involvement in any organization or entity with financial or non-financial interest in the subject matter and materials discussed in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 2.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. doi: 10.1002/art.34453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bapat S, Hubbard D, Munjal A, Hunter M, Fulzele S. Pros and cons of mouse models for studying osteoarthritis. Clin Transl Med. 2018;7(1):36. doi: 10.1186/s40169-018-0215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson CI, Argyle DJ, Clements DN. In vitro models for the study of osteoarthritis. Vet J. 2016;209:40–49. doi: 10.1016/j.tvjl.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 5.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14(1):R7. doi: 10.1186/ar3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent TL. IL-1 in osteoarthritis: time for a critical review of the literature. F1000. 2019;8:934. doi:10.12688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleischmann RM, Bliddal H, Blanco FJ, Schnitzer TJ, Peterfy C, Chen S, et al. A phase II trial of lutikizumab, an anti-interleukin-1α/β dual variable domain immunoglobulin, in knee osteoarthritis patients with synovitis. Arthritis Rheumatol. 2019;71(7):1056–1069. doi: 10.1002/art.40840 [DOI] [PubMed] [Google Scholar]
- 8.Kloppenburg M, Ramonda R, Bobacz K, Kwok W-Y, Elewaut D, Huizinga TWJ, et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): a multicentre, randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2018;77(12):1757–1764. doi: 10.1136/annrheumdis-2018-213202 [DOI] [PubMed] [Google Scholar]
- 9.Homandberg GA. Potential regulation of cartilage metabolism in osteoarthritis by fibronectin fragments. Front Biosci. 1999;4:D713-D730. doi: 10.2741/homandberg [DOI] [PubMed] [Google Scholar]
- 10.Zack MD, Arner EC, Anglin CP, Alston JT, Malfait A-M, Tortorella MD. Identification of fibronectin neoepitopes present in human osteoarthritic cartilage. Arthritis Rheum. 2006;54(9):2912–2922. doi: 10.1002/art.22045 [DOI] [PubMed] [Google Scholar]
- 11.Carnemolla B, Cutolo M, Castellani P, Balza E, Raffanti S, Zardi L. Characterization of synovial fluid fibronectin from patients with rheumatic inflammatory diseases and healthy subjects. Arthritis Rheum. 1984;27(8):913–921. doi: 10.1002/art.1780270811 [DOI] [PubMed] [Google Scholar]
- 12.Xie DL, Meyers R, Homandberg GA. Fibronectin fragments in osteoarthritic synovial fluid. J Rheumatol. 1992;19(9):1448–1452. https://www.ncbi.nlm.nih.gov/pubmed/1433014 [PubMed] [Google Scholar]
- 13.Homandberg GA, Wen C, Hui F. Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthritis Cartilage. 1998;6(4):231–244. doi: 10.1053/joca.1998.0116 [DOI] [PubMed] [Google Scholar]
- 14.Homandberg GA, Meyers R, Williams JM. Intraarticular injection of fibronectin fragments causes severe depletion of cartilage proteoglycans in vivo. J Rheumatol. 1993;20(8):1378–1382. https://www.ncbi.nlm.nih.gov/pubmed/8230023 [PubMed] [Google Scholar]
- 15.Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46(9):2368–2376. doi: 10.1002/art.10502 [DOI] [PubMed] [Google Scholar]
- 16.Pulai JI, Chen H, Im H-J, Kumar S, Hanning C, Hegde PS, et al. NF-kappa B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J Immunol. 2005;174(9):5781–5788. doi: 10.4049/jimmunol.174.9.5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48(8):2188–2196. doi: 10.1002/art.11209 [DOI] [PubMed] [Google Scholar]
- 18.Wood ST, Long DL, Reisz JA, Yammani RR, Burke EA, Klomsiri C, et al. Cysteine-mediated redox regulation of cell signaling in chondrocytes stimulated with fibronectin fragments. Arthritis Rheumatol. 2016;68(1):117–126. doi: 10.1002/art.39326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17(1):10–12. https://journal.embnet.org/index.php/embnetjournal/article/view/200/479 [Google Scholar]
- 20.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14(4):417–419. doi: 10.1038/nmeth.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soneson C, Love MI, Robinson MD. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 2015;4:1521. doi: 10.12688/f1000research.7563.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu A, Ibrahim JG, Love MI. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. Bioinformatics. 2019;35(12):2084–2092. doi: 10.1093/bioinformatics/bty895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos YFM, den Hollander W, Bovée JVMG, Bomer N, van der Breggen R, Lakenberg N, et al. Genes involved in the osteoarthritis process identified through genome wide expression analysis in articular cartilage; the RAAK study. PLoS One. 2014;9(7):e103056. doi: 10.1371/journal.pone.0103056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–589. doi: 10.1016/j.molcel.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metz KS, Deoudes EM, Berginski ME, Jimenez-Ruiz I, Aksoy BA, Hammerbacher J, et al. Coral: clear and customizable visualization of human kinome data. Cell Syst. 2018;7(3):347–350.e1. doi: 10.1016/j.cels.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–1934. doi: 10.1126/science.1075762 [DOI] [PubMed] [Google Scholar]
- 29.Daheshia M, Yao JQ. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35(12):2306–2312. doi: 10.3899/jrheum.080346 [DOI] [PubMed] [Google Scholar]
- 30.Ji Q, Xu X, Zhang Q, Kang L, Xu Y, Zhang K, et al. The IL-1β/AP-1/miR-30a/ADAMTS-5 axis regulates cartilage matrix degradation in human osteoarthritis. J Mol Med. 2016;94(7):771–785. doi: 10.1007/s00109-016-1418-z [DOI] [PubMed] [Google Scholar]
- 31.Arner EC, Tortorella MD. Signal transduction through chondrocyte integrin receptors induces matrix metalloproteinase synthesis and synergizes with interleukin-1. Arthritis Rheum. 1995;38(9):1304–1314. doi: 10.1002/art.1780380919 [DOI] [PubMed] [Google Scholar]
- 32.Fernandes JC, Martel-Pelletier J, Pelletier J-P. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39(1–2):237–246. https://www.ncbi.nlm.nih.gov/pubmed/12082286 [PubMed] [Google Scholar]
- 33.Li H, Wang D, Yuan Y, Min J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Res Ther. 2017;19(1):248. doi: 10.1186/s13075-017-1454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji B, Ma Y, Wang H, Fang X, Shi P. Activation of the P38/CREB/MMP13 axis is associated with osteoarthritis. Drug Des Devel Ther. 2019;13:2195–2204. doi: 10.2147/DDDT.S209626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leijten JCH, Bos SD, Landman EBM, Georgi N, Jahr H, Meulenbelt I, et al. GREM1, FRZB and DKK1 mRNA levels correlate with osteoarthritis and are regulated by osteoarthritis-associated factors. Arthritis Res Ther. 2013;15(5):R126. doi: 10.1186/ar4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tachmazidou I, Hatzikotoulas K, Southam L, Esparza-Gordillo J, Haberland V, Zheng J, et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet. 2019;51(2):230–236. doi: 10.1038/s41588-018-0327-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill RE, Lettice LA. Alterations to the remote control of Shh gene expression cause congenital abnormalities. Philos Trans R Soc Lond B Biol Sci. 2013;368(1620):20120357. doi: 10.1098/rstb.2012.0357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kragesteen BK, Spielmann M, Paliou C, Heinrich V, Schöpflin R, Esposito A, et al. Dynamic 3D chromatin architecture contributes to enhancer specificity and limb morphogenesis. Nat Genet. 2018;50(10):1463–1473. doi: 10.1038/s41588-018-0221-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palstra R-J, Tolhuis B, Splinter E, Nijmeijer R, Grosveld F, de Laat W. The beta-globin nuclear compartment in development and erythroid differentiation. Nat Genet. 2003;35(2):190–194. doi: 10.1038/ng1244 [DOI] [PubMed] [Google Scholar]
- 40.Stadhouders R, Thongjuea S, Andrieu-Soler C, Palstra R-J, Bryne JC, van den Heuvel A, et al. Dynamic long-range chromatin interactions control Myb proto-oncogene transcription during erythroid development. EMBO J. 2012;31(4):986–999. doi: 10.1038/emboj.2011.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489(7414):109–113. doi: 10.1038/nature11279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoenfelder S, Fraser P. Long-range enhancer-promoter contacts in gene expression control. Nat Rev Genet. 2019;20(8):437–455. doi: 10.1038/s41576-019-0128-0 [DOI] [PubMed] [Google Scholar]
- 43.Choi M-C, Jo J, Park J, Kang HK, Park Y. NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells. 2019;8(7). doi: 10.3390/cells8070734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haag J, Gebhard PM, Aigner T. SOX gene expression in human osteoarthritic cartilage. Pathobiology. 2008;75(3):195–199. doi: 10.1159/000124980 [DOI] [PubMed] [Google Scholar]
- 45.Chou C-H, Lee MTM, Song I-W, Lu L-S, Shen H-C, Lee C-H, et al. Insights into osteoarthritis progression revealed by analyses of both knee tibiofemoral compartments. Osteoarthritis Cartilage. 2015;23(4):571–580. doi: 10.1016/j.joca.2014.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.arcOGEN Consortium, arcOGEN Collaborators, Zeggini E, Panoutsopoulou K, Southam L, Rayner NW, et al. Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. Lancet. 2012;380(9844):815–823. doi: 10.1016/S0140-6736(12)60681-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatakeyama Y, Tuan RS, Shum L. Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis. J Cell Biochem. 2004;91(6):1204–1217. doi: 10.1002/jcb.20019 [DOI] [PubMed] [Google Scholar]
- 48.Yao X, Zhang J, Jing X, Ye Y, Guo J, Sun K, et al. Fibroblast growth factor 18 exerts anti-osteoarthritic effects through PI3K-AKT signaling and mitochondrial fusion and fission. Pharmacol Res. 2019;139:314–324. doi: 10.1016/j.phrs.2018.09.026 [DOI] [PubMed] [Google Scholar]
- 49.Caron MMJ, Emans PJ, Surtel DAM, van der Kraan PM, van Rhijn LW, Welting TJM. BAPX-1/NKX-3.2 acts as a chondrocyte hypertrophy molecular switch in osteoarthritis. Arthritis & Rheumatology. 2015;67(11):2944–2956. doi: 10.1002/art.39293 [DOI] [PubMed] [Google Scholar]
- 50.Motomura H, Seki S, Shiozawa S, Aikawa Y, Nogami M, Kimura T. A selective c-Fos/AP-1 inhibitor prevents cartilage destruction and subsequent osteophyte formation. Biochem Biophys Res Commun. 2018;497(2):756–761. doi: 10.1016/j.bbrc.2018.02.147 [DOI] [PubMed] [Google Scholar]
- 51.Wang T, He C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi: 10.1016/j.cytogfr.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 52.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun H-Y, Hu K-Z, Yin Z-S. Inhibition of the p38-MAPK signaling pathway suppresses the apoptosis and expression of proinflammatory cytokines in human osteoarthritis chondrocytes. Cytokine. 2017;90:135–143. doi: 10.1016/j.cyto.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 54.Pelletier JP, McCollum R, Cloutier JM, Martel-Pelletier J. Synthesis of metalloproteases and interleukin 6 (IL-6) in human osteoarthritic synovial membrane is an IL-1 mediated process. J Rheumatol Suppl. 1995;43:109–114. https://www.ncbi.nlm.nih.gov/pubmed/7752112 [PubMed] [Google Scholar]
- 55.Kapoor Mohit, Johanne Martel-Pelletier, Lajeunesse Daniel, Pelletier Jean-Pierre, Fahmi Hassan. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nature Reviews Rheumatology. Published November 30, 2010. Accessed April 13, 2020 https://www.nature.com/articles/nrrheum.2010.196 [DOI] [PubMed] [Google Scholar]
- 56.Hwang HS, Lee MH, Choi MH, Kim HA. NOD2 signaling pathway is involved in fibronectin fragment-induced pro-catabolic factor expressions in human articular chondrocytes. BMB Rep. 2019;52(6):373–378. https://www.ncbi.nlm.nih.gov/pubmed/30760380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chou C-H, Lee C-H, Lu L-S, Song I-W, Chuang H-P, Kuo S-Y, et al. Direct assessment of articular cartilage and underlying subchondral bone reveals a progressive gene expression change in human osteoarthritic knees. Osteoarthritis Cartilage. 2013;21(3):450–461. doi: 10.1016/j.joca.2012.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verma P, Dalal K. ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis. J Cell Biochem. 2011;112(12):3507–3514. doi: 10.1002/jcb.23298 [DOI] [PubMed] [Google Scholar]
- 59.Kim HA, Cho M-L, Choi HY, Yoon CS, Jhun JY, Oh HJ, et al. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006;54(7):2152–2163. doi: 10.1002/art.21951 [DOI] [PubMed] [Google Scholar]
- 60.van den Bosch MHJ, van Lent PLEM, van der Kraan PM3. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthritis Cartilage. 2020;28(5):532–543. doi: 10.1016/j.joca.2020.01.016 [DOI] [PubMed] [Google Scholar]
- 61.Lu X, Gilbert L, He X, Rubin J, Nanes MS. Transcriptional regulation of the osterix (Osx, Sp7) promoter by tumor necrosis factor identifies disparate effects of mitogen-activated protein kinase and NFκB pathways. J Biol Chem. 2006;281(10):6297–6306. doi: 10.1074/jbc.M507804200 [DOI] [PubMed] [Google Scholar]
- 62.Ferrari D, Kosher RA. Dlx5 is a positive regulator of chondrocyte differentiation during endochondral ossification. Dev Biol. 2002;252(2):257–270. doi: 10.1006/dbio.2002.0862 [DOI] [PubMed] [Google Scholar]
- 63.Lefebvre V, Bhattaram P. SoxC transcription factors in skeletogenesis and cartilage differentiation. Osteoarthritis Cartilage. 2015;23:A23. doi: 10.1016/j.joca.2015.02.055 [DOI] [Google Scholar]
- 64.Murphy G, Knäuper V, Atkinson S, Butler G, English W, Hutton M, et al. Matrix metalloproteinases in arthritic disease. Arthritis Res. 2002;4 Suppl 3:S39–S49. doi: 10.1186/ar572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qin H-J, Xu T, Wu H-T, Yao Z-L, Hou Y-L, Xie Y-H, et al. SDF-1/CXCR4 axis coordinates crosstalk between subchondral bone and articular cartilage in osteoarthritis pathogenesis. Bone. 2019;125:140–150. doi: 10.1016/j.bone.2019.05.010 [DOI] [PubMed] [Google Scholar]
- 66.Huang G, Chubinskaya S, Liao W, Loeser RF. Wnt5a induces catabolic signaling and matrix metalloproteinase production in human articular chondrocytes. Osteoarthritis Cartilage. 2017;25(9):1505–1515. doi: 10.1016/j.joca.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, Xiao W, Sun M, Deng Z, Zeng C, Li H, et al. The expression of osteopontin and Wnt5a in articular cartilage of patients with knee osteoarthritis and its correlation with disease severity. Biomed Res Int. 2016;2016:9561058. doi: 10.1155/2016/9561058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lambert C, Dubuc J-E, Montell E, Vergés J, Munaut C, Noël A, et al. Gene expression pattern of cells from inflamed and normal areas of osteoarthritis synovial membrane. Arthritis Rheumatol. 2014;66(4):960–968. doi: 10.1002/art.38315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, et al. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1(1):87–105. doi: 10.1016/j.gendis.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van der Kraan PM, Blaney Davidson EN, van den Berg WB. Bone morphogenetic proteins and articular cartilage: To serve and protect or a wolf in sheep clothing’s? Osteoarthritis Cartilage. 2010;18(6):735–741. doi: 10.1016/j.joca.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 71.Karlsson C, Dehne T, Lindahl A, Brittberg M, Pruss A, Sittinger M, et al. Genome-wide expression profiling reveals new candidate genes associated with osteoarthritis. Osteoarthritis Cartilage. 2010;18(4):581–592. doi: 10.1016/j.joca.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 72.Zeltz C, Gullberg D. The integrin-collagen connection--a glue for tissue repair? J Cell Sci. 2016;129(4):653–664. doi: 10.1242/jcs.180992 [DOI] [PubMed] [Google Scholar]
- 73.Korostynski M, Malek N, Piechota M, Starowicz K. Cell-type-specific gene expression patterns in the knee cartilage in an osteoarthritis rat model. Funct Integr Genomics. 2018;18(1):79–87. doi: 10.1007/s10142-017-0576-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, Weiss T, et al. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54(11):3533–3544. doi: 10.1002/art.22174 [DOI] [PubMed] [Google Scholar]
- 75.Homandberg GA, Hui F, Wen C, Purple C, Bewsey K, Koepp H, et al. Fibronect-infragment-induced cartilage chondrolysis is associated with release of catabolic cytokines. Biochem J. 1997;321 ( Pt 3):751–757. doi: 10.1042/bj3210751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19(1):18. doi: 10.1186/s13075-017-1229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Lange-Brokaar BJE, Ioan-Facsinay A, van Osch GJVM, Zuurmond A-M, Schoones J, Toes REM, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20(12):1484–1499. doi: 10.1016/j.joca.2012.08.027 [DOI] [PubMed] [Google Scholar]
- 78.Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14(9):839–848. doi: 10.1016/j.joca.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 79.Li J, Cao F, Yin H-L, Huang Z-J, Lin Z-T, Mao N, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. doi: 10.1038/s41419-020-2298-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Collins JA, Arbeeva L, Chubinskaya S, Loeser RF. Articular chondrocytes isolated from the knee and ankle joints of human tissue donors demonstrate similar redox-regulated MAP kinase and Akt signaling. Osteoarthritis Cartilage. 2019;27(4):703–711. doi: 10.1016/j.joca.2018.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li T, Chubinskaya S, Esposito A, Jin X, Tagliafierro L, Loeser R, et al. TGF-β type 2 receptor-mediated modulation of the IL-36 family can be therapeutically targeted in osteoarthritis. Sci Transl Med. 2019;11(491). doi: 10.1126/scitranslmed.aan2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang Q, Ding W, Cao Y, Zhou Y, Ni S, Shi T, et al. Interferonregulatoryfactor-8(IRF-8) regulates the expression of matrix metalloproteinase-13 (MMP-13) in chondrocytes. Cell Stress Chaperones. 2018;23(3):393–398. doi: 10.1007/s12192-017-0849-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sandoval J, Pereda J, Pérez S, Finamor I, Vallet-Sánchez A, Rodríguez JL, et al. Epigenetic regulation of early- and late-response genes in acute pancreatitis. J Immunol. 2016;197(10):4137–4150. doi: 10.4049/jimmunol.1502378 [DOI] [PubMed] [Google Scholar]
- 84.Nilsson R, Bajic VB, Suzuki H, di Bernardo D, Björkegren J, Katayama S, et al. Transcriptional network dynamics in macrophage activation. Genomics. 2006;88(2):133–142. doi: 10.1016/j.ygeno.2006.03.022 [DOI] [PubMed] [Google Scholar]
- 85.Tsukahara Y, Lian Z, Zhang X, Whitney C, Kluger Y, Tuck D, et al. Gene expression in human neutrophils during activation and priming by bacterial lipopolysaccharide. J Cell Biochem. 2003;89(4):848–861. doi: 10.1002/jcb.10526 [DOI] [PubMed] [Google Scholar]
- 86.Smale ST. Selective transcription in response to an inflammatory stimulus. Cell. 2010;140(6):833–844. doi: 10.1016/j.cell.2010.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208(3):417–420. doi: 10.1084/jem.20110367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Serrat N, Sebastian C, Pereira-Lopes S, Valverde-Estrella L, Lloberas J, Celada A. The response of secondary genes to lipopolysaccharides in macrophages depends on histone deacetylase and phosphorylation of C/EBPβ. J Immunol. 2014;192(1):418–426. doi: 10.4049/jimmunol.1203500 [DOI] [PubMed] [Google Scholar]
- 89.Tullai JW, Schaffer ME, Mullenbrock S, Sholder G, Kasif S, Cooper GM. Immediate-early and delayed primary response genes are distinct in function and genomic architecture. J Biol Chem. 2007;282(33):23981–23995. doi: 10.1074/jbc.M702044200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Donnell VB, Aldrovandi M, Murphy RC, Krönke G. Enzymatically oxidized phospholipids assume center stage as essential regulators of innate immunity and cell death. Sci Signal. 2019;12(574). doi: 10.1126/scisignal.aau2293 [DOI] [PubMed] [Google Scholar]
- 91.Shah R, Raska K Jr, Tiku ML. The presence of molecular markers of in vivo lipid peroxidation in osteoarthritic cartilage: a pathogenic role in osteoarthritis. Arthritis Rheum. 2005;52(9):2799–2807. doi: 10.1002/art.21239 [DOI] [PubMed] [Google Scholar]
- 92.Carlo MD Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48(12):3419–3430. doi: 10.1002/art.11338 [DOI] [PubMed] [Google Scholar]
- 93.Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7(3):161–169. doi: 10.1038/nrrheum.2010.213 [DOI] [PubMed] [Google Scholar]
- 94.Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier J-P. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2003;62(6):501–509. doi: 10.1136/ard.62.6.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carroll GJ, Breidahl WH, Bulsara MK, Olynyk JK. Hereditary hemochromatosis is characterized by a clinically definable arthropathy that correlates with iron load. Arthritis Rheum. 2011;63(1):286–294. doi: 10.1002/art.30094 [DOI] [PubMed] [Google Scholar]
- 96.Hochberg MC, Guermazi A, Guehring H, Aydemir A, Wax S, Fleuranceau-Morel P, et al. Effect of intra-articular sprifermin vs placebo on femorotibial joint cartilage thickness in patients with osteoarthritis: The FORWARD randomized clinical trial. JAMA. 2019;322(14):1360–1370. doi: 10.1001/jama.2019.14735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Southam L, Rodriguez-Lopez J, Wilkins JM, Pombo-Suarez M, Snelling S, Gomez-Reino JJ, et al. An SNP in the 5’-UTR of GDF5 is associated with osteoarthritis susceptibility in Europeans and with in vivo differences in allelic expression in articular cartilage. Hum Mol Genet. 2007;16(18):2226–2232. doi: 10.1093/hmg/ddm174 [DOI] [PubMed] [Google Scholar]
- 98.Ishida W, Hamamoto T, Kusanagi K, Yagi K, Kawabata M, Takehara K, et al. Smad6 is a Smad1/5-induced smad inhibitor. Characterization of bone morphogenetic protein-responsive element in the mouse Smad6 promoter. J Biol Chem. 2000;275(9):6075–6079. doi: 10.1074/jbc.275.9.6075 [DOI] [PubMed] [Google Scholar]
- 99.Scharstuhl A, Vitters EL, van der Kraan PM, van den Berg WB. Reduction of osteophyte formation and synovial thickening by adenoviral overexpression of transforming growth factor beta/bone morphogenetic protein inhibitors during experimental osteoarthritis. Arthritis Rheum. 2003;48(12):3442–3451. doi: 10.1002/art.11328 [DOI] [PubMed] [Google Scholar]
- 100.D’Costa S, Rich MJ, Diekman BO. Engineered Cartilage from Human Chondrocytes with Homozygous Knockout of Cell Cycle Inhibitor p21. Tissue Eng Part A. 2020;26(7–8):441–449. doi: 10.1089/ten.TEA.2019.0214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S5. KEGG pathways enriched among FN-f differential response genes
This table includes the full KEGG pathway enrichment results from HOMER. The results from each response class (as determined by k-means clustering) are on a separate tab.
Table S3. Osteoarthritis genes in response to FN-f treatment
This table includes all OA-responsive genes from the RAAK study with a p-value less than 0.01 and an absolute L2FC greater than 0.585 (n=87), and details how they change in response to FN-f treatment. For each gene, we report: the p-value (LRT); the FN-f response class (as determined by k-means clustering of all differential genes); the L2FC between control and FN-f treated samples at each time point; the difference between FN-f treated and control z-score normalized counts at each time point; the raw counts from each sample; and the original p-value and L2FC from the RAAK study24.
Table S4. Gene ontology terms enriched among FN-f differential response genes
This table includes the full gene ontology (GO) term enrichment results from HOMER, for terms within the “biological process” category. The results from each response class (as determined by k-means clustering) are on a separate tab.
Table S2. Differential genes in response 823 to FN-f treatment
This table lists all differential genes (n=3,914) that change in response to FN-f treatment. For each gene, we report: the p-value (LRT); the FN-f response class (as determined by k-means clustering of all differential genes); the L2FC between control and FN-f treated samples at each time point; the difference between FN-f treated and control z-score normalized counts for each time point; and the raw counts for each sample.
Table S1. Differential genes in response to time in culture
This table lists all genes (n=748) that changed significantly due to time in culture. For each gene, we report: the p-value (LRT); the FN-f response class (as determined by k-means clustering of all differential genes); the L2FC between control samples at different time points; L2FC between control and FN-f treated at each time point; the difference between FN-f treated and control z-score normalized counts for each time point; and the raw counts for each sample.
Figure S3. Unannotated de novo motifs identified in differential gene promoters.
When the promoters of differential genes among up- and downregulated genes were analyzed for enriched transcription factor motifs, several had no convincing match to known motifs (motif match score < 0.9). These unannotated de novo motifs represent potential binding sites of transcription factors that have yet to be characterized. For each motif, the motif logo, p-value of enrichment, and cluster in which it was enriched are listed.
Figure S2. Gene length distributions among genes up- and downregulated by FN-f.
Boxplots depicting the lengths of early-response genes (both up and down, n=2,161) and late response genes (both up and down, n=1,753) reveal that late-response genes are statistically significantly longer (Mann-Whitney U test, p-value <0.01). Outliers are excluded from this plot.
Figure S1. Experimental design of fibronectin fragment 848 (FN-f) treatment
Primary articular chondrocytes were isolated from normal human femoral cartilage obtained from three tissue donors aged 50-61 years, each without osteoarthritis. Six samples from each donor were cultured and treated with either 1 ¼M fibronectin fragments (FN-f) or PBS. After 3, 6, or 18 hours, RNA was extracted and used to create RNA-seq libraries. This design allowed for the comparison of gene expression in FN-f-treated samples compared to time-matched untreated controls, accounting for changes occurring as a result of isolation, time in culture, and donor genotype.
Data Availability Statement
Data is made publicly available at GEO accession GSE150411, including; raw sequencing data; transcript-level quantification output from Salmon; and a table containing gene-level summaries of read counts in each sample, cluster assignments and FDR-adjusted p-values (LRT) for each gene, as well as L2FC and the difference between FN-f treated and control normalized count z-score at each time point.